Exploring the Pharmacokinetics of Drugs in Disabled Saudi Patients: A Systematic Review
Abstract
1. Introduction
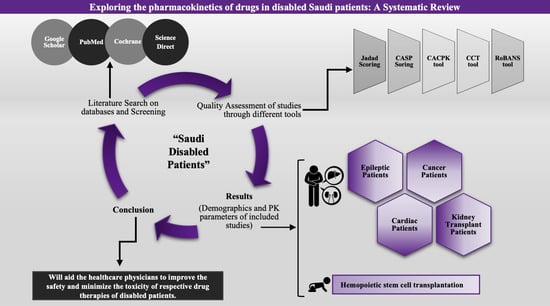
2. Materials and Methods
2.1. Study Protocol and Screening Strategy for Literature Review
2.2. Eligibility Criteria
2.3. Data Extraction:
2.4. Evaluation of the Quality of the Included Articles
2.5. Risk of Bias Assessment
3. Results
3.1. Outcomes of the Literature Search
3.2. Characteristics of Included Research Articles
3.3. Evaluation of Quality and Risk of Bias Findings
3.4. PK of Drugs in Adult Disabled Patients
3.5. PKs of Drugs in Pediatric Disabled Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Classification of Functioning, Disability and Health (ICF). Available online: https://www.who.int/classifications/international-classification-of-functioning-disability-and-health (accessed on 8 December 2024).
- Definition of Disability. Available online: https://www.dundee.ac.uk/corporate-information/definition-disability (accessed on 8 November 2024).
- Hedaya, M.A. Basic Pharmacokinetics; Routledge: London, UK, 2023. [Google Scholar]
- Kuper, H.; Rotenberg, S.; Azizatunnisa’, L.; Banks, L.M.; Smythe, T. The association between disability and mortality: A mixed-methods study. Lancet Public Health 2024, 9, e306–e315. [Google Scholar] [CrossRef] [PubMed]
- Awofisayo, S.O.; Akpabio, A.; Olorunsola, E.O.; Arhewoh, M.I. Lifestyle Biopharmaceutics and Mechanistic Basis of Drug Clinical Outcomes: A Review. J. Adv. Pharm. Res. 2024, 8, 63–76. [Google Scholar] [CrossRef]
- Saudi Arabia. Available online: https://webapps.ilo.org/ilostat-files/SSM/SSM11/SAU.pdf (accessed on 18 December 2024).
- Alali, M.; Ismail Al-khalil, W.; Rijjal, S.; Al-Salhi, L.; Saifo, M.; Youssef, L.A. Frequencies of CYP2D6 genetic polymorphisms in Arab populations. Hum. Genom. 2022, 16, 6. [Google Scholar] [CrossRef]
- Mirghani, R.A.; Chowdhary, G.; Elghazali, G. Distribution of the major cytochrome P450 (CYP) 2C9 genetic variants in a Saudi population. Basic Clin. Pharmacol. Toxicol. 2011, 109, 111–114. [Google Scholar] [CrossRef]
- Binmahfouz, L.S.; Bagher, A.M. Genetic polymorphism of the drug-metabolizing enzyme Cytochrome P4502E1 (CYP2E1) in a healthy Saudi population. Saudi Pharm. J. 2021, 29, 1355–1360. [Google Scholar] [CrossRef]
- Tayeb, M.T.; Clark, C.; Ameyaw, M.M.; Haites, N.E.; Evans, D.A.; Tariq, M.; Mobarek, A.; Ofori-Adjei, D.; McLeod, H.L. CYP3A4 promoter variant in Saudi, Ghanaian and Scottish Caucasian populations. Pharmacogenetics 2000, 10, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, L.M.; Marok, F.Z.; Hanke, N.; Selzer, D.; Lehr, T. Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug-Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics 2021, 13, 270. [Google Scholar] [CrossRef]
- Wen, X.; Wang, J.S.; Kivistö, K.T.; Neuvonen, P.J.; Backman, J.T. In vitro evaluation of valproic acid as an inhibitor of human cytochrome P450 isoforms: Preferential inhibition of cytochrome P450 2C9 (CYP2C9). Br. J. Clin. Pharmacol. 2001, 52, 547–553. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269, w64. [Google Scholar] [CrossRef]
- Rubin, K.; Sullivan, D.; Sadhasivam, S. Are peripheral and neuraxial blocks with ultrasound guidance more effective and safe in children? Pediatr. Anesth. 2009, 19, 92–96. [Google Scholar] [CrossRef]
- Long, H.A.; French, D.P.; Brooks, J.M. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res. Methods Med. Health Sci. 2020, 1, 31–42. [Google Scholar] [CrossRef]
- Soliman, A.B.E.; Pawluk, S.A.; Wilby, K.J.; Rachid, O. The use of a modified Delphi technique to develop a critical appraisal tool for clinical pharmacokinetic studies. Int. J. Clin. Pharm. 2022, 44, 894–903. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Kim, S.Y.; Lee, Y.J.; Park, J.E. RoBANS 2: A Revised Risk of Bias Assessment Tool for Nonrandomized Studies of Interventions. Korean J. Fam. Med. 2023, 44, 249–260. [Google Scholar] [CrossRef]
- Alsultan, A.; Albassam, A.A.; Alturki, A.; Alsultan, A.; Essa, M.; Almuzzaini, B.; Alfadhel, S. Population pharmacokinetics of busulfan in Saudi pediatric patients undergoing hematopoietic stem cell transplantation. Int. J. Clin. Pharm. 2020, 42, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alandas, N.; Alsultan, A. Estimation of apparent clearance of valproic acid in adult Saudi patients. Int. J. Clin. Pharm. 2019, 41, 1056–1061. [Google Scholar] [CrossRef]
- Islam, S.; Al Aidarous, R.; Jan, M.; Dehlawi, F. Population pharmacokinetics of Carbamazepine and optimising its use in Saudi epileptic children. Int. Res. J. Med. Med. Sci. 2013, 1, 85–93. [Google Scholar]
- El-Yazigi, A.; el-Baage, T.; al-Humaidan, A.; Yusuf, A. Steady state pharmacokinetics of propranolol in Saudi Arabian patients and comparison with data for different populations. J. Clin. Pharmacol. 1990, 30, 144–150. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Alsultan, A.S.; Alqattan, H.M.; Eldemerdash, A.; Albacker, T.B. Population Pharmacokinetic Model for Vancomycin Used in Open Heart Surgery: Model-Based Evaluation of Standard Dosing Regimens. Antimicrob. Agents Chemother. 2018, 62, e00088-18. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Alsultan, A.S.; Alqattan, H.M.; Eldemerdash, A.; Albacker, T.B. Population Pharmacokinetic Model-Based Evaluation of Standard Dosing Regimens for Cefuroxime Used in Coronary Artery Bypass Graft Surgery with Cardiopulmonary Bypass. Antimicrob. Agents Chemother. 2018, 62, e02241-17. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alenazi, M.; Alsultan, A.; Alsarhani, E. Estimation of Tacrolimus Clearance in Saudi Adult Kidney Transplant Recipients. Saudi J. Kidney Dis. Transplantat. 2021, 32, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Almatrafi, A.; Bin Aydan, N.; Alqahtani, M.; Alzamil, F.; Alsultan, A.; Asiri, Y. Optimization of Vancomycin Dosing Regimen in Cancer Patients using Pharmacokinetic/Pharmacodynamic Modeling. Pharmacotherapy 2020, 40, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alfarhan, A.; Alsultan, A.; Alsarhani, E.; Alsubaie, A.; Asiri, Y. Assessment of Micafungin Dosage Regimens in Patients with Cancer Using Pharmacokinetic/Pharmacodynamic Modeling and Monte Carlo Simulation. Antibiotics 2021, 10, 1363. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Boussery, K.; Rowland-Yeo, K.; Tucker, G.T.; Rostami-Hodjegan, A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin. Pharmacokinet. 2010, 49, 189–206. [Google Scholar] [CrossRef]
- Terman, S.W.; Hill, C.E.; Burke, J.F. Disability in people with epilepsy: A nationally representative cross-sectional study. Epilepsy Behav. 2020, 112, 107429. [Google Scholar] [CrossRef]
- Desoky, E.S.E.; Fuseau, E.; Amry, S.E.D.; Cosson, V. Pharmacokinetic modelling of valproic acid from routine clinical data in Egyptian epileptic patients. Eur. J. Clin. Pharmacol. 2004, 59, 783–790. [Google Scholar] [CrossRef]
- Jiang, D.; Bai, X.; Zhang, Q.; Lu, W.; Wang, Y.; Li, L.; Müller, M. Effects of CYP2C19 and CYP2C9 genotypes on pharmacokinetic variability of valproic acid in Chinese epileptic patients: Nonlinear mixed-effect modeling. Eur. J. Clin. Pharmacol. 2009, 65, 1187–1193. [Google Scholar] [CrossRef]
- Cohen, H.; Howland, M.A.; Luciano, D.J.; Rubin, R.N.; Kutt, H.; Hoffman, R.S.; Leung, L.K.; Devinsky, O.; Goldfrank, L.R. Feasibility and pharmacokinetics of carbamazepine oral loading doses. Am. J. Health-Syst. Pharm. 1998, 55, 1134–1140. [Google Scholar] [CrossRef]
- Veal, G.J.; Nguyen, L.; Paci, A.; Riggi, M.; Amiel, M.; Valteau-Couanet, D.; Brock, P.; Ladenstein, R.; Vassal, G. Busulfan pharmacokinetics following intravenous and oral dosing regimens in children receiving high-dose myeloablative chemotherapy for high-risk neuroblastoma as part of the HR-NBL-1/SIOPEN trial. Eur. J. Cancer 2012, 48, 3063–3072. [Google Scholar] [CrossRef]
- Cotter, L.M.; Eadie, M.J.; Hooper, W.D.; Lander, C.M.; Smith, G.A.; Tyrer, J.H. The pharmacokinetics of carbamazepine. Eur. J. Clin. Pharmacol. 1977, 12, 451–456. [Google Scholar] [CrossRef]
- Fagan, T.C.; Walle, T.; Oexmann, M.J.; Walle, U.K.; Bai, S.A.; Gaffney, T.E. Increased clearance of propranolol and theophylline by high-protein compared with high-carbohydrate diet. Clin. Pharmacol. Ther. 1987, 41, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kuzuya, T.; Baba, H.; Yamada, K.; Nabeshima, T. Population pharmacokinetic analysis of vancomycin in patients with gram-positive infections and the influence of infectious disease type. J. Clin. Pharm. Ther. 2009, 34, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Asín-Prieto, E.; Soraluce, A.; Trocóniz, I.F.; Campo Cimarras, E.; Sáenz de Ugarte Sobrón, J.; Rodríguez-Gascón, A.; Isla, A. Population pharmacokinetic models for cefuroxime and metronidazole used in combination as prophylactic agents in colorectal surgery: Model-based evaluation of standard dosing regimens. Int. J. Antimicrob. Agents 2015, 45, 504–511. [Google Scholar] [CrossRef]
- Viberg, A.; Lannergård, A.; Larsson, A.; Cars, O.; Karlsson, M.O.; Sandström, M. A population pharmacokinetic model for cefuroxime using cystatin C as a marker of renal function. Br. J. Clin. Pharmacol. 2006, 62, 297–303. [Google Scholar] [CrossRef]
- Golubović, B.; Vučićević, K.; Radivojević, D.; Kovačević, S.V.; Prostran, M.; Miljković, B. Total plasma protein effect on tacrolimus elimination in kidney transplant patients--population pharmacokinetic approach. Eur. J. Pharm. Sci. 2014, 52, 34–40. [Google Scholar] [CrossRef]
- Al-Kofide, H.; Zaghloul, I.; Al-Naim, L. Pharmacokinetics of vancomycin in adult cancer patients. J. Oncol. Pharm. Pract. 2010, 16, 245–250. [Google Scholar] [CrossRef]
- Grau, S.; Luque, S.; Campillo, N.; Samsó, E.; Rodríguez, U.; García-Bernedo, C.A.; Salas, E.; Sharma, R.; Hope, W.W.; Roberts, J.A. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J. Antimicrob. Chemother. 2015, 70, 2854–2861. [Google Scholar] [CrossRef]
- Jullien, V.; Azoulay, E.; Schwebel, C.; Le Saux, T.; Charles, P.E.; Cornet, M.; Souweine, B.; Klouche, K.; Jaber, S.; Trouillet, J.L.; et al. Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. J. Antimicrob. Chemother. 2017, 72, 181–189. [Google Scholar] [CrossRef]
- Martial, L.C.; Ter Heine, R.; Schouten, J.A.; Hunfeld, N.G.; van Leeuwen, H.J.; Verweij, P.E.; de Lange, D.W.; Pickkers, P.; Brüggemann, R.J. Population Pharmacokinetic Model and Pharmacokinetic Target Attainment of Micafungin in Intensive Care Unit Patients. Clin. Pharmacokinet. 2017, 56, 1197–1206. [Google Scholar] [CrossRef]
- Sun, L.Y.; Tu, J.V.; Lee, D.S.; Beanlands, R.S.; Ruel, M.; Austin, P.C.; Eddeen, A.B.; Liu, P.P. Disability-free survival after coronary artery bypass grafting in women and men with heart failure. Open Heart 2018, 5, e000911. [Google Scholar] [CrossRef]
- Sun, L.Y.; Eddeen, A.B.; Mesana, T.G. Disability-free survival after major cardiac surgery: A population-based retrospective cohort study. CMAJ Open 2021, 9, E384–E393. [Google Scholar] [CrossRef] [PubMed]
- Joshy, G.; Thandrayen, J.; Koczwara, B.; Butow, P.; Laidsaar-Powell, R.; Rankin, N.; Canfell, K.; Stubbs, J.; Grogan, P.; Bailey, L.; et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: Population-Based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med. 2020, 18, 372. [Google Scholar] [CrossRef]


| S. No. | Reference | Population | Disabled Condition | Gender | Age (Years) | N | Analytical Method | Drug | Dose | Route | Frequency | Weight (kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alsultan et al. (2020) [19] | Pediatrics | Hematopoietic stem cell transplantation | N/S | 6.10 ± 3.17 | 59 | LC-MS/MS | Busulphan | 0.99 ± 0.15 mg/kg | IV infusion | QID for 4 days | N/S |
| 2 | Alqahtani et al. (2019) [20] | Adults | Epilepsy | 42.5% M/57.5% F | 36.3 ± 13.5 | 54 | Fluorescence polarization immunoassay | Valproic acid | 867 ± 514.2 mg | PO | OD | 82.5 ± 26.8 |
| 3 | Islam et al. (2013) [21] | Pediatrics | Partial or generalized epilepsy | 50% M/50% F | 8.23 ± 3.90 | 12 | N/S | Carbamazepine | 4.19 ± 1.64 mg/kg | PO | OD | 26.18 ± 12.10 |
| 4 | El-Yazigi et al. (1990) [22] | Adults | Heart disease, hypertension, depressive disorder, migraine | 64.5% M/35.5% F | 45.7 ± 1.8 | 48 | N/S | Propranolol | 85.8 ± 5.0 mg | PO | 40 mg every 8 hr | 73.3 ± 2.4 |
| 5 | Alqahtani et al. (2018) [23] | Adults | Open heart surgery | 61% M/39% F | 51.7 ± 15.9 | 28 | Architect i4000SR immunoassay analyzer | Vancomycin | 1 g | IV infusion | q12 hr for 2 days | 79.6 ± 17 |
| 6 | Alqahtani et al. (2018) [24] | Adults | Coronary artery bypass graft surgery | 76% M/24% F | 54.2 ± 13.2 | 78 | HPLC | Cefuroxime | 1.5 g | IV infusion | TID | 76.7 ± 14.7 |
| 7 | Alqahtani et al. (2021) [25] | Adults | Kidney transplant | 67%M/33% F | 43.9 ± 13.5 | 139 | Architect tacrolimus assay | Tacrolimus | 2.7 ± 1.2 mg/kg | PO | OD | 74.2 ± 19.5 |
| 8 | Alqahtani et al. (2020) [26] | Adults | Cancer patients | 58% M/42% F | 53.8 ± 15.7 | 147 | Architect iVancomycin i4000SR immunoassay analyzer | Vancomycin | 1 g | IV infusion | BID | 72.7 ± 16.2 |
| 9 | Alqahtani et al. (2021) [27] | Adults | Cancer patients | 60% M/40% F | 47.3 ± 12.3 | 10 | HPLC with UV detection | Micafungin | 100–150 mg/day | IV infusion | OD | 63.4 ± 18.2 |
| Sr. No. | Reference | Drug | Route | CL (L/h) | Vd (L) |
|---|---|---|---|---|---|
| 1 | Alqahtani et al. (2019) [20] | Valproic acid | PO | 0.14 (12) a | 37.7 |
| 2 | El-Yazigi et al. (1990) [22] | Propranolol | PO | 3.15 ± 0.37 b | N/S |
| 3 | Alqahtani et al. (2018) [23] | Vancomycin | IV Infusion | 6.13 (19) a | 40 (15) a |
| 4 | Alqahtani et al. (2018) [24] | Cefuroxime | IV Infusion | 2.23 (17) a | N/S |
| 5 | Alqahtani et al. (2021) [25] | Tacrolimus | PO | 9.1 (6) a | 912 (3) a |
| 6 | Alqahtani et al. (2020) [26] | Vancomycin | IV Infusion | 7.4 (20) a | 45 (15) a |
| 7 | Alqahtani et al. (2021) [27] | Micafungin | IV infusion | 1.2 (11.6) | N/S |
| Sr. No. | Reference | Drug | Route | Dose (mg/kg) | AUC0–∞ (μg h/mL) | Cmax (μg/mL) | CL (L/h) | T1/2 (h) | Vd (L/kg) | Tmax (h) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alsultan et al. (2020) [19] | Busulphan | IV Infusion | 0.99 ± 0.15 | 4392.5 ± 1354.65 | 1 ± 0.21 | N/S | 2.26 ± 0.47 | N/S | N/S | |
| 2 | Islam et al. (2013) [21] | Carbamazepine | PO | Group 1 a | 4.19 ± 1.64 | 13.63 ± 5.03 | 2.83 ± 1.12 | 0.37 ± 0.15 | 12.35 ± 9.22 | 7.91 ± 7.59 | 5.0 ± 2.58 |
| Group 2 b | 13.27 ± 7.79 | 2.64 ± 2.01 | 0.30 ± 0.13 | 10.48 ± 8.70 | 5.28 ± 6.53 | 5.5 ± 1.91 | |||||
| Group 3 c | 13.58 ± 9.41 | 2.09 ± 1.39 | 0.38 ± 0.39 | 12.21 ± 3.20 | 5.28 ± 6.53 | 4.0 ± 1.63 |
| Sr. No. | Author | Drug | EV of SA | EV from Previous Reports | EV of SA | EV from Previous Reports | EV of SA | EV from Previous Reports |
|---|---|---|---|---|---|---|---|---|
| Cmax (μg/mL) | AUC0–∞ (μg∙h/mL) | CL (L/h) | ||||||
| 1 | Alsultan et al. [19] | Busulphan | N/S | N/S | 4392.35 ± 1354.65 | 4704.33 ± 767.63 [33] | N/S | N/S |
| 2 | Alqahtani et al. [20] | Valproic acid | N/S | N/S | N/S | N/S | 0.14 | 0.09 [31] |
| 0.58 [30] | ||||||||
| 3 | Islam et al. [21] | Carbamazepine | 2.52 ± 1.447 | 7.10–9.92 [32] | 13.49 ± 6.90 | 54.85–82.83 [32] | 0.35 ± 0.23 | 0.01 ± 0.006 a [34] |
| 4 | El-Yazigi et al. [22] | Propranolol | N/S | N/S | N/S | N/S | 3.15 ± 0.37 | 3.29 (0.54) b [35] |
| 5 | Alqahtani et al. [23] | Vancomycin | N/S | N/S | N/S | N/S | 6.13 (19) b | 3.83 [36] |
| 6 | Alqahtani et al. [24] | Cefuroxime | N/S | N/S | N/S | N/S | 2.23 (17) b | 7.27 (6) b [37] |
| 6.00 (3.2) b [38] | ||||||||
| 7 | Alqahtani et al. [25] | Tacrolimus | N/S | N/S | N/S | N/S | 9.1 (6) b | 10.01 [39] |
| 8 | Alqahtani et al. [26] | Vancomycin | N/S | N/S | N/S | N/S | 7.4 (20) b | 71.2 + 22.2 c [40] |
| 9 | Alqahtani et al. [27] | Micafungin | N/S | N/S | N/S | N/S | 1.2 (11.6) b | 1.27 [41] |
| 1.34 [42] | ||||||||
| 1.10 [43] | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, F.; Al Awadh, S.A.; Rasool, M.F. Exploring the Pharmacokinetics of Drugs in Disabled Saudi Patients: A Systematic Review. Pharmaceuticals 2025, 18, 582. https://doi.org/10.3390/ph18040582
Alqahtani F, Al Awadh SA, Rasool MF. Exploring the Pharmacokinetics of Drugs in Disabled Saudi Patients: A Systematic Review. Pharmaceuticals. 2025; 18(4):582. https://doi.org/10.3390/ph18040582
Chicago/Turabian StyleAlqahtani, Faleh, Saeed A. Al Awadh, and Muhammad Fawad Rasool. 2025. "Exploring the Pharmacokinetics of Drugs in Disabled Saudi Patients: A Systematic Review" Pharmaceuticals 18, no. 4: 582. https://doi.org/10.3390/ph18040582
APA StyleAlqahtani, F., Al Awadh, S. A., & Rasool, M. F. (2025). Exploring the Pharmacokinetics of Drugs in Disabled Saudi Patients: A Systematic Review. Pharmaceuticals, 18(4), 582. https://doi.org/10.3390/ph18040582