Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design
Abstract
1. Introduction
2. Unveiling Endogenous Quinolines in the Kynurenine (KYN) Pathway: A Gateway to Kynurenic Acid (KYNA)-Driven Neuroprotection and Rational Drug Design
2.1. Structural and Functional Synergy
2.2. Key Gaps in Translation
3. Expanding the Quinoline Landscape: Derivatives Beyond the Kynurenine (KYN) Metabolic Pathway
3.1. Repurposing Potential
3.2. Bridging to Kynurenic Acid (KYNA)-Based Targets
3.3. Innovative Directions
4. Exogenous Horizons: Synthetic Quinoline Scaffolds in Rational Drug Design
4.1. Rational Design Successes and Failure
4.2. Mechanistic Trade-Offs
5. Next-Generation Kynurenic Acid (KYNA) Analogues: The SZR Series
5.1. SAR-Driven Innovations
5.2. Translational Barriers
5.3. Path to Lead Optimization
6. Discussion
7. Conclusions
8. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | blood–brain barrier |
| 7-CKA | 7-chlorokynurenic acid |
| DPA | dipicolinic acid |
| GPR35 | G-protein-coupled receptor 35 |
| HDAC | histone deacetylase |
| IDOs | indoleamine 2,3-dioxygenases |
| INA | isonicotinic acid |
| KMO | kynurenine 3-monooxygenase |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| NA | nicotinic acid |
| NMDA | N-methyl-D-aspartate |
| QUIN | quinolinic acid |
| SAR | structure–activity relationship |
| Trp | tryptophan |
| TDO | tryptophan 2,3-dioxygenase |
| XA | xanthurenic acid |
References
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a ‘reflex’ feature of neuroimmune communication: Sensor and effector functions for the indoleamine-2,3-dioxygenase kynurenine pathway. J. Neurochem. 2024, 168, 3333–3357. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Lennon, M.J.; Lim, C.K.; Jacobs, K.; Guillemin, G.J.; Brew, B.J. Recent evidence for an expanded role of the kynurenine pathway of tryptophan metabolism in neurological diseases. Neuropharmacology 2017, 112, 373–388. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan metabolism and gut-brain homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef]
- Badawy, A.A. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.-H. Deregulated tryptophan-kynurenine pathway is linked to inflammation, oxidative stress, and immune activation pathway in cardiovascular diseases. Front. Biosci. 2015, 20, 1116. [Google Scholar]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining roles: A paradigm shift in tryptophan–kynurenine metabolism for innovative clinical applications. Int. J. Mol. Sci. 2024, 25, 12767. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Li, X.; Guo, H.-Y.; Shen, Q.-K.; Quan, Z.-S.; Luan, T. Application of quinoline ring in structural modification of natural products. Molecules 2023, 28, 6478. [Google Scholar] [CrossRef]
- Ajani, O.O.; Iyaye, K.T.; Ademosun, O.T. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs—A review. RSC Adv. 2022, 12, 18594–18614. [Google Scholar] [CrossRef]
- Sehlangia, S.; Nayak, N.; Garg, N.; Pradeep, C.P. Substituent-controlled structural, supramolecular, and cytotoxic properties of a series of 2-Styryl-8-nitro and 2-Styryl-8-hydroxy quinolines. ACS Omega 2022, 7, 24838–24850. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ayala, L.F.; Guzmán-López, E.G.; Galano, A. Quinoline Derivatives: Promising Antioxidants with Neuroprotective Potential. Antioxidants 2023, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; La Monica, G.; Lauria, A. Quinoline-based molecules targeting c-Met, EGF, and VEGF receptors and the proteins involved in related carcinogenic pathways. Molecules 2020, 25, 4279. [Google Scholar] [CrossRef]
- Shoji, T.; Takeuchi, M.; Uda, M.; Ariga, Y.; Yamazaki, A.; Sekiguchi, R.; Ito, S. Synthesis of Azuleno [2,1-b] quinolones and Quinolines via Brønsted Acid-Catalyzed Cyclization of 2-Arylaminoazulenes. Molecules 2023, 28, 5785. [Google Scholar] [CrossRef]
- Yadav, V.; Reang, J.; Sharma, V.; Majeed, J.; Sharma, P.C.; Sharma, K.; Giri, N.; Kumar, A.; Tonk, R.K. Quinoline-derivatives as privileged scaffolds for medicinal and pharmaceutical chemists: A comprehensive review. Chem. Biol. Drug Des. 2022, 100, 389–418. [Google Scholar] [CrossRef]
- Lanz, T.V.; Williams, S.K.; Stojic, A.; Iwantscheff, S.; Sonner, J.K.; Grabitz, C.; Becker, S.; Böhler, L.-I.; Mohapatra, S.R.; Sahm, F. Tryptophan-2, 3-Dioxygenase (TDO) deficiency is associated with subclinical neuroprotection in a mouse model of multiple sclerosis. Sci. Rep. 2017, 7, 41271. [Google Scholar] [CrossRef]
- Maya, S.; Prakash, T.; Goli, D. Effect of wedelolactone and gallic acid on quinolinic acid-induced neurotoxicity and impaired motor function: Significance to sporadic amyotrophic lateral sclerosis. Neurotoxicology 2018, 68, 1–12. [Google Scholar]
- Platten, M.; Friedrich, M.; Wainwright, D.A.; Panitz, V.; Opitz, C.A. Tryptophan metabolism in brain tumors—IDO and beyond. Curr. Opin. Immunol. 2021, 70, 57–66. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Franco, N.F.; Ng, M.L.; Pai, S.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Current evidence for a role of the kynurenine pathway of tryptophan metabolism in multiple sclerosis. Front. Immunol. 2016, 7, 246. [Google Scholar] [CrossRef]
- Krause, D.; Suh, H.S.; Tarassishin, L.; Cui, Q.L.; Durafourt, B.A.; Choi, N.; Bauman, A.; Cosenza-Nashat, M.; Antel, J.P.; Zhao, M.L.; et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: Role of hemeoxygenase-1. Am. J. Pathol. 2011, 179, 1360–1372. [Google Scholar] [CrossRef]
- Zádori, D.; Veres, G.; Szalárdy, L.; Klivényi, P.; Vécsei, L. Alzheimer’s disease: Recent concepts on the relation of mitochondrial disturbances, excitotoxicity, neuroinflammation, and kynurenines. J. Alzheimer’s Dis. 2018, 62, 523–547. [Google Scholar] [CrossRef]
- Phillips, R.S.; Iradukunda, E.C.; Hughes, T.; Bowen, J.P. Modulation of enzyme activity in the kynurenine pathway by kynurenine monooxygenase inhibition. Front. Mol. Biosci. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Valotto Neto, L.J.; Reverete de Araujo, M.; Moretti Junior, R.C.; Mendes Machado, N.; Joshi, R.K.; Dos Santos Buglio, D.; Barbalho Lamas, C.; Direito, R.; Fornari Laurindo, L.; Tanaka, M.; et al. Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis. Antioxidants 2024, 13, 393. [Google Scholar] [CrossRef] [PubMed]
- Michaudel, C.; Danne, C.; Agus, A.; Magniez, A.; Aucouturier, A.; Spatz, M.; Lefevre, A.; Kirchgesner, J.; Rolhion, N.; Wang, Y. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases. Gut 2023, 72, 1296–1307. [Google Scholar] [CrossRef]
- Opitz, C.A.; Somarribas Patterson, L.F.; Mohapatra, S.R.; Dewi, D.L.; Sadik, A.; Platten, M.; Trump, S. The therapeutic potential of targeting tryptophan catabolism in cancer. Br. J. Cancer 2020, 122, 30–44. [Google Scholar] [CrossRef]
- Tang, K.; Wu, Y.-H.; Song, Y.; Yu, B. Indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- El-Zahabi, M.A.A.; Abouzeid, M.Y.; Eissa, I.H.; Taghour, M.S. Dual Inhibitors of Indoleamine-2,3-Dioxygenase (IDO) and Tryptophan-2,3-Dioxygenase (TDO) as Anti-Tumor Immune Modulators. Al-Azhar J. Pharm. Sci. 2024, 69, 38–61. [Google Scholar] [CrossRef]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G. Kynurenic acid in schizophrenia: A systematic review and meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J. Indoleamine 2,3-dioxygenase (IDO) activity: A perspective biomarker for laboratory determination in tumor immunotherapy. Biomedicines 2023, 11, 1988. [Google Scholar] [CrossRef]
- Stone, T.W. Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J. Neurochem. 2020, 152, 627–649. [Google Scholar] [CrossRef]
- Fukuwatari, T. Possibility of amino acid treatment to prevent the psychiatric disorders via modulation of the production of tryptophan metabolite kynurenic acid. Nutrients 2020, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Bauzon, J.; Lee, G.; Cummings, J. Repurposed agents in the Alzheimer’s disease drug development pipeline. Alzheimer’s Res. Ther. 2020, 12, 98. [Google Scholar] [CrossRef]
- Saranraj, K.; Kiran, P.U. Drug repurposing: Clinical practices and regulatory pathways. Perspect. Clin. Res. 2025, 16, 61–68. [Google Scholar] [CrossRef]
- Patrignani, P.; Contursi, A.; Tacconelli, S.; Steinhilber, D. The future of pharmacology and therapeutics of the arachidonic acid cascade in the next decade: Innovative advancements in drug repurposing. Front. Pharmacol. 2024, 15, 1472396. [Google Scholar] [CrossRef]
- Panchal, N.B.; Vaghela, V.M. From Molecules to Medicine: The Remarkable Pharmacological Odyssey of Quinoline and It’s Derivatives. Orient. J. Chem. 2023, 39, 546–567. [Google Scholar] [CrossRef]
- Atukuri, D.; Vijayalaxmi, S.; Sanjeevamurthy, R.; Vidya, L.; Prasannakumar, R.; Raghavendra, M. Identification of quinoline-chalcones and heterocyclic chalcone-appended quinolines as broad-spectrum pharmacological agents. Bioorganic Chem. 2020, 105, 104419. [Google Scholar] [CrossRef]
- Elebiju, O.F.; Ajani, O.O.; Oduselu, G.O.; Ogunnupebi, T.A.; Adebiyi, E. Recent advances in functionalized quinoline scaffolds and hybrids—Exceptional pharmacophore in therapeutic medicine. Front. Chem. 2023, 10, 1074331. [Google Scholar] [CrossRef]
- Fukushima, T.; Umino, M.; Sakamoto, T.; Onozato, M. A review of chromatographic methods for bioactive tryptophan metabolites, kynurenine, kynurenic acid, quinolinic acid, and others, in biological fluids. Biomed. Chromatogr. 2022, 36, e5308. [Google Scholar] [CrossRef]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders—Unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Kynurenines in the pathogenesis of multiple sclerosis: Therapeutic perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.D. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Tóth, F.; Cseh, E.K.; Vécsei, L. Natural molecules and neuroprotection: Kynurenic acid, pantethine and α-lipoic acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T. Intrastriatal quinolinic acid administration impairs redox homeostasis and induces inflammatory changes: Prevention by kynurenic acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef]
- Maitre, M.; Taleb, O.; Jeltsch-David, H.; Klein, C.; Mensah-Nyagan, A.G. Xanthurenic acid: A role in brain intercellular signaling. J. Neurochem. 2024, 168, 2303–2315. [Google Scholar] [CrossRef]
- Skorobogatov, K.; Autier, V.; Foiselle, M.; Richard, J.-R.; Boukouaci, W.; Wu, C.-L.; Raynal, S.; Carbonne, C.; Laukens, K.; Meysman, P. Kynurenine pathway abnormalities are state-specific but not diagnosis-specific in schizophrenia and bipolar disorder. Brain Behav. Immun. Health 2023, 27, 100584. [Google Scholar] [CrossRef]
- Bonaparte, J.; Cook, M.; Dixon, S.; Wright, C.; Hill, W.; Wang, G. Kynurenine Metabolites Induce Microglial Cell Senescence and Stimulate Neuroinflammation. Innov. Aging 2023, 7, 1073. [Google Scholar] [CrossRef]
- Chen, C.-M.; Huang, C.-Y.; Lai, C.-H.; Chen, Y.-C.; Hwang, Y.-T.; Lin, C.-Y. Neuroprotection effects of kynurenic acid-loaded micelles for the Parkinson’s disease models. J. Liposome Res. 2024, 34, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Hertelendy, P.; Toldi, J.; Fülöp, F.; Vécsei, L. Ischemic stroke and kynurenines: Medicinal chemistry aspects. Curr. Med. Chem. 2018, 25, 5945–5957. [Google Scholar] [CrossRef]
- Martín-Hernández, D.; Tendilla-Beltrán, H.; Madrigal, J.L.; García-Bueno, B.; Leza, J.C.; Caso, J.R. Chronic mild stress alters kynurenine pathways changing the glutamate neurotransmission in frontal cortex of rats. Mol. Neurobiol. 2019, 56, 490–501. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic acid: The Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Savitz, J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry 2020, 25, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Lőrinczi, B.; Fazakas, C.; Szatmári, I.; Fülöp, F.; Kmetykó, N.; Berkecz, R.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I. Szr-104, a novel kynurenic acid analogue with high permeability through the blood–brain barrier. Pharmaceutics 2021, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Juhász, Á.; Ungor, D.; Varga, N.; Katona, G.; Balogh, G.T.; Csapó, E. Lipid-Based nanocarriers for delivery of neuroprotective kynurenic acid: Preparation, characterization, and BBB transport. Int. J. Mol. Sci. 2023, 24, 14251. [Google Scholar] [CrossRef]
- Varga, N.; Csapó, E.; Majláth, Z.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; Knapp, L.; Toldi, J.; Vécsei, L.; Dékány, I. Targeting of the kynurenic acid across the blood–brain barrier by core-shell nanoparticles. Eur. J. Pharm. Sci. 2016, 86, 67–74. [Google Scholar] [CrossRef]
- Hara, N.; Yamada, K.; Shibata, T.; Osago, H.; Hashimoto, T.; Tsuchiya, M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J. Biol. Chem. 2007, 282, 24574–24582. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD (H) and NADP (H) redox couples and cellular energy metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Oyama, T.; Yamamoto, T.; Kameda, T.; Kamiya, T.; Abe, H.; Abe, T.; Tanuma, S.-I. Supplementation of nicotinic acid and its derivatives up-regulates cellular NAD+ level rather than nicotinamide derivatives in cultured normal human epidermal keratinocytes. Life 2024, 14, 413. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.A. Nicotinic acid: The broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 2005, 258, 94–114. [Google Scholar] [CrossRef]
- Schachter, M. Strategies for modifying high-density lipoprotein cholesterol: A role for nicotinic acid. Cardiovasc. Drugs Ther. 2005, 19, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Figge, H.L.; Figge, J.; Souney, P.F.; Mutnick, A.H.; Sacks, F. Nicotinic acid: A review of its clinical use in the treatment of lipid disorders. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988, 8, 287–294. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, T.-J.; Wu, K.K.; Sturino, C.; Metters, K.; Gottesdiener, K.; Wright, S.D.; Wang, Z.; O’Neill, G.; Lai, E. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc. Natl. Acad. Sci. USA 2006, 103, 6682–6687. [Google Scholar] [CrossRef]
- Benyo, Z.; Gille, A.; Bennett, C.L.; Clausen, B.E.; Offermanns, S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol. Pharmacol. 2006, 70, 1844–1849. [Google Scholar] [CrossRef]
- Benyó, Z.; Gille, A.; Kero, J.; Csiky, M.; Suchánková, M.C.; Nüsing, R.M.; Moers, A.; Pfeffer, K.; Offermanns, S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid–induced flushing. J. Clin. Investig. 2005, 115, 3634–3640. [Google Scholar] [CrossRef]
- Braidy, N.; Grant, R.; Adams, S.; Brew, B.J.; Guillemin, G.J. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox. Res. 2009, 16, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxidative Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef]
- Schurr, A.; West, C.A.; Rigor, B. Neurotoxicity of quinolinic acid and its derivatives in hypoxic rat hippocampal slices. Brain Res. 1991, 568, 199–204. [Google Scholar] [CrossRef]
- La Cruz, V.P.-D.; Carrillo-Mora, P.; Santamaría, A. Quinolinic acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012, 5, IJTR.S8158. [Google Scholar] [CrossRef] [PubMed]
- Leipnitz, G.; Schumacher, C.; Scussiato, K.; Dalcin, K.B.; Wannmacher, C.M.; Wyse, A.T.; Dutra-Filho, C.S.; Wajner, M.; Latini, A. Quinolinic acid reduces the antioxidant defenses in cerebral cortex of young rats. Int. J. Dev. Neurosci. 2005, 23, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Severiano, F.; Rodríguez-Pérez, M.; Pedraza-Chaverrí, J.; Maldonado, P.D.; Medina-Campos, O.N.; Ortíz-Plata, A.; Sánchez-García, A.; Villeda-Hernández, J.; Galván-Arzate, S.; Aguilera, P. S-Allylcysteine, a garlic-derived antioxidant, ameliorates quinolinic acid-induced neurotoxicity and oxidative damage in rats. Neurochem. Int. 2004, 45, 1175–1183. [Google Scholar] [CrossRef]
- Schwarcz, R.; Whetsell, W.O., Jr.; Mangano, R.M. Quinolinic acid: An endogenous metabolite that produces axon-sparing lesions in rat brain. Science 1983, 219, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The role of tryptophan dysmetabolism and quinolinic acid in depressive and neurodegenerative diseases. Biomolecules 2022, 12, 998. [Google Scholar] [CrossRef]
- Seal, C.J.; Heaton, F.W. Effect of dietary picolinic acid on the metabolism of exogenous and endogenous zinc in the rat. J. Nutr. 1985, 115, 986–993. [Google Scholar] [CrossRef]
- Testa, U.; Louache, F.; Titeux, M.; Thomopoulos, P.; Rochant, H. The iron-chelating agent picolinic acid enhances transferrin receptors expression in human erythroleukaemic cell lines. Br. J. Haematol. 1985, 60, 491–502. [Google Scholar] [CrossRef]
- Prodinger, J.; Loacker, L.J.; Schmidt, R.L.; Ratzinger, F.; Greiner, G.; Witzeneder, N.; Hoermann, G.; Jutz, S.; Pickl, W.F.; Steinberger, P. The tryptophan metabolite picolinic acid suppresses proliferation and metabolic activity of CD4+ T cells and inhibits c-Myc activation. J. Leucoc. Biol. 2016, 99, 583–594. [Google Scholar] [CrossRef]
- Tomioka, H.; Shimizu, T.; Tatano, Y. Effects of picolinic acid on the antimicrobial functions of host macrophages against Mycobacterium avium complex. Int. J. Antimicrob. Agents 2007, 29, 460–464. [Google Scholar] [CrossRef]
- Cai, S.; Sato, K.; Shimizu, T.; Yamabe, S.; Hiraki, M.; Sano, C.; Tomioka, H. Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin and fluoroquinolones. J. Antimicrob. Chemother. 2006, 57, 85–93. [Google Scholar] [CrossRef]
- Bosco, M.C.; Rapisarda, A.; Massazza, S.; Melillo, G.; Young, H.; Varesio, L. The tryptophan catabolite picolinic acid selectively induces the chemokines macrophage inflammatory protein-1α and-1β in macrophages. J. Immunol. 2000, 164, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Coggan, S.; Smythe, G.A. The physiological action of picolinic acid in the human brain. Int. J. Tryptophan Res. 2009, 2, IJTR.S2469. [Google Scholar] [CrossRef] [PubMed]
- Cockhill, J.; Jhamandas, K.; Boegman, R.; Beninger, R. Action of picolinic acid and structurally related pyridine carboxylic acids on quinolinic acid-induced cortical cholinergic damage. Brain Res. 1992, 599, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Kalisch, B.E.; Jhamandas, K.; Boegman, R.J.; Beninger, R.J. Picolinic acid protects against quinolinic acid-induced depletion of NADPH diaphorase containing neurons in the rat striatum. Brain Res. 1994, 668, 1–8. [Google Scholar] [CrossRef]
- Taleb, O.; Maammar, M.; Klein, C.; Maitre, M.; Mensah-Nyagan, A.G. A role for xanthurenic acid in the control of brain dopaminergic activity. Int. J. Mol. Sci. 2021, 22, 6974. [Google Scholar] [CrossRef]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Copeland, C.S.; Neale, S.A.; Bruno, V.; Battaglia, G.; Salt, T.E.; Nicoletti, F. Cinnabarinic acid and xanthurenic acid: Two kynurenine metabolites that interact with metabotropic glutamate receptors. Neuropharmacology 2017, 112, 365–372. [Google Scholar] [CrossRef]
- Murakami, K.; Haneda, M.; Yoshino, M. Prooxidant action of xanthurenic acid and quinoline compounds: Role of transition metals in the generation of reactive oxygen species and enhanced formation of 8-hydroxy-2′-deoxyguanosine in DNA. Biometals 2006, 19, 429–435. [Google Scholar] [CrossRef]
- Murakami, K.; Ito, M.; Yoshino, M. Xanthurenic acid inhibits metal ion-induced lipid peroxidation and protects NADP-isocitrate dehydrogenase from oxidative inactivation. J. Nutr. Sci. Vitaminol. 2001, 47, 306–310. [Google Scholar] [CrossRef]
- Lima, V.L.; Dias, F.; Nunes, R.D.; Pereira, L.O.; Santos, T.S.; Chiarini, L.B.; Ramos, T.D.; Silva-Mendes, B.J.; Perales, J.; Valente, R.H. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS ONE 2012, 7, e38349. [Google Scholar] [CrossRef]
- Nowicka-Stążka, P.; Langner, E.; Turski, W.; Rzeski, W.; Parada-Turska, J. Quinaldic acid in synovial fluid of patients with rheumatoid arthritis and osteoarthritis and its effect on synoviocytes in vitro. Pharmacol. Rep. 2018, 70, 277–283. [Google Scholar] [CrossRef]
- Okamoto, H.; Miyamoto, S.; Mabuchi, H.; Yoneyama, Y.; Takeda, R. Insulin-releasing effect of quinaldic acid and its relatives on isolated Langerhans islets. Biochem. Biophys. Res. Commun. 1973, 53, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, K.H.; Boegman, R.J.; Beninger, R.J.; Miranda, A.F.; Lipic, K.A. Excitotoxicity of quinolinic acid: Modulation by endogenous antagonists. Neurotox. Res. 2000, 2, 139–155. [Google Scholar] [CrossRef]
- Bala, M.; Radhakrishnan, T.; Kumar, A.; Mishra, G.P.; Dobraia, J.R.; Kirti, P.B. Erratum: Overexpression of a fusion defensin gene from radish and fenugreek improves resistance against leaf spot diseases caused by Cercospora arachidicola and Phaeoisariopsis personata in peanut. Turk. J. Biol. 2019, 43, 154. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, Q.; Wang, S.; Wu, J.; Gao, Q.; Liu, W. Thiopeptide Antibiotics Exhibit a Dual Mode of Action against Intracellular Pathogens by Affecting Both Host and Microbe. Chem. Biol. 2015, 22, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, K.; Boegman, R.J.; Beninger, R.J.; Bialik, M. Quinolinate-induced cortical cholinergic damage: Modulation by tryptophan metabolites. Brain Res. 1990, 529, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.W.; Fishbein, J.; Hong, J.; Roeser, J.; Furie, R.A.; Aranow, C.; Volpe, B.T.; Diamond, B.; Mackay, M. Quinolinic acid, a kynurenine/tryptophan pathway metabolite, associates with impaired cognitive test performance in systemic lupus erythematosus. Lupus Sci. Med. 2021, 8, e000559. [Google Scholar] [CrossRef]
- Sawada, Y.; Kayakiri, H.; Abe, Y.; Mizutani, T.; Inamura, N.; Asano, M.; Hatori, C.; Aramori, I.; Oku, T.; Tanaka, H. Discovery of the first non-peptide full agonists for the human bradykinin B2 receptor incorporating 4-(2-picolyloxy)quinoline and 1-(2-picolyl)benzimidazole frameworks. J. Med. Chem. 2004, 47, 2853–2863. [Google Scholar] [CrossRef]
- Antosiewicz, J.M.; Kane, P.M. Editorial: Intracellular Molecular Processes Affected by pH. Front. Mol. Biosci. 2022, 9, 891533. [Google Scholar] [CrossRef]
- Ghorai, P.; Pal, K.; Karmakar, P.; Saha, A. The development of two fluorescent chemosensors for the selective detection of Zn2+ and Al3+ ions in a quinoline platform by tuning the substituents in the receptor part: Elucidation of the structures of the metal-bound chemosensors and biological studies. Dalton Trans. 2020, 49, 4758–4773. [Google Scholar] [CrossRef]
- Mikata, Y. Quinoline- and isoquinoline-derived ligand design on TQEN (N,N,N′,N′-tetrakis(2-quinolylmethyl)ethylenediamine) platform for fluorescent sensing of specific metal ions and phosphate species. Dalton Trans. 2020, 49, 17494–17504. [Google Scholar] [CrossRef]
- Peterson, L.R. Quinolone molecular structure-activity relationships: What we have learned about improving antimicrobial activity. Clin. Infect. Dis. 2001, 33 (Suppl. S3), S180–S186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, F.; Jin, G. Structural basis of quinolone derivatives, inhibition of type I and II topoisomerases and inquiry into the relevance of bioactivity in odd or even branches with molecular docking study. J. Mol. Struct. 2020, 1221, 128869. [Google Scholar] [CrossRef]
- Cramer, J.; Sager, C.P.; Ernst, B. Hydroxyl Groups in Synthetic and Natural-Product-Derived Therapeutics: A Perspective on a Common Functional Group. J. Med. Chem. 2019, 62, 8915–8930. [Google Scholar] [CrossRef] [PubMed]
- Fray, M.J.; Bull, D.J.; Carr, C.L.; Gautier, E.C.; Mowbray, C.E.; Stobie, A. Structure-activity relationships of 1,4-dihydro-(1H,4H)-quinoxaline-2,3-diones as N-methyl-D-aspartate (glycine site) receptor antagonists. 1. Heterocyclic substituted 5-alkyl derivatives. J. Med. Chem. 2001, 44, 1951–1962. [Google Scholar] [CrossRef]
- Wei, L.; Hou, T.; Li, J.; Zhang, X.; Zhou, H.; Wang, Z.; Cheng, J.; Xiang, K.; Wang, J.; Zhao, Y.; et al. Structure-Activity Relationship Studies of Coumarin-like Diacid Derivatives as Human G Protein-Coupled Receptor-35 (hGPR35) Agonists and a Consequent New Design Principle. J. Med. Chem. 2021, 64, 2634–2647. [Google Scholar] [CrossRef]
- Hou, Z.; Vanecek, A.S.; Tepe, J.J.; Odom, A.L. Synthesis, structure, properties, and cytotoxicity of a (quinoline)RuCp+ complex. Dalton Trans. 2023, 52, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Wellen, K.E. Advances into understanding metabolites as signaling molecules in cancer progression. Curr. Opin. Cell Biol. 2020, 63, 144–153. [Google Scholar] [CrossRef]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024, 164, 105791. [Google Scholar] [CrossRef]
- Boros, F.; Vécsei, L. Progress in the development of kynurenine and quinoline-3-carboxamide-derived drugs. Expert Opin. Investig. Drugs 2020, 29, 1223–1247. [Google Scholar] [CrossRef]
- Stone, T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993, 45, 309–379. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001, 64, 185–218. [Google Scholar] [CrossRef]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lamson, A.R.; Shelley, M.; Troyanskaya, O. Interpretable neural architecture search and transfer learning for understanding CRISPR-Cas9 off-target enzymatic reactions. Nat. Comput. Sci. 2023, 3, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.A.; Shin, C.B.; Arroyo-Currás, N.; Ortega, G.; Li, W.; Keller, A.A.; Plaxco, K.W.; Kippin, T.E. Ultra-High-Precision, in-vivo Pharmacokinetic Measurements Highlight the Need for and a Route Toward More Highly Personalized Medicine. Front. Mol. Biosci. 2019, 6, 69. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Yang, L.; He, D.; Li, Y.; Shi, M.; Zhang, J. Metabolic pathways and pharmacokinetics of natural medicines with low permeability. Drug Metab. Rev. 2017, 49, 464–476. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.M.; Hansen, J.M.; Abou-Kassem, D.; Hansted, A.K.; Ubhayasekera, K.; Bergquist, J.; Vécsei, L.; Jansen-Olesen, I.; Ashina, M. Phase 1 study to access safety, tolerability, pharmacokinetics, and pharmacodynamics of kynurenine in healthy volunteers. Pharmacol. Res. Perspect. 2021, 9, e00741. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef]
- Siddique, S.; Hussain, K.; Shehzadi, N.; Arshad, M.; Arshad, M.N.; Iftikhar, S.; Saghir, F.; Shaukat, A.; Sarfraz, M.; Ahmed, N. Design, synthesis, biological evaluation and molecular docking studies of quinoline-anthranilic acid hybrids as potent anti-inflammatory drugs. Org. Biomol. Chem. 2024, 22, 3708–3724. [Google Scholar] [CrossRef]
- Arabiyat, S.; Alzoubi, A.; Al-Daghistani, H.; Al-Hiari, Y.; Kasabri, V.; Alkhateeb, R. Evaluation of Quinoline-Related Carboxylic Acid Derivatives as Prospective Differentially Antiproliferative, Antioxidative, and Anti-Inflammatory Agents. Chem. Biol. Drug Des. 2024, 104, e14615. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xie, Y.; Liu, X.; Xu, H.; Ren, H.; Tang, S.; Liu, Q.; Huang, M.; Shao, X.; Li, C.; et al. Discovery of Novel Imidazo[4,5-c]quinoline Derivatives to Treat Inflammatory Bowel Disease (IBD) by Inhibiting Multiple Proinflammatory Signaling Pathways and Restoring Intestinal Homeostasis. J. Med. Chem. 2022, 65, 11949–11969. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, C.; Liu, W.; Guo, Z.; Zhang, Y.; Wu, Y.; Wei, C.; Wu, J.; Yang, X. Quinolines: A Promising Heterocyclic Scaffold for Cancer Therapeutics. Curr. Med. Chem. 2025, 32, 958–973. [Google Scholar] [CrossRef]
- Park, L.T.; Kadriu, B.; Gould, T.D.; Zanos, P.; Greenstein, D.; Evans, J.W.; Yuan, P.; Farmer, C.A.; Oppenheimer, M.; George, J.M. A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int. J. Neuropsychopharmacol. 2020, 23, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Heyes, M.P.; Saito, K.; Crowley, J.S.; Davis, L.E.; Demitrack, M.A.; Der, M.; Dilling, L.A.; Elia, J.; Kruesi, M.J.; Lackner, A.; et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 1992, 115 Pt 5, 1249–1273. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E. Lipophilicity and ADMET Analysis of Quinoline-1,4-quinone Hybrids. Pharmaceutics 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Jastrzębska, M.; Chrobak, E.; Bębenek, E.; Latocha, M. Hybrids of 1,4-Quinone with Quinoline Derivatives: Synthesis, Biological Activity, and Molecular Docking with DT-Diaphorase (NQO1). Molecules 2022, 27, 6206. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Chiarugi, A.; Meli, E.; Moroni, F. Similarities and differences in the neuronal death processes activated by 3OH-kynurenine and quinolinic acid. J. Neurochem. 2001, 77, 1310–1318. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Fang, X.; Guo, L.; Hu, N.; Guo, Z.; Li, X.; Yang, S.; He, J.C.; Kuang, C.; et al. N-Benzyl/Aryl Substituted Tryptanthrin as Dual Inhibitors of Indoleamine 2,3-Dioxygenase and Tryptophan 2,3-Dioxygenase. J. Med. Chem. 2019, 62, 9161–9174. [Google Scholar] [CrossRef] [PubMed]
- Dolšak, A.; Gobec, S.; Sova, M. Indoleamine and tryptophan 2,3-dioxygenases as important future therapeutic targets. Pharmacol. Ther. 2021, 221, 107746. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Leme Boaro, B.; da Silva Camarinha Oliveira, J.; Patočka, J.; Barbalho Lamas, C.; Tanaka, M.; Laurindo, L.F. Molecular Mechanisms Underlying Neuroinflammation Intervention with Medicinal Plants: A Critical and Narrative Review of the Current Literature. Pharmaceuticals 2025, 18, 133. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the Redox Status in Multiple Sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Gericke, B. Novel Intrinsic Mechanisms of Active Drug Extrusion at the Blood-Brain Barrier: Potential Targets for Enhancing Drug Delivery to the Brain? Pharmaceutics 2020, 12, 966. [Google Scholar] [CrossRef]
- Zuchero, Y.J.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Terstappen, G.C.; Meyer, A.H.; Bell, R.D.; Zhang, W. Strategies for delivering therapeutics across the blood-brain barrier. Nat. Rev. Drug Discov. 2021, 20, 362–383. [Google Scholar] [CrossRef]
- Dounay, A.B.; Tuttle, J.B.; Verhoest, P.R. Challenges and Opportunities in the Discovery of New Therapeutics Targeting the Kynurenine Pathway. J. Med. Chem. 2015, 58, 8762–8782. [Google Scholar] [CrossRef]
- Shen, L.L.; Pernet, A.G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: The target of the drugs is DNA. Proc. Natl. Acad. Sci. USA 1985, 82, 307–311. [Google Scholar] [CrossRef]
- Walker, H.A.; Wilson, S.; Farrar, C.; Richardson, A.P. The reflex respiratory and circulatory actions of some cinchoninic acid derivatives and a number of unrelated compounds. J. Pharmacol. Exp. Ther. 1952, 104, 211–218. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Guillén, H.; González-Peña, D.; Arán, V.J. Antimalarial Quinoline Drugs Inhibit β-Hematin and Increase Free Hemin Catalyzing Peroxidative Reactions and Inhibition of Cysteine Proteases. Sci. Rep. 2019, 9, 15398. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Staalsø, T.; Yang, Y.; Lee, J.; Pérez-Berná, A.J.; Pereiro, E.; Yang, Y.; Werner, S.; Guttmann, P.; Leiserowitz, L.; et al. Mode of action of quinoline antimalarial drugs in red blood cells infected by Plasmodium falciparum revealed in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 22946–22952. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.; Tilley, L. Quinoline antimalarials: Mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar] [CrossRef]
- Currie, J.P.; Brown, R.A.; Will, G. Observations on the treatment of rheumatoid arthritis with butazolidin. Ann. Rheum. Dis. 1953, 12, 88–94. [Google Scholar] [CrossRef]
- Gelfman, D.M. Reflections on quinine and its importance in dermatology today. Clin. Dermatol. 2021, 39, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Mukusheva, G.K.; Zhasymbekova, A.R.; Seidakhmetova, R.B.; Nurkenov, O.A.; Akishina, E.A.; Petkevich, S.K.; Dikusar, E.A.; Potkin, V.I. Quinine Esters with 1,2-Azole, Pyridine and Adamantane Fragments. Molecules 2022, 27, 3476. [Google Scholar] [CrossRef]
- Man-Son-Hing, M.; Wells, G.; Lau, A. Quinine for nocturnal leg cramps: A meta-analysis including unpublished data. J. Gen. Intern. Med. 1998, 13, 600–606. [Google Scholar] [CrossRef]
- Gisselmann, G.; Alisch, D.; Welbers-Joop, B.; Hatt, H. Effects of Quinine, Quinidine and Chloroquine on Human Muscle Nicotinic Acetylcholine Receptors. Front. Pharmacol. 2018, 9, 1339. [Google Scholar] [CrossRef]
- Li, Z.; Jin, X.; Wu, T.; Huang, G.; Wu, K.; Lei, J.; Pan, X.; Yan, N. Structural Basis for Pore Blockade of the Human Cardiac Sodium Channel Nav1.5 by the Antiarrhythmic Drug Quinidine. Angew. Chem. Int. Ed. Engl. 2021, 60, 11474–11480. [Google Scholar] [CrossRef]
- Hill, R.J.; Duff, H.J.; Sheldon, R.S. Class I antiarrhythmic drug receptor: Biochemical evidence for state-dependent interaction with quinidine and lidocaine. Mol. Pharmacol. 1989, 36, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Kodama, I.; Toyama, J.; Yamada, K. Open and inactivated sodium channel block by class-I antiarrhythmic drugs. Jpn. Heart J. 1986, 27 (Suppl. 1), 83–89. [Google Scholar] [CrossRef] [PubMed]
- Conn, H.L., Jr.; Luchi, R.J. Some Cellular and Metabolic Considerations Relating to the Action of Quinidine as a Prototype Antiarrhythmic Agent. Am. J. Med. 1964, 37, 685–699. [Google Scholar] [CrossRef]
- Yan, M.; Fan, P.; Shi, Y.; Feng, L.; Wang, J.; Zhan, G.; Li, B. Stereoselective Blockage of Quinidine and Quinine in the hERG Channel and the Effect of Their Rescue Potency on Drug-Induced hERG Trafficking Defect. Int. J. Mol. Sci. 2016, 17, 1648. [Google Scholar] [CrossRef]
- Brocks, D.R.; Mehvar, R. Stereoselectivity in the pharmacodynamics and pharmacokinetics of the chiral antimalarial drugs. Clin. Pharmacokinet. 2003, 42, 1359–1382. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Camm, A.J. Evaluation of drug-induced QT interval prolongation: Implications for drug approval and labelling. Drug Saf. 2001, 24, 323–351. [Google Scholar] [CrossRef]
- Haeusler, I.L.; Chan, X.H.S.; Guérin, P.J.; White, N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: A systematic review. BMC Med. 2018, 16, 200. [Google Scholar] [CrossRef]
- Reiter, M.J.; Higgins, S.L.; Payne, A.G.; Mann, D.E. Effects of quinidine versus procainamide on the QT interval. Am. J. Cardiol. 1986, 58, 512–516. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Y.; Sheng, L.; Larson, P.; Liu, X.; Zou, X.; Wang, S.; Guo, K.; Ma, C.; Zhang, G.; et al. A Cinchona Alkaloid Antibiotic That Appears To Target ATP Synthase in Streptococcus pneumoniae. J. Med. Chem. 2019, 62, 2305–2332. [Google Scholar] [CrossRef]
- Boratyński, P.J.; Zielińska-Błajet, M.; Skarżewski, J. Cinchona Alkaloids-Derivatives and Applications. Alkaloids Chem. Biol. 2019, 82, 29–145. [Google Scholar] [CrossRef]
- Chajdaś, Z.; Kucharska, M.; Wesełucha-Birczyńska, A. Two-Dimensional Correlation Spectroscopy (2D-COS) Tracking of the Formation of Selected Transition Metal Compounds Cu(II) and Cd(II) with Cinchonine and Their Impact on Model Components of Erythrocytes. Appl. Spectrosc. 2024, 79, 37028241279434. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Świderski, G.; Lewandowski, W. Biological Activity of New Cichoric Acid-Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Nutrients 2020, 12, 154. [Google Scholar] [CrossRef]
- Ramić, A.; Skočibušić, M.; Odžak, R.; Čipak Gašparović, A.; Milković, L.; Mikelić, A.; Sović, K.; Primožič, I.; Hrenar, T. Antimicrobial Activity of Quasi-Enantiomeric Cinchona Alkaloid Derivatives and Prediction Model Developed by Machine Learning. Antibiotics 2021, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Maurya, N.; Meena, A.; Luqman, S. Cinchonine: A Versatile Pharmacological Agent Derived from Natural Cinchona Alkaloids. Curr. Top. Med. Chem. 2024, 24, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Kalluraya, B.; Sreenivasa, S. Synthesis and pharmacological properties of some quinoline derivatives. Farmaco 1998, 53, 399–404. [Google Scholar] [CrossRef]
- Chen, C.R.; Malik, M.; Snyder, M.; Drlica, K. DNA gyrase and topoisomerase IV on the bacterial chromosome: Quinolone-induced DNA cleavage. J. Mol. Biol. 1996, 258, 627–637. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar] [CrossRef]
- Spencer, A.C.; Panda, S.S. DNA Gyrase as a Target for Quinolones. Biomedicines 2023, 11, 371. [Google Scholar] [CrossRef]
- Fu, H.G.; Li, Z.W.; Hu, X.X.; Si, S.Y.; You, X.F.; Tang, S.; Wang, Y.X.; Song, D.Q. Synthesis and Biological Evaluation of Quinoline Derivatives as a Novel Class of Broad-Spectrum Antibacterial Agents. Molecules 2019, 24, 548. [Google Scholar] [CrossRef]
- Khamkhenshorngphanuch, T.; Kulkraisri, K.; Janjamratsaeng, A.; Plabutong, N.; Thammahong, A.; Manadee, K.; Na Pombejra, S.; Khotavivattana, T. Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-quinolone Analogs. Molecules 2020, 25, 3059. [Google Scholar] [CrossRef]
- Kania, A.; Tejchman, W.; Pawlak, A.M.; Mokrzyński, K.; Różanowski, B.; Musielak, B.M.; Greczek-Stachura, M. Preliminary Studies of Antimicrobial Activity of New Synthesized Hybrids of 2-Thiohydantoin and 2-Quinolone Derivatives Activated with Blue Light. Molecules 2022, 27, 1069. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Tzani, A.; Chronaki, K.; Prousis, K.C.; Pontiki, E.; Hadjiplavlou-Litina, D.; Detsi, A. Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules 2023, 29, 190. [Google Scholar] [CrossRef]
- Gach-Janczak, K.; Piekielna-Ciesielska, J.; Waśkiewicz, J.; Krakowiak, K.; Wtorek, K.; Janecka, A. Quinolin-4-ones: Methods of Synthesis and Application in Medicine. Molecules 2025, 30, 163. [Google Scholar] [CrossRef]
- Ahmed, M.; Kelley, S.O. Enhancing the Potency of Nalidixic Acid toward a Bacterial DNA Gyrase with Conjugated Peptides. ACS Chem. Biol. 2017, 12, 2563–2569. [Google Scholar] [CrossRef]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.B. Mode of action, and in vitro and in vivo activities of the fluoroquinolones. J. Clin. Pharmacol. 1988, 28, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, G.J.; Williams, J.D. Frequency of appearance of resistant variants to norfloxacin and nalidixic acid. J. Antimicrob. Chemother. 1984, 13 (Suppl. B), 33–38. [Google Scholar] [CrossRef]
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings or Curses. Curr. Drug Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef]
- Barlow, A.M. Nalidixic Acid in Infections of Urinary Tract. Laboratory and Clinical Investigations. Br. Med. J. 1963, 2, 1308–1310. [Google Scholar] [CrossRef]
- Reeves, D.S.; Lacey, R.W.; Mummery, R.V.; Mahendra, M.; Bint, A.J.; Newsom, S.W. Treatment of acute urinary infection by norfloxacin or nalidixic acid/citrate: A multi-centre comparative study. J. Antimicrob. Chemother. 1984, 13 (Suppl. B), 99–105. [Google Scholar] [CrossRef]
- Engle, E.C.; Manes, S.H.; Drlica, K. Differential effects of antibiotics inhibiting gyrase. J. Bacteriol. 1982, 149, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Mizuuchi, K.; O’Dea, M.H.; Gellert, M. DNA gyrase: Subunit structure and ATPase activity of the purified enzyme. Proc. Natl. Acad. Sci. USA 1978, 75, 5960–5963. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Heller, K.; Gellert, M.; Zubay, G. Differential sensitivity of gene expression in vitro to inhibitors of DNA gyrase. Proc. Natl. Acad. Sci. USA 1979, 76, 3304–3308. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef]
- Martinez, M.N.; Watts, J.L.; Gilbert, J.M. Questions associated with the development of novel drugs intended for the treatment of bacterial infections in veterinary species. Vet. J. 2019, 248, 79–85. [Google Scholar] [CrossRef]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; Van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef]
- Wolfson, J.S.; Hooper, D.C. The fluoroquinolones: Structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob. Agents Chemother. 1985, 28, 581–586. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Li, Z. Room-Temperature Phosphorescence of Nicotinic Acid and Isonicotinic Acid: Efficient Intermolecular Hydrogen-Bond Interaction in Molecular Array. J. Phys. Chem. Lett. 2022, 13, 1652–1659. [Google Scholar] [CrossRef]
- Rajan, K.; Jaw, R.; Grecz, N. Role of chelation and water binding of calcium in dormancy and heat resistance of bacterial endospores. Bioinorg. Chem. 1978, 8, 477–491. [Google Scholar] [CrossRef]
- Ragab, A.E.; Badawy, E.T.; Aboukhatwa, S.M.; Abdel-Aziz, M.M.; Kabbash, A.; Abo Elseoud, K.A. Isonicotinic acid N-oxide, from isoniazid biotransformation by Aspergillus niger, as an InhA inhibitor antituberculous agent against multiple and extensively resistant strains supported by in silico docking and ADME prediction. Nat. Prod. Res. 2023, 37, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; Macdonald, I.K.; Murphy, E.J.; Brown, K.A.; Raven, E.L.; Moody, P.C. The tuberculosis prodrug isoniazid bound to activating peroxidases. J. Biol. Chem. 2008, 283, 6193–6200. [Google Scholar] [CrossRef]
- Volynets, G.P.; Tukalo, M.A.; Bdzhola, V.G.; Derkach, N.M.; Gumeniuk, M.I.; Tarnavskiy, S.S.; Yarmoluk, S.M. Novel isoniazid derivative as promising antituberculosis agent. Future Microbiol. 2020, 15, 869–879. [Google Scholar] [CrossRef]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of N2-acyl isonicotinic acid hydrazide derivatives. Med. Chem. 2013, 9, 53–76. [Google Scholar] [CrossRef]
- Lewis, J.C. Germination of bacterial spores by calcium chelates of dipicolinic acid analogues. J. Biol. Chem. 1972, 247, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Hintze, P.E.; Nicholson, W.L. Single-spore elemental analyses indicate that dipicolinic acid-deficient Bacillus subtilis spores fail to accumulate calcium. Arch. Microbiol. 2010, 192, 493–497. [Google Scholar] [CrossRef]
- Chen, A.Y.; Thomas, P.W.; Stewart, A.C.; Bergstrom, A.; Cheng, Z.; Miller, C.; Bethel, C.R.; Marshall, S.H.; Credille, C.V.; Riley, C.L.; et al. Dipicolinic Acid Derivatives as Inhibitors of New Delhi Metallo-β-lactamase-1. J. Med. Chem. 2017, 60, 7267–7283. [Google Scholar] [CrossRef] [PubMed]
- Church, B.D.; Halvorson, H. Dependence of the heat resistance of bacterial endospores on their dipicolinic acid content. Nature 1959, 183, 124–125. [Google Scholar] [CrossRef]
- Grecz, N.; Tang, T. Relation of dipicolinic acid to heat resistance of bacterial spores. J. Gen. Microbiol. 1970, 63, 303–310. [Google Scholar] [CrossRef]
- Setlow, B.; Atluri, S.; Kitchel, R.; Koziol-Dube, K.; Setlow, P. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective alpha/beta-type small acid-soluble proteins. J. Bacteriol. 2006, 188, 3740–3747. [Google Scholar] [CrossRef]
- Summers, K.L.; Pushie, M.J.; Sopasis, G.J.; James, A.K.; Dolgova, N.V.; Sokaras, D.; Kroll, T.; Harris, H.H.; Pickering, I.J.; George, G.N. Solution Chemistry of Copper(II) Binding to Substituted 8-Hydroxyquinolines. Inorg. Chem. 2020, 59, 13858–13874. [Google Scholar] [CrossRef]
- Pierre, J.L.; Baret, P.; Serratrice, G. Hydroxyquinolines as iron chelators. Curr. Med. Chem. 2003, 10, 1077–1084. [Google Scholar] [CrossRef]
- Pape, V.F.S.; May, N.V.; Gál, G.T.; Szatmári, I.; Szeri, F.; Fülöp, F.; Szakács, G.; Enyedy, É.A. Impact of copper and iron binding properties on the anticancer activity of 8-hydroxyquinoline derived Mannich bases. Dalton Trans. 2018, 47, 17032–17045. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, H.; Wang, J.; Ji, Z.; Fang, T.; Lu, H.; Yan, L.; Shen, F.; Zhang, D.; Jiang, Y.; et al. Discovery of Novel 8-Hydroxyquinoline Derivatives with Potent In Vitro and In Vivo Antifungal Activity. J. Med. Chem. 2023, 66, 16364–16376. [Google Scholar] [CrossRef] [PubMed]
- Shen, A.Y.; Chen, C.P.; Roffler, S. A chelating agent possessing cytotoxicity and antimicrobial activity: 7-morpholinomethyl-8-hydroxyquinoline. Life Sci. 1999, 64, 813–825. [Google Scholar] [CrossRef]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Bergamini, C.; Fato, R.; Soukup, O.; Korabecny, J.; Andrisano, V.; Bartolini, M.; Bolognesi, M.L. Novel 8-Hydroxyquinoline Derivatives as Multitarget Compounds for the Treatment of Alzheimer’s Disease. ChemMedChem 2016, 11, 1284–1295. [Google Scholar] [CrossRef]
- Fernández-Bachiller, M.I.; Pérez, C.; González-Muñoz, G.C.; Conde, S.; López, M.G.; Villarroya, M.; García, A.G.; Rodríguez-Franco, M.I. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J. Med. Chem. 2010, 53, 4927–4937. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef]
- Fazio, F.; Lionetto, L.; Curto, M.; Iacovelli, L.; Cavallari, M.; Zappulla, C.; Ulivieri, M.; Napoletano, F.; Capi, M.; Corigliano, V.; et al. Xanthurenic Acid Activates mGlu2/3 Metabotropic Glutamate Receptors and is a Potential Trait Marker for Schizophrenia. Sci. Rep. 2015, 5, 17799. [Google Scholar] [CrossRef]
- Wu, Q.; Li, L.; Zhang, Y.; Ming, X.; Feng, N. Measurement methods, influencing factors and applications of intercellular receptor-ligand binding kinetics in diseases. Prog. Biophys. Mol. Biol. 2024, 194, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Taleb, O.; Maammar, M.; Brumaru, D.; Bourguignon, J.J.; Schmitt, M.; Klein, C.; Kemmel, V.; Maitre, M.; Mensah-Nyagan, A.G. Xanthurenic acid binds to neuronal G-protein-coupled receptors that secondarily activate cationic channels in the cell line NCB-20. PLoS ONE 2012, 7, e48553. [Google Scholar] [CrossRef]
- Haruki, H.; Hovius, R.; Pedersen, M.G.; Johnsson, K. Tetrahydrobiopterin Biosynthesis as a Potential Target of the Kynurenine Pathway Metabolite Xanthurenic Acid. J. Biol. Chem. 2016, 291, 652–657. [Google Scholar] [CrossRef]
- Nilsson, L.; Larsson, A.; Begum, A.; Iakovleva, I.; Carlsson, M.; Brännström, K.; Sauer-Eriksson, A.E.; Olofsson, A. Modifications of the 7-Hydroxyl Group of the Transthyretin Ligand Luteolin Provide Mechanistic Insights into Its Binding Properties and High Plasma Specificity. PLoS ONE 2016, 11, e0153112. [Google Scholar] [CrossRef] [PubMed]
- Kontermann, R.E. Half-life extended biotherapeutics. Expert Opin. Biol. Ther. 2016, 16, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Contella, L.; Farrell, C.L.; Boccuto, L.; Litwin, A.; Snyder, M.L. Gene Variant Frequencies of IDO1, IDO2, TDO, and KMO in Substance Use Disorder Cohorts. Genes 2024, 15, 1388. [Google Scholar] [CrossRef]
- Procaccini, C.; Santopaolo, M.; Faicchia, D.; Colamatteo, A.; Formisano, L.; de Candia, P.; Galgani, M.; De Rosa, V.; Matarese, G. Role of metabolism in neurodegenerative disorders. Metabolism 2016, 65, 1376–1390. [Google Scholar] [CrossRef]
- Andrade, J.C.O.; do Vale, T.M.; Gomes, R.L.M.; Forezi, L.; de Souza, M.; Batalha, P.N.; Boechat, F. Exploring 4-quinolone-3-carboxamide derivatives: A versatile framework for emerging biological applications. Bioorg. Chem. 2025, 157, 108240. [Google Scholar] [CrossRef]
- Verma, S.; Lal, S.; Narang, R.; Sudhakar, K. Quinoline Hydrazide/Hydrazone Derivatives: Recent Insights on Antibacterial Activity and Mechanism of Action. ChemMedChem 2023, 18, e202200571. [Google Scholar] [CrossRef]
- Stone, T.W.; Forrest, C.M.; Darlington, L.G. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012, 279, 1386–1397. [Google Scholar] [CrossRef]
- Liu, X.; Lv, H.; Guo, Y.; Teka, T.; Wang, X.; Huang, Y.; Han, L.; Pan, G. Structure-Based Reactivity Profiles of Reactive Metabolites with Glutathione. Chem. Res. Toxicol. 2020, 33, 1579–1593. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 2017, 12, 583–597. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Y.; Wang, Z.; Li, H.; Dong, C.; Zhang, X. Recent Advances in Pro-PROTAC Development to Address On-Target Off-Tumor Toxicity. J. Med. Chem. 2023, 66, 8428–8440. [Google Scholar] [CrossRef]
- Robles, O.; Romo, D. Chemo- and site-selective derivatizations of natural products enabling biological studies. Nat. Prod. Rep. 2014, 31, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Ramann, G.A.; Cowen, B.J. Recent Advances in Metal-Free Quinoline Synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, J.J.; Wang, Z.Y.; Xie, Y.P.; Lu, X. Structural diversity of copper(I) alkynyl cluster-based coordination polymers utilizing bifunctional pyridine carboxylic acid ligands. Nanoscale 2024, 16, 17817–17824. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C. Tuning receptor signaling through ligand engineering. FASEB J. 2020, 34, 1-1. [Google Scholar] [CrossRef]
- Abraham, M.H.; Acree, W.E., Jr. On the solubility of nicotinic acid and isonicotinic acid in water and organic solvents. J. Chem. Thermodyn. 2013, 61, 74–78. [Google Scholar] [CrossRef]
- Kotammagari, T.K.; Saleh, L.Y.; Lönnberg, T. Organometallic modification confers oligonucleotides new functionalities. Chem. Commun. 2024, 60, 3118–3128. [Google Scholar] [CrossRef]
- Murakami, K.; Ueda, T.; Morikawa, R.; Ito, M.; Haneda, M.; Yoshino, M. Antioxidant effect of dipicolinic acid on the metal-catalyzed lipid peroxidation and enzyme inactivation. Biomed. Res. 1998, 19, 205–208. [Google Scholar] [CrossRef][Green Version]
- Dattatray Shinde, S.; Kumar Behera, S.; Kulkarni, N.; Dewangan, B.; Sahu, B. Bifunctional backbone modified squaramide dipeptides as amyloid beta (Aβ) aggregation inhibitors. Bioorg. Med. Chem. 2024, 97, 117538. [Google Scholar] [CrossRef]
- Bhat, S.A.; Henry, R.J.; Blanchard, A.C.; Stoica, B.A.; Loane, D.J.; Faden, A.I. Enhanced Akt/GSK-3β/CREB signaling mediates the anti-inflammatory actions of mGluR5 positive allosteric modulators in microglia and following traumatic brain injury in male mice. J. Neurochem. 2021, 156, 225–248. [Google Scholar] [CrossRef]
- Jiang, J.; Dingledine, R. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol. Sci. 2013, 34, 413–423. [Google Scholar] [CrossRef]
- Falcucci, R.M.; Wertz, R.; Green, J.L.; Meucci, O.; Salvino, J.; Fontana, A.C.K. Novel Positive Allosteric Modulators of Glutamate Transport Have Neuroprotective Properties in an in Vitro Excitotoxic Model. ACS Chem. Neurosci. 2019, 10, 3437–3453. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, W.X.; Dai, S.X.; Guo, Y.C.; Han, F.F.; Zheng, J.J.; Li, G.H.; Huang, J.F. Meta-Analysis of Parkinson’s Disease and Alzheimer’s Disease Revealed Commonly Impaired Pathways and Dysregulation of NRF2-Dependent Genes. J. Alzheimer’s Dis. 2017, 56, 1525–1539. [Google Scholar] [CrossRef]
- Yang, L.; Nao, J.; Dong, X. The Therapeutic Potential of Hydroxycinnamic Acid Derivatives in Parkinson’s Disease: Focus on In Vivo Research Advancements. J. Agric. Food Chem. 2023, 71, 10932–10951. [Google Scholar] [CrossRef]
- Pagotto, G.L.d.O.; Santos, L.M.O.d.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.d.; Goulart, R.d.A.; Catharin, V.M.S. Ginkgo biloba: A leaf of hope in the fight against Alzheimer’s dementia: Clinical trial systematic review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Salomé, C.; Salomé-Grosjean, E.; Park, K.D.; Morieux, P.; Swendiman, R.; DeMarco, E.; Stables, J.P.; Kohn, H. Synthesis and anticonvulsant activities of (R)-N-(4’-substituted)benzyl 2-acetamido-3-methoxypropionamides. J. Med. Chem. 2010, 53, 1288–1305. [Google Scholar] [CrossRef]
- Sharifi, M.; Buzatu, D.; Harris, S.; Wilkes, J. Development of models for predicting Torsade de Pointes cardiac arrhythmias using perceptron neural networks. BMC Bioinform. 2017, 18, 497. [Google Scholar] [CrossRef]
- Assis de Oliveira, B.; Gonçalves de Oliveira, F.; de Assis Cruz, O.; Mendonça de Assis, P.; Glanzmann, N.; David da Silva, A.; Rezende Barbosa Raposo, N.; Zabala Capriles Goliatt, P.V. In Silico and In Vitro Evaluation of Quinoline Derivatives as Potential Inhibitors of AChE, BACE1, and GSK3β for Neurodegenerative Diseases Treatment. Chem. Biodivers. 2025, 22, e202401629. [Google Scholar] [CrossRef] [PubMed]
- Zindo, F.T.; Joubert, J.; Malan, S.F. Propargylamine as functional moiety in the design of multifunctional drugs for neurodegenerative disorders: MAO inhibition and beyond. Future Med. Chem. 2015, 7, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.D.; Terre′Blanche, G.; van der Walt, M.M.; Legoabe, L.J. 5-Substituted 2-benzylidene-1-tetralone analogues as A(1) and/or A(2A) antagonists for the potential treatment of neurological conditions. Bioorg. Chem. 2017, 74, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bongarzone, S.; Bolognesi, M.L. The concept of privileged structures in rational drug design: Focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin. Drug Discov. 2011, 6, 251–268. [Google Scholar] [CrossRef]
- Jun, J.V.; Petersson, E.J.; Chenoweth, D.M. Rational Design and Facile Synthesis of a Highly Tunable Quinoline-Based Fluorescent Small-Molecule Scaffold for Live Cell Imaging. J. Am. Chem. Soc. 2018, 140, 9486–9493. [Google Scholar] [CrossRef]
- Foster, A.C.; Kemp, J.A.; Leeson, P.D.; Grimwood, S.; Donald, A.E.; Marshall, G.R.; Priestley, T.; Smith, J.D.; Carling, R.W. Kynurenic acid analogues with improved affinity and selectivity for the glycine site on the N-methyl-D-aspartate receptor from rat brain. Mol. Pharmacol. 1992, 41, 914–922. [Google Scholar] [CrossRef]
- Kemp, J.A.; Priestley, T. Effects of (+)-HA-966 and 7-chlorokynurenic acid on the kinetics of N-methyl-D-aspartate receptor agonist responses in rat cultured cortical neurons. Mol. Pharmacol. 1991, 39, 666–670. [Google Scholar] [CrossRef]
- Kloog, Y.; Lamdani-Itkin, H.; Sokolovsky, M. The glycine site of the N-methyl-D-aspartate receptor channel: Differences between the binding of HA-966 and of 7-chlorokynurenic acid. J. Neurochem. 1990, 54, 1576–1583. [Google Scholar] [CrossRef]
- Wu, H.Q.; Salituro, F.G.; Schwarcz, R. Enzyme-catalyzed production of the neuroprotective NMDA receptor antagonist 7-chlorokynurenic acid in the rat brain in vivo. Eur. J. Pharmacol. 1997, 319, 13–20. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg. Chem. 2021, 109, 104639. [Google Scholar] [CrossRef]
- Dolšak, A.; Švajger, U.; Lešnik, S.; Konc, J.; Gobec, S.; Sova, M. Selective Toll-like receptor 7 agonists with novel chromeno[3,4-d]imidazol-4(1H)-one and 2-(trifluoromethyl)quinoline/ quinazoline-4-amine scaffolds. Eur. J. Med. Chem. 2019, 179, 109–122. [Google Scholar] [CrossRef]
- Konieczny, J.; Ossowska, K.; Schulze, G.; Coper, H.; Wolfarth, S. L-701,324, a selective antagonist at the glycine site of the NMDA receptor, counteracts haloperidol-induced muscle rigidity in rats. Psychopharmacology 1999, 143, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Foster, A.C.; Leeson, P.D.; Priestley, T.; Tridgett, R.; Iversen, L.L.; Woodruff, G.N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc. Natl. Acad. Sci. USA 1988, 85, 6547–6550. [Google Scholar] [CrossRef]
- Lehmann, J.C.; Procureur, D.; Wood, P.L. 7-Chlorokynurenate prevents NMDA-induced and kainate-induced striatal lesions. Brain Res. 1993, 620, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.L.; Wang, S.J.; Liu, M.M.; Shi, H.S.; Zhang, R.X.; Liu, J.F.; Ding, Z.B.; Lu, L. Glycine site N-methyl-D-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. J. Psychiatry Neurosci. 2013, 38, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Koek, W.; Colpaert, F.C. Selective blockade of N-methyl-D-aspartate (NMDA)-induced convulsions by NMDA antagonists and putative glycine antagonists: Relationship with phencyclidine-like behavioral effects. J. Pharmacol. Exp. Ther. 1990, 252, 349–357. [Google Scholar] [CrossRef]
- Wood, E.R.; Bussey, T.J.; Phillips, A.G. A glycine antagonist 7-chlorokynurenic acid attenuates ischemia-induced learning deficits. Neuroreport 1993, 4, 151–154. [Google Scholar] [CrossRef]
- AdisInsight. Drug Profile AV101. Available online: https://adisinsight.springer.com/drugs/800019948 (accessed on 18 April 2025).
- Baron, B.M.; Harrison, B.L.; Miller, F.P.; McDonald, I.A.; Salituro, F.G.; Schmidt, C.J.; Sorensen, S.M.; White, H.S.; Palfreyman, M.G. Activity of 5,7-dichlorokynurenic acid, a potent antagonist at the N-methyl-D-aspartate receptor-associated glycine binding site. Mol. Pharmacol. 1990, 38, 554–561. [Google Scholar] [CrossRef]
- Baron, B.M.; Siegel, B.W.; Slone, A.L.; Harrison, B.L.; Palfreyman, M.G.; Hurt, S.D. [3H]5,7-dichlorokynurenic acid, a novel radioligand labels NMDA receptor-associated glycine binding sites. Eur. J. Pharmacol. 1991, 206, 149–154. [Google Scholar] [CrossRef]
- McNamara, D.; Smith, E.C.; Calligaro, D.O.; O’Malley, P.J.; McQuaid, L.A.; Dingledine, R. 5,7-Dichlorokynurenic acid, a potent and selective competitive antagonist of the glycine site on NMDA receptors. Neurosci. Lett. 1990, 120, 17–20. [Google Scholar] [CrossRef]
- Hurt, S.D.; Baron, B.M. [3H] 5,7-dichlorokynurenic acid, a high affinity ligand for the NMDA receptor glycine regulatory site. J. Recept. Res. 1991, 11, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Corbett, R.; Dunn, R.W. Effects of 5,7 dichlorokynurenic acid on conflict, social interaction and plus maze behaviors. Neuropharmacology 1993, 32, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Plaznik, A.; Palejko, W.; Nazar, M.; Jessa, M. Effects of antagonists at the NMDA receptor complex in two models of anxiety. Eur. Neuropsychopharmacol. 1994, 4, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Hlinák, Z.; Krejci, I. Kynurenic acid and 5,7-dichlorokynurenic acids improve social and object recognition in male rats. Psychopharmacology 1995, 120, 463–469. [Google Scholar] [CrossRef]
- Priestley, T.; Laughton, P.; Macaulay, A.J.; Hill, R.G.; Kemp, J.A. Electrophysiological characterisation of the antagonist properties of two novel NMDA receptor glycine site antagonists, L-695,902 and L-701,324. Neuropharmacology 1996, 35, 1573–1581. [Google Scholar] [CrossRef]
- Dannhardt, G.; Kohl, B.K. The glycine site on the NMDA receptor: Structure-activity relationships and possible therapeutic applications. Curr. Med. Chem. 1998, 5, 253–263. [Google Scholar] [CrossRef]
- Kotlinska, J.; Liljequist, S. A characterization of anxiolytic-like actions induced by the novel NMDA/glycine site antagonist, L-701,324. Psychopharmacology 1998, 135, 175–181. [Google Scholar] [CrossRef]
- Potschka, H.; Löscher, W.; Wlaź, P.; Behl, B.; Hofmann, H.P.; Treiber, H.J.; Szabo, L. LU 73068, a new non-NMDA and glycine/NMDA receptor antagonist: Pharmacological characterization and comparison with NBQX and L-701,324 in the kindling model of epilepsy. Br. J. Pharmacol. 1998, 125, 1258–1266. [Google Scholar] [CrossRef]
- Bristow, L.J.; Flatman, K.L.; Hutson, P.H.; Kulagowski, J.J.; Leeson, P.D.; Young, L.; Tricklebank, M.D. The atypical neuroleptic profile of the glycine/N-methyl-D-aspartate receptor antagonist, L-701,324, in rodents. J. Pharmacol. Exp. Ther. 1996, 277, 578–585. [Google Scholar] [CrossRef]
- Christoph, T.; Reissmüller, E.; Schiene, K.; Englberger, W.; Chizh, B.A. Antiallodynic effects of NMDA glycine(B) antagonists in neuropathic pain: Possible peripheral mechanisms. Brain Res. 2005, 1048, 218–227. [Google Scholar] [CrossRef]
- Lees, K.R.; Asplund, K.; Carolei, A.; Davis, S.M.; Diener, H.-C.; Kaste, M.; Orgogozo, J.-M.; Whitehead, J. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: A randomised controlled trial. Lancet 2000, 355, 1949–1954. [Google Scholar] [CrossRef]
- Banks, P.; Franks, N.P.; Dickinson, R. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor mediates xenon neuroprotection against hypoxia-ischemia. Anesthesiology 2010, 112, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Benneyworth, M.A.; Basu, A.C.; Coyle, J.T. Discordant behavioral effects of psychotomimetic drugs in mice with altered NMDA receptor function. Psychopharmacology 2011, 213, 143–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haley, E.C., Jr.; Thompson, J.L.; Levin, B.; Davis, S.; Lees, K.R.; Pittman, J.G.; DeRosa, J.T.; Ordronneau, P.; Brown, D.L.; Sacco, R.L. Gavestinel does not improve outcome after acute intracerebral hemorrhage: An analysis from the GAIN International and GAIN Americas studies. Stroke 2005, 36, 1006–1010. [Google Scholar] [CrossRef]
- Sacco, R.L.; DeRosa, J.T.; Haley, E.C., Jr.; Levin, B.; Ordronneau, P.; Phillips, S.J.; Rundek, T.; Snipes, R.G.; Thompson, J.L.; Investigators, G.A. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: A randomized controlled trial. JAMA 2001, 285, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, W.; Wang, Z. Laquinimod protects against TNF-α-induced attachment of monocytes to human aortic endothelial cells (HAECs) by increasing the expression of KLF2. Drug Des. Dev. Ther. 2020, 14, 1683–1691. [Google Scholar] [CrossRef]
- Nessler, S.; Ott, M.; Wegner, C.; Hayardeny, L.; Ullrich, E.; Bruck, W. Laquinimod suppresses CNS autoimmunity by activation of natural killer cells (P1. 161). Neurology 2015, 84, P1–P161. [Google Scholar] [CrossRef]
- Ott, M.; Avendaño-Guzmán, E.; Ullrich, E.; Dreyer, C.; Strauss, J.; Harden, M.; Schön, M.; Schön, M.P.; Bernhardt, G.; Stadelmann, C. Laquinimod, a prototypic quinoline-3-carboxamide and aryl hydrocarbon receptor agonist, utilizes a CD155-mediated natural killer/dendritic cell interaction to suppress CNS autoimmunity. J. Neuroinflammation 2019, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Ellrichmann, G.; Blusch, A.; Fatoba, O.; Brunner, J.; Reick, C.; Hayardeny, L.; Hayden, M.; Sehr, D.; Winklhofer, K.F.; Saft, C. Laquinimod treatment in the R6/2 mouse model. Sci. Rep. 2017, 7, 4947. [Google Scholar] [CrossRef]
- Rothhammer, V.; Kenison, J.E.; Li, Z.; Tjon, E.; Takenaka, M.C.; Chao, C.-C.; Alves de Lima, K.; Borucki, D.M.; Kaye, J.; Quintana, F.J. Aryl hydrocarbon receptor activation in astrocytes by laquinimod ameliorates autoimmune inflammation in the CNS. Neurol. Neuroimmunol. Neuroinflammation 2021, 8, e946. [Google Scholar] [CrossRef]
- Comi, G.; Jeffery, D.; Kappos, L.; Montalban, X.; Boyko, A.; Rocca, M.A.; Filippi, M. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N. Engl. J. Med. 2012, 366, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Sorensen, P.; Selmaj, K.; Zipp, F.; Havrdova, E.; Cohen, J.; Sasson, N.; Gilgun-Sherki, Y.; Arnold, D.; Group, B.S. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J. Neurol. 2014, 261, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; Dadon, Y.; Sasson, N.; Steinerman, J.R.; Knappertz, V.; Vollmer, T.L.; Boyko, A.; Vermersch, P.; Ziemssen, T.; Montalban, X. CONCERTO: A randomized, placebo-controlled trial of oral laquinimod in relapsing-remitting multiple sclerosis. Mult. Scler. J. 2022, 28, 608–619. [Google Scholar] [CrossRef]
- Isaacs, J.T. The long and winding road for the development of tasquinimod as an oral second-generation quinoline-3-carboxamide antiangiogenic drug for the treatment of prostate cancer. Expert Opin. Investig. Drugs 2010, 19, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Sundstedt, A.; Ciesielski, M.; Miles, K.M.; Celander, M.; Adelaiye, R.; Orillion, A.; Ciamporcero, E.; Ramakrishnan, S.; Ellis, L. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol. Res. 2015, 3, 136–148. [Google Scholar] [CrossRef]
- Wobus, M.; Balaian, E.; Oelschlaegel, U.; Towers, R.; Möbus, K.; Habermann, I.; Wehner, R.; Stölzel, F.; Chavakis, T.; Törngren, M. Targeting the Inflammatory Niche in MDS By Tasquinimod Restores Hematopoietic Support and Suppresses Immune-Checkpoint Expression in Vitro. Blood 2021, 138, 2596. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liu, C.; Peng, Q.; Wu, Y.; Wen, Y.; Zheng, R.; Yan, Q.; Ma, J. Macrophage-Biomimetic Nanoparticles Ameliorate Ulcerative Colitis through Reducing Inflammatory Factors Expression. J. Innate Immun. 2022, 14, 380–392. [Google Scholar] [CrossRef]
- Cristóvão, J.S.; Gomes, C.M. S100 Proteins in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 463. [Google Scholar] [CrossRef]
- Leimkühler, N.B.; Gleitz, H.F.E.; Ronghui, L.; Snoeren, I.A.M.; Fuchs, S.N.R.; Nagai, J.S.; Banjanin, B.; Lam, K.H.; Vogl, T.; Kuppe, C.; et al. Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell 2021, 28, 637–652.e638. [Google Scholar] [CrossRef]
- Xu, K.; Dai, X.L.; Huang, H.C.; Jiang, Z.F. Targeting HDACs: A promising therapy for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2011, 2011, 143269. [Google Scholar] [CrossRef]
- Ganai, S.A.; Ramadoss, M.; Mahadevan, V. Histone Deacetylase (HDAC) Inhibitors—Emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration. Curr. Neuropharmacol. 2016, 14, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Bhatia, N.; Biharee, A.; Kulkarni, S.; Thareja, S.; Monga, V. Developing our knowledge of the quinolone scaffold and its value to anticancer drug design. Expert Opin. Drug Discov. 2023, 18, 1151–1167. [Google Scholar] [CrossRef]
- Kleckner, N.; Dingledine, R. Selectivity of quinoxalines and kynurenines as antagonists of the glycine site on N-methyl-D-aspartate receptors. Mol. Pharmacol. 1989, 36, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Culletta, G.; Tutone, M.; Zappalà, M.; Almerico, A.M. Sulfonamide moiety as “molecular chimera” in the design of new drugs. Curr. Med. Chem. 2023, 30, 128–163. [Google Scholar] [CrossRef]
- Brnardic, E.J.; Ye, G.; Brooks, C.; Donatelli, C.; Barton, L.; McAtee, J.; Sanchez, R.M.; Shu, A.; Erhard, K.; Terrell, L. Discovery of pyrrolidine sulfonamides as selective and orally bioavailable antagonists of transient receptor potential vanilloid-4 (TRPV4). J. Med. Chem. 2018, 61, 9738–9755. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, C.; Zhang, X.; Zhu, J.; Wang, L.; Ji, M.; Zhang, Z.; Ji, X.-M.; Wang, S.-L. Perfluorooctane sulfonate disrupts the blood brain barrier through the crosstalk between endothelial cells and astrocytes in mice. Environ. Pollut. 2020, 256, 113429. [Google Scholar] [CrossRef]
- Ma, H.; Brust, T.; Frankowski, K.J.; Lovell, K.M.; Cameron, M.D.; Bohn, L.M.; Aube, J. Advances in Sulfonamide Kappa Opioid Receptor Antagonists: Structural Refinement and Evaluation of CNS Clearance. ACS Chem. Neurosci. 2022, 13, 1315–1332. [Google Scholar] [CrossRef]
- Zong, P.; Feng, J.; Yue, Z.; Li, Y.; Wu, G.; Sun, B.; He, Y.; Miller, B.; Albert, S.Y.; Su, Z. Functional coupling of TRPM2 and extrasynaptic NMDARs exacerbates excitotoxicity in ischemic brain injury. Neuron 2022, 110, 1944–1958.e1948. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef]
- Butcher, S.P.; Henshall, D.C.; Teramura, Y.; Iwasaki, K.; Sharkey, J. Neuroprotective actions of FK506 in experimental stroke: In vivo evidence against an antiexcitotoxic mechanism. J. Neurosci. 1997, 17, 6939–6946. [Google Scholar] [CrossRef]
- Singh, D.; Wasan, H.; Reeta, K. Preclinical stroke research and translational failure: A bird’s eye view on preventable variables. Cell. Mol. Neurobiol. 2022, 42, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.l.; Golubczyk, D.; Tolppanen, A.M.; Boltze, J.; Jolkkonen, J. Cell therapy for ischemic stroke: Are differences in preclinical and clinical study design responsible for the translational loss of efficacy? Ann. Neurol. 2019, 86, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Fisher, M. International, multicenter randomized preclinical trials in translational stroke research: It’s time to act. J. Cereb. Blood Flow Metab. 2012, 32, 933–935. [Google Scholar] [CrossRef]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2020, 9, 1777625. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, B.J.; van Baren, N.; Baurain, J.-F. Is there a clinical future for IDO1 inhibitors after the failure of epacadostat in melanoma? Annu. Rev. Cancer Biol. 2020, 4, 241–256. [Google Scholar] [CrossRef]
- Muller, A.J.; Manfredi, M.G.; Zakharia, Y.; Prendergast, G.C. Inhibiting IDO pathways to treat cancer: Lessons from the ECHO-301 trial and beyond. Semin. Immunopathol. 2019, 41, 41–48. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Yan, Y.; Kumar, A.B.; Finnes, H.; Markovic, S.N.; Park, S.; Dronca, R.S.; Dong, H. Combining immune checkpoint inhibitors with conventional cancer therapy. Front. Immunol. 2018, 9, 1739. [Google Scholar] [CrossRef]
- Desnoyer, A.; Broutin, S.; Delahousse, J.; Maritaz, C.; Blondel, L.; Mir, O.; Chaput, N.; Paci, A. Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 2, immune checkpoint inhibitor antibodies. Eur. J. Cancer 2020, 128, 119–128. [Google Scholar] [CrossRef]
- Metropulos, A.E.; Munshi, H.G.; Principe, D.R. The difficulty in translating the preclinical success of combined TGFβ and immune checkpoint inhibition to clinical trial. EBioMedicine 2022, 86, 104380. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Lab to Life: Exploring Cutting-Edge Models for Neurological and Psychiatric Disorders. Biomedicines 2024, 12, 613. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, P.S.; Bar-Or, A. Laquinimod in multiple sclerosis. Clin. Immunol. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Brück, W.; Zamvil, S.S. Laquinimod, a once-daily oral drug in development for the treatment of relapsing–remitting multiple sclerosis. Expert Rev. Clin. Pharmacol. 2012, 5, 245–256. [Google Scholar] [CrossRef]
- Haggiag, S.; Ruggieri, S.; Gasperini, C. Efficacy and safety of laquinimod in multiple sclerosis: Current status. Ther. Adv. Neurol. Disord. 2013, 6, 343–352. [Google Scholar] [CrossRef]
- Ahmad, S.R.; Zeyaullah, M.; Khan, M.S.; AlShahrani, A.M.; Altijani, A.A.G.; Ali, H.; Dawria, A.; Mohieldin, A.; Alam, M.S.; Mohamed, A.O.A. Pharmacogenomics for neurodegenerative disorders—A focused review. Front. Pharmacol. 2024, 15, 1478964. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef]
- Hutson, P.H.; Barton, C.L. L-701,324, a glycine/NMDA receptor antagonist, blocks the increase of cortical dopamine metabolism by stress and DMCM. Eur. J. Pharmacol. 1997, 326, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zellinger, C.; Salvamoser, J.D.; Soerensen, J.; van Vliet, E.A.; Aronica, E.; Gorter, J.; Potschka, H. Pre-treatment with the NMDA receptor glycine-binding site antagonist L-701,324 improves pharmacosensitivity in a mouse kindling model. Epilepsy Res. 2014, 108, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.K.; Dedek, A.; Bonin, R.P.; Salter, M.W.; Snutch, T.P.; Hildebrand, M.E. The T-type calcium channel antagonist, Z944, reduces spinal excitability and pain hypersensitivity. Br. J. Pharmacol. 2021, 178, 3517–3532. [Google Scholar] [CrossRef]
- Obrenovitch, T.P.; Hardy, A.M.; Zilkha, E. Effects of L-701,324, a high-affinity antagonist at the N-methyl-D-aspartate (NMDA) receptor glycine site, on the rat electroencephalogram. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 355, 779–786. [Google Scholar] [CrossRef]
- Egunlusi, A.O.; Joubert, J. NMDA receptor antagonists: Emerging insights into molecular mechanisms and clinical applications in neurological disorders. Pharmaceuticals 2024, 17, 639. [Google Scholar] [CrossRef] [PubMed]
- Lotti, J.S.; Jones, J.; Farnsworth, J.C.; Yi, F.; Zhao, F.; Menniti, F.S.; Volkmann, R.A.; Clausen, R.P.; Hansen, K.B. Evaluation of allosteric N-methyl-d-aspartate receptor modulation by GluN2A-selective antagonists using pharmacological equilibrium modeling. Mol. Pharmacol. 2025, 107, 100004. [Google Scholar] [CrossRef]
- Ahmed, H.; Haider, A.; Ametamey, S.M. N-Methyl-D-Aspartate (NMDA) receptor modulators: A patent review (2015-present). Expert Opin. Ther. Pat. 2020, 30, 743–767. [Google Scholar] [CrossRef]
- Verma, S.; Ambatwar, R.; Datusalia, A.K.; Khatik, G.L. Convenient One-Pot Synthesis of Kynurenic Acid Ethyl Ester and Exploration to Direct Synthesis of Neuroprotective Kynurenic Acid and Amide Derivatives. J. Org. Chem. 2023, 88, 10494–10500. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: A recent perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T.; Antony, L.; Dalrymple, S.L.; Brennen, W.N.; Gerber, S.; Hammers, H.; Wissing, M.; Kachhap, S.; Luo, J.; Xing, L.; et al. Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013, 73, 1386–1399. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dalgleish, A.; Damber, J.-E.; Smith, M.; Pili, R. Mechanisms of action of tasquinimod on the tumour microenvironment. Cancer Chemother. Pharmacol. 2014, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Gonzalez-Lopez, M.; Galang, C.; Garcia, C.; Lemar, H.; Lu, J.; Vaidya, K.; Fischer, M.; Frey, C.; Alam, M. Small-molecule antagonists of the aryl hydrocarbon receptor (AhR) promote activation of human PBMCs in vitro and demonstrate significant impact on tumor growth and immune modulation in vivo. Cancer Res. 2018, 78, 4719. [Google Scholar] [CrossRef]
- Acharya, A.; Yadav, M.; Nagpure, M.; Kumaresan, S.; Guchhait, S.K. Molecular medicinal insights into scaffold hopping-based drug discovery success. Drug Discov. Today 2024, 29, 103845. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, Q.; Yuan, Z.; Fan, T.; Zhang, C.; Liu, Z.; Chu, B.; Sun, Q.; Chen, Y.; Jiang, Y. Quinazoline-based hydroxamic acid derivatives as dual histone methylation and deacetylation inhibitors for potential anticancer agents. Bioorganic Med. Chem. 2022, 53, 116524. [Google Scholar] [CrossRef]
- Ma, C.; Taghour, M.S.; Belal, A.; Mehany, A.B.M.; Mostafa, N.; Nabeeh, A.; Eissa, I.H.; Al-Karmalawy, A.A. Design and Synthesis of New Quinoxaline Derivatives as Potential Histone Deacetylase Inhibitors Targeting Hepatocellular Carcinoma: In Silico, In Vitro, and SAR Studies. Front. Chem. 2021, 9, 725135. [Google Scholar] [CrossRef] [PubMed]
- Sforzini, L.; Nettis, M.A.; Mondelli, V.; Pariante, C.M. Inflammation in cancer and depression: A starring role for the kynurenine pathway. Psychopharmacology 2019, 236, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Maddison, D.C.; Giorgini, F. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 2015, 40, 134–141. [Google Scholar] [CrossRef]
- Li, T.; Kung, H.J.; Mack, P.C.; Gandara, D.R. Genotyping and genomic profiling of non-small-cell lung cancer: Implications for current and future therapies. J. Clin. Oncol. 2013, 31, 1039–1049. [Google Scholar] [CrossRef]
- Correia, A.C.; Monteiro, A.R.; Silva, R.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: Crossing or circumventing the blood-brain barrier (BBB) to manage neurological disorders. Adv. Drug Deliv. Rev. 2022, 189, 114485. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Edgecomb, S.X.; Hamadani, C.M.; Tanner, E.E.L.; Manickam, D.S. Lipid nanoparticle-mediated drug delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114861. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-modified lipid nanocarriers for crossing the blood-brain barrier (BBB): A current overview of active targeting in brain diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999. [Google Scholar] [CrossRef]
- Rahimi, S.; Khoee, S.; Ghandi, M. Preparation and characterization of rod-like chitosan-quinoline nanoparticles as pH-responsive nanocarriers for quercetin delivery. Int. J. Biol. Macromol. 2019, 128, 279–289. [Google Scholar] [CrossRef]
- Ren, B.; Guo, C.; Liu, R.-Z.; Bian, Z.-Y.; Liu, R.-C.; Huang, L.-F.; Tang, J.-J. Imidazolylacetophenone oxime-based multifunctional neuroprotective agents: Discovery and structure-activity relationships. Eur. J. Med. Chem. 2022, 228, 114031. [Google Scholar] [CrossRef]
- Knyihar-Csillik, E.; Mihaly, A.; Krisztin-Peva, B.; Robotka, H.; Szatmari, I.; Fulop, F.; Toldi, J.; Csillik, B.; Vecsei, L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: Comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008, 61, 429–432. [Google Scholar] [CrossRef]
- Kassai, F.; Kedves, R.; Gyertyán, I.; Tuka, B.; Fülöp, F.; Toldi, J.; Lendvai, B.; Vécsei, L. Effect of a kynurenic acid analog on home-cage activity and body temperature in rats. Pharmacol. Rep. 2015, 67, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. The impact of C-3 side chain modifications on Kynurenic Acid: A behavioral analysis of its analogs in the Motor Domain. Int. J. Mol. Sci. 2024, 25, 3394. [Google Scholar] [CrossRef] [PubMed]
- Poles, M.Z.; Nászai, A.; Gulácsi, L.; Czakó, B.L.; Gál, K.G.; Glenz, R.J.; Dookhun, D.; Rutai, A.; Tallósy, S.P.; Szabó, A. Kynurenic acid and its synthetic derivatives protect against sepsis-associated neutrophil activation and brain mitochondrial dysfunction in rats. Front. Immunol. 2021, 12, 717157. [Google Scholar] [CrossRef]
- Mándi, Y.; Endrész, V.; Mosolygó, T.; Burián, K.; Lantos, I.; Fülöp, F.; Szatmári, I.; Lőrinczi, B.; Balog, A.; Vécsei, L. The opposite effects of kynurenic acid and different kynurenic acid analogs on tumor necrosis factor-α (TNF-α) production and tumor necrosis factor-stimulated gene-6 (TSG-6) expression. Front. Immunol. 2019, 10, 1406. [Google Scholar] [CrossRef]
- Knapp, L.; Szita, B.; Kocsis, K.; Vécsei, L.; Toldi, J. Nitroglycerin enhances the propagation of cortical spreading depression: Comparative studies with sumatriptan and novel kynurenic acid analogues. Drug Des. Dev. Ther. 2016, 11, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Lőrinczi, B.; Szatmári, I.; Fülöp, F.; Vécsei, L. Antidepressant-like Effects of Kynurenic Acid Analogues. In Proceedings of the 1st International Electronic Conference on Biomedicine, Online, 1–26 March 2021; p. 26. [Google Scholar]
- Evelin, H.L.F. Kinurénsav-Analógok In Vitro Elektrofiziológiai Vizsgálata az Ischaemiás Stroke Szempontjából; Szegedi Tudomanyegyetem: Szeged, Hungary, 2022. [Google Scholar]
- Leeson, P.D.; Baker, R.; Carling, R.W.; Curtis, N.R.; Moore, K.W.; Williams, B.J.; Foster, A.C.; Donald, A.E.; Kemp, J.A.; Marshall, G.R. Kynurenic acid derivatives. Structure-activity relationships for excitatory amino acid antagonism and identification of potent and selective antagonists at the glycine site on the N-methyl-D-aspartate receptor. J. Med. Chem. 1991, 34, 1243–1252. [Google Scholar] [CrossRef]
- Vasilopoulos, A.; Krska, S.W.; Stahl, S.S. C(sp3)–H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling. Science 2021, 372, 398–403. [Google Scholar] [CrossRef]
- Comeau, C.; Ries, B.; Stadelmann, T.; Tremblay, J.; Poulet, S.; Frohlich, U.; Côté, J.; Boudreault, P.-L.; Derbali, R.M.; Sarret, P. Modulation of the passive permeability of semipeptidic macrocycles: N-and C-methylations fine-tune conformation and properties. J. Med. Chem. 2021, 64, 5365–5383. [Google Scholar] [CrossRef]
- Jóźwiak, K.; Płazińska, A. Structural insights into ligand—Receptor interactions involved in biased agonism of G-protein coupled receptors. Molecules 2021, 26, 851. [Google Scholar] [CrossRef]
- Liu, L.; Ge, J.; Fu, H.; Wang, J.; Chen, Y.; Sun, Y.; Zhao, C.; Li, Y.; Li, Y. A fluorinated prodrug strategy enhances the therapeutic index of nanoparticle-delivered hydrophilic drugs. Colloids Surf. B Biointerfaces 2025, 249, 114539. [Google Scholar] [CrossRef]
- Chen, K.J.; Plaunt, A.J.; Leifer, F.G.; Kang, J.Y.; Cipolla, D. Recent advances in prodrug-based nanoparticle therapeutics. Eur. J. Pharm. Biopharm. 2021, 165, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tuka, B.; Vécsei, L. Navigating the Neurobiology of Migraine: From pathways to potential therapies. Cells 2024, 13, 1098. [Google Scholar] [CrossRef] [PubMed]
- Juhász, L.; Spisák, K.; Szolnoki, B.G.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J. Neurochem. 2025; in press. [Google Scholar]
- Deora, G.S.; Kantham, S.; Chan, S.; Dighe, S.N.; Veliyath, S.K.; McColl, G.; Parat, M.O.; McGeary, R.P.; Ross, B.P. Multifunctional Analogs of Kynurenic Acid for the Treatment of Alzheimer’s Disease: Synthesis, Pharmacology, and Molecular Modeling Studies. ACS Chem. Neurosci. 2017, 8, 2667–2675. [Google Scholar] [CrossRef]
- Yoshida, Y.; Fujigaki, H.; Kato, K.; Yamazaki, K.; Fujigaki, S.; Kunisawa, K.; Yamamoto, Y.; Mouri, A.; Oda, A.; Nabeshima, T.; et al. Selective and competitive inhibition of kynurenine aminotransferase 2 by glycyrrhizic acid and its analogues. Sci. Rep. 2019, 9, 10243. [Google Scholar] [CrossRef] [PubMed]
- Agrafiotis, D.K.; Shemanarev, M.; Connolly, P.J.; Farnum, M.; Lobanov, V.S. SAR maps: A new SAR visualization technique for medicinal chemists. J. Med. Chem. 2007, 50, 5926–5937. [Google Scholar] [CrossRef]
- Lipinski, C.A. Overview of hit to lead: The medicinal chemist’s role from HTS retest to lead optimization hand off. In Lead-Seeking Approaches; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–24. [Google Scholar]
- Segall, M.D.; Beresford, A.P.; Gola, J.M.; Hawksley, D.; Tarbit, M.H. Focus on success: Using a probabilistic approach to achieve an optimal balance of compound properties in drug discovery. Expert Opin. Drug Metab. Toxicol. 2006, 2, 325–337. [Google Scholar] [CrossRef]
- Ward, K.W. Optimizing pharmacokinetic properties and attaining candidate selection. In Reducing Drug Attrition; Springer: Berlin/Heidelberg, Germany, 2014; pp. 73–95. [Google Scholar]
- Qin, D.; Lin, X.; Liu, Z.; Chen, Y.; Zhang, Z.; Wu, C.; Liu, L.; Pan, Y.; Laquerre, S.; Emery, J. Discovery of orally bioavailable ligand efficient quinazolindiones as potent and selective tankyrases inhibitors. ACS Med. Chem. Lett. 2021, 12, 1005–1010. [Google Scholar] [CrossRef]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef]
- Mager, D.E. Quantitative structure–pharmacokinetic/pharmacodynamic relationships. Adv. Drug Deliv. Rev. 2006, 58, 1326–1356. [Google Scholar] [CrossRef]
- Guha, R. On exploring structure–activity relationships. In In Silico Models for Drug Discovery; Humana Press: Totowa, NJ, USA, 2013; pp. 81–94. [Google Scholar]
- Yan, G.; Rose, J.; Ellison, C.; Mudd, A.M.; Zhang, X.; Wu, S. Refine and strengthen SAR-based read-across by considering bioactivation and modes of action. Chem. Res. Toxicol. 2023, 36, 1532–1548. [Google Scholar] [CrossRef]
- Brogi, S.; Tabanelli, R.; Calderone, V. Combinatorial approaches for novel cardiovascular drug discovery: A review of the literature. Expert Opin. Drug Discov. 2022, 17, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.-Y.; Chen, S.; Wang, Y.; Yuan, Y.; Yang, X.; Hu, W.; Chen, B.; Qi, Z.; Huse, J.T. Characterization of an Enhancer RNA Signature Reveals Treatment Strategies for Improving Immunotherapy Efficacy in Cancer. Cancer Res. 2025, 85, 1530–1543. [Google Scholar] [CrossRef]
- Günther, J.; Däbritz, J.; Wirthgen, E. Limitations and off-target effects of tryptophan-related IDO inhibitors in cancer treatment. Front. Immunol. 2019, 10, 1801. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, M.; Chen, Q.; He, Y.; Wang, W.; Wang, Z. IDO as a drug target for cancer immunotherapy: Recent developments in IDO inhibitors discovery. RSC Adv. 2016, 6, 7575–7581. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohar, Z.; Vecsei, L. Are kynurenines accomplices or principal villains in dementia? Maintenance of kynurenine metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef]
- Papathanasiou, T.; Strathe, A.; Hooker, A.C.; Lund, T.M.; Overgaard, R.V. Feasibility of exposure-response analyses for clinical dose-ranging studies of drug combinations. AAPS J. 2018, 20, 64. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Michor, F. Pharmacokinetics and drug interactions determine optimum combination strategies in computational models of cancer evolution. Cancer Res. 2017, 77, 3908–3921. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Weiss, A.; Ding, X.; Dyson, P.J.; Van Den Bergh, H.; Griffioen, A.W.; Ho, C.-M. Optimization of drug combinations using Feedback System Control. Nat. Protoc. 2016, 11, 302–315. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Perez-Castro, L.; Garcia, R.; Venkateswaran, N.; Barnes, S.; Conacci-Sorrell, M. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J. 2023, 290, 7–27. [Google Scholar] [CrossRef]
- Zuccarello, E.; Zhang, H.; Acquarone, E.; Pham, D.; Staniszewski, A.; Deng, S.X.; Landry, D.W.; Arancio, O.; Fiorito, J. Optimizing metabolic stability of phosphodiesterase 5 inhibitors: Discovery of a potent N-(pyridin-3-ylmethyl)quinoline derivative targeting synaptic plasticity. Bioorg. Med. Chem. Lett. 2023, 92, 129409. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.; Tanaka, M.; Lamas, C.; Quesada, K.; Detregiachi, C.; Araújo, A.; Guiguer, E.; Catharin, V.; de Castro, M.; Junior, E. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Giménez-Llort, L.; Chen, C.; Hepsomali, P.; Avenanti, A.; Vécsei, L. Innovation at the Intersection: Emerging Translational Research in Neurology and Psychiatry. Cells 2024, 13, 790. [Google Scholar] [CrossRef]
- Jena, S.; Gonzalez, G.; Vítek, D.; Kvasnicová, M.; Štěpánková, Š.; Strnad, M.; Voller, J.; Chanda, K. Novel neuroprotective 5,6-dihydropyrido[2’,1’:2,3]imidazo[4,5-c]quinoline derivatives acting through cholinesterase inhibition and CB2 signaling modulation. Eur. J. Med. Chem. 2024, 276, 116592. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Zengin, G.; Bumbu, A.G.; Andronie-Cioara, F.L.; Nechifor, A.C.; et al. The Footprint of Kynurenine Pathway in Neurodegeneration: Janus-Faced Role in Parkinson’s Disorder and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6737. [Google Scholar] [CrossRef]
- Shen, H.; Xu, X.; Bai, Y.; Wang, X.; Wu, Y.; Zhong, J.; Wu, Q.; Luo, Y.; Shang, T.; Shen, R.; et al. Therapeutic potential of targeting kynurenine pathway in neurodegenerative diseases. Eur. J. Med. Chem. 2023, 251, 115258. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, N.; Kofinas, A.; Papanikolaou, M.G.; Miras, H.N.; Drouza, C.; Kalampounias, A.G.; Kabanos, T.A.; Konstandi, M.; Leondaritis, G. Synthesis, characterization and pharmacological evaluation of quinoline derivatives and their complexes with copper(ΙΙ) in in vitro cell models of Alzheimer’s disease. J. Inorg. Biochem. 2021, 217, 111393. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E. AdipoRon’s Impact on Alzheimer’s Disease—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 484. [Google Scholar] [CrossRef]
- Adlard, P.A.; Cherny, R.A.; Finkelstein, D.I.; Gautier, E.; Robb, E.; Cortes, M.; Volitakis, I.; Liu, X.; Smith, J.P.; Perez, K.; et al. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron 2008, 59, 43–55. [Google Scholar] [CrossRef]
- Huang, W.; Liang, M.; Li, Q.; Zheng, X.; Zhang, C.; Wang, Q.; Tang, L.; Zhang, Z.; Wang, B.; Shen, Z. Development of the “hidden” multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 247–258. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Körtési, T.; Szok, D.; Tajti, J.; Vécsei, L. From CGRP to PACAP, VIP, and Beyond: Unraveling the Next Chapters in Migraine Treatment. Cells 2023, 12, 2649. [Google Scholar] [CrossRef]
- Sarkar, S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem. Soc. Trans. 2013, 41, 1103–1130. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Liu, L.; Gan, W. The Roles of Post-Translational Modifications on mTOR Signaling. Int. J. Mol. Sci. 2021, 22, 1784. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Watts Alexander, C.S.; Dhanasekaran, M.; Moore, T. The Influence of Kynurenine Metabolites on Neurodegenerative Pathologies. Int. J. Mol. Sci. 2024, 25, 853. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007, 257, 221–239. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J. Neural Transm. 2024, 131, 1367–1387. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in Cognitive Impairment and Mood Disorders for Experimental, Clinical and Translational Neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural Correlates and Molecular Mechanisms of Memory and Learning. Int. J. Mol. Sci. 2024, 25, 2724. [Google Scholar] [CrossRef]
- Jászberényi, M.; Thurzó, B.; Bagosi, Z.; Vécsei, L.; Tanaka, M. The Orexin/Hypocretin System, the Peptidergic Regulator of Vigilance, Orchestrates Adaptation to Stress. Biomedicines 2024, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Manetti, F.; Maresca, L.; Crivaro, E.; Pepe, S.; Cini, E.; Singh, S.; Governa, P.; Maramai, S.; Giannini, G.; Stecca, B.; et al. Quinolines and Oxazino-quinoline Derivatives as Small Molecule GLI1 Inhibitors Identified by Virtual Screening. ACS Med. Chem. Lett. 2022, 13, 1329–1336. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front. Biosci. 2025, 30, 25706. [Google Scholar] [CrossRef]
- Wang, S.W.; Gao, C.; Zheng, Y.M.; Yi, L.; Lu, J.C.; Huang, X.Y.; Cai, J.B.; Zhang, P.F.; Cui, Y.H.; Ke, A.W. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, F.; Wang, Y.; Sun, X.; Chen, H.; Chen, T.; Gao, Y.; Chen, H. Synthesis, structure-activity relationship, and biological evaluation of quinolines for development of anticancer agents. Arch. Pharm. 2023, 356, e2200673. [Google Scholar] [CrossRef]
- Behan, F.M.; Iorio, F.; Picco, G.; Gonçalves, E.; Beaver, C.M.; Migliardi, G.; Santos, R.; Rao, Y.; Sassi, F.; Pinnelli, M.; et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 2019, 568, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Jeng, E.E.; Hess, G.T.; Morgens, D.W.; Li, A.; Bassik, M.C. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat. Biotechnol. 2017, 35, 463–474. [Google Scholar] [CrossRef]
- Ngai, C.; Sevian, H. Probing the Relevance of Chemical Identity Thinking in Biochemical Contexts. CBE Life Sci. Educ. 2018, 17, ar58. [Google Scholar] [CrossRef]
- Günther, D.; Boto, R.A.; Contreras-Garcia, J.; Piquemal, J.P.; Tierny, J. Characterizing Molecular Interactions in Chemical Systems. IEEE Trans. Vis. Comput. Graph. 2014, 20, 2476–2485. [Google Scholar] [CrossRef]
- Gallegos, L.C.; Luchini, G.; St John, P.C.; Kim, S.; Paton, R.S. Importance of Engineered and Learned Molecular Representations in Predicting Organic Reactivity, Selectivity, and Chemical Properties. Acc. Chem. Res. 2021, 54, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Wang, H.; Zhuang, L.; Sun, Y. Multi-granularity physicochemical-inspired molecular representation learning for property prediction. Expert Syst. Appl. 2025, 266, 126115. [Google Scholar] [CrossRef]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2020, 10, 20784–20793. [Google Scholar] [CrossRef]
- Palani, V.; Wendlandt, A.E. Strain-Inducing Positional Alkene Isomerization. J. Am. Chem. Soc. 2023, 145, 20053–20061. [Google Scholar] [CrossRef]
- Ishida, K.; Higashino, T.; Wada, Y.; Kaji, H.; Saeki, A.; Imahori, H. Thiophene-Fused Naphthodiphospholes: Modulation of the Structural and Electronic Properties of Polycyclic Aromatics by Precise Fusion of Heteroles. Chempluschem 2021, 86, 130–136. [Google Scholar] [CrossRef]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 2023, 44, 442–456. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Schwieler, L.; Imbeault, S.; Engberg, G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology 2017, 112, 297–306. [Google Scholar] [CrossRef]
- González Esquivel, D.; Ramírez-Ortega, D.; Pineda, B.; Castro, N.; Ríos, C.; Pérez de la Cruz, V. Kynurenine pathway metabolites and enzymes involved in redox reactions. Neuropharmacology 2017, 112 Pt B, 331–345. [Google Scholar] [CrossRef]
- Sutherland, J.J.; Yonchev, D.; Fekete, A.; Urban, L. A preclinical secondary pharmacology resource illuminates target-adverse drug reaction associations of marketed drugs. Nat. Commun. 2023, 14, 4323. [Google Scholar] [CrossRef]
- Casas, A.I.; Hassan, A.A.; Larsen, S.J.; Gomez-Rangel, V.; Elbatreek, M.; Kleikers, P.W.M.; Guney, E.; Egea, J.; López, M.G.; Baumbach, J.; et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Lian, X.; Li, F.; Wang, C.; Zhu, F.; Qiu, Y.; Chen, Y. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022, 50, D1398–D1407. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Resasco, A.; Di Virgilio, A.L.; Ayala, M.; Cavaco, I.; Cabrera, S.; Aleman, J.; León, I.E. In vitro and in vivo anticancer effects of two quinoline-platinum(II) complexes on human osteosarcoma models. Cancer Chemother. Pharmacol. 2019, 83, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Majee, S.; Ray, D.; Saha, B. Inhibition of cancer cells by Quinoline-Based compounds: A review with mechanistic insights. Bioorg. Med. Chem. 2024, 103, 117681. [Google Scholar] [CrossRef]
- Pich, O.; Bailey, C.; Watkins, T.B.K.; Zaccaria, S.; Jamal-Hanjani, M.; Swanton, C. The translational challenges of precision oncology. Cancer Cell 2022, 40, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Kim, B.Y.S.; Trounson, A. How to design preclinical studies in nanomedicine and cell therapy to maximize the prospects of clinical translation. Nat. Biomed. Eng. 2018, 2, 797–809. [Google Scholar] [CrossRef]
- Lin, J.; Sahakian, D.C.; de Morais, S.M.; Xu, J.J.; Polzer, R.J.; Winter, S.M. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr. Top. Med. Chem. 2003, 3, 1125–1154. [Google Scholar] [CrossRef]
- Liu, J.; Gui, Y.; Rao, J.; Sun, J.; Wang, G.; Ren, Q.; Qu, N.; Niu, B.; Chen, Z.; Sheng, X.; et al. In silico off-target profiling for enhanced drug safety assessment. Acta Pharm. Sin. B 2024, 14, 2927–2941. [Google Scholar] [CrossRef]
- David, P.O. Abstract A001: Navigating the future of therapeutic development: Innovations in safety and efficacy through advanced research methodologies and nanotechnology. Mol. Cancer Ther. 2024, 23, A001. [Google Scholar] [CrossRef]
- Son, A.; Park, J.; Kim, W.; Yoon, Y.; Lee, S.; Ji, J.; Kim, H. Recent Advances in Omics, Computational Models, and Advanced Screening Methods for Drug Safety and Efficacy. Toxics 2024, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Ciwun, M.; Lewkowicz, J.; Pawlak, D. Kynurenines as a Novel Target for the Treatment of Inflammatory Disorders. Cells 2024, 13, 1259. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv. Clin. Exp. Med. 2024, 33, 1295–1301. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. A decade of dedication: Pioneering perspectives on neurological diseases and mental illnesses. Biomedicines 2024, 12, 1083. [Google Scholar] [CrossRef]
- Ryan, K.M.; Corrigan, M.; Murphy, T.M.; McLoughlin, D.M.; Harkin, A. Gene expression of kynurenine pathway enzymes in depression and following electroconvulsive therapy. Acta Neuropsychiatr. 2025, 37, e34. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13, 167. [Google Scholar] [CrossRef]
- Cadilla, R.; Deaton, D.N.; Do, Y.; Elkins, P.A.; Ennulat, D.; Guss, J.H.; Holt, J.; Jeune, M.R.; King, A.G.; Klapwijk, J.C.; et al. The exploration of aza-quinolines as hematopoietic prostaglandin D synthase (H-PGDS) inhibitors with low brain exposure. Bioorg. Med. Chem. 2020, 28, 115791. [Google Scholar] [CrossRef]
- Boros, F.A.; Bohár, Z.; Vécsei, L. Genetic alterations affecting the genes encoding the enzymes of the kynurenine pathway and their association with human diseases. Mutat. Res. Rev. Mutat. Res. 2018, 776, 32–45. [Google Scholar] [CrossRef]
- Tamimou, R.; Montout, C.; Mura, T.; Conejero, I.; Evrard, A.; Courtet, P.; Bonilla-Escribano, P.; Riaza, C.; Vaquero-Lorenzo, C.; Baca-Garcia, E.; et al. Genetic association of the kynurenine pathway to suicidal behavior. Brain Behav. Immun. Health 2024, 42, 100903. [Google Scholar] [CrossRef] [PubMed]
- Nemkov, T.; Stephenson, D.; Erickson, C.; Dzieciatkowska, M.; Key, A.; Moore, A.; Earley, E.J.; Page, G.P.; Lacroix, I.S.; Stone, M.; et al. Regulation of kynurenine metabolism by blood donor genetics and biology impacts red cell hemolysis in vitro and in vivo. Blood 2024, 143, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Galettis, P.; Flynn, A.; Schneider, J. Phenotype versus genotype to optimize cancer dosing in the clinical setting-focus on 5-fluorouracil and tyrosine kinase inhibitors. Pharmacol. Res. Perspect. 2024, 12, e1182. [Google Scholar] [CrossRef] [PubMed]
- Swenberg, J.A.; Fryar-Tita, E.; Jeong, Y.C.; Boysen, G.; Starr, T.; Walker, V.E.; Albertini, R.J. Biomarkers in toxicology and risk assessment: Informing critical dose-response relationships. Chem. Res. Toxicol. 2008, 21, 253–265. [Google Scholar] [CrossRef]
- Cores, Á.; Carmona-Zafra, N.; Clerigué, J.; Villacampa, M.; Menéndez, J.C. Quinones as Neuroprotective Agents. Antioxidants 2023, 12, 1464. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Yang, X.; Feng, X.; Li, X.; Huang, L.; Chan, A.S.C. Design, Synthesis, and Evaluation of Orally Bioavailable Quinoline-Indole Derivatives as Innovative Multitarget-Directed Ligands: Promotion of Cell Proliferation in the Adult Murine Hippocampus for the Treatment of Alzheimer’s Disease. J. Med. Chem. 2018, 61, 1871–1894. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.; dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P. Curcumin: A golden approach to healthy aging: A systematic review of the evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Venkanna, A.; Cho, K.H.; Dhorma, L.P.; Kumar, D.N.; Hah, J.M.; Park, H.G.; Kim, S.Y.; Kim, M.H. Chemistry-oriented synthesis (ChOS) and target deconvolution on neuroprotective effect of a novel scaffold, oxaza spiroquinone. Eur. J. Med. Chem. 2019, 163, 453–480. [Google Scholar] [CrossRef]
- Karale, U.B.; Shinde, A.; Gaikwad, V.R.; Kalari, S.; Gourishetti, K.; Radhakrishnan, M.; Poornachandra, Y.; Amanchy, R.; Chakravarty, S.; Andugulapati, S.B.; et al. Iron mediated reductive cyclization/oxidation for the generation of chemically diverse scaffolds: An approach in drug discovery. Bioorg. Chem. 2023, 139, 106698. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, D.; Hong, S.Y.; Chang, S. Tuning Orbital Symmetry of Iridium Nitrenoid Enables Catalytic Diastereo- and Enantioselective Alkene Difunctionalizations. J. Am. Chem. Soc. 2021, 143, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Ruiz, A.; Guo, T.; Sokolovska, A.; Miller, P.F.; Collins, J.J.; Lu, T.K.; Lora, J.M. Engineering living therapeutics with synthetic biology. Nat. Rev. Drug Discov. 2021, 20, 941–960. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, S.; Liu, M.; Kang, W.; Xia, J. Self-Assembled Multienzyme Nanostructures on Synthetic Protein Scaffolds. ACS Nano 2019, 13, 11343–11352. [Google Scholar] [CrossRef] [PubMed]
- Siefen, T.; Lokhnauth, J.; Liang, A.; Larsen, C.C.; Lamprecht, A. An ex-vivo model for transsynovial drug permeation of intraarticular injectables in naive and arthritic synovium. J. Control. Release 2021, 332, 581–591. [Google Scholar] [CrossRef]
- Lavallie, E.R.; Dorner, A.J.; Burczynski, M.E. Use of ex vivo systems for biomarker discovery. Curr. Opin. Pharmacol. 2008, 8, 647–653. [Google Scholar] [CrossRef]
- Stefaniak, J.; Huber, K.V.M. Importance of Quantifying Drug-Target Engagement in Cells. ACS Med. Chem. Lett. 2020, 11, 403–406. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Stefanov, B.A.; Fussenegger, M. Biomarker-driven feedback control of synthetic biology systems for next-generation personalized medicine. Front. Bioeng. Biotechnol. 2022, 10, 986210. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Atyabi, F.; Dinarvand, R. Technical and engineering considerations for designing therapeutics and delivery systems. J. Control. Release 2023, 353, 411–422. [Google Scholar] [CrossRef]

| Compounds | Main Characteristics | Ref. |
|---|---|---|
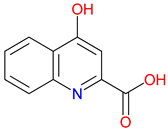 Kynurenic Acid (KYNA) | Neuroprotective—Blocks NMDA and α7nAChR to reduce excitotoxicity KYN pathway metabolite—Modulates neurotransmission and immune response Poor BBB permeability—Limited CNS access, driving prodrug development | [30,51,52] [53,54,55] [56,57,58] |
 Nicotinic Acid (NA) | Essential vitamin (B3)—Crucial for NAD+/NADP+ synthesis, supporting cellular metabolism Lipid-lowering effects—Reduces LDL and triglycerides while increasing HDL cholesterol Modulation of nicotinic acid-induced flushing—GPR109A and Langerhans cells drive flushing; DP1 antagonists suppress it | [59,60,61] [62,63,64] [65,66,67] |
 Quinolinic Acid (QUIN) | Excitotoxic agent—Acts as a potent NMDA receptor agonist, leading to neuronal overexcitation and potential cell death Oxidative stress contributor—Promotes free radical generation, exacerbating cellular damage Neurodegenerative implications—Linked to the progression of neurological disorders through its detrimental effects on brain tissue | [68,69,70] [71,72,73] [69,74,75] |
 Picolinic Acid | Metal ion chelator—Binds zinc, iron, and other metals, influencing cellular metabolism Immune modulator—Enhances macrophage activity and antimicrobial defense Neuroactive compound—Involved in neurotransmission and potential neuroprotective effects | [76,77,78] [79,80,81] [82,83,84] |
 Xanthurenic Acid (XA) | Neuromodulatory effects: Influences neuronal signaling and may modulate receptor activity Putative neuroactive role—Modulates glutamate signaling and may influence neurotransmission Metal chelating properties—Binds with zinc and other metal ions, potentially affecting oxidative stress | [48,85,86] [48,85,86] [87,88,89] |
 Quinaldic Acid | Quinoline derivative—Structurally related to other KYN pathway metabolites Antimicrobial properties—Exhibits activity against certain bacteria and fungi Potential neuroactive role—May influence neurotransmission, though its biological significance remains underexplored | [90,91,92] [93,94] [92,95] |
| Compounds | Main Characteristics | Ref. |
|---|---|---|
| Quinoline and quinolone derivatives | ||
 Quinine  Quinidine | Quinine: Antimalarial agent—Inhibits Plasmodium pp. by interfering with heme detoxification Historically used to reduce fever and pain Muscle relaxant—Helps alleviate nocturnal leg cramps by modulating ion channels Quinidine: Class I antiarrhythmic—Blocks sodium channels, stabilizing cardiac rhythm Chiral isomer of quinine—Shares structural similarities but has distinct pharmacological effects Proarrhythmic risk—Can prolong QT interval 1, requiring careful clinical use | [142,143,144] [145,146,147] [148,149] [150,151,152] [153,154,155] [156,157,158] |
 Cinchoninic Acid | Quinoline derivative—Structurally related to quinine and other cinchona alkaloids Metal chelation—Binds with metal ions, potentially influencing enzymatic activity Pharmacological potential—Investigated for antimicrobial and neuroactive properties | [149,159,160] [161,162] [163,164,165] |
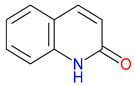 2-Quinolinone  4-Quinolinone | Core scaffold for quinolone antibiotics—Forms the backbone of fluoroquinolones, targeting bacterial DNA gyrase and topoisomerase IV Broad-spectrum antimicrobial activity—Effective against Gram-positive and Gram-negative bacteria Synthetic versatility—Modifiable structure allows for improved potency, pharmacokinetics, and resistance mitigation | [166,167,168] [169,170,171] [170,172,173] |
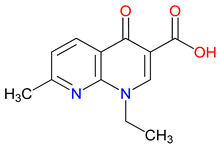 Nalidixic Acid | First-generation quinolone antibiotic—Inhibits DNA gyrase, primarily effective against Gram-negative bacteria Limited spectrum and rapid resistance—Narrow activity and high bacterial resistance limit its clinical use Urinary tract infection treatment—Historically used for urinary tract infections, though largely replaced by newer fluoroquinolones | [174,175,176] [177,178] [179,180,181] |
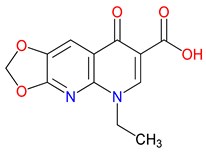 Oxolinic Acid | Early quinolone antibiotic—Inhibits DNA gyrase, effective against Gram-negative bacteria Used in veterinary medicine—Primarily employed for treating bacterial infections in animals Limited clinical use—Replaced by newer fluoroquinolones due to resistance and pharmacokinetic limitations | [182,183,184] [185,186,187] [188] |
| Pyridinecarboxylic Acids | ||
 Isonicotinic Acid (INA) | Pyridine carboxylic acid derivative—Structurally related to NA (vitamin B3) Key precursor for isoniazid—Used in the synthesis of isoniazid, a frontline anti-tuberculosis drug Pharmacological potential—Investigated for antimicrobial and metabolic regulatory properties | [189,190] [191,192] [191,193,194] |
 Dipicolinic Acid (DPA) | Metal ion chelator—Strongly binds calcium and other metal ions, playing a role in metal homeostasis Bacterial spore component—Essential for bacterial endospore resistance and heat stability Potential neuroprotective role—Explored for its effects on metal-related oxidative stress in neurodegeneration | [195,196] [197] [198,199,200] |
| Hydroxy-substituted derivatives | ||
 8-Hydroxyquinoline | Metal chelator—Strongly binds iron, copper, and zinc, influencing redox balance and enzymatic activity Antimicrobial and antifungal agent—Exhibits broad-spectrum activity against bacteria and fungi Potential neuroprotective role—Investigated for treating neurodegenerative diseases by regulating metal toxicity | [201,202,203] [204,205,206] [207,208,209] |
| Compounds | Main Characteristics | Ref. |
|---|---|---|
 7-Chlorokynurenic Acid (7-CKA) | NMDA receptor antagonist—Blocks the glycine-binding site, reducing excitotoxicity Preclinical behavioral actions—Elicits antidepressant-like effects, blocks NMDA-induced convulsions, and attenuates ischemia-induced learning deficits Phase II clinical trials have demonstrated that 4-chlorokynurenine, a prodrug of 7-CKA, lacks significant antidepressant efficacy in individuals with treatment-resistant depression. It is currently undergoing Phase III evaluation for hypertension and Phase I trials for neurological disorders and neuropathic pain. Additional clinical studies are planned to investigate its potential utility in epilepsy, Huntington’s disease, Parkinson’s disease, various psychiatric conditions, and suicidal ideation. | [253,254] [255,256,257] [125,258] |
 5,7-Dichlorokynurenic Acid | NMDA receptor antagonist—Blocks the glycine site to reduce excitotoxicity Enhanced potency—More effective than KYNA at inhibiting NMDA receptor activity Preclinical behavioral actions—Shows anxiolytic effects and enhances short-term memory and recognition | [259,260,261] [246,259,261,262] [263,264,265] |
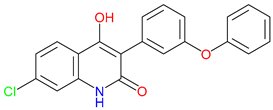 L-701,324 | NMDA receptor antagonist—Blocks the glycine site, reducing excitotoxicity Preclinical behavioral evidence—Demonstrates antidepressant-like activity, reduces anxiety-like behavior Potential clinical application–Epilepsy, schizophrenia, and chronic pain | [266,267] [265,268] [269,270,271] |
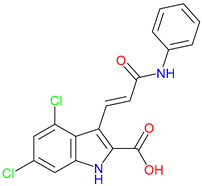 Gavestinel | Glycine site NMDA receptor antagonist—Blocks NMDA receptor activity to reduce excitotoxicity Preclinical behavioral findings—Potential in reducing ischemic damage and modulating certain NMDA-receptor-mediated schizophrenia-like behaviors Stroke neuroprotection candidate—Investigated for acute stroke, primary intracerebral hemorrhage, and acute ischemic stroke, but failed in clinical trials | [272,273] [272,274] [272,275,276] |
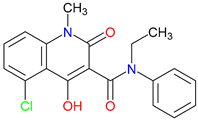 Laquinimod | Immunomodulatory agent—Reduces pro-inflammatory cytokines and modulates immune cell activity Preclinical findings—Improves motor function in a Huntington’s disease model, activates the AhR in the EAE Model of MS Clinical trials—Modestly reduces relapse rates and disability progression and significantly reduces brain volume change atrophy in relapsing–remitting MS, and shows limited efficacy in active non-infectious intermediate, posterior, or panuveitis (NCT02720102) | [277,278,279] [280,281] [282,283,284] |
 Tasquinimod | Anti-cancer properties—Inhibits tumor angiogenesis, suppresses myeloid-derived suppressor cells, downregulates immune suppressive pathways and inflammatory cytokine signaling S100A9 modulation—Suppresses inflammatory factor expression, potentially inhibist the upregulation of S100A9 in AD, and inhibits MDSC recruitment HDAC4 modulation—potentially influences epigenetic regulation in neurodegenerative or cognitive disorders and potentially controls neuronal memory, plasticity, and learning | [285,286,287] [288,289,290] [291,292] |
| Recommendation | Approach | Illustrative Examples |
|---|---|---|
| Create a Unified SAR Overview | Gather scattered substituent data (chlorine, fluorine, sulfonamides, etc.) in a dedicated section or table. | Summarize each motif (e.g., Cl, F, sulfonamide) and its known effects on receptor affinity or metabolism. |
| Cross-Reference Key Examples and Motifs | Explicitly reference primary compounds (7-CKA, Gavestinel) to illustrate general rules about halogenation or sulfonamide additions. | For halogens (Cl, F): provide examples showing increased receptor blockade or enhanced lipophilicity (7-CKA, 5,7-DCKA). |
| Compounds | Main Characteristics | Ref. |
|---|---|---|
 SZR-72 | BBB penetration—effectively crosses the blood–brain barrier, enhancing its therapeutic potential in the CNS Neuroprotective effect—Offers enhanced neuroprotection, reduces brainstem c-fos, and induces therapeutic hypothermia Behavioral effects—Alters motor and exploratory behaviors, reducing vertical activity and affecting curiosity-linked emotional and motor responses | [342,343] [341,342] [343] |
 SZR-73 | Mitochondrial function enhancement—Enhances mitochondrial respiration and ATP production, improving complex I- and II-linked OXPHOS in a rodent sepsis model Systemic inflammatory activation reduction—Reduces systemic inflammation in sepsis, lowering ET-1, IL-6, and NT levels and XOR activity Microcirculatory effects—Improves mitochondrial function but does not restore microcirculation, unlike KYNA, which improves microvascular perfusion in sepsis | [229] [229] [229] |
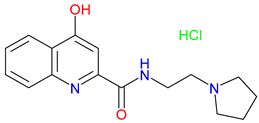 SZR-81 | Antidepressant-like effects—Reduces immobility and increasing swimming in FST via serotonin 5-HT system involvement BBB permeability—Poor BBB penetration or metabolic alteration before reaching the CNS Neuroprotective potential–To be explored for use in stroke, neurodegenerative diseases, and inflammatory conditions | [347] [347] [348] |
 SZR-104 | High permeability through the blood—Shows high BBB permeability, surpassing KYNA and analogues Neuroprotective effects in sepsis—Demonstrates neuroprotection in septic rodents, reducing BBB disruption and CNS mitochondrial dysfunction Influence on motor behavior—Increases horizontal exploration, indicating BBB penetration and retention of KYNA-like effects on motor-related curiosity and emotional behavior | [56] [344] [343] |
 SZR-105 | High BBB penetrability—Significantly better BBB permeability than KYNA and earlier analogues due to its dual cationic side chains Potent anti-inflammatory effects—Suppresses TNF-α production and strongly induces TSG-6 Neuroprotective activity in CNS models—Reduces cortical spreading depression propagation in migraine models | [56] [345] [346] |
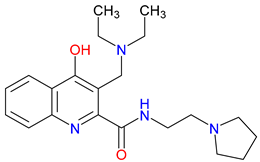 SZR-109 | Enhanced BBB penetration—penetrates the BBB more effectively than KYNA Neuroprotective effects—Suppresses pro-inflammatory TNF-α production and upregulates TSG-6 Anti-convulsant activity—prevents or diminishes seizure-like activity in the brain | [56] [345] [346] |
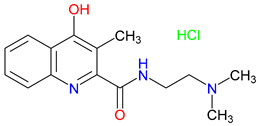 SZRG-21 | BBB penetration—Demonstrated efficacy in reducing cytokine levels in colitis models Impact on motor activity—Alters motor activity and exploratory behavior Emotional and behavioral modulation—Affects emotional functions, such as motor-associated curiosity and emotions | [343] [343] [343] |
| Pillar | Primary Objectives | Illustrative Approaches |
|---|---|---|
| Pinpoint Disease Pathways | Link structural motifs to specific therapeutic outcomes. | Combat excitotoxicity in Alzheimer’s |
| Refine SAR Strategies | Optimize structure–activity to boost efficacy and reduce risks. | Use computational docking |
| Validate in Context-Specific Models | Confirm mechanistic relevance in robust disease models. | Test in transgenic mice (e.g., Parkinson’s) |
| Incorporate Biomarker-Based Selection | Use metabolic/genomic markers for targeted therapy. | Stratify patients by IDO/KMO polymorphisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals 2025, 18, 607. https://doi.org/10.3390/ph18050607
Tanaka M, Szatmári I, Vécsei L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals. 2025; 18(5):607. https://doi.org/10.3390/ph18050607
Chicago/Turabian StyleTanaka, Masaru, István Szatmári, and László Vécsei. 2025. "Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design" Pharmaceuticals 18, no. 5: 607. https://doi.org/10.3390/ph18050607
APA StyleTanaka, M., Szatmári, I., & Vécsei, L. (2025). Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals, 18(5), 607. https://doi.org/10.3390/ph18050607










