Effects of Various Muscle Disuse States and Countermeasures on Muscle Molecular Signaling
Abstract
:1. Introduction
2. Models of Decreased Activity of Skeletal Muscles and Their Functional Consequences
3. Molecular Signaling Alterations in Skeletal Muscles under Disuse Conditions
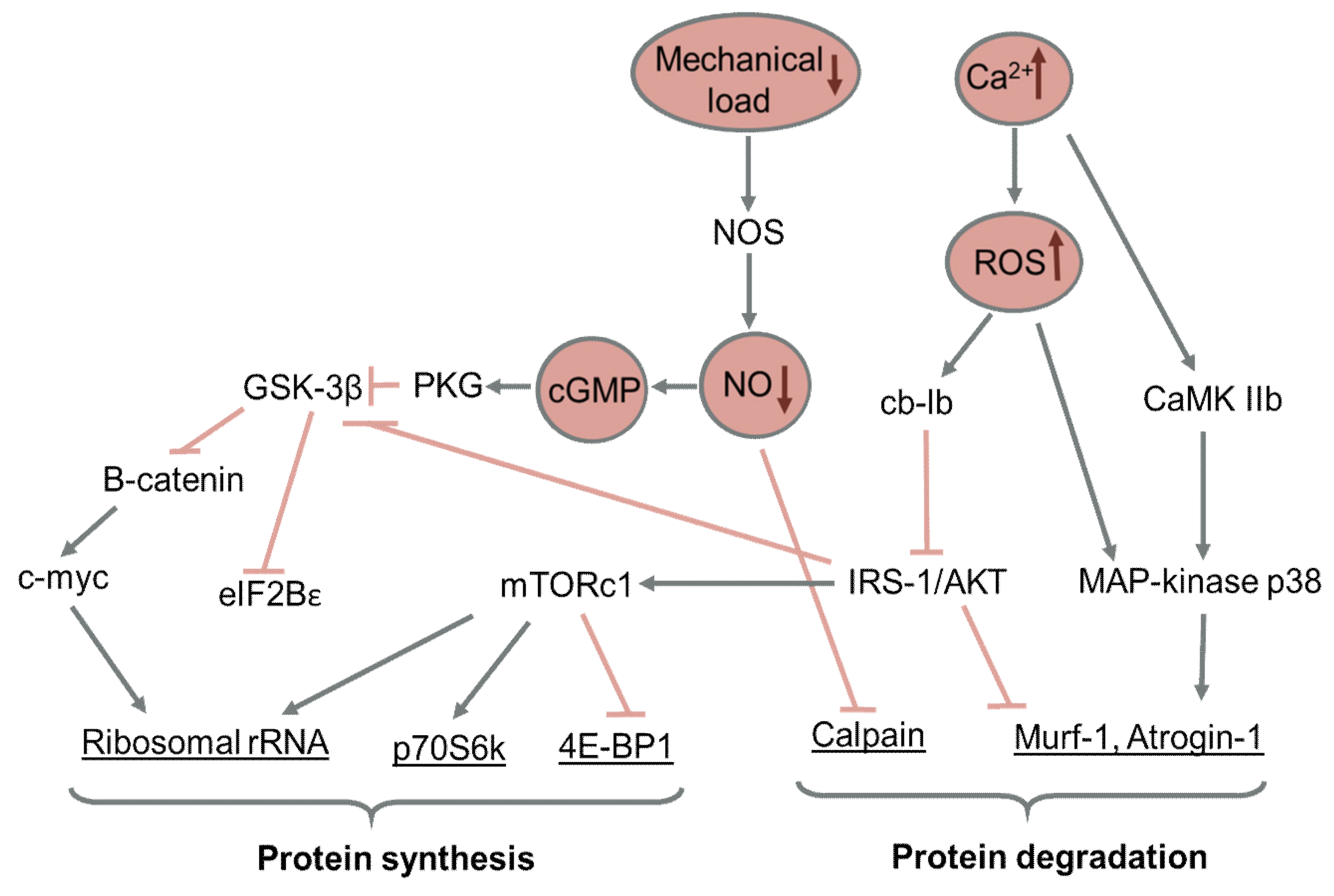
3.1. Effect of Reduced Motor Activity on Protein Synthesis in Skeletal Muscle
3.2. Effect of Reduced Motor Activity on Protein Degradation in Skeletal Muscle
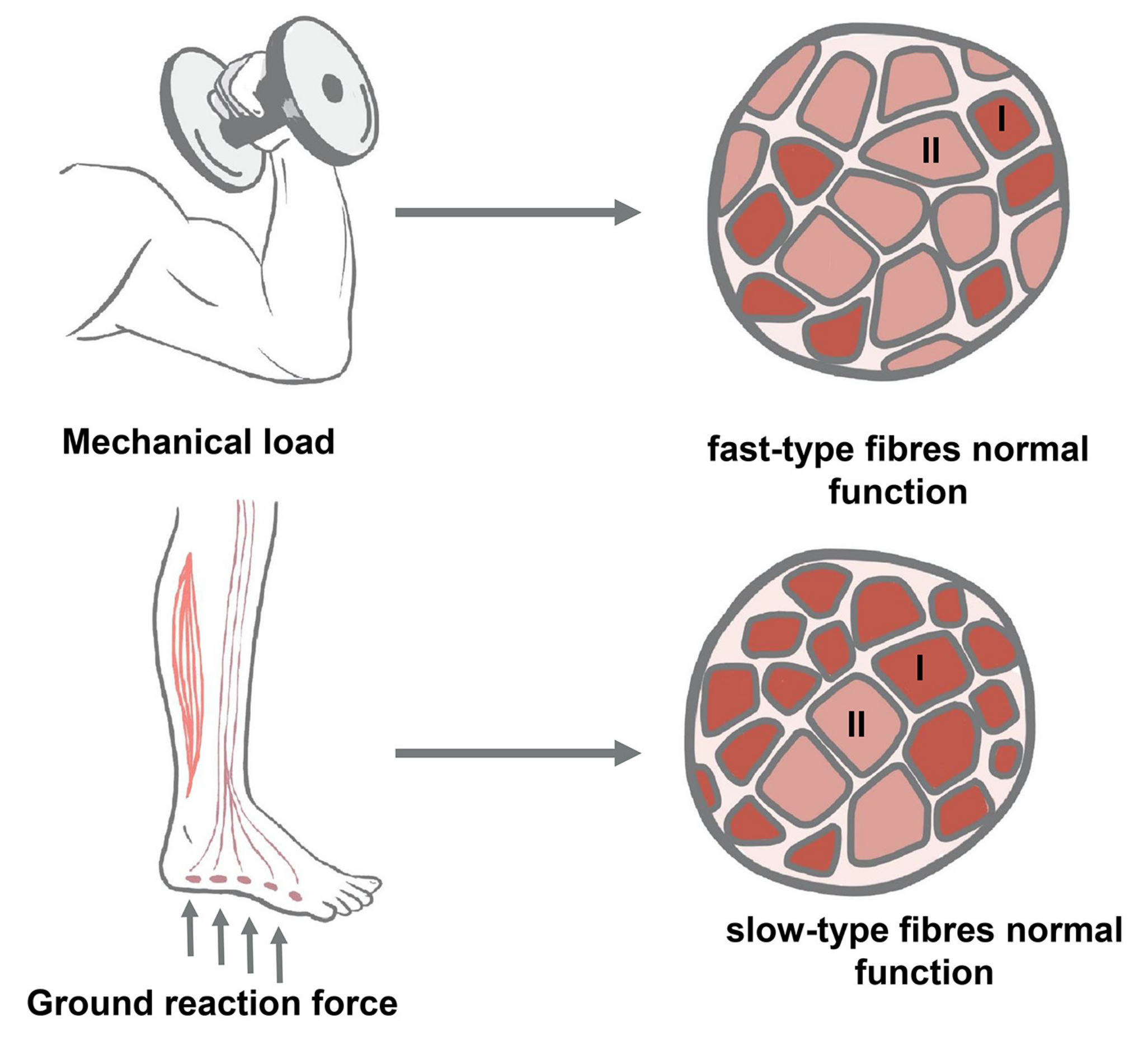
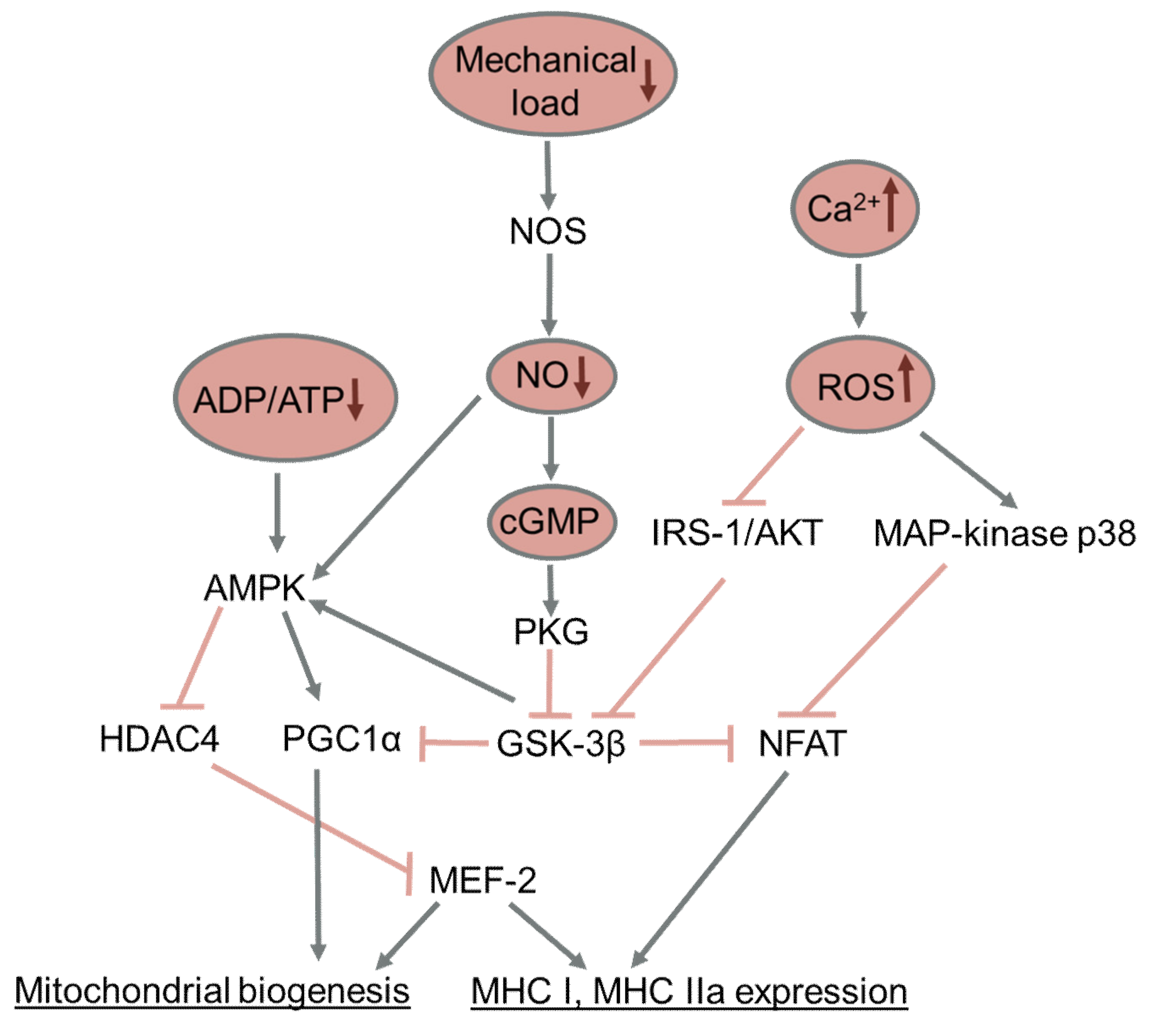
3.3. Effect of Reduced Activity on Changes in Muscle Fiber Types
3.4. Effect of Reduced Muscle Activity on Muscle Oxidative Capacity
4. Countermeasures to Changes Caused by Reduced Motor Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4E-BP1 | eukaryotic initiation factor 4E binding protein |
| AKT | protein kinase B |
| AMPK | AMP-activated protein kinase |
| cGMP | cyclic guanosine monophosphate |
| c-Myc | c-myelocytomatosis oncogene (transcription factor) |
| CSA | cross-sectional area |
| eIF2B | eukaryotic initiation factor 2B |
| FoxO | forkhead box O protein |
| GS1 | glycogen synthase-1 |
| GSK-3β | glycogen synthase kinase-3β |
| HDT BR | head down tilt bed rest |
| HS | hindlimb suspension |
| HU | hindlimb unloading |
| IGF-1 | insulin-like growth factor 1 |
| L-NAME | N(gamma)-nitro-L-arginine methyl ester |
| MAFbx | muscle atrophy F-box protein/atrogin 1 |
| MAPK | mitogen-activated protein kinase |
| MCIP 1.4 | modulatory calcineurin-interaction protein 1.4 |
| MEF-2 | myocyte enhancer factor 2 |
| mir-208 | micro-RNA 208 |
| mTORC1 | mammalian/mechanistic target of rapamycin complex 1 |
| MuRF1 | muscle RING finger protein |
| MyHC | myosin heavy chain |
| NFAT | nuclear factor of activated T-cells |
| NO | nitric oxide |
| NRF | nuclear respiratory factor |
| p70S6K | ribosomal protein S6 kinase p70 |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | phosphatidylinositol 3-kinase |
| PKA | protein kinase A |
| PKB | protein kinase B (AKT) |
| PKC | protein kinase C |
| PKD1 | protein kinase D1 |
| PKG | protein kinase G |
| PMS | plantar mechanical stimulation |
| PuRA | purine Rich Element Binding Protein A |
| PuRB | purine Rich Element Binding Protein B |
| rRNA | ribosomal RNA |
| SOX-6 | transcription factor |
| TFAM | mitochondrial transcription factor A |
References
- Falempin, M.; Mounier, Y. Muscle atrophy associated with microgravity in rat: Basic data for countermeasures. Acta Astronaut. 1998, 42, 489–502. [Google Scholar] [CrossRef]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-Induced Skeletal Muscle Atrophy and Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Grigoriev, A.I.; Kozlovskaya, I.B. Gravity mechanisms in tonic motor system. Neurophysiological and muscle aspects. Hum. Physiol. 2017, 43, 578–590. [Google Scholar] [CrossRef]
- Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. The plasticity of skeletal muscle: Effects of neuromuscular activity. Exerc. Sport Sci. Rev. 1991, 19, 269–312. [Google Scholar] [CrossRef]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Bodine, S.C. Disuse-induced muscle wasting. Int. J. Biochem. Cell. Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koryak, Y.A. Influence of simulated microgravity on mechanical properties in the human triceps surae muscle in vivo. I: Effect of 120 days of bed-rest without physical training on human muscle musculo-tendinous stiffness and contractile properties in young women. Eur. J. Appl. Physiol. 2014, 114, 1025–1036. [Google Scholar] [CrossRef] [Green Version]
- Holt, J.A.; Macias, B.R.; Schneider, S.M.; Watenpaugh, D.E.; Lee, S.M.C.; Chang, D.G.; Hargens, A.R. WISE 2005: Aerobic and resistive countermeasures prevent paraspinal muscle deconditioning during 60-day bed rest in women. J. Appl. Physiol. 2016, 120, 1215–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trappe, S.; Trappe, T.; Gallagher, P.; Harber, M.; Alkner, B.; Tesch, P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J. Physiol. 2004, 557, 501–513. [Google Scholar] [CrossRef]
- Rittweger, J.; Frost, H.M.; Schiessl, H.; Ohshima, H.; Alkner, B.; Tesch, P.; Felsenberg, D. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: Results from the LTBR study. Bone 2005, 36, 1019–1029. [Google Scholar] [CrossRef]
- Brocca, L.; Cannavino, J.; Coletto, L.; Biolo, G.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms. J. Physiol. 2012, 590, 5211–5230. [Google Scholar] [CrossRef]
- Akima, H.; Kubo, K.; Imai, M.; Kanehisa, H.; Suzuki, Y.; Gunji, A.; Fukunaga, T. Inactivity and muscle: Effect of resistance training during bed rest on muscle size in the lower limb. Acta Physiol. Scand. 2001, 172, 269–278. [Google Scholar] [CrossRef]
- Miokovic, T.; Armbrecht, G.; Gast, U.; Rawer, R.; Roth, H.J.; Runge, M.; Felsenberg, D.; Belavy, D.L. Muscle atrophy, pain, and damage in bed rest reduced by resistive (vibration) exercise. Med. Sci. Sports Exerc. 2014, 46, 1506–1516. [Google Scholar] [CrossRef] [Green Version]
- Glover, E.I.; Phillips, S.M.; Oates, B.R.; Tang, J.E.; Tarnopolsky, M.A.; Selby, A.; Smith, K.; Rennie, M.J. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J. Physiol. 2008, 586, 6049–6061. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Hvid, L.G.; Justesen, L.; Christensen, U.; Neergaard, K.; Simonsen, L.; Ortenblad, N.; Magnusson, S.P.; Kjaer, M.; Aagaard, P. Effects of aging on human skeletal muscle after immobilization and retraining. J. Appl. Physiol. 2009, 107, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; Senden, J.M.; Dolmans, J.; van Loon, L.J. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. 2014, 210, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Dirks, M.L.; Wall, B.T.; Snijders, T.; Ottenbros, C.L.; Verdijk, L.B.; van Loon, L.J. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. 2014, 210, 628–641. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.; Lin, C.; Shackelford, L.; Sinitsyn, V.; Evans, H.; Belichenko, O.; Schenkman, B.; Kozlovskaya, I.; Oganov, V.; Bakulin, A.; et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 2000, 89, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.M.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588, 3567–3592. [Google Scholar] [CrossRef] [PubMed]
- Шульженкo, Е.Б.; Виль-Вильямс, И.Ф. Вoзмoжнoсть прoведения длительнoй вoднoй иммерсии метoдoм «сухoгo» пoгружения. Кoсмическая Биoлoгия Авиакoсмическая Медицина 1976, 10, 82–84. [Google Scholar]
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry Immersion as a Ground-Based Model of Microgravity Physiological Effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Shenkman, B.S.; Kozlovskaya, I.B. Cellular Responses of Human Postural Muscle to Dry Immersion. Front. Physiol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Litvinova, K.S.; Vikhlyantsev, I.M.; Kozlovskaya, I.B.; Podlubnaya, Z.A.; Shenkman, B.S. Effects of artificial support stimulation on fiber and molecular characteristics of soleus muscle in men exposed to 7-day dry immersion. J. Gravit. Physiol. 2004, 11, P131–P132. [Google Scholar] [PubMed]
- Moukhina, A.; Shenkman, B.; Blottner, D.; Nemirovskaya, T.; Lemesheva, Y.; Puttmann, B.; Kozlovskaya, I. Effects of support stimulation on human soleus fiber characteristics during exposure to “dry” immersion. J. Gravit. Physiol. 2004, 11, P137–P138. [Google Scholar] [PubMed]
- Ogneva, I.V.; Shenkman, B.S.; Kozlovskaya, I.B. The contents of desmin and alpha-actinin-1 in the human soleus muscle after seven-day “dry” immersion. Dokl. Biol. Sci. 2011, 436, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.F.; Saenko, I.V.; Popov, D.V.; Vinogradova, O.L.; Kozlovskaya, I.B. Effect of mechanical stimulation of the support zones of soles on the muscle stiffness in 7-day dry immersion. J. Gravit. Physiol. 2004, 11, P135–P136. [Google Scholar] [PubMed]
- Kirenskaia, A.V.; Kozlovskaia, I.B.; Sirota, M.G. [Effect of immersion hypokinesia on the characteristics of the rhythmic activity of the motor units of the soleus muscle]. Fiziol. Cheloveka 1986, 12, 627–632. [Google Scholar] [PubMed]
- Bamman, M.M.; Clarke, M.S.; Feeback, D.L.; Talmadge, R.J.; Stevens, B.R.; Lieberman, S.A.; Greenisen, M.C. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J. Appl. Physiol. 1998, 84, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Hikida, R.S.; Gollnick, P.D.; Dudley, G.A.; Convertino, V.A.; Buchanan, P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat. Space Env. Med. 1989, 60, 664–670. [Google Scholar]
- Shenkman, B.S.; Podlubnaya, Z.A.; Vikhlyantsev, I.M.; Litvinova, K.S.; Udaltsov, S.N.; Nemirovskaya, T.L.; Lemesheva, Y.S.; Mukhina, A.M.; Kozlovskaya, I.B. Contractile characteristics and sarcomeric cytoskeletal proteins of human soleus fibers in muscle unloading: Role of mechanical stimulation from the support surface. Biophysics 2004, 49, 807–815. [Google Scholar]
- Hernandez Corvo, R.; Kozlovskaia, I.B.; Kreidich Iu, V.; Martinez Fernandez, S.; Rakhamanov, A.S. Effect of a 7-day space flight on the structure and function of the human locomotor apparatus. Kosm. Biol. I Aviakosmicheskaia Meditsina 1983, 17, 37–44. [Google Scholar]
- Kozlovskaia, I.B.; Grigor’eva, L.S.; Gevlich, G.I. [Comparative analysis of the effect of weightlessness and its model on the velocity-strength properties and tonus of human skeletal muscles]. Kosm. Biol. I Aviakosmicheskaia Meditsina 1984, 18, 22–26. [Google Scholar]
- Friedrich, O.; Diermeier, S.; Larsson, L. Weak by the machines: Muscle motor protein dysfunction—A side effect of intensive care unit treatment. Acta Physiol. 2018, 222, e12885. [Google Scholar] [CrossRef]
- Giangregorio, L.; McCartney, N. Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction, and rehabilitation strategies. J. Spinal Cord. Med. 2006, 29, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Cisterna, B.A.; Cardozo, C.; Saez, J.C. Neuronal involvement in muscular atrophy. Front. Cell. Neurosci. 2014, 8, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, S.Y.; McGlory, C.; D’Souza, L.K.; Morgan, A.K.; Saddler, N.I.; Baker, S.K.; Parise, G.; Phillips, S.M. A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am. J. Clin. Nutr. 2018, 108, 1060–1068. [Google Scholar] [CrossRef] [Green Version]
- Krogh-Madsen, R.; Thyfault, J.P.; Broholm, C.; Mortensen, O.H.; Olsen, R.H.; Mounier, R.; Plomgaard, P.; van Hall, G.; Booth, F.W.; Pedersen, B.K. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J. Appl. Physiol. 2010, 108, 1034–1040. [Google Scholar] [CrossRef] [Green Version]
- Fisher, S.R.; Goodwin, J.S.; Protas, E.J.; Kuo, Y.F.; Graham, J.E.; Ottenbacher, K.J.; Ostir, G.V. Ambulatory activity of older adults hospitalized with acute medical illness. J. Am. Geriatr. Soc. 2011, 59, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, S.Y.; Holloway, T.M.; Phillips, S.M. The Impact of Step Reduction on Muscle Health in Aging: Protein and Exercise as Countermeasures. Front. Nutr. 2019, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Shad, B.J.; Thompson, J.L.; Holwerda, A.M.; Stocks, B.; Elhassan, Y.S.; Philp, A.; LJC, V.A.N.L.; Wallis, G.A. One Week of Step Reduction Lowers Myofibrillar Protein Synthesis Rates in Young Men. Med. Sci. Sports Exerc. 2019, 51, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Breen, L.; Von Allmen, M.; MacDonald, M.J.; Moore, D.R.; Offord, E.A.; Horcajada, M.N.; Breuille, D.; Phillips, S.M. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, K.F.; Pasha, E.; Doros, G.; Clark, D.J.; Patten, C.; Phillips, E.M.; Frontera, W.R.; Fielding, R.A. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: Influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur. J. Appl. Physiol. 2014, 114, 29–39. [Google Scholar] [CrossRef]
- Hitomi, Y.; Kizaki, T.; Watanabe, S.; Matsumura, G.; Fujioka, Y.; Haga, S.; Izawa, T.; Taniguchi, N.; Ohno, H. Seven skeletal muscles rich in slow muscle fibers may function to sustain neutral position in the rodent hindlimb. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [Green Version]
- Ilin, E.A.; Novikov, V.E. Stand for modelling the physiological effects of weightlessness in laboratory experiments with rats. Kosm. Biol. I Aviakosmicheskaia Meditsina 1980, 14, 79–80. [Google Scholar]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Lawler, J.M.; Song, W.; Demaree, S.R. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic. Biol. Med. 2003, 35, 9–16. [Google Scholar] [CrossRef]
- Ohira, Y.; Yoshinaga, T.; Nomura, T.; Kawano, F.; Ishihara, A.; Nonaka, I.; Roy, R.R.; Edgerton, V.R. Gravitational unloading effects on muscle fiber size, phenotype and myonuclear number. Adv. Space. Res. Off. J. Comm. Space. Res. 2002, 30, 777–781. [Google Scholar] [CrossRef]
- Zhong, H.; Roy, R.R.; Siengthai, B.; Edgerton, V.R. Effects of inactivity on fiber size and myonuclear number in rat soleus muscle. J. Appl. Physiol. 2005, 99, 1494–1499. [Google Scholar] [CrossRef] [Green Version]
- Thomason, D.B.; Booth, F.W. Atrophy of the soleus muscle by hindlimb unweighting. J. Appl. Physiol. 1990, 68, 1–12. [Google Scholar] [CrossRef]
- Григoрьев, А.И.; Кoзлoвская, И.Б.; Шенкман, Б.С. Рoль oпoрнoй афферентации в oрганизации тoническoй мышечнoй системы. Рoссийский Физиoлoгический Журнал им. И.М. Сеченoва 2004, 90, 508–521. [Google Scholar]
- Gardetto, P.R.; Schluter, J.M.; Fitts, R.H. Contractile function of single muscle fibers after hindlimb suspension. J. Appl. Physiol. 1989, 66, 2739–2749. [Google Scholar] [CrossRef]
- Stevens, L.; Mounier, Y.; Holy, X.; Falempin, M. Contractile properties of rat soleus muscle after 15 days of hindlimb suspension. J. Appl. Physiol. 1990, 68, 334–340. [Google Scholar] [CrossRef]
- Ponomareva, E.V.; Kravtsova, V.V.; Kachaeva, E.V.; Altaeva, E.G.; Vikhliantsev, I.M.; Podlubnaia, Z.A.; Krivoi, I.I.; Shenkman, B.S. [Contractile properties of the isolated rat musculus soleus and single skinned soleus fibers at the early stage of gravitational unloading: Facts and hypotheses]. Biofizika 2008, 53, 1087–1094. [Google Scholar]
- Pierotti, D.J.; Roy, R.R.; Flores, V.; Edgerton, V.R. Influence of 7 days of hindlimb suspension and intermittent weight support on rat muscle mechanical properties. Aviat. Space Environ. Med. 1990, 61, 205–210. [Google Scholar] [PubMed]
- Wang, X.D.; Kawano, F.; Matsuoka, Y.; Fukunaga, K.; Terada, M.; Sudoh, M.; Ishihara, A.; Ohira, Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol. 2006, 290, C981–C989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemura, A.; Roy, R.R.; Edgerton, V.R.; Ishihara, A. Biochemical Adaptations in a Slow and a Fast Plantarflexor Muscle of Rats Housed in Small Cages. Aerosp. Med. Hum. Perform. 2016, 87, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.P.; Tyganov, S.A.; Mochalova, E.P.; Shenkman, B.S. Restricted Activity and Protein Synthesis in Postural and Locomotor Muscles. Ross. Fiziol. Zhurnal. Im. I.M. Sechenova 2021, 107, 842–853. [Google Scholar] [CrossRef]
- Belozerova, I.N.; Nemirovskaya, T.L.; Shenkman, B.S.; Kozlovskaya, I.B. Characteristic of changes in the structure and metabolism of the vastus lateralis muscles in monkeys after space flight. Neurosci. Behav. Physiol. 2003, 33, 735–740. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Belozerova, I.N.; Lee, P.; Nemirovskaya, T.L.; Kozlovskaya, I.B. Effects of weightlessness and movement restriction on the structure and metabolism of the soleus muscle in monkeys after space flight. Neurosci. Behav. Physiol. 2003, 33, 717–722. [Google Scholar] [CrossRef]
- Fischbach, G.D.; Robbins, N. Changes in contractile properties of disused soleus muscles. J. Physiol. 1969, 201, 305–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hnik, P.; Vejsada, R.; Goldspink, D.F.; Kasicki, S.; Krekule, I. Quantitative evaluation of electromyogram activity in rat extensor and flexor muscles immobilized at different lengths. Exp. Neurol. 1985, 88, 515–528. [Google Scholar] [CrossRef]
- Jokl, P.; Konstadt, S. The effect of limb immobilization on muscle function and protein composition. Clin. Orthop. Relat. Res. 1983, 222–229. [Google Scholar] [CrossRef]
- Simard, C.P.; Spector, S.A.; Edgerton, V.R. Contractile properties of rat hind limb muscles immobilized at different lengths. Exp. Neurol. 1982, 77, 467–482. [Google Scholar] [CrossRef]
- Booth, F.W. Effect of limb immobilization on skeletal muscle. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 1113–1118. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Barnard, R.J.; Peter, J.B.; Maier, P.A.; Simpson, D.R. Properties of immobilized hind-limb muscles of the Galago senegalensis. Exp. Neurol. 1975, 46, 115–131. [Google Scholar] [CrossRef]
- Elder, G.C.; Toner, L.V. Muscle shortening induced by tenotomy does not reduce activity levels in rat soleus. J. Physiol. 1998, 512 (Pt. 1), 251–265. [Google Scholar] [CrossRef]
- Nelson, P.G. Functional consequences of tenotomy in hind limb muscles of the cat. J. Physiol. 1969, 201, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Jakubiec-Puka, A.; Catani, C.; Carraro, U. Myosin heavy-chain composition in striated muscle after tenotomy. Biochem. J. 1992, 282 (Pt 1), 237–242. [Google Scholar] [CrossRef] [Green Version]
- Buller, A.J.; Lewis, D.M. Further Observations on the Differentiation of Skeletal Muscles in the Kitten Hind Limb. J. Physiol. 1965, 176, 355–370. [Google Scholar] [CrossRef] [Green Version]
- Jamali, A.A.; Afshar, P.; Abrams, R.A.; Lieber, R.L. Skeletal muscle response to tenotomy. Muscle Nerve 2000, 23, 851–862. [Google Scholar] [CrossRef]
- Krivoi, I.I.; Kravtsova, V.V.; Altaeva, E.G.; Kubasov, I.V.; Prokof’ev, A.V.; Drabkina, T.M.; Nikol’skii, E.E.; Shenkman, B.S. [Decrease in the electrogenic contribution of Na,K-ATPase and resting membrane potential as a possible mechanism of calcium ion accumulation in filaments of the rat musculus soleus subjected to the short-term gravity unloading]. Biofizika 2008, 53, 1051–1057. [Google Scholar] [PubMed]
- Vilchinskaya, N.A.; Krivoi, I.I.; Shenkman, B.S. AMP-Activated Protein Kinase as a Key Trigger for the Disuse-Induced Skeletal Muscle Remodeling. Int. J. Mol. Sci. 2018, 19, 3558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenkman, B.S. How Postural Muscle Senses Disuse? Early Signs and Signals. Int. J. Mol. Sci. 2020, 21, 5037. [Google Scholar] [CrossRef]
- De-Doncker, L.; Kasri, M.; Picquet, F.; Falempin, M. Physiologically adaptive changes of the L5 afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J. Exp. Biol. 2005, 208, 4585–4592. [Google Scholar] [CrossRef] [Green Version]
- McKay, W.B.; Ovechkin, A.V.; Vitaz, T.W.; Terson de Paleville, D.G.; Harkema, S.J. Long-lasting involuntary motor activity after spinal cord injury. Spinal Cord 2011, 49, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.; Tyganov, S.; Vilchinskaya, N.; Lomonosova, Y.; Shenkman, B. Key Markers of mTORC1-Dependent and mTORC1-Independent Signaling Pathways Regulating Protein Synthesis in Rat Soleus Muscle During Early Stages of Hindlimb Unloading. Cell Physiol. Biochem. 2016, 39, 1011–1020. [Google Scholar] [CrossRef]
- Paramonova, I.I.; Sharlo, K.A.; Vilchinskaya, N.A.; Shenkman, B.S. The Time Course of Muscle Nuclear Content of Transcription Factors Regulating the MyHC I(β) Expression in the Rat Soleus Muscle under Gravitational Unloading. Biol. Membr. 2020, 37. [Google Scholar] [CrossRef]
- Belova, S.P.; Vilchinskaya, N.A.; Mochalova, E.P.; Mirzoev, T.M.; Nemirovskaya, T.L.; Shenkman, B.S. Elevated p70S6K phosphorylation in rat soleus muscle during the early stage of unloading: Causes and consequences. Arch. Biochem. Biophys. 2019, 674, 108105. [Google Scholar] [CrossRef] [PubMed]
- Sharlo, K.; Paramonova, I.; Turtikova, O.; Tyganov, S.; Shenkman, B. Plantar mechanical stimulation prevents calcineurin-NFATc1 inactivation and slow-to-fast fiber type shift in rat soleus muscle under hindlimb unloading. J. Appl. Physiol. 2019, 126, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Zaripova, K.A.; Kalashnikova, E.P.; Belova, S.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. Role of Pannexin 1 ATP-Permeable Channels in the Regulation of Signaling Pathways during Skeletal Muscle Unloading. Int. J. Mol. Sci. 2021, 22, 10444. [Google Scholar] [CrossRef]
- Tyganov, S.A.; Mochalova, E.P.; Belova, S.P.; Sharlo, K.A.; Rozhkov, S.V.; Vilchinskaya, N.A.; Paramonova, I.I.; Mirzoev, T.M.; Shenkman, B.S. Effects of Plantar Mechanical Stimulation on Anabolic and Catabolic Signaling in Rat Postural Muscle Under Short-Term Simulated Gravitational Unloading. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Rozhkov, S.V.; Sharlo, K.A.; Mirzoev, T.M.; Shenkman, B.S. Temporal changes in the markers of ribosome biogenesis in rat soleus muscle under simulated microgravity. Acta Astronaut. 2021, 186, 252–258. [Google Scholar] [CrossRef]
- Tyganov, S.A.; Mochalova, E.; Belova, S.; Sharlo, K.; Rozhkov, S.; Kalashnikov, V.; Turtikova, O.; Mirzoev, T.; Shenkman, B. Plantar mechanical stimulation attenuates protein synthesis decline in disused skeletal muscle via modulation of nitric oxide level. Sci. Rep. 2021, 11, 9806. [Google Scholar] [CrossRef]
- Loughna, P.; Goldspink, G.; Goldspink, D.F. Effect of inactivity and passive stretch on protein turnover in phasic and postural rat muscles. J. Appl. Physiol. 1986, 61, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Fluckey, J.D.; Dupont-Versteegden, E.E.; Montague, D.C.; Knox, M.; Tesch, P.; Peterson, C.A.; Gaddy-Kurten, D. A rat resistance exercise regimen attenuates losses of musculoskeletal mass during hindlimb suspension. Acta. Physiol. Scand. 2002, 176, 293–300. [Google Scholar] [CrossRef]
- Fluckey, J.D.; Dupont-Versteegden, E.E.; Knox, M.; Gaddy, D.; Tesch, P.A.; Peterson, C.A. Insulin facilitation of muscle protein synthesis following resistance exercise in hindlimb-suspended rats is independent of a rapamycin-sensitive pathway. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1070–E1075. [Google Scholar] [CrossRef] [Green Version]
- You, J.S.; Anderson, G.B.; Dooley, M.S.; Hornberger, T.A. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis. Models Mech. 2015, 8, 1059–1069. [Google Scholar] [CrossRef] [Green Version]
- Gibson, J.N.; Halliday, D.; Morrison, W.L.; Stoward, P.J.; Hornsby, G.A.; Watt, P.W.; Murdoch, G.; Rennie, M.J. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin. Sci. 1987, 72, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, A.A.; Lane, H.W.; Stuart, C.A.; Davis-Street, J.; Wolfe, R.R. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am. J. Physiol. 1996, 270, E627–E633. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Zhang, X.J.; Volpi, E.; Wolf, S.E.; Aarsland, A.; Ferrando, A.A.; Wolfe, R.R. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E321–E328. [Google Scholar] [CrossRef] [PubMed]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, T.; Kirby, T.J.; McCarthy, J.J. Ribosome biogenesis: Emerging evidence for a central role in the regulation of skeletal muscle mass. J. Cell. Physiol. 2014, 229, 1584–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzoev, T.M.; Shenkman, B.S. Regulation of protein synthesis in inactivated skeletal muscle: Signal inputs, protein kinase cascades, and ribosome biogenesis. Biochemistry 2018, 83, 1299–1317. [Google Scholar] [CrossRef]
- Baehr, L.M.; West, D.W.D.; Marshall, A.G.; Marcotte, G.R.; Baar, K.; Bodine, S.C. Muscle-specific and age-related changes in protein synthesis and protein degradation in response to hindlimb unloading in rats. J. Appl. Physiol. 2017, 122, 1336–1350. [Google Scholar] [CrossRef] [Green Version]
- Hornberger, T.A. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int. J. Biochem. Cell. Biol. 2011, 43, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Wackerhage, H.; Ratkevicius, A. Signal transduction pathways that regulate muscle growth. Essays Biochem. 2008, 44, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Tong, J.; Zhu, M.J.; Ma, C.; Du, M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol. Reprod. Dev. 2008, 75, 810–817. [Google Scholar] [CrossRef]
- Erbay, E.; Park, I.H.; Nuzzi, P.D.; Schoenherr, C.J.; Chen, J. IGF-II transcription in skeletal myogenesis is controlled by mTOR and nutrients. J. Cell Biol. 2003, 163, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Jheng, H.F.; Tsai, P.J.; Guo, S.M.; Kuo, L.H.; Chang, C.S.; Su, I.J.; Chang, C.R.; Tsai, Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell. Biol. 2012, 32, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell. Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef]
- Goodman, C.A.; Miu, M.H.; Frey, J.W.; Mabrey, D.M.; Lincoln, H.C.; Ge, Y.; Chen, J.; Hornberger, T.A. A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol. Biol. Cell. 2010, 21, 3258–3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, M.; McCarthy, J.J.; Fedele, M.J.; Esser, K.A. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J. Physiol. 2011, 589, 1831–1846. [Google Scholar] [CrossRef]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of muscle protein synthesis and the effects of catabolic states. Int. J. Biochem. Cell Biol. 2013, 45, 2147–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, T.; Sakashita, Y.; Kitahata, K.; Yamashita, Y.; Tomida, C.; Kimori, Y.; Komatsu, A.; Hirasaka, K.; Ohno, A.; Nakao, R.; et al. Reactive oxygen species upregulate expression of muscle atrophy-associated ubiquitin ligase Cbl-b in rat L6 skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2018, 314, C721–C731. [Google Scholar] [CrossRef]
- Welsh, G.I.; Miller, C.M.; Loughlin, A.J.; Price, N.T.; Proud, C.G. Regulation of eukaryotic initiation factor eIF2B: Glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett. 1998, 421, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Chibalin, A.V.; Benziane, B.; Zakyrjanova, G.F.; Kravtsova, V.V.; Krivoi, I.I. Early endplate remodeling and skeletal muscle signaling events following rat hindlimb suspension. J. Cell. Physiol. 2018, 233, 6329–6336. [Google Scholar] [CrossRef]
- Dupont, E.; Cieniewski-Bernard, C.; Bastide, B.; Stevens, L. Electrostimulation during hindlimb unloading modulates PI3K-AKT downstream targets without preventing soleus atrophy and restores slow phenotype through ERK. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Bajotto, G.; Sato, Y.; Kitaura, Y.; Shimomura, Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur. J. Appl. Physiol. 2011, 111, 1815–1828. [Google Scholar] [CrossRef]
- Gwag, T.; Lee, K.; Ju, H.; Shin, H.; Lee, J.W.; Choi, I. Stress and signaling responses of rat skeletal muscle to brief endurance exercise during hindlimb unloading: A catch-up process for atrophied muscle. Cell. Physiol. Biochem. 2009, 24, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.R.; Kimball, S.R.; Dennis, M.D.; Schilder, R.J.; Jefferson, L.S. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E229–E236. [Google Scholar] [CrossRef] [Green Version]
- Cannavino, J.; Brocca, L.; Sandri, M.; Bottinelli, R.; Pellegrino, M.A. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J. Physiol. 2014, 592, 4575–4589. [Google Scholar] [CrossRef]
- Han, B.; Zhu, M.J.; Ma, C.; Du, M. Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.M.; Tyganov, S.A.; Lomonosova, Y.N.; Musienko, P.E.; Shenkman, B.S. Signaling Pathways Regulating Protein Synthesis in Rat Soleus Muscle during Early Stages of Hindlimb Unloading. Ross. Fiziol. Zhurnal. Im. I.M. Sechenova 2015, 101, 1299–1308. [Google Scholar]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilchinskaya, N.A.; Mochalova, E.P.; Nemirovskaya, T.L.; Mirzoev, T.M.; Turtikova, O.V.; Shenkman, B.S. Rapid decline in MyHC I(beta) mRNA expression in rat soleus during hindlimb unloading is associated with AMPK dephosphorylation. J. Physiol. 2017, 595, 7123–7134. [Google Scholar] [CrossRef] [Green Version]
- Vilchinskaya, N.A.; Mirzoev, T.M.; Lomonosova, Y.N.; Kozlovskaya, I.B.; Shenkman, B.S. Human muscle signaling responses to 3-day head-out dry immersion. J. Musculoskelet. Neuronal Interact 2015, 15, 286–293. [Google Scholar]
- Wall, B.T.; Snijders, T.; Senden, J.M.; Ottenbros, C.L.; Gijsen, A.P.; Verdijk, L.B.; van Loon, L.J. Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J. Clin. Endocrinol. Metab. 2013, 98, 4872–4881. [Google Scholar] [CrossRef] [Green Version]
- de Boer, M.D.; Selby, A.; Atherton, P.; Smith, K.; Seynnes, O.R.; Maganaris, C.N.; Maffulli, N.; Movin, T.; Narici, M.V.; Rennie, M.J. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J. Physiol. 2007, 585, 241–251. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dickinson, J.M.; Fry, C.S.; Walker, D.K.; Gundermann, D.M.; Reidy, P.T.; Timmerman, K.L.; Markofski, M.M.; Paddon-Jones, D.; Rasmussen, B.B.; et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1113–E1122. [Google Scholar] [CrossRef] [Green Version]
- Glover, E.I.; Yasuda, N.; Tarnopolsky, M.A.; Abadi, A.; Phillips, S.M. Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl. Physiol. Nutr. Metab. 2010, 35, 125–133. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Shangraw, R.E.; Stuart, C.A.; Prince, M.J.; Peters, E.J.; Wolfe, R.R. Insulin responsiveness of protein metabolism in vivo following bedrest in humans. Am. J. Physiol. 1988, 255, E548–E558. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.A.; Shangraw, R.E.; Prince, M.J.; Peters, E.J.; Wolfe, R.R. Bed-rest-induced insulin resistance occurs primarily in muscle. Metab. Clin. Exp. 1988, 37, 802–806. [Google Scholar] [CrossRef]
- Richter, E.A.; Kiens, B.; Mizuno, M.; Strange, S. Insulin action in human thighs after one-legged immobilization. J. Appl. Physiol. 1989, 67, 19–23. [Google Scholar] [CrossRef]
- Allen, D.L.; Linderman, J.K.; Roy, R.R.; Grindeland, R.E.; Mukku, V.; Edgerton, V.R. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J. Appl. Physiol. 1997, 83, 1857–1861. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Baldwin, K.M.; Tesch, P.A. Pretranslational markers of contractile protein expression in human skeletal muscle: Effect of limb unloading plus resistance exercise. J. Appl. Physiol. 2005, 98, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamrin, L.; Berg, H.E.; Essen, P.; Tesch, P.A.; Hultman, E.; Garlick, P.J.; McNurlan, M.A.; Wernerman, J. The effect of unloading on protein synthesis in human skeletal muscle. Acta Physiol. Scand. 1998, 163, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Petersson, B.; Wernerman, J.; Waller, S.O.; von der Decken, A.; Vinnars, E. Elective abdominal surgery depresses muscle protein synthesis and increases subjective fatigue: Effects lasting more than 30 days. Br. J. Surg. 1990, 77, 796–800. [Google Scholar] [CrossRef]
- Gibson, J.N.; Smith, K.; Rennie, M.J. Prevention of disuse muscle atrophy by means of electrical stimulation: Maintenance of protein synthesis. Lancet 1988, 2, 767–770. [Google Scholar] [CrossRef]
- Anderson, J.E.; Zhu, A.; Mizuno, T.M. Nitric oxide treatment attenuates muscle atrophy during hind limb suspension in mice. Free Radic. Biol. Med. 2018, 115, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B.; Judge, A.R.; Bodine, S.C. CrossTalk opposing view: The dominant mechanism causing disuse muscle atrophy is proteolysis. J. Physiol. 2014, 592, 5345–5347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesch, P.A.; von Walden, F.; Gustafsson, T.; Linnehan, R.M.; Trappe, T.A. Skeletal muscle proteolysis in response to short-term unloading in humans. J. Appl. Physiol. 2008, 105, 902–906. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.M.; McGlory, C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J. Physiol. 2014, 592, 5341–5343. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Faraldi, M.; Musaro, A. The Proteolytic Systems of Muscle Wasting. Recent. Adv. DNA Gene Seq. 2015, 9, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayton, W.R.; Goll, D.E.; Zeece, M.G.; Robson, R.M.; Reville, W.J. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry 1976, 15, 2150–2158. [Google Scholar] [CrossRef]
- Arutyunyan, R.S.; Kozlovskaya, I.B.; Nasledov, G.A.; Nemirovskaya, T.L.; Radzyukevitch, T.L.; Shenkman, B.S. The contraction of unweighted fast and slow rat muscles in calcium-free solution. Basic Appl. Myol. 1995, 5, 169–175. [Google Scholar]
- Ingalls, C.P.; Warren, G.L.; Armstrong, R.B. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J. Appl. Physiol. 1999, 87, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Shenkman, B.S.; Nemirovskaya, T.L. Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J. Muscle Res. Cell Motil. 2008, 29, 221–230. [Google Scholar] [CrossRef]
- Ingalls, C.P.; Wenke, J.C.; Armstrong, R.B. Time course changes in [Ca2+]i, force, and protein content in hindlimb-suspended mouse soleus muscles. Aviat. Space Env. Med. 2001, 72, 471–476. [Google Scholar]
- Tidball, J.G.; Spencer, M.J. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J. Physiol. 2002, 545, 819–828. [Google Scholar] [CrossRef]
- Shenkman, B.S.; Belova, S.P.; Lomonosova, Y.N.; Kostrominova, T.Y.; Nemirovskaya, T.L. Calpain-dependent regulation of the skeletal muscle atrophy following unloading. Arch. Biochem. Biophys. 2015, 584, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Petrova, I.O.; Tyganov, S.A.; Mirzoev, T.M.; Tsaturyan, A.K.; Kozlovskaya, I.B.; Shenkman, B.S. Early Desmall es, Cyrillicline in Rat Soleus Passive Tension with Hindlimb Unloading: Inactivation of Cross-bridges or Activation of Calpains? Dokl. Biochem. Biophys. 2018, 481, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Aweida, D.; Rudesky, I.; Volodin, A.; Shimko, E.; Cohen, S. GSK3-beta promotes calpain-1-mediated desmin filament depolymerization and myofibril loss in atrophy. J. Cell. Biol. 2018, 217, 3698–3714. [Google Scholar] [CrossRef] [Green Version]
- Tyganov, S.A.; Mochalova, E.P.; Melnikov, I.Y.; Vikhlyantsev, I.M.; Ulanova, A.D.; Sharlo, K.A.; Mirzoev, T.M.; Shenkman, B.S. NOS-dependent effects of plantar mechanical stimulation on mechanical characteristics and cytoskeletal proteins in rat soleus muscle during hindlimb suspension. FASEB J. 2021, 35, e21905. [Google Scholar] [CrossRef]
- Lechado, I.T.A.; Vitadello, M.; Traini, L.; Namuduri, A.V.; Gastaldello, S.; Gorza, L. Sarcolemmal loss of active nNOS (Nos1) is an oxidative stress-dependent, early event driving disuse atrophy. J. Pathol. 2018, 246, 433–446. [Google Scholar] [CrossRef]
- Lomonosova, Y.N.; Kalamkarov, G.R.; Bugrova, A.E.; Shevchenko, T.F.; Kartashkina, N.L.; Lysenko, E.A.; Shvets, V.I.; Nemirovskaya, T.L. Protective effect of L-Arginine administration on proteins of unloaded m. soleus. Biochemistry 2011, 76, 571–580. [Google Scholar] [CrossRef]
- Sharlo, K.A.; Paramonova, I.I.; Lvova, I.D.; Vilchinskaya, N.A.; Bugrova, A.E.; Shevchenko, T.F.; Kalamkarov, G.R.; Shenkman, B.S. NO-Dependent Mechanisms of Myosin Heavy Chain Transcription Regulation in Rat Soleus Muscle After 7-Days Hindlimb Unloading. Front. Physiol. 2020, 11, 814. [Google Scholar] [CrossRef]
- Suzuki, N.; Motohashi, N.; Uezumi, A.; Fukada, S.; Yoshimura, T.; Itoyama, Y.; Aoki, M.; Miyagoe-Suzuki, Y.; Takeda, S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J. Clin. Investig. 2007, 117, 2468–2476. [Google Scholar] [CrossRef] [Green Version]
- Sharlo, K.A.; Paramonova, I.I.; Lvova, I.D.; Mochalova, E.P.; Kalashnikov, V.E.; Vilchinskaya, N.A.; Tyganov, S.A.; Konstantinova, T.S.; Shevchenko, T.F.; Kalamkarov, G.R.; et al. Plantar Mechanical Stimulation Maintains Slow Myosin Expression in Disused Rat Soleus Muscle via NO-Dependent Signaling. Int. J. Mol. Sci. 2021, 22, 1372. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, X. The molecular mechanisms of calpains action on skeletal muscle atrophy. Physiol. Res. 2016, 65, 547–560. [Google Scholar] [CrossRef]
- Talbert, E.E.; Smuder, A.J.; Min, K.; Kwon, O.S.; Powers, S.K. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J. Appl. Physiol. 2013, 114, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M. Autophagy in skeletal muscle. FEBS Lett. 2010, 584, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Leger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflug. Arch. Eur. J. Physiol. 2011, 461, 325–335. [Google Scholar] [CrossRef]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Murton, A.J.; Constantin, D.; Greenhaff, P.L. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim. Biophys. Acta 2008, 1782, 730–743. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.L.; Bandstra, E.R.; Harrison, B.C.; Thorng, S.; Stodieck, L.S.; Kostenuik, P.J.; Morony, S.; Lacey, D.L.; Hammond, T.G.; Leinwand, L.L.; et al. Effects of spaceflight on murine skeletal muscle gene expression. J. Appl. Physiol. 2009, 106, 582–595. [Google Scholar] [CrossRef] [Green Version]
- Gambara, G.; Salanova, M.; Ciciliot, S.; Furlan, S.; Gutsmann, M.; Schiffl, G.; Ungethuem, U.; Volpe, P.; Gunga, H.C.; Blottner, D. Microgravity-Induced Transcriptome Adaptation in Mouse Paraspinal longissimus dorsi Muscle Highlights Insulin Resistance-Linked Genes. Front. Physiol. 2017, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Labeit, S.; Kohl, C.H.; Witt, C.C.; Labeit, D.; Jung, J.; Granzier, H. Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J. Biomed. Biotechnol. 2010, 2010, 693741. [Google Scholar] [CrossRef] [Green Version]
- Belova, S.P.; Mochalova, E.P.; Kostrominova, T.Y.; Shenkman, B.S.; Nemirovskaya, T.L. P38alpha-MAPK Signaling Inhibition Attenuates Soleus Atrophy during Early Stages of Muscle Unloading. Int. J. Mol. Sci. 2020, 21, 2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derbre, F.; Ferrando, B.; Gomez-Cabrera, M.C.; Sanchis-Gomar, F.; Martinez-Bello, V.E.; Olaso-Gonzalez, G.; Diaz, A.; Gratas-Delamarche, A.; Cerda, M.; Vina, J. Inhibition of xanthine oxidase by allopurinol prevents skeletal muscle atrophy: Role of p38 MAPKinase and E3 ubiquitin ligases. PLoS ONE 2012, 7, e46668. [Google Scholar] [CrossRef]
- Casas, M.; Buvinic, S.; Jaimovich, E. ATP signaling in skeletal muscle: From fiber plasticity to regulation of metabolism. Exerc. Sport Sci. Rev. 2014, 42, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Mochalova, E.P.; Belova, S.P.; Mirzoev, T.M.; Shenkman, B.S.; Nemirovskaya, T.L. Atrogin-1/MAFbx mRNA expression is regulated by histone deacetylase 1 in rat soleus muscle under hindlimb unloading. Sci. Rep. 2019, 9, 10263. [Google Scholar] [CrossRef]
- Abadi, A.; Glover, E.I.; Isfort, R.J.; Raha, S.; Safdar, A.; Yasuda, N.; Kaczor, J.J.; Melov, S.; Hubbard, A.; Qu, X.; et al. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS ONE 2009, 4, e6518. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, T.; Osterlund, T.; Flanagan, J.N.; von Walden, F.; Trappe, T.A.; Linnehan, R.M.; Tesch, P.A. Effects of 3 days unloading on molecular regulators of muscle size in humans. J. Appl. Physiol. 2010, 109, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Furochi, H.; Mameoka, M.; Hirasaka, K.; Onishi, Y.; Suzue, N.; Oarada, M.; Akamatsu, M.; Akima, H.; Fukunaga, T.; et al. Ubiquitin ligase gene expression in healthy volunteers with 20-day bedrest. Muscle Nerve 2006, 34, 463–469. [Google Scholar] [CrossRef]
- Jones, S.W.; Hill, R.J.; Krasney, P.A.; O’Conner, B.; Peirce, N.; Greenhaff, P.L. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004, 18, 1025–1027. [Google Scholar] [CrossRef]
- Salanova, M.; Schiffl, G.; Puttmann, B.; Schoser, B.G.; Blottner, D. Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J. Anat. 2008, 212, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Milan, G.; Romanello, V.; Pescatore, F.; Armani, A.; Paik, J.H.; Frasson, L.; Seydel, A.; Zhao, J.; Abraham, R.; Goldberg, A.L.; et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat. Commun. 2015, 6, 6670. [Google Scholar] [CrossRef] [Green Version]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef] [Green Version]
- Stitt, T.N.; Drujan, D.; Clarke, B.A.; Panaro, F.; Timofeyva, Y.; Kline, W.O.; Gonzalez, M.; Yancopoulos, G.D.; Glass, D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 2004, 14, 395–403. [Google Scholar] [CrossRef]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Brault, J.J.; Schild, A.; Cao, P.; Sandri, M.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell. Metab. 2007, 6, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Machida, S. Changes in FOXO and proinflammatory cytokines in the late stage of immobilized fast and slow muscle atrophy. Biomed. Res. 2017, 38, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.; Biswas, C.; Dierov, J.; Barsotti, R.; Shrager, J.B.; Nguyen, T.; Sonnad, S.; Kucharchzuk, J.C.; Kaiser, L.R.; Singhal, S.; et al. Increased proteolysis, myosin depletion, and atrophic AKT-FOXO signaling in human diaphragm disuse. Am. J. Respir. Crit. Care Med. 2011, 183, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Giresi, P.G.; Stevenson, E.J.; Theilhaber, J.; Koncarevic, A.; Parkington, J.; Fielding, R.A.; Kandarian, S.C. Identification of a molecular signature of sarcopenia. Physiol. Genom. 2005, 21, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Sacheck, J.M.; Hyatt, J.P.; Raffaello, A.; Jagoe, R.T.; Roy, R.R.; Edgerton, V.R.; Lecker, S.H.; Goldberg, A.L. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007, 21, 140–155. [Google Scholar] [CrossRef]
- Brocca, L.; Toniolo, L.; Reggiani, C.; Bottinelli, R.; Sandri, M.; Pellegrino, M.A. FoxO-dependent atrogenes vary among catabolic conditions and play a key role in muscle atrophy induced by hindlimb suspension. J. Physiol. 2017, 595, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; Sandesara, P.B.; Senf, S.M.; Judge, A.R. Inhibition of FoxO transcriptional activity prevents muscle fiber atrophy during cachexia and induces hypertrophy. FASEB J. 2012, 26, 987–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senf, S.M.; Dodd, S.L.; McClung, J.M.; Judge, A.R. Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J. 2008, 22, 3836–3845. [Google Scholar] [CrossRef]
- Mammucari, C.; Milan, G.; Romanello, V.; Masiero, E.; Rudolf, R.; Del Piccolo, P.; Burden, S.J.; Di Lisi, R.; Sandri, C.; Zhao, J.; et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007, 6, 458–471. [Google Scholar] [CrossRef]
- Grigor’ev, A.I.; Kozlovskaia, I.B.; Shenkman, B.S. [The role of support afferents in organisation of the tonic muscle system]. Ross. Fiziol. Zh. Im. I. M. Sechenova. 2004, 90, 508–521. [Google Scholar]
- Trappe, S.; Costill, D.; Gallagher, P.; Creer, A.; Peters, J.R.; Evans, H.; Riley, D.A.; Fitts, R.H. Exercise in space: Human skeletal muscle after 6 months aboard the International Space Station. J. Appl. Physiol. 2009, 106, 1159–1168. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.Y.; Klitgaard, H.; Saltin, B.; Roy, R.R.; Edgerton, V.R.; Gollnick, P.D. Myosin heavy chain isoforms of human muscle after short-term spaceflight. J. Appl. Physiol. 1995, 78, 1740–1744. [Google Scholar] [CrossRef] [PubMed]
- Borina, E.; Pellegrino, M.A.; D’Antona, G.; Bottinelli, R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand. J. Med. Sci. Sports. 2010, 20, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Malisoux, L.; Jamart, C.; Delplace, K.; Nielens, H.; Francaux, M.; Theisen, D. Effect of long-term muscle paralysis on human single fiber mechanics. J. Appl. Physiol. 2007, 102, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Giger, J.M.; Bodell, P.W.; Zeng, M.; Baldwin, K.M.; Haddad, F. Rapid muscle atrophy response to unloading: Pretranslational processes involving MHC and actin. J. Appl. Physiol. 2009, 107, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Stevens, L.; Sultan, K.R.; Peuker, H.; Gohlsch, B.; Mounier, Y.; Pette, D. Time-dependent changes in myosin heavy chain mRNA and protein isoforms in unloaded soleus muscle of rat. Am. J. Physiol. 1999, 277, C1044–C1049. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in muscle mass and contractile phenotype in response to unloading models: Role of transcriptional/pretranslational mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lomonosova, Y.N.; Turtikova, O.V.; Shenkman, B.S. Reduced expression of MyHC slow isoform in rat soleus during unloading is accompanied by alterations of endogenous inhibitors of calcineurin/NFAT signaling pathway. J. Muscle Res. Cell Motil. 2016, 37, 7–16. [Google Scholar] [CrossRef]
- Matoba, H.; Wakatuti, Y.; Ohira, Y. 879β-Guanidinopropionic acid suppresses suspension-induced changes in myosin expression in rat skeletal muscle. Med. Sci. Sports Exerc. 1993, 25, 157. [Google Scholar] [CrossRef]
- Braz, J.C.; Bueno, O.F.; Liang, Q.; Wilkins, B.J.; Dai, Y.S.; Parsons, S.; Braunwart, J.; Glascock, B.J.; Klevitsky, R.; Kimball, T.F.; et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Investig. 2003, 111, 1475–1486. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Bueno, O.F.; Wilkins, B.J.; Kuan, C.Y.; Xia, Y.; Molkentin, J.D. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J. 2003, 22, 5079–5089. [Google Scholar] [CrossRef] [Green Version]
- McCullagh, K.J.; Calabria, E.; Pallafacchina, G.; Ciciliot, S.; Serrano, A.L.; Argentini, C.; Kalhovde, J.M.; Lomo, T.; Schiaffino, S. NFAT is a nerve activity sensor in skeletal muscle and controls activity-dependent myosin switching. Proc. Natl. Acad. Sci. USA 2004, 101, 10590–10595. [Google Scholar] [CrossRef] [Green Version]
- Sharlo, C.A.; Turtikova, Y.N.; Turtikova, O.V.; Mitrofanova, O.V.; Kalamkarov, G.R.; Bugrova, A.E.; Shevchenko, T.F.; Shenkman, B.S. The Role of GSK-3β Phosphorylation in the Regulation of Slow Myosin Expression in Soleus Muscle during Functional Unloading. Biochem. Suppl. Ser. A Membr. Cell. Biol. 2018, 12, 85–91. [Google Scholar] [CrossRef]
- Sharlo, K.A.; Mochalova, E.P.; Belova, S.P.; Lvova, I.D.; Nemirovskaya, T.L.; Shenkman, B.S. The role of MAP-kinase p38 in the m. soleus slow myosin mRNA transcription regulation during short-term functional unloading. Arch. Biochem. Biophys. 2020, 695, 108622. [Google Scholar] [CrossRef]
- Popov, D.V.; Vinogradova, O.L.; A.I., G. Human aerobic performance. (book in russian).
- Gifford, J.R.; Weavil, J.C.; Nelson, A.D. Symmorphosis in patients with chronic heart failure? J. Appl. Physiol. 2016, 121, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, H.E.; Dudley, G.A.; Hather, B.; Tesch, P.A. Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading. Clin. Physiol. 1993, 13, 337–347. [Google Scholar] [CrossRef]
- Vigelso, A.; Gram, M.; Wiuff, C.; Andersen, J.L.; Helge, J.W.; Dela, F. Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass and aerobic capacity but does not fully rehabilitate leg strength in young and older men. J. Rehabil. Med. 2015, 47, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Puthucheary, Z.A.; Astin, R.; McPhail, M.J.W.; Saeed, S.; Pasha, Y.; Bear, D.E.; Constantin, D.; Velloso, C.; Manning, S.; Calvert, L.; et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax 2018, 73, 926–935. [Google Scholar] [CrossRef]
- Kramer, A.; Kummel, J.; Mulder, E.; Gollhofer, A.; Frings-Meuthen, P.; Gruber, M. High-Intensity Jump Training Is Tolerated during 60 Days of Bed Rest and Is Very Effective in Preserving Leg Power and Lean Body Mass: An Overview of the Cologne RSL Study. PLoS ONE 2017, 12, e0169793. [Google Scholar] [CrossRef] [Green Version]
- Blottner, D.; Hastermann, M.; Weber, R.; Lenz, R.; Gambara, G.; Limper, U.; Rittweger, J.; Bosutti, A.; Degens, H.; Salanova, M. Reactive Jumps Preserve Skeletal Muscle Structure, Phenotype, and Myofiber Oxidative Capacity in Bed Rest. Front. Physiol. 2019, 10, 1527. [Google Scholar] [CrossRef] [Green Version]
- Falempin, M.; In-Albon, S.F. Influence of brief daily tendon vibration on rat soleus muscle in non-weight-bearing situation. J. Appl. Physiol. 1999, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Arentson-Lantz, E.J.; English, K.L.; Paddon-Jones, D.; Fry, C.S. Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults. J. Appl. Physiol. 2016, 120, 965–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammers, G.; van Duijnhoven, N.T.; Hoenderop, J.G.; Horstman, A.M.; de Haan, A.; Janssen, T.W.; de Graaf, M.J.; Pardoel, E.M.; Verwiel, E.T.; Thijssen, D.H.; et al. The identification of genetic pathways involved in vascular adaptations after physical deconditioning versus exercise training in humans. Exp. Physiol. 2013, 98, 710–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackney, K.J.; Ploutz-Snyder, L.L. Unilateral lower limb suspension: Integrative physiological knowledge from the past 20 years (1991–2011). Eur. J. Appl. Physiol. 2012, 112, 9–22. [Google Scholar] [CrossRef]
- Hyatt, H.; Deminice, R.; Yoshihara, T.; Powers, S.K. Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: A review of the causes and effects. Arch. Biochem. Biophys. 2019, 662, 49–60. [Google Scholar] [CrossRef]
- Moriggi, M.; Vasso, M.; Fania, C.; Capitanio, D.; Bonifacio, G.; Salanova, M.; Blottner, D.; Rittweger, J.; Felsenberg, D.; Cerretelli, P.; et al. Long term bed rest with and without vibration exercise countermeasures: Effects on human muscle protein dysregulation. Proteomics 2010, 10, 3756–3774. [Google Scholar] [CrossRef] [Green Version]
- Dillon, E.L.; Soman, K.V.; Wiktorowicz, J.E.; Sur, R.; Jupiter, D.; Danesi, C.P.; Randolph, K.M.; Gilkison, C.R.; Durham, W.J.; Urban, R.J.; et al. Proteomic investigation of human skeletal muscle before and after 70 days of head down bed rest with or without exercise and testosterone countermeasures. PLoS ONE 2019, 14, e0217690. [Google Scholar] [CrossRef] [Green Version]
- Chopard, A.; Lecunff, M.; Danger, R.; Lamirault, G.; Bihouee, A.; Teusan, R.; Jasmin, B.J.; Marini, J.F.; Leger, J.J. Large-scale mRNA analysis of female skeletal muscles during 60 days of bed rest with and without exercise or dietary protein supplementation as countermeasures. Physiol. Genom. 2009, 38, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibegovic, A.C.; Sonne, M.P.; Hojbjerre, L.; Bork-Jensen, J.; Jacobsen, S.; Nilsson, E.; Faerch, K.; Hiscock, N.; Mortensen, B.; Friedrichsen, M.; et al. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E752–E763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevino, M.B.; Zhang, X.; Standley, R.A.; Wang, M.; Han, X.; Reis, F.C.G.; Periasamy, M.; Yu, G.; Kelly, D.P.; Goodpaster, B.H.; et al. Loss of mitochondrial energetics is associated with poor recovery of muscle function but not mass following disuse atrophy. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E899–E910. [Google Scholar] [CrossRef] [Green Version]
- Rullman, E.; Fernandez-Gonzalo, R.; Mekjavic, I.B.; Gustafsson, T.; Eiken, O. MEF2 as upstream regulator of the transcriptome signature in human skeletal muscle during unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R799–R809. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Gonzalo, R.; Tesch, P.A.; Lundberg, T.R.; Alkner, B.A.; Rullman, E.; Gustafsson, T. Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. FASEB J. 2020, 34, 7958–7969. [Google Scholar] [CrossRef] [Green Version]
- Mahmassani, Z.S.; Reidy, P.T.; McKenzie, A.I.; Stubben, C.; Howard, M.T.; Drummond, M.J. Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy. J. Appl. Physiol. 2019, 126, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Wiggs, M.P.; Duarte, J.A.; Zergeroglu, A.M.; Demirel, H.A. Mitochondrial signaling contributes to disuse muscle atrophy. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E31–E39. [Google Scholar] [CrossRef]
- Max, S.R. Disuse atrophy of skeletal muscle: Loss of functional activity of mitochondria. Biochem. Biophys. Res. Commun. 1972, 46, 1394–1398. [Google Scholar] [CrossRef]
- Memme, J.M.; Slavin, M.; Moradi, N.; Hood, D.A. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int. J. Mol. Sci. 2021, 22, 5179. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Ozdemir, M.; Yoshihara, T.; Nguyen, B.L.; Deminice, R.; Powers, S.K. Calpains play an essential role in mechanical ventilation-induced diaphragmatic weakness and mitochondrial dysfunction. Redox Biol. 2021, 38, 101802. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Mitochondrial dysregulation and muscle disuse atrophy. F1000Research 2019, 8. [Google Scholar] [CrossRef]
- Standley, R.A.; Distefano, G.; Trevino, M.B.; Chen, E.; Narain, N.R.; Greenwood, B.; Kondakci, G.; Tolstikov, V.V.; Kiebish, M.A.; Yu, G.; et al. Skeletal Muscle Energetics and Mitochondrial Function Are Impaired Following 10 Days of Bed Rest in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1744–1753. [Google Scholar] [CrossRef] [Green Version]
- Buso, A.; Comelli, M.; Picco, R.; Isola, M.; Magnesa, B.; Pisot, R.; Rittweger, J.; Salvadego, D.; Simunic, B.; Grassi, B.; et al. Mitochondrial Adaptations in Elderly and Young Men Skeletal Muscle Following 2 Weeks of Bed Rest and Rehabilitation. Front. Physiol. 2019, 10, 474. [Google Scholar] [CrossRef]
- Jiroutkova, K.; Krajcova, A.; Ziak, J.; Fric, M.; Waldauf, P.; Dzupa, V.; Gojda, J.; Nemcova-Furstova, V.; Kovar, J.; Elkalaf, M.; et al. Mitochondrial function in skeletal muscle of patients with protracted critical illness and ICU-acquired weakness. Crit. Care 2015, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Theeuwes, W.F.; Gosker, H.R.; Langen, R.C.J.; Verhees, K.J.P.; Pansters, N.A.M.; Schols, A.; Remels, A.H.V. Inactivation of glycogen synthase kinase-3beta (GSK-3beta) enhances skeletal muscle oxidative metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 3075–3086. [Google Scholar] [CrossRef]
- Oishi, Y.; Ogata, T.; Yamamoto, K.I.; Terada, M.; Ohira, T.; Ohira, Y.; Taniguchi, K.; Roy, R.R. Cellular adaptations in soleus muscle during recovery after hindlimb unloading. Acta Physiol. 2008, 192, 381–395. [Google Scholar] [CrossRef]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; Lebrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef] [Green Version]
- Tadaishi, M.; Miura, S.; Kai, Y.; Kano, Y.; Oishi, Y.; Ezaki, O. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS ONE 2011, 6, e28290. [Google Scholar] [CrossRef] [Green Version]
- Theeuwes, W.F.; Gosker, H.R.; Schols, A.; Langen, R.C.J.; Remels, A.H.V. Regulation of PGC-1alpha expression by a GSK-3beta-TFEB signaling axis in skeletal muscle. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118610. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Chen, D.; Yu, B.; Li, M.; He, J.; Huang, Z. MicroRNA-499–5p regulates skeletal myofiber specification via NFATc1/MEF2C pathway and Thrap1/MEF2C axis. Life Sci. 2018, 215, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, I.B.; Sayenko, I.V.; Sayenko, D.G.; Miller, T.F.; Khusnutdinova, D.R.; Melnik, K.A. Role of support afferentation in control of the tonic muscle activity. Acta Astronaut. 2007, 60, 285–294. [Google Scholar] [CrossRef]
- Mirzoev, T.M. Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth. Int. J. Mol. Sci. 2020, 21, 7940. [Google Scholar] [CrossRef]
- Clement, G. International roadmap for artificial gravity research. NPJ Microgravity 2017, 3, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layne, C.S.; Mulavara, A.P.; Pruett, C.J.; McDonald, P.V.; Kozlovskaya, I.B.; Bloomberg, J.J. The use of in-flight foot pressure as a countermeasure to neuromuscular degradation. Acta Astronaut. 1998, 42, 231–246. [Google Scholar] [CrossRef]
- Yarmanova, E.N.; Kozlovskaya, I.B.; Khimoroda, N.N.; Fomina, E.V. Evolution of Russian Microgravity Countermeasures. Aerosp. Med. Hum. Perform. 2015, 86, A32–A37. [Google Scholar] [CrossRef] [PubMed]
- Vitadello, M.; Germinario, E.; Ravara, B.; Libera, L.D.; Danieli-Betto, D.; Gorza, L. Curcumin counteracts loss of force and atrophy of hindlimb unloaded rat soleus by hampering neuronal nitric oxide synthase untethering from sarcolemma. J. Physiol. 2014, 592, 2637–2652. [Google Scholar] [CrossRef] [PubMed]
- Fomina, E.V.; Lysova, N.Y.; Chernova, M.V.; Khustnudinova, D.R.; Kozlovskaya, I.B. [Comparative Analisys of Efficacy of Countermeasure Provided by Different Modes of Locomotor Training in Space Flight.]. Fiziol. Cheloveka 2016, 42, 84–91. [Google Scholar] [PubMed]
- English, K.L.; Downs, M.; Goetchius, E.; Buxton, R.; Ryder, J.W.; Ploutz-Snyder, R.; Guilliams, M.; Scott, J.M.; Ploutz-Snyder, L.L. High intensity training during spaceflight: Results from the NASA Sprint Study. NPJ Microgravity 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, I.B.; Grigoriev, A.I. Russian system of countermeasures on board of the International Space Station (ISS): The first results. Acta Astronaut. 2004, 55, 233–237. [Google Scholar] [CrossRef]
- Hotta, N.; Ishida, K.; Sato, K.; Koike, T.; Katayama, K.; Akima, H. The effect of intense interval cycle-training on unloading-induced dysfunction and atrophy in the human calf muscle. J. Physiol. Anthr. 2011, 30, 29–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohira, Y.; Yoshinaga, T.; Ohara, M.; Nonaka, I.; Yoshioka, T.; Yamashita-Goto, K.; Shenkman, B.S.; Kozlovskaya, I.B.; Roy, R.R.; Edgerton, V.R. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J. Appl. Physiol. 1999, 87, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Usuki, F.; Fujimura, M.; Nakamura, A.; Nakano, J.; Okita, M.; Higuchi, I. Local Vibration Stimuli Induce Mechanical Stress-Induced Factors and Facilitate Recovery From Immobilization-Induced Oxidative Myofiber Atrophy in Rats. Front. Physiol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, M.M.; Van Pelt, D.W.; Confides, A.L.; Hunt, E.R.; Hettinger, Z.R.; Laurin, J.L.; Reid, J.J.; Peelor, F.F., 3rd; Butterfield, T.A.; Dupont-Versteegden, E.E.; et al. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol. 2020, 229, e13460. [Google Scholar] [CrossRef]
- Symons, T.B.; Sheffield-Moore, M.; Chinkes, D.L.; Ferrando, A.A.; Paddon-Jones, D. Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J. Appl. Physiol. 2009, 107, 34–38. [Google Scholar] [CrossRef]
- Caiozzo, V.J.; Haddad, F.; Lee, S.; Baker, M.; Paloski, W.; Baldwin, K.M. Artificial gravity as a countermeasure to microgravity: A pilot study examining the effects on knee extensor and plantar flexor muscle groups. J. Appl. Physiol. 2009, 107, 39–46. [Google Scholar] [CrossRef]
- D’Aunno, D.S.; Robinson, R.R.; Smith, G.S.; Thomason, D.B.; Booth, F.W. Intermittent acceleration as a countermeasure to soleus muscle atrophy. J. Appl. Physiol. 1992, 72, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Clement, G.; Paloski, W.H.; Rittweger, J.; Linnarsson, D.; Bareille, M.P.; Mulder, E.; Wuyts, F.L.; Zange, J. Centrifugation as a countermeasure during bed rest and dry immersion: What has been learned? J. Musculoskelet Neuronal. Interact. 2016, 16, 84–91. [Google Scholar] [PubMed]
- Mirzoev, T.; Tyganov, S.; Petrova, I.; Gnyubkin, V.; Laroche, N.; Vico, L.; Shenkman, B. Divergent Anabolic Signalling responses of Murine Soleus and Tibialis Anterior Muscles to Chronic 2G Hypergravity. Sci. Rep. 2017, 7, 3514. [Google Scholar] [CrossRef] [Green Version]
- Shenkman, B.S.; Podlubnaia, Z.A.; Vikhliantsev, I.M.; Litvinova, K.S.; Udal’tsov, S.N.; Nemirovskaia, T.L.; Lemesheva Iu, S.; Mukhina, A.M.; Kozlovskaia, I.B. Human soleus fibers contractile characteristics and sarcomeric cytoskeletal proteins after gravitational unloading. Contribution of support stimulus]. Biofizika 2004, 49, 881–890. [Google Scholar] [PubMed]
- Kyparos, A.; Feeback, D.L.; Layne, C.S.; Martinez, D.A.; Clarke, M.S. Mechanical stimulation of the plantar foot surface attenuates soleus muscle atrophy induced by hindlimb unloading in rats. J. Appl. Physiol. 2005, 99, 739–746. [Google Scholar] [CrossRef]
- Vanderthommen, M.; Crielaard, J.M. [Muscle electric stimulation in sports medicine]. Rev. Med. Liege 2001, 56, 391–395. [Google Scholar]
- Gerovasili, V.; Stefanidis, K.; Vitzilaios, K.; Karatzanos, E.; Politis, P.; Koroneos, A.; Chatzimichail, A.; Routsi, C.; Roussos, C.; Nanas, S. Electrical muscle stimulation preserves the muscle mass of critically ill patients: A randomized study. Crit. Care 2009, 13, R161. [Google Scholar] [CrossRef] [Green Version]
- Toth, M.J.; Voigt, T.B.; Tourville, T.W.; Prior, S.M.; Guigni, B.A.; Schlosberg, A.V.; Smith, I.B.; Forest, T.J.; Kaufman, P.A.; Wood, M.E.; et al. Effect of neuromuscular electrical stimulation on skeletal muscle size and function in patients with breast cancer receiving chemotherapy. J. Appl. Physiol. 2020, 128, 1654–1665. [Google Scholar] [CrossRef]
- Morrissey, M.C.; Brewster, C.E.; Shields, C.L., Jr.; Brown, M. The effects of electrical stimulation on the quadriceps during postoperative knee immobilization. Am. J. Sports Med. 1985, 13, 40–45. [Google Scholar] [CrossRef]
- Kwong, W.H.; Vrbova, G. Effects of low-frequency electrical stimulation on fast and slow muscles of the rat. Pflug. Arch. 1981, 391, 200–207. [Google Scholar] [CrossRef]
- Scott, O.M.; Vrbova, G.; Hyde, S.A.; Dubowitz, V. Effects of chronic low frequency electrical stimulation on normal human tibialis anterior muscle. J. Neurol. Neurosurg. Psychiatry 1985, 48, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, M.; Suneja, M.; Shields, R.K. Low-frequency stimulation regulates metabolic gene expression in paralyzed muscle. J. Appl. Physiol. 2015, 118, 723–731. [Google Scholar] [CrossRef] [Green Version]
- Nuhr, M.J.; Pette, D.; Berger, R.; Quittan, M.; Crevenna, R.; Huelsman, M.; Wiesinger, G.F.; Moser, P.; Fialka-Moser, V.; Pacher, R. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur. Heart J. 2004, 25, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Cherepakhin, M.A.; Kakurin, L.I.; Il’ina-Kakueva, E.I.; Fedorenko, G.T. [Evaluation of the effectiveness of electrostimulation of the muscles in preventing disorders related to prolonged limited motor activity in man]. Kosm. Biol. Aviakosm. Med. 1977, 11, 64–68. [Google Scholar]
- Shenkman, B.S.; Liubaeva, E.V.; Popov, D.V.; Netreba, A.I.; Tarasova, O.S.; Vdovina, A.B.; Tarakin, P.P.; Lemesheva Iu, S.; Belichenko, O.I.; Sinitsyn, V.E. [Effects of chronic low-frequency electrical stimulation of human knee extensor muscles exposed to static passive stretching]. Fiziol. Cheloveka 2006, 32, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Conjard, A.; Peuker, H.; Pette, D. Energy state and myosin heavy chain isoforms in single fibres of normal and transforming rabbit muscles. Pflug. Arch. 1998, 436, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Ausoni, S.; Gorza, L.; Schiaffino, S.; Gundersen, K.; Lomo, T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. J. Neurosci. 1990, 10, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Kubis, H.P.; Scheibe, R.J.; Meissner, J.D.; Hornung, G.; Gros, G. Fast-to-slow transformation and nuclear import/export kinetics of the transcription factor NFATc1 during electrostimulation of rabbit muscle cells in culture. J. Physiol. 2002, 541, 835–847. [Google Scholar] [CrossRef]
- Canon, F.; Goubel, F.; Guezennec, C.Y. Effects of chronic low frequency stimulation on contractile and elastic properties of hindlimb suspended rat soleus muscle. Eur. J. Appl. Physiol. Occup. Physiol. 1998, 77, 118–124. [Google Scholar] [CrossRef]
- Leterme, D.; Falempin, M. Compensatory effects of chronic electrostimulation on unweighted rat soleus muscle. Pflug. Arch. 1994, 426, 155–160. [Google Scholar] [CrossRef]
- Stein, T.P.; Blanc, S. Does protein supplementation prevent muscle disuse atrophy and loss of strength? Crit. Rev. Food Sci. Nutr. 2011, 51, 828–834. [Google Scholar] [CrossRef]
- Stuart, C.A.; Shangraw, R.E.; Peters, E.J.; Wolfe, R.R. Effect of dietary protein on bed-rest-related changes in whole-body-protein synthesis. Am. J. Clin. Nutr. 1990, 52, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Paddon-Jones, D. Interplay of stress and physical inactivity on muscle loss: Nutritional countermeasures. J. Nutr. 2006, 136, 2123–2126. [Google Scholar] [CrossRef]
- Brooks, N.; Cloutier, G.J.; Cadena, S.M.; Layne, J.E.; Nelsen, C.A.; Freed, A.M.; Roubenoff, R.; Castaneda-Sceppa, C. Resistance training and timed essential amino acids protect against the loss of muscle mass and strength during 28 days of bed rest and energy deficit. J. Appl. Physiol. 2008, 105, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Kavazis, A.N.; McClung, J.M. Oxidative stress and disuse muscle atrophy. J. Appl. Physiol. 2007, 102, 2389–2397. [Google Scholar] [CrossRef]
- Appell, H.J.; Duarte, J.A.; Soares, J.M. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int. J. Sports Med. 1997, 18, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Desaphy, J.F.; Pierno, S.; Liantonio, A.; Giannuzzi, V.; Digennaro, C.; Dinardo, M.M.; Camerino, G.M.; Ricciuti, P.; Brocca, L.; Pellegrino, M.A.; et al. Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles. Pharm. Res. 2010, 61, 553–563. [Google Scholar] [CrossRef]
- Powers, S.K. Can antioxidants protect against disuse muscle atrophy? Sports Med. 2014, 44 (Suppl. 2), S155–S165. [Google Scholar] [CrossRef] [Green Version]
- Whitley, K.C.; Hamstra, S.I.; Baranowski, R.W.; Watson, C.J.F.; MacPherson, R.E.K.; MacNeil, A.J.; Roy, B.D.; Vandenboom, R.; Fajardo, V.A. GSK3 inhibition with low dose lithium supplementation augments murine muscle fatigue resistance and specific force production. Physiol. Rep. 2020, 8, e14517. [Google Scholar] [CrossRef] [PubMed]
- Mirzoev, T.M.; Sharlo, K.A.; Shenkman, B.S. The Role of GSK-3β in the Regulation of Protein Turnover, Myosin Phenotype, and Oxidative Capacity in Skeletal Muscle under Disuse Conditions. Int. J. Mol. Sci. 2021, 22, 5081. [Google Scholar] [CrossRef] [PubMed]
| Slow-Type Fibers | Fast-Type Fibers | |||
|---|---|---|---|---|
| Inactivation of Muscles of Systemic Genesis | CSA Decrease | Force Decrease | CSA Decrease | Force Decrease |
| A. With limitation or elimination of anti-gravity component | ||||
| a. With complete or almost complete elimination of anti-gravity component | ||||
| - Space flight/Kepler parabola flight | +++ | +++ | ++ | ++ |
| - Immersion | +++ | +++ | ++ | ++ |
| - Unloading of all limbs or only hindlimbs of rats | +++ | +++ | ++ | ++ |
| - Spinal transection or spinal isolation | ++++ | ++++ | ++++ | ++++ |
| - Systemic muscle inactivation with artificial life support (“intensive care unit” model) | ++++ | ++++ | ++++ | ++++ |
| b. With partial elimination or limitation of anti-gravity component | ||||
| - Unilateral lower limb suspension | +++ | +++ | ++ | ++ |
| - Bed rest | ++ | ++ | ++ | ++ |
| B. Without limitation or elimination of anti-gravity component | ||||
| - Restriction of locomotor activity | - | - | ++ | ++ |
| Inactivation of muscles of local genesis | ||||
| - Immobilization of joint with cast | ++ | ++ | ++ | ++ |
| - Denervation | +++ | +++ | ++ | ++ |
| - Tenotomy | +++ | +++ | ++ | ++ |
| - Inactivation of diaphragm by forced ventilation | ++++ | ++++ | ++++ | ++++ |
| Countermeasure | Sensory Structures |
|---|---|
| Exercise | whole body |
| Plantar stimulation | plantar mechanoreceptors |
| Neuromuscular electrostimulation | motor nerve voltage-operated channels |
| Muscular electrostimulation | muscle voltage-operated channels |
| Axial load | mechanosensitive structures of muscles, tendons and bones |
| Centrifuge | mechanosensitive structures of muscles, tendons and bones; vestibular system |
| Vibration stimulation | mechanosensitive structures of muscles, tendons and bones |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharlo, K.; Tyganov, S.A.; Tomilovskaya, E.; Popov, D.V.; Saveko, A.A.; Shenkman, B.S. Effects of Various Muscle Disuse States and Countermeasures on Muscle Molecular Signaling. Int. J. Mol. Sci. 2022, 23, 468. https://doi.org/10.3390/ijms23010468
Sharlo K, Tyganov SA, Tomilovskaya E, Popov DV, Saveko AA, Shenkman BS. Effects of Various Muscle Disuse States and Countermeasures on Muscle Molecular Signaling. International Journal of Molecular Sciences. 2022; 23(1):468. https://doi.org/10.3390/ijms23010468
Chicago/Turabian StyleSharlo, Kristina, Sergey A. Tyganov, Elena Tomilovskaya, Daniil V. Popov, Alina A. Saveko, and Boris S. Shenkman. 2022. "Effects of Various Muscle Disuse States and Countermeasures on Muscle Molecular Signaling" International Journal of Molecular Sciences 23, no. 1: 468. https://doi.org/10.3390/ijms23010468






