An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review
Abstract
:1. Introduction
2. Valorization Mediated by Hydrothermal (HT) Treatment Preceding Biological Pretreatments
2.1. Analysis of Combinatorial Effects of HTL Pretreatment and Biocatalytic Hydrolysis
2.1.1. Pretreatment with Hot Compressed Water (HCW) and Its Effect on Enzymatic Hydrolysis
2.1.2. Pretreatment Methods Implemented through Basic Catalysis
2.1.3. Effect of Organic Solvent and Ionic Liquids on Subsequent Enzymatic Hydrolysis
2.1.4. Effect of Eutectic Solvents and HT Treatments on Subsequent Enzymatic Hydrolysis
2.2. Combinatorial HT and Biological Pretreatment
2.2.1. Effect of Anaerobic Digestion, Fermentation, and HT Pretreatment on Biomass
| Biomass Type | Temperature for AD | Conditions for HT Treatment | Outcome | Ref. |
|---|---|---|---|---|
| Manure and straw (maize) | 41 °C | H2O, 2 h at 270 °C | Zeolite improved the HT treatment performance with decreased oxygen/carbon and hydrogen/carbon ratios in biochar. | [127] |
| Maize (silage) | 55 °C | H2O, 5–10 h at 270 °C | Cumulative yield of biochar was 50–80% of starting carbon. | [128] |
| Maize (silage) | 55 °C | H2O, 5–8 h at 200–280 °C | Total biochar production was 60–70% at the end of 8 h. | [129] |
| Manure | Ambient temperature | H2O, 30 min at 350 °C | Production of biocrude increased by 20% with energy recovery up to 70%. | [130] |
| Straw + silage + manure | 55 and 37 °C | H2O, 350 min at 240 °C | Nutrient recovery after carbonization. | [131] |
| Manure | mesophilic | Acid/ H2O, 40 min at 300 °C | Biocrude production at 60% of carbon recovery with production of cyclic compounds. | [132] |
| Sunflower biomass (stalks) | mesophilic | Acid/ H2O, 30–60 min at 150–200 °C | Partial lignin removal with complete eradication of hemicellulose with 2.5-fold increase in methane yield. | [127] |
| Microalgae | mesophilic | H2O, 15 min at 100–130 °C | Forty percent increase in methane yield. | [117] |
| Straw (sunflower) | mesophilic | H2O, 5 h at 180 °C | Complete removal of hemicellulose and lignin with increase in methane yield. | [133] |
| Grass (energy) | mesophilic | H2O-CaOH (calcium hydroxide), 30 min at 75 °C | Increase in cellulose and lignin ratio with high VFA production. | [134] |
| Grass (energy) | mesophilic | H2O/GVL, 90 min at 180 °C | Thirty percent lignin removal with simultaneous biomethane and lignin nanoparticle. | [125] |
| Food waste | mesophilic | H2O, 30 min at 90 °C | Increased methane yield and energy efficiency. | [116] |
2.2.2. Biochar and Biocrude Production Following HT Liquefaction and Carbonization
3. Summation of the Findings and Insights
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agency, I.E. World Energy Outlook; OECD: Paris, France, 1995. [Google Scholar]
- Tumuluru, J.S.; Wright, C.T.; Boardman, R.D.; Yancey, N.A.; Sokhansanj, S. A review on biomass classification and composition, co-firing issues and pretreatment methods. In Proceedings of the 2011 Louisville, Louisville, KY, USA, 7–10 August 2011; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011; p. 1. [Google Scholar]
- Frei, C.; General, S. Global Transport Scenarios 2050; World Energy Council: London, UK, 2011. [Google Scholar]
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Bórawski, P.; Bełdycka-Bórawska, A.; Szymańska, E.J.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of renewable energy sources market and biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Rahikainen, J.L.; Johansson, L.-S.; Marjamaa, K.; Laine, J.; Kruus, K.; Rojas, O.J. Preferential adsorption and activity of monocomponent cellulases on lignocellulose thin films with varying lignin content. Biomacromolecules 2013, 14, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Lamers, P.; Stichnothe, H.; Beermann, M.; Jungmeier, G. Bioeconomy strategies. In Developing the Global Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–9. [Google Scholar]
- Meyer, R. Bioeconomy strategies: Contexts, visions, guiding implementation principles and resulting debates. Sustainability 2017, 9, 1031. [Google Scholar] [CrossRef] [Green Version]
- de Jong, E.; Jungmeier, G. Biorefinery concepts in comparison to petrochemical refineries. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar]
- Zhou, X.; Xu, Y. Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour. Technol. 2019, 282, 81–87. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Fu, Y.; Guo, C.; Li, Y.; Jiang, N.; Yin, C. Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/acetone wastewater. Bioresour. Technol. 2018, 260, 130–134. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Hydrothermal processing of lignocellulosic materials. Holz Roh- Werkst. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar]
- Tsui, T.-H.; Chen, L.; Hao, T.; Chen, G.-H. A super high-rate sulfidogenic system for saline sewage treatment. Water Res. 2016, 104, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yan, H.; Quan, Y.; Zhao, H.; Jiang, N.; Yin, C. Recent progress and perspectives in biotrickling filters for VOCs and odorous gases treatment. J. Environ. Manag. 2018, 222, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Vazquez, I.; Sanchez, A. Proposal for biorefineries based on mixed cultures for lignocellulosic biofuel production: A techno-economic analysis. Biofuels Bioprod. Biorefining 2017, 12, 56–67. [Google Scholar] [CrossRef]
- Saraiva, A.B. System boundary setting in life cycle assessment of biorefineries: A review. Int. J. Environ. Sci. Technol. 2016, 14, 435–452. [Google Scholar] [CrossRef]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.-F.; Jing, J.; Rasmussen, J.; Søegaard, K.; Eriksen, J. Forbs enhance productivity of unfertilised grass-clover leys and support low-carbon bioenergy. Sci. Rep. 2017, 7, 1422. [Google Scholar] [CrossRef]
- Mauser, W.; Klepper, G.; Zabel, F.; Delzeit, R.; Hank, T.; Putzenlechner, B.; Calzadilla, A. Global biomass production potentials exceed expected future demand without the need for cropland expansion. Nat. Commun. 2015, 6, 8946. [Google Scholar] [CrossRef] [Green Version]
- Woodward, F.I.; Lomas, M.R.; Kelly, C.K. Global climate and the distribution of plant biomes. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 1465–1476. [Google Scholar] [CrossRef] [Green Version]
- Green, J.K.; Seneviratne, S.I.; Berg, A.M.; Findell, K.L.; Hagemann, S.; Lawrence, D.M.; Gentine, P. Large influence of soil moisture on long-term terrestrial carbon uptake. Nature 2019, 565, 476–479. [Google Scholar]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of Fuels and Chemicals from Biomass: Condensation Reactions and Beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Mousdale, D.M. Biofuels: Biotechnology, Chemistry, and Sustainable Development; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Rulli, M.C.; Saviori, A.; D’Odorico, P. Global land and water grabbing. Proc. Natl. Acad. Sci. USA 2013, 110, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Holkar, C.R.; Jain, S.S.; Jadhav, A.J.; Pinjari, D.V. Valorization of keratin based waste. Process Saf. Environ. Prot. 2018, 115, 85–98. [Google Scholar] [CrossRef]
- Sivasamy, A.; Cheah, K.Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. Catalytic Applications in the Production of Biodiesel from Vegetable Oils. ChemSusChem 2009, 2, 278–300. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from Waste cooking oil. Renew. Sustain. Energy Rev. 2012, 18, 184–193. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Schutyser, W.; Sels, B.F. Lignin-first biomass fractionation: The advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Meng, X.; Pu, Y.; Yoo, C.G.; Li, M.; Bali, G.; Park, D.-Y.; Gjersing, E.; Davis, M.F.; Muchero, W.; Tuskan, G.A.; et al. An In-Depth Understanding of Biomass Recalcitrance Using Natural Poplar Variants as the Feedstock. ChemSusChem 2016, 10, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.-F.; Renders, T.; De Meester, B.; Huijgen, W.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Binder, J.B.; Raines, R.T. Fermentable sugars by chemical hydrolysis of biomass. Proc. Natl. Acad. Sci. USA 2010, 107, 4516–4521. [Google Scholar] [CrossRef] [Green Version]
- Bodachivskyi, I.; Kuzhiumparambil, U.; Williams, D.B.G. The role of the molecular formula of ZnCl2·nH2O on its catalyst activity: A systematic study of zinc chloride hydrates in the catalytic valorisation of cellulosic biomass. Catal. Sci. Technol. 2019, 9, 4693–4701. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 1995, 41, 1723–1778. [Google Scholar] [CrossRef] [Green Version]
- Dutta, N.; Mukhopadhyay, A.; Dasgupta, A.K.; Chakrabarti, K. Improved production of reducing sugars from rice husk and rice straw using bacterial cellulase and xylanase activated with hydroxyapatite nanoparticles. Bioresour. Technol. 2014, 153, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef]
- Lai, C.; Tu, M.; Xia, C.; Shi, Z.; Sun, S.; Yong, Q.; Yu, S. Lignin Alkylation Enhances Enzymatic Hydrolysis of Lignocellulosic Biomass. Energy Fuels 2017, 31, 12317–12326. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sustain. Energy Rev. 2018, 81, 1742–1770. [Google Scholar] [CrossRef]
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging renewable and sustainable energy technologies: State of the art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Sun, Z.-J.; Shi, Z.-J.; Xu, F.; Sun, R.-C. Impact of hot compressed water pretreatment on the structural changes of woody biomass for bioethanol production. BioResources 2011, 6, 1576–1598. [Google Scholar]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Orozco, R.; Redwood, M.; Leeke, G.; Bahari, A.; Santos, R.; Macaskie, L. Hydrothermal hydrolysis of starch with CO2 and detoxification of the hydrolysates with activated carbon for bio-hydrogen fermentation. Int. J. Hydrogen Energy 2012, 37, 6545–6553. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Dogaris, I.; Karapati, S.; Mamma, D.; Kalogeris, E.; Kekos, D. Hydrothermal processing and enzymatic hydrolysis of sorghum bagasse for fermentable carbohydrates production. Bioresour. Technol. 2009, 100, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.; Carrier, D.J. Insights into exo-Cellulase Inhibition by the Hot Water Hydrolyzates of Rice Straw. ACS Sustain. Chem. Eng. 2016, 4, 3627–3633. [Google Scholar] [CrossRef]
- Qi, W.; He, C.; Wang, Q.; Liu, S.; Yu, Q.; Wang, W.; Leksawasdi, N.; Wang, C.; Yuan, Z. Carbon-based solid acid pretreatment in corncob saccharification: Specific xylose production and efficient enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2018, 6, 3640–3648. [Google Scholar] [CrossRef]
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Enzymatic hydrolysis of liquid hot water-pretreated switchgrass at high solid content. Energy Fuels 2019, 33, 4361–4368. [Google Scholar] [CrossRef]
- Xiao, X.; Bian, J.; Li, M.-F.; Xu, H.; Xiao, B.; Sun, R.-C. Enhanced enzymatic hydrolysis of bamboo (Dendrocalamus giganteus Munro) culm by hydrothermal pretreatment. Bioresour. Technol. 2014, 159, 41–47. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Choli-Papadopoulou, T.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of hardwood and softwood lignocellulosic residues for selective hemicellulose recovery and improved cellulose enzymatic hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 4529–4544. [Google Scholar] [CrossRef]
- Yu, Q.; Zhuang, X.; Yuan, Z.; Wang, Q.; Qi, W.; Wang, W.; Zhang, Y.; Xu, J.; Xu, H. Two-step liquid hot water pretreatment of Eucalyptus grandis to enhance sugar recovery and enzymatic digestibility of cellulose. Bioresour. Technol. 2010, 101, 4895–4899. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yamauchi, K.; Takabe, K.; Awano, T.; Yoshinaga, A.; Kato, M.; Kobayashi, T.; Asada, T.; Furujyo, A.; Fukushima, K. Enzymatic saccharification of Eucalyptus bark using hydrothermal pre-treatment with carbon dioxide. Bioresour. Technol. 2010, 101, 4936–4939. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.; Mitchinson, C.; Saddler, J.N. Comparative sugar recovery and fermentation data following pretreatment of poplar wood by leading technologies. Biotechnol. Prog. 2009, 25, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zong, M.-H.; Wu, H.; Li, N. Efficient pretreatment of wheat straw using novel renewable cholinium ionic liquids to improve enzymatic saccharification. Ind. Eng. Chem. Res. 2016, 55, 1788–1795. [Google Scholar] [CrossRef]
- Petrik, S.; Kádár, Z.; Márová, I. Utilization of hydrothermally pretreated wheat straw for production of bioethanol and carotene-enriched biomass. Bioresour. Technol. 2013, 133, 370–377. [Google Scholar] [CrossRef]
- Xu, J.; Zong, M.-H.; Fu, S.-Y.; Li, N. Correlation between physicochemical properties and enzymatic digestibility of rice straw pretreated with cholinium ionic liquids. ACS Sustain. Chem. Eng. 2016, 4, 4340–4345. [Google Scholar] [CrossRef]
- Li, M.-F.; Chen, C.-Z.; Sun, R.-C. Effect of pretreatment severity on the enzymatic hydrolysis of bamboo in hydrothermal deconstruction. Cellulose 2014, 21, 4105–4117. [Google Scholar] [CrossRef]
- Djioleu, A.; Carrier, D.J. Effects of dilute acid pretreatment parameters on sugar production during biochemical conversion of switchgrass using a full factorial design. ACS Sustain. Chem. Eng. 2016, 4, 4124–4130. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Icaza-Herrera, J.P.; Méndoza-Pérez, J.A.; González-Álvarez, V.; Méndez-Acosta, H.O.; Arreola-Vargas, J. Mild reaction conditions induce high sugar yields during the pretreatment of Agave tequilana bagasse with 1-ethyl-3-methylimidazolium acetate. Bioresour. Technol. 2019, 275, 78–85. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, A.; Wang, W.; Chen, L.; Bai, R.; Zhuang, X.; Wang, Q.; Wang, Z.; Yuan, Z. Deep eutectic solvents from hemicellulose-derived acids for the cellulosic ethanol refining of Akebia’herbal residues. Bioresour. Technol. 2018, 247, 705–710. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2012, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Lucia, L.A.; Ghiladi, R.A. Development of a highly efficient pretreatment sequence for the enzymatic saccharification of loblolly pine wood. ACS Sustain. Chem. Eng. 2016, 4, 3669–3678. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Chen, H.-Z. Biomass–water interaction and its correlations with enzymatic hydrolysis of steam-exploded corn stover. ACS Sustain. Chem. Eng. 2016, 4, 1274–1285. [Google Scholar] [CrossRef]
- Vu, A.; Wickramasinghe, S.R.; Qian, X. Polymeric solid acid catalysts for lignocellulosic biomass fractionation. Ind. Eng. Chem. Res. 2018, 57, 4514–4525. [Google Scholar] [CrossRef]
- Hattori, H. ChemInform Abstract: Heterogeneous Basic Catalysis. ChemInform 2010, 26, 37. [Google Scholar] [CrossRef]
- Bing, W.; Wei, M. Recent advances for solid basic catalysts: Structure design and catalytic performance. J. Solid State Chem. 2019, 269, 184–194. [Google Scholar] [CrossRef]
- Figueras, F. Base catalysis in the synthesis of fine chemicals. Top. Catal. 2004, 29, 189–196. [Google Scholar] [CrossRef]
- Tichit, D.; Coq, B. Catalysis by Hydrotalcites and Related Materials. CATTECH 2003, 7, 206–217. [Google Scholar] [CrossRef]
- Gardy, J.; Rehan, M.; Hassanpour, A.; Lai, X.; Nizami, A.-S. Advances in nano-catalysts based biodiesel production from non-food feedstocks. J. Environ. Manag. 2019, 249, 109316. [Google Scholar] [CrossRef]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of Liquid Alkanes by Aqueous-Phase Processing of Biomass-Derived Carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- West, R.M.; Liu, Z.Y.; Peter, M.; Gärtner, C.A.; Dumesic, J.A. Carbon–carbon bond formation for biomass-derived furfurals and ketones by aldol condensation in a biphasic system. J. Mol. Catal. A Chem. 2008, 296, 18–27. [Google Scholar] [CrossRef]
- Bohre, A.; Saha, B.; Abu-Omar, M.M. Catalytic Upgrading of 5-Hydroxymethylfurfural to Drop-in Biofuels by Solid Base and Bifunctional Metal-Acid Catalysts. ChemSusChem 2015, 8, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.J.F. Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. ACS Sustain. Chem. Eng. 2008, 87, 3572–3578. [Google Scholar]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Wang, W.; Yu, Q.; Wang, Q.; Miao, C.; Guo, Y.; Zhuang, X.; Yuan, Z. Screening solvents based on Hansen solubility parameter theory to depolymerize lignocellulosic biomass efficiently under low temperature. ACS Sustain. Chem. Eng. 2019, 7, 8678–8686. [Google Scholar] [CrossRef]
- Lynd, L.R.; Cushman, J.H.; Nichols, R.J.; Wyman, C.E. Fuel Ethanol from Cellulosic Biomass. Science 1991, 251, 1318–1323. [Google Scholar] [CrossRef]
- Pan, X.; Xie, D.; Yu, R.W.; Lam, D.; Saddler, J.N. Pretreatment of lodgepole pine killed by mountain pine beetle using the ethanol organosolv process: Fractionation and process optimization. Ind. Eng. Chem. Res. 2007, 46, 2609–2617. [Google Scholar] [CrossRef]
- Santo, M.E.; Rezende, C.A.; Bernardinelli, O.D.; Pereira, N., Jr.; Curvelo, A.A.; Deazevedo, E.R.; Guimarães, F.E.; Polikarpov, I. Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crops Prod. 2018, 113, 64–74. [Google Scholar] [CrossRef]
- Luterbacher, J.S.; Rand, J.M.; Alonso, D.M.; Han, J.; Youngquist, J.T.; Maravelias, C.T.; Pfleger, B.F.; Dumesic, J.A. Nonenzymatic Sugar Production from Biomass Using Biomass-Derived γ-Valerolactone. Science 2014, 343, 277–280. [Google Scholar] [CrossRef]
- Qi, L.; Mui, Y.F.; Lo, S.W.; Lui, M.Y.; Akien, G.R.; Horváth, I.n.T. Catalytic conversion of fructose, glucose, and sucrose to 5-(hydroxymethyl) furfural and levulinic and formic acids in γ-valerolactone as a green solvent. Acs Catal. 2014, 4, 1470–1477. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Alonso, D.M.; Chong, Y.; Dumesic, J.A. Production of levulinic acid and gamma-valerolactone (GVL) from cellulose using GVL as a solvent in biphasic systems. Energy Environ. Sci. 2012, 5, 8199–8203. [Google Scholar] [CrossRef]
- Song, B.; Yu, Y.; Wu, H. Insights into hydrothermal decomposition of cellobiose in gamma-valerolactone/water mixtures. Ind. Eng. Chem. Res. 2017, 56, 7957–7963. [Google Scholar] [CrossRef]
- Song, B.; Yu, Y.; Wu, H. Solvent effect of gamma-valerolactone (GVL) on cellulose and biomass hydrolysis in hot-compressed GVL/water mixtures. Fuel 2018, 232, 317–322. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Fort, D.A.; Remsing, R.C.; Swatloski, R.P.; Moyna, P.; Moyna, G.; Rogers, R.D. Can ionic liquids dissolve wood? Processing and analysis of lignocellulosic materials with 1-n-butyl-3-methylimidazolium chloride. Green Chem. 2006, 9, 63–69. [Google Scholar] [CrossRef]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Hallett, J.P.; Leak, D.J.; Murphy, R.J.; Welton, T. The effect of the ionic liquid anion in the pretreatment of pine wood chips. Green Chem. 2010, 12, 672–679. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2012, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- An, Y.-X.; Zong, M.-H.; Wu, H.; Li, N. Pretreatment of lignocellulosic biomass with renewable cholinium ionic liquids: Biomass fractionation, enzymatic digestion and ionic liquid reuse. Bioresour. Technol. 2015, 192, 165–171. [Google Scholar] [CrossRef]
- Lê, H.Q.; Sixta, H.; Hummel, M.J.C.O.i.G.; Chemistry, S. Ionic liquids and gamma-valerolactone as case studies for green solvents in the deconstruction and refining of biomass. Bioresour. Technol. 2019, 18, 20–24. [Google Scholar] [CrossRef]
- Mumme, J.; Titirici, M.-M.; Pfeiffer, A.; Lüder, U.; Reza, M.T.; Mašek, O.e. Hydrothermal carbonization of digestate in the presence of zeolite: Process efficiency and composite properties. ACS Sustain. Chem. Eng. 2015, 3, 2967–2974. [Google Scholar] [CrossRef]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef]
- Reza, M.T.; Mumme, J.; Ebert, A. Characterization of hydrochar obtained from hydrothermal carbonization of wheat straw digestate. Biomass-Convers. Biorefinery 2015, 5, 425–435. [Google Scholar] [CrossRef]
- Eboibi, B.E.; Lewis, D.M.; Ashman, P.J.; Chinnasamy, S. Integrating anaerobic digestion and hydrothermal liquefaction for renewable energy production: An experimental investigation. Environ. Prog. Sustain. Energy 2015, 34, 1662–1673. [Google Scholar] [CrossRef]
- Funke, A. Fate of Plant Available Nutrients during Hydrothermal Carbonization of Digestate. Chem. Ing. Tech. 2015, 87, 1713–1719. [Google Scholar] [CrossRef]
- Posmanik, R.; Martinez, C.M.; Cantero-Tubilla, B.; Cantero, D.A.; Sills, D.; Cocero, M.J.; Tester, J.W. Acid and alkali catalyzed hydrothermal liquefaction of dairy manure digestate and food waste. ACS Sustain. Chem. Eng. 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of hydrothermal pretreatment on microalgal biomass anaerobic digestion and bioenergy production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.S.; Karimi, K.; Nosratpour, M.J.; Sárvári Horváth, I. Efficient biogas and ethanol production from safflower straw using sodium carbonate pretreatment. Energy Fuels 2016, 30, 10592–10601. [Google Scholar] [CrossRef]
- Wang, S.; Tao, X.; Zhang, G.; Zhang, P.; Wang, H.; Ye, J.; Li, F.; Zhang, Q.; Nabi, M. Benefit of solid-liquid separation on volatile fatty acid production from grass clipping with ultrasound-calcium hydroxide pretreatment. Bioresour. Technol. 2019, 274, 97–104. [Google Scholar] [CrossRef]
- Wu, P.; Li, L.; Sun, Y.; Song, B.; Yu, Y.; Liu, H. Near complete valorisation of Hybrid pennisetum to biomethane and lignin nanoparticles based on gamma-valerolactone/water pretreatment. Bioresour. Technol. 2020, 305, 123040. [Google Scholar] [CrossRef]
- Jia, X.; Xi, B.; Li, M.; Xia, T.; Hao, Y.; Liu, D.; Hou, J. Evaluation of biogasification and energy consumption from food waste using short-term hydrothermal pretreatment coupled with different anaerobic digestion processes. J. Clean. Prod. 2017, 152, 364–368. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Ionic Liquids and Deep Eutectic Solvents in Natural Products Research: Mixtures of Solids as Extraction Solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural deep eutectic solvent mediated pretreatment of rice straw: Bioanalytical characterization of lignin extract and enzymatic hydrolysis of pretreated biomass residue. Environ. Sci. Pollut. Res. 2015, 23, 9265–9275. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Campanile, A.G.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bai, X.; Wan, C. High-solid lignocellulose processing enabled by natural deep eutectic solvent for lignin extraction and industrially relevant production of renewable chemicals. ACS Sustain. Chem. Eng. 2018, 6, 12205–12216. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Sun, J.; Simmons, B.; Singh, S. Biomass pretreatment using deep eutectic solvents from lignin derived phenols. Green Chem. 2018, 20, 809–815. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Das, P.; Bhagia, S.; Pu, Y.; Puri, S.K.; Ramakumar, S.; Ragauskas, A.J. Assessing the facile pretreatments of bagasse for efficient enzymatic conversion and their impacts on structural and chemical properties. ACS Sustain. Chem. Eng. 2018, 7, 1095–1104. [Google Scholar] [CrossRef]
- Chen, L.; Yu, Q.; Wang, Q.; Wang, W.; Qi, W.; Zhuang, X.; Wang, Z.; Yuan, Z. A novel deep eutectic solvent from lignin-derived acids for improving the enzymatic digestibility of herbal residues from cellulose. Cellulose 2019, 26, 1947–1959. [Google Scholar] [CrossRef]
- Zhang, C.-W.; Xia, S.-Q.; Ma, P.-S. Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2014, 17, 1728–1734. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Silva, E.L.; Varesche, M.B.A. Hydrothermal processing of biomass for anaerobic digestion–A review. Renew. Sustain. Energy Rev. 2018, 98, 108–124. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew. Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Şenol, H.; Açıkel, Ü.; Demir, S.; Oda, V. Anaerobic digestion of cattle manure, corn silage and sugar beet pulp mixtures after thermal pretreatment and kinetic modeling study. Fuel 2019, 263, 116651. [Google Scholar] [CrossRef]
- Yang, D.; Dai, X.; Song, L.; Dai, L.; Dong, B. Effects of stepwise thermal hydrolysis and solid-liquid separation on three different sludge organic matter solubilization and biodegradability. Bioresour. Technol. 2019, 290, 121753. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef]
- Yuan, H.; Song, X.; Guan, R.; Zhang, L.; Li, X.; Zuo, X. Effect of low severity hydrothermal pretreatment on anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 294, 122238. [Google Scholar] [CrossRef]
- Li, L.; Kong, X.; Yang, F.; Li, D.; Yuan, Z.; Sun, Y. Biogas Production Potential and Kinetics of Microwave and Conventional Thermal Pretreatment of Grass. Appl. Biochem. Biotechnol. 2011, 166, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhang, Y.; Lin, R.; Li, L.; Zhen, F.; Kong, X.; Sun, Y.; Yuan, Z. Optimization of liquid hot water pretreatment on Hybrid Pennisetum anaerobic digestion and its effect on energy efficiency. Energy Convers. Manag. 2020, 210, 112718. [Google Scholar] [CrossRef]
- Kang, X.; Sun, Y.; Li, L.; Kong, X.; Yuan, Z. Improving methane production from anaerobic digestion of Pennisetum Hybrid by alkaline pretreatment. Bioresour. Technol. 2018, 255, 205–212. [Google Scholar] [CrossRef]
- Kang, X.; Zhang, Y.; Song, B.; Sun, Y.; Li, L.; He, Y.; Kong, X.; Luo, X.; Yuan, Z. The effect of mechanical pretreatment on the anaerobic digestion of Hybrid Pennisetum. Fuel 2019, 252, 469–474. [Google Scholar] [CrossRef]
- Ezeji, T.; Qureshi, N.; Blaschek, H.P. Production of acetone–butanol–ethanol (ABE) in a continuous flow bioreactor using degermed corn and Clostridium beijerinckii. Process Biochem. 2007, 42, 34–39. [Google Scholar] [CrossRef]
- Remón, J.; Santomauro, F.; Chuck, C.J.; Matharu, A.S.; Clark, J.H. Production of fermentable species by microwave-assisted hydrothermal treatment of biomass carbohydrates: Reactivity and fermentability assessments. Green Chem. 2018, 20, 4507–4520. [Google Scholar] [CrossRef] [Green Version]
- Eskicioglu, C.; Monlau, F.; Barakat, A.; Ferrer, I.; Kaparaju, P.; Trably, E.; Carrere, H. Assessment of hydrothermal pretreatment of various lignocellulosic biomass with CO2 catalyst for enhanced methane and hydrogen production. Water Res. 2017, 120, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Lin, R.; Deng, C.; Ding, L.; Bose, A.; Murphy, J.D. Improving gaseous biofuel production from seaweed Saccharina latissima: The effect of hydrothermal pretreatment on energy efficiency. Energy Convers. Manag. 2019, 196, 1385–1394. [Google Scholar] [CrossRef]
- Cheng, J.; Yue, L.; Ding, L.; Li, Y.-Y.; Ye, Q.; Zhou, J.; Cen, K.; Lin, R. Improving fermentative hydrogen and methane production from an algal bloom through hydrothermal/steam acid pretreatment. Int. J. Hydrogen Energy 2019, 44, 5812–5820. [Google Scholar] [CrossRef]
- Matsakas, L.; Kekos, D.; Loizidou, M.; Christakopoulos, P. Utilization of household food waste for the production of ethanol at high dry material content. Biotechnol. Biofuels 2014, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.-Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef]
- Nakasaki, K.; Mimoto, H.; Tran, Q.N.M.; Oinuma, A. Composting of food waste subjected to hydrothermal pretreatment and inoculated with Paecilomyces sp. FA13. Bioresour. Technol. 2015, 180, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Masebinu, S.; Akinlabi, E.; Muzenda, E.; Aboyade, A. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.-G.; Gu, Y.-L.; Xu, Y.; Zeng, G.-M.; Hu, X.-J.; Liu, S.-B.; Wang, X.; Liu, S.-M.; Li, J. Biochar-based nano-composites for the decontamination of wastewater: A review. Bioresour. Technol. 2016, 212, 318–333. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling- optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef] [Green Version]
- Aragón-Briceño, C.; Ross, A.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, B.H. Effect of thermochemically fractionation before hydrothermal liquefaction of herbaceous biomass on biocrude characteristics. Renew. Energy 2020, 160, 612–622. [Google Scholar] [CrossRef]
- Ji, M.; Wang, X.; Usman, M.; Liu, F.; Dan, Y.; Zhou, L.; Campanaro, S.; Luo, G.; Sang, W. Effects of different feedstocks-based biochar on soil remediation: A review. Environ. Pollut. 2022, 294, 118655. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Lyu, X.; Xu, L.; Wang, J.; Lu, X. Hydrothermal conversion of the hyperaccumulator Sedum alfredii Hance for efficiently recovering heavy metals and bio-oil. J. Environ. Chem. Eng. 2019, 7, 103321. [Google Scholar] [CrossRef]
- Muratori, M.; Ledna, C.; McJeon, H.; Kyle, P.; Patel, P.; Kim, S.H.; Wise, M.; Kheshgi, H.S.; Clarke, L.E.; Edmonds, J. Cost of power or power of cost: A US modeling perspective. Renew. Sustain. Energy Rev. 2017, 77, 861–874. [Google Scholar] [CrossRef]
- Jones, S.B.; Zhu, Y.; Anderson, D.B.; Hallen, R.T.; Elliott, D.C.; Schmidt, A.J.; Albrecht, K.O.; Hart, T.R.; Butcher, M.G.; Drennan, C. Process Design and Economics for the Conversion of Algal Biomass to Hydrocarbons: Whole Algae Hydrothermal Liquefaction and Upgrading; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2014. [Google Scholar]
- Campbell, R.M.; Anderson, N.M.; Daugaard, D.E.; Naughton, H.T. Financial viability of biofuel and biochar production from forest biomass in the face of market price volatility and uncertainty. Appl. Energy 2018, 230, 330–343. [Google Scholar] [CrossRef]
- Boukis, N.; Hauer, E.; Herbig, S.; Sauer, J.; Vogel, F. Catalytic gasification of digestate sludge in supercritical water on the pilot plant scale. Biomass-Convers. Biorefinery 2017, 7, 415–424. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Zimmerman, J.B.; de Winter, T.M.; Petitjean, L.; Melnikov, F.; Lam, C.H.; Lounsbury, A.W.; Mellor, K.E.; Janković, N.Z.; Tu, Q. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. [Google Scholar] [CrossRef]
- Hu, F.; Jung, S.; Ragauskas, A. Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour. Technol. 2012, 117, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.D.; Meng, X.; Kumar, R.; Ragauskas, A.J. Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 2018, 20, 2192–2205. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.K.; Ekielski, A. The Self-Assembly of Lignin and Its Application in Nanoparticle Synthesis: A Short Review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Song, B.; Long, Y.; Wu, H. Mass spectrometry analysis of sugar and anhydrosugar oligomers from biomass thermochemical processing. Energy Fuels 2016, 30, 8787–8789. [Google Scholar] [CrossRef]
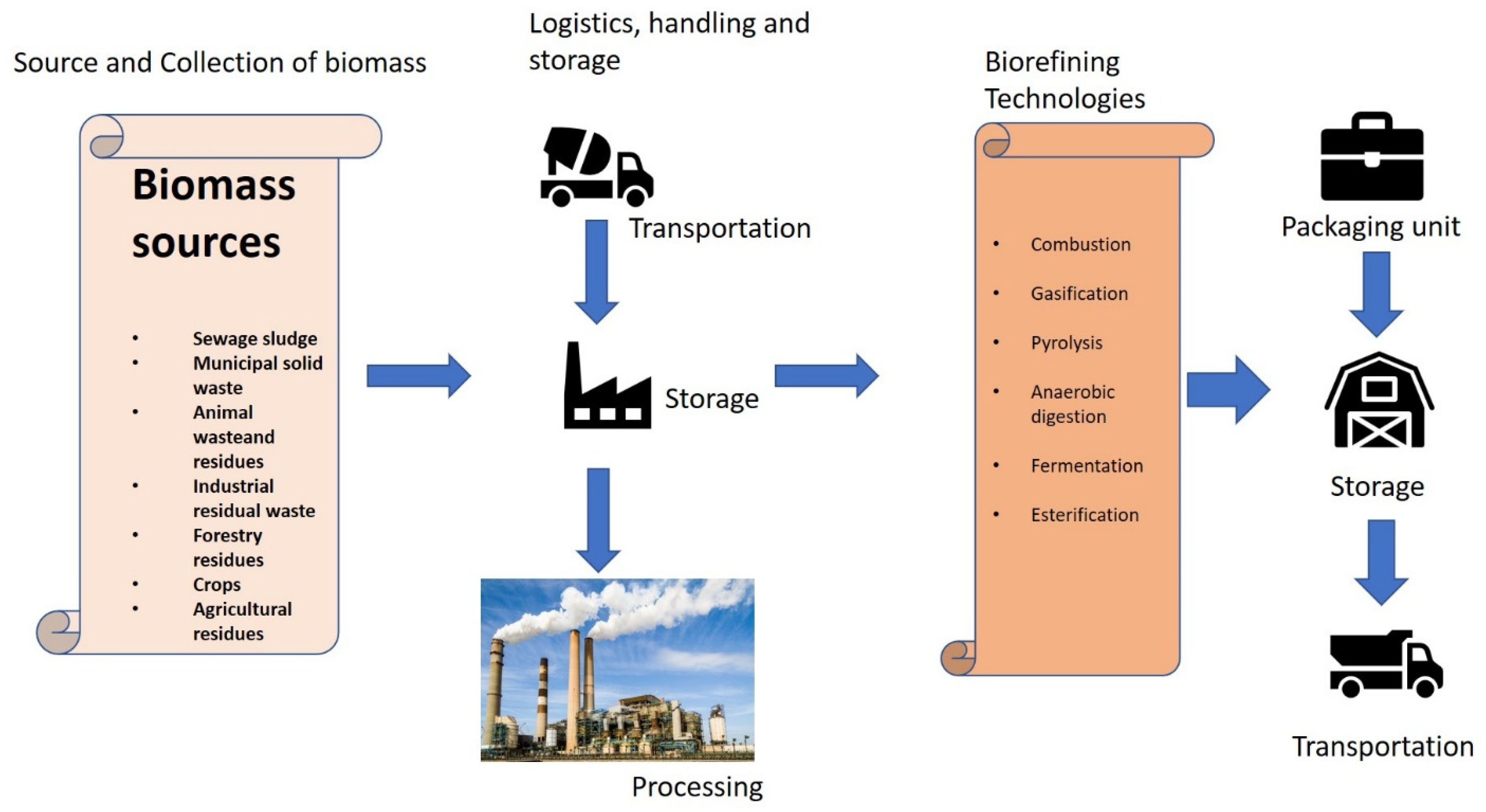
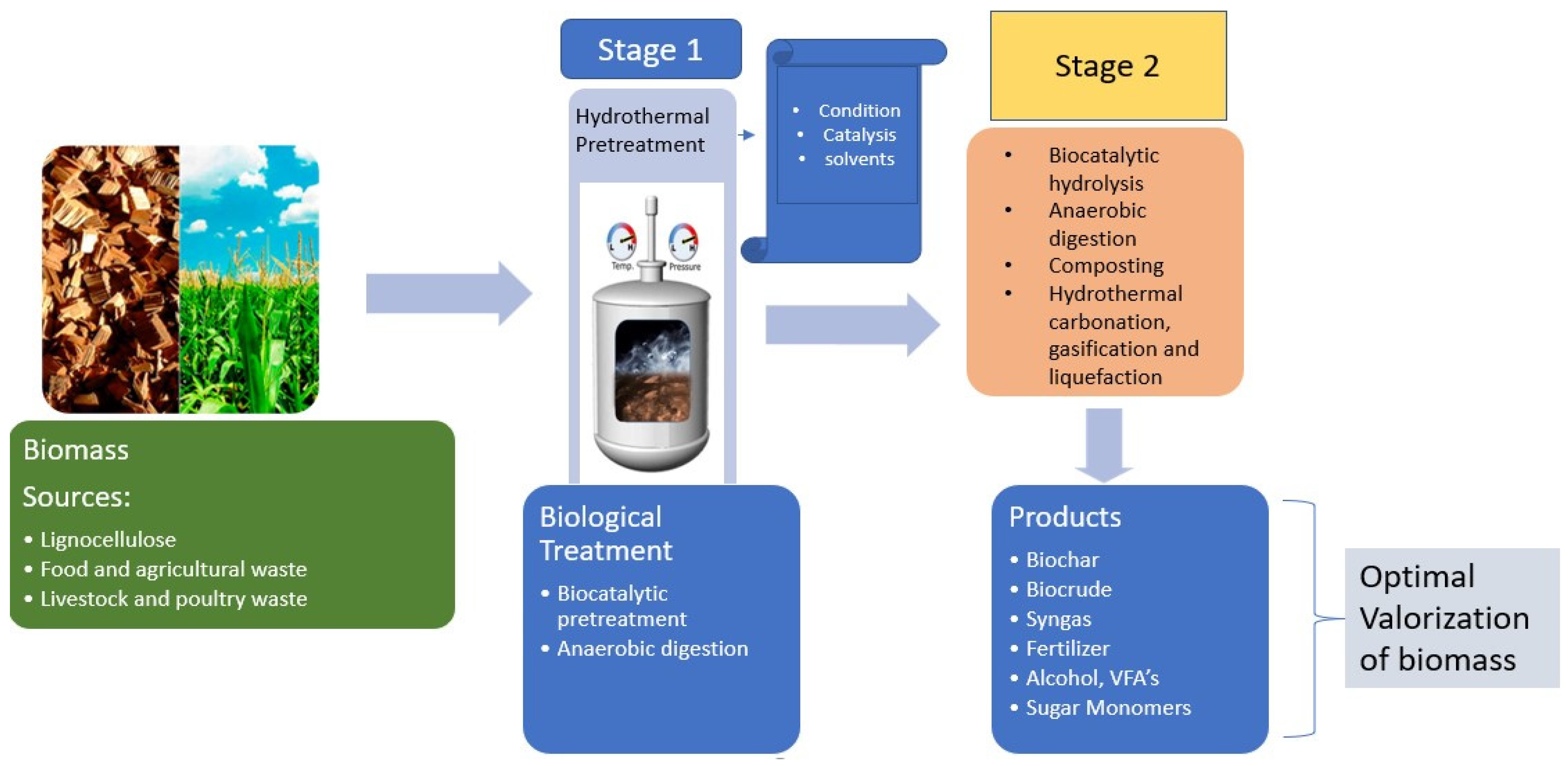
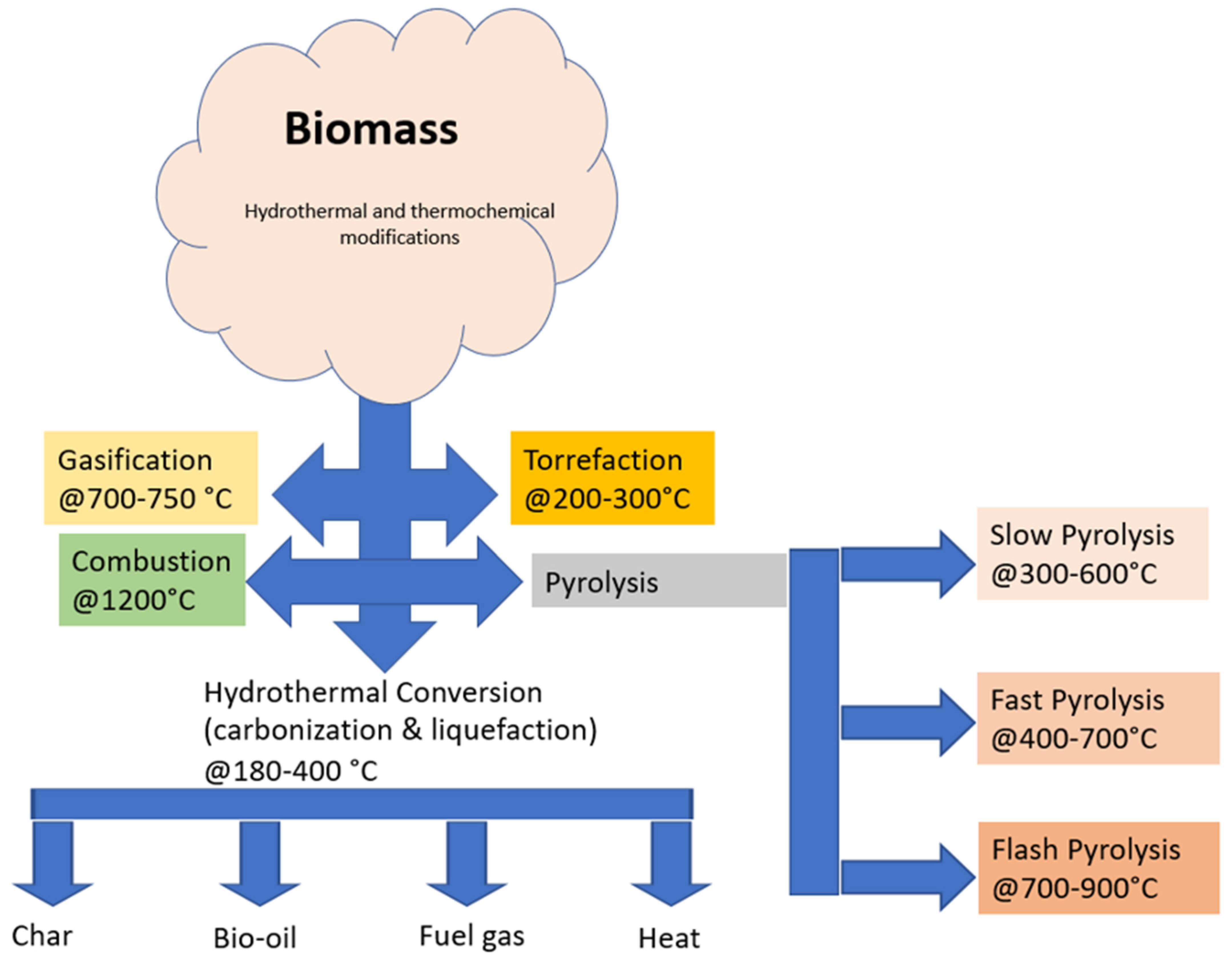
| Type of Biomass | HT Treatment | Biocatalytic Hydrolysis Phase | Outcome | Ref. |
|---|---|---|---|---|
| Eucalyptus barks | Water, 150–200 °C, 20 min–4 h | Cellulase and xylanase loading amount: 12.5–40 FPU/g for 24–72 h | Biocatalytic digestibility increased by more than 91%, and ~10% glucose was lost during the HT process. | [48,49] |
| Poplar lumber wood | H2SO4 and/water followed by ~190 °C for 50–60 s | Cellulase loading at 20–60 FPU/g for 120 h | Acid pretreatment elucidated hemicellulose release. | [50] |
| Wheat straw | Water/IL/Acetic acid 80–200 °C for 10 min–6 h | Ctec 2+Htec 2 at 11–15 FPU/g for ~50 h | Hemicellulose removal with glucose yield of ~90% at 190 °C. ILs removed 50% lignin with increased digestibility of the raw biomass. | [51,52] |
| Rice straw | Water/IL at 90–220 °C for 50 min–6 h | Cellulase loading at 42 U/mL | Identification of cellulase inhibitors and analysis correlation between biocatalytic digestibility and lignin content. | [53,54] |
| Bamboo | Water at 120–240 °C for 10–120 min | Cellulase loading at 14.5–20 FPU/g for 96–120 h | Enzymatic conversion of >80% of the pretreatment material with increment in cellulosic crystalline index. | [55,56] |
| Sugarcane bagasse | Water/ethanol at 140–180 °C for 40 min | Cellulase loading at 15 FPU/g | Increased enzymatic digestibility with enhanced digestibility of cellulose. | [57,58] |
| Corncob | Acid/water at 120–160 °C for 4–6 h | Cellulase loading at 20–40 FPU/g | Eighty percent hemicellulose released during HT. | [59] |
| Residues from akebia | ES/water/acid at 80–120 °C for ~8 h | Cellulase loading at 10–40 FPU/g for 72 h | Optimized conditions of lignin removal were ascertained. | [60] |
| Solvent | Advantages | Shortcomings |
|---|---|---|
| Water | (1) Dearth of pollutants. (2) Helps in the removal of hemicellulose. | (1) Lignin fractionations remain unaffected and produce inhibitors. (2) Lower sugar recovery with increased energy input. |
| Water (acid-catalyzed) | Highly efficient hemicellulose removal and lowered energy consumption w.r.t water. | (1) Generates toxic catalysts, corrosive in nature. (2) Unable to remove lignin. |
| Water and ethanol | Highly efficient hemicellulose removal with easier recovery of ethanol. | (1) Use of ethanol increases the overall economy. (2) Low concentration of lignin with high treatment severity. |
| Water and GVL | (1) Simple recovery of solvent and economically viable. (2) High recovery of sugars with increased fractionation of RLB. | Increased cost of GVL recovery. |
| Water and ILs | High recovery of sugars with increased fractionation of RLB. | Highly toxic solvents and not a cost-effective process with the cumulative process incurring high cost. |
| Water and ES | Emerging alternatives for ILs as biodegradable in nature. | The process is still in its infancy and has high viscosity. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, N.; Usman, M.; Luo, G.; Zhang, S. An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustain. Chem. 2022, 3, 35-55. https://doi.org/10.3390/suschem3010003
Dutta N, Usman M, Luo G, Zhang S. An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustainable Chemistry. 2022; 3(1):35-55. https://doi.org/10.3390/suschem3010003
Chicago/Turabian StyleDutta, Nalok, Muhammad Usman, Gang Luo, and Shicheng Zhang. 2022. "An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review" Sustainable Chemistry 3, no. 1: 35-55. https://doi.org/10.3390/suschem3010003
APA StyleDutta, N., Usman, M., Luo, G., & Zhang, S. (2022). An Insight into Valorization of Lignocellulosic Biomass by Optimization with the Combination of Hydrothermal (HT) and Biological Techniques: A Review. Sustainable Chemistry, 3(1), 35-55. https://doi.org/10.3390/suschem3010003









