Effect of Porcine Whole Blood Protein Hydrolysate on Slow-Twitch Muscle Fiber Expression and Mitochondrial Biogenesis via the AMPK/SIRT1 Pathway
Abstract
:1. Introduction
2. Results
2.1. PWBPH Increases Endurance Performance and Relative Muscle Weight
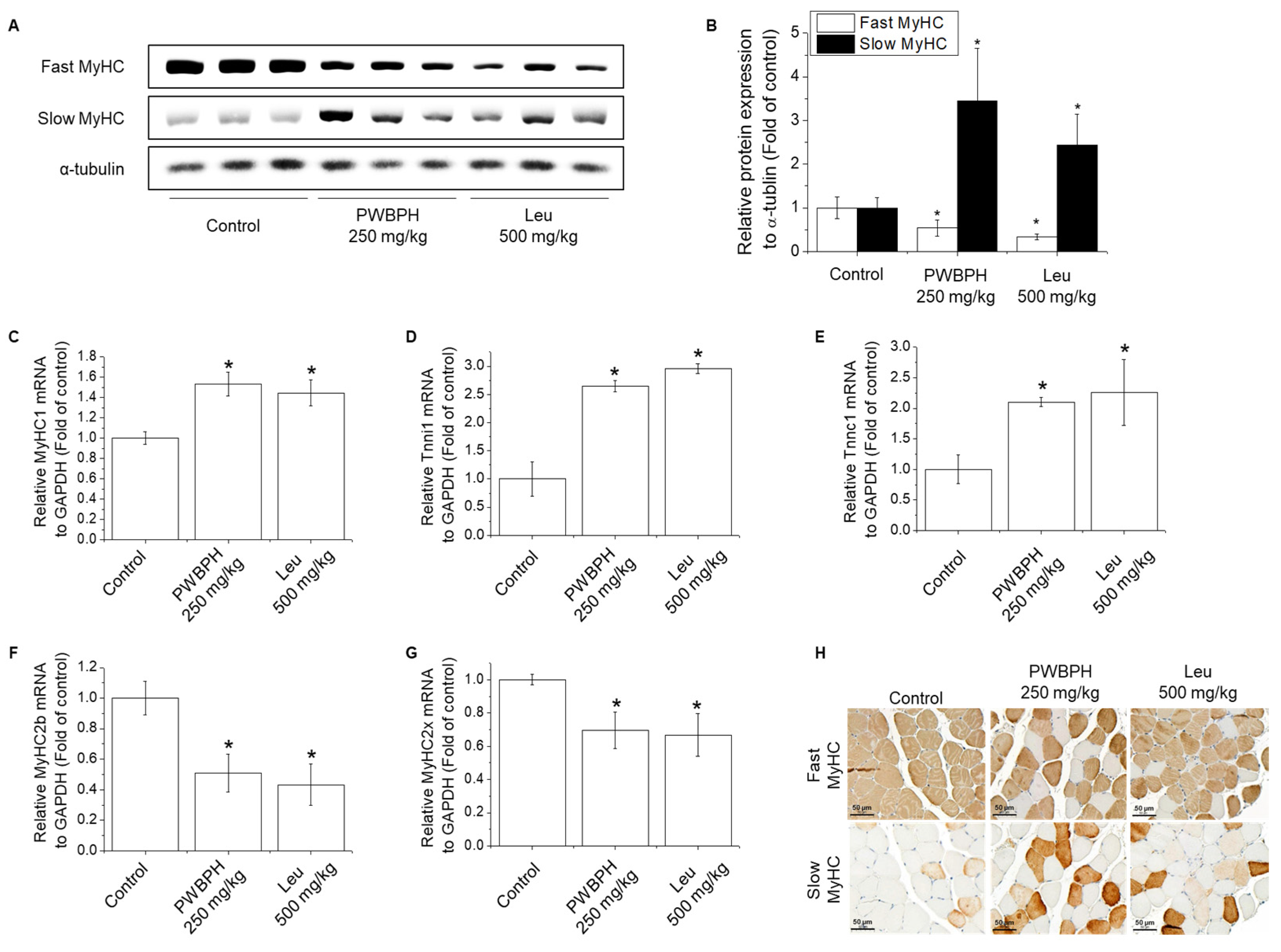
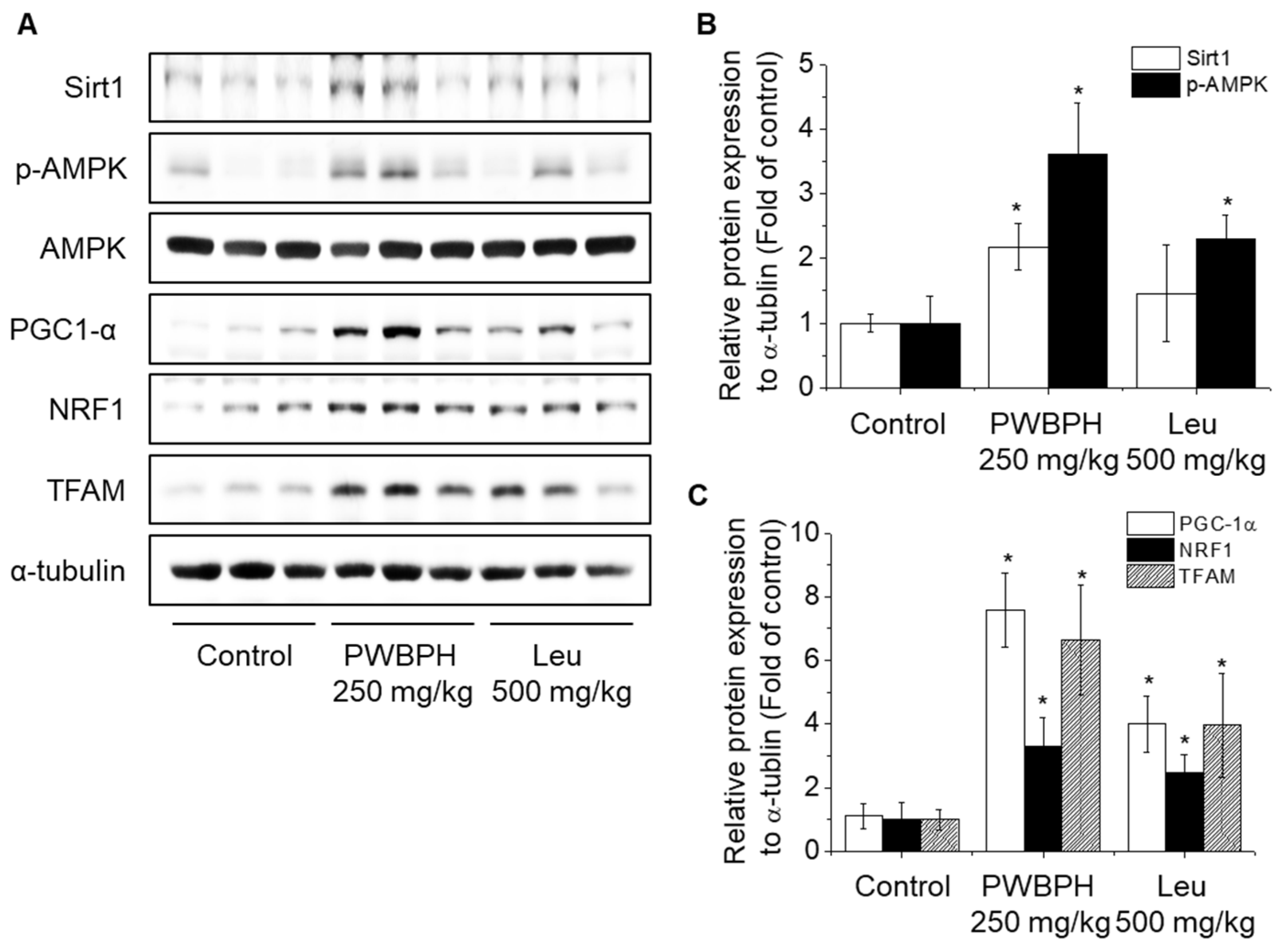
2.2. PWBPH Increases Slow-Twitch Muscle Fibers and Mitochondrial Function-Related Markers In Vivo
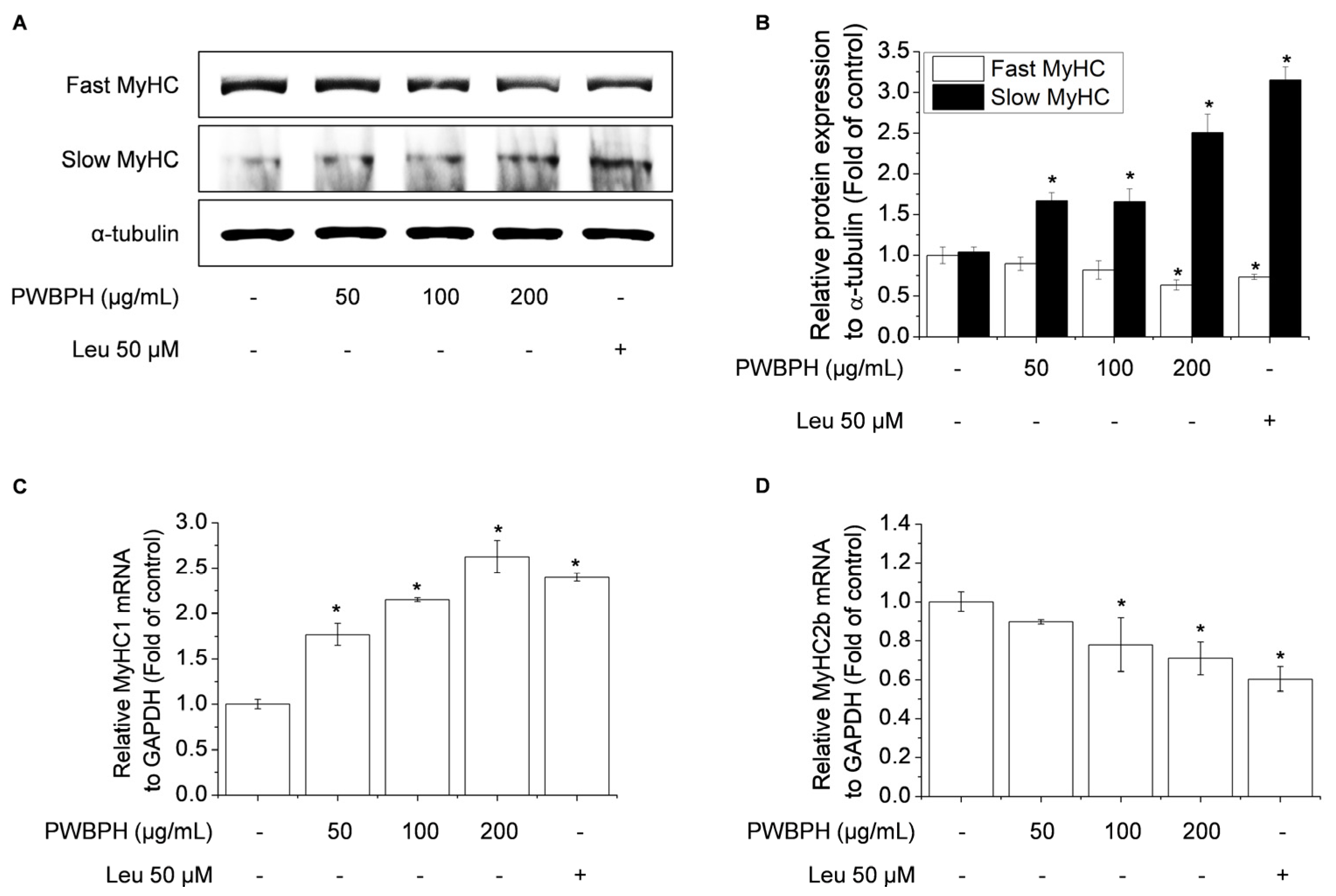
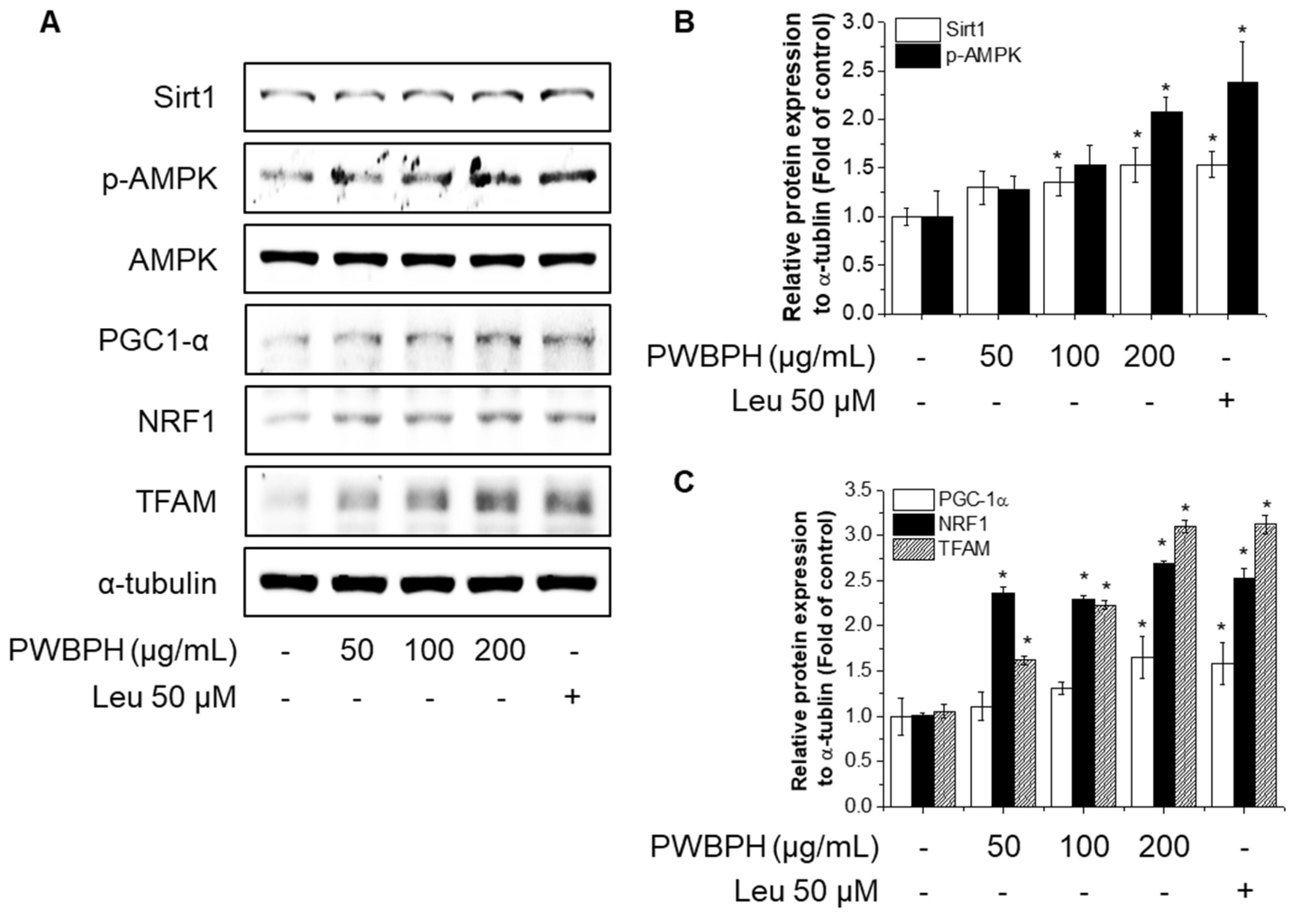
2.3. PWBPH Increases Slow-Twitch Muscle Fibers and Mitochondrial Function-Related Markers in C2C12 Myotubes
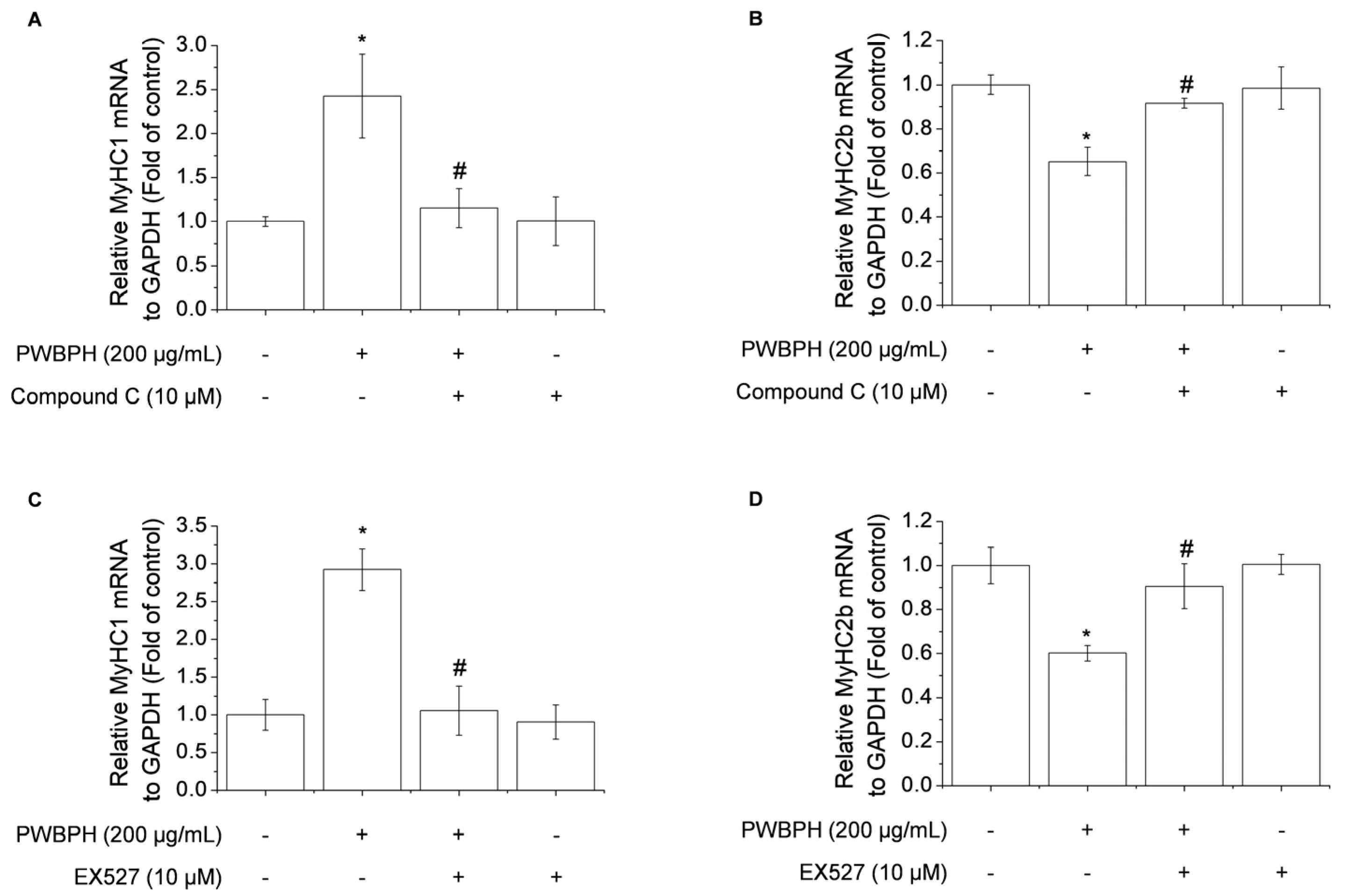
2.4. Inhibition of AMPK/SIRT1 Signaling Blocks the Effect of PWBPH on Slow-Twitch Muscle Fiber-Related Gene Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Treatment
4.3. Exercise Performance
4.4. Serum Biochemistry
4.5. Enzyme Assays
4.6. Cell Culture and Treatment
4.7. Western Blotting
4.8. RNA Extraction and Quantitative Reverse Transcriptase-Polymerase Chain Reaction
4.9. Myosin Heavy-Chain Immunohistochemical Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassel-Duby, R.; Olson, E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Mech, A.M.; Brown, A.L.; Schiavo, G.; Sleigh, J.N. Morphological variability is greater at developing than mature mouse neuromuscular junctions. J. Anat. 2020, 237, 603–617. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Zheng, C.; Hu, Y.; Wang, L.; Yang, X.; Jiang, Z. Dietary L-arginine supplementation affects the skeletal longissimus muscle proteome in finishing pigs. PLoS ONE 2015, 10, e0117294. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- De Vuono, M.; Penteado, C.; Lajolo, F.M.; dos Santos, N.P. Functional and nutritional properties of isolated bovine blood proteins. J. Sci. Food Agric. 1979, 30, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Salvador, P.; Saguer, E.; Parés, D.; Carretero, C.; Toldrà, M. Foaming and emulsifying properties of porcine red cell protein concentrate. Food Sci. Technol. Int. 2010, 16, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, M.Y.; Jin, J.Y. Changes in physicochemical characteristics of porcine blood under various conditions of enzyme hydrolysis. Korean J. Food Preserv. 2016, 23, 413–421. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, P.; Bianchini, G.; Cappellari, O.; Boccanegra, B.; Conte, E.; Sanarica, F.; Mele, A.; Camerino, G.M.; Brandolini, L.; Allegretti, M.; et al. Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise. Nutrients 2020, 12, 2295. [Google Scholar] [CrossRef]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [Green Version]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2017, 174, 1366–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haegens, A.; Schols, A.M.; van Essen, A.L.; van Loon, L.J.; Langen, R.C. Leucine induces myofibrillar protein accretion in cultured skeletal muscle through mTOR dependent and -independent control of myosin heavy chain mRNA levels. Mol. Nutr. Food Res. 2012, 56, 741–752. [Google Scholar] [CrossRef]
- Waldron, M.; Whelan, K.; Jeffries, O.; Burt, D.; Howe, L.; Patterson, S.D. The effects of acute branched-chain amino acid supplementation on recovery from a single bout of hypertrophy exercise in resistance-trained athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 630–636. [Google Scholar] [CrossRef]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Bak, K.H.; Petersen, M.A.; Lametsch, R.; Hansen, E.T.; Ruiz-Carrascal, J. Development of Volatile Compounds during Hydrolysis of Porcine Hemoglobin with Papain. Molecules 2018, 23, 357. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.K.; Choi, J.S.; Yim, D.G. Hydrolysis Conditions of Porcine Blood Proteins and Antimicrobial Effects of Their Hydrolysates. Food Sci. Anim. Resour. 2020, 40, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Dijk, F.J.; van Dijk, M.; Walrand, S.; van Loon, L.J.C.; van Norren, K.; Luiking, Y.C. Differential effects of leucine and leucine-enriched whey protein on skeletal muscle protein synthesis in aged mice. Clin. Nutr. ESPEN 2018, 24, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.S.; Nascimento, F.E.L. Isolated branched-chain amino acid intake and muscle protein synthesis in humans: A biochemical review. Einstein (Sao Paulo) 2019, 17, eRB4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, N.L.; Banks, G.B.; Tsang, M.; Margineantu, D.; Gu, H.; Djukovic, D.; Chan, J.; Torres, M.; Liggitt, H.D.; Hirenallur-S, D.K.; et al. Fnip1 regulates skeletal muscle fiber type specification, fatigue resistance, and susceptibility to muscular dystrophy. Proc. Natl. Acad. Sci. USA 2015, 112, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Chen, H.; Luo, Y.; He, J.; Zheng, P.; Yu, J.; Yu, B. Resveratrol regulates muscle fiber type conversion via miR-22-3p and AMPK/SIRT1/PGC-1α pathway. J. Nutr. Biochem. 2020, 77, 108297. [Google Scholar] [CrossRef]
- Men, X.M.; Deng, B.; Tao, X.; Qi, K.K.; Xu, Z.W. Association Analysis of Myosin Heavy-chain Genes mRNA Transcription with the Corresponding Proteins Expression of Longissimus Muscle in Growing Pigs. Asian-Australas. J. Anim. Sci. 2016, 29, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Booth, F.W.; Ruegsegger, G.N.; Toedebusch, R.G.; Yan, Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 129–151. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, K.Z.; Chu, C.T. After the banquet: Mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 2013, 9, 1663–1676. [Google Scholar] [CrossRef] [Green Version]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [Green Version]
- Chau, M.D.; Gao, J.; Yang, Q.; Wu, Z.; Gromada, J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 12553–12558. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Xu, Y.Q.; Yuan, Y.X.; Xu, P.W.; Zhang, C.; Li, F.; Wang, L.N.; Yin, C.; Zhang, L.; Cai, X.C.; et al. Succinate induces skeletal muscle fiber remodeling via SUCNR1 signaling. EMBO Rep. 2021, 22, e53027. [Google Scholar] [CrossRef]
- Lantier, L.; Fentz, J.; Mounier, R.; Leclerc, J.; Treebak, J.T.; Pehmøller, C.; Sanz, N.; Sakakibara, I.; Saint-Amand, E.; Rimbaud, S.; et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 2014, 28, 3211–3224. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Zheng, P.; He, J.; Chen, H.; Yu, J.; Luo, Y.; Yu, B. miR-22-3p regulates muscle fiber-type conversion through inhibiting AMPK/SIRT1/PGC-1α pathway. Anim. Biotechnol. 2021, 32, 254–261. [Google Scholar] [CrossRef]
- Liang, C.; Curry, B.J.; Brown, P.L.; Zemel, M.B. Leucine Modulates Mitochondrial Biogenesis and SIRT1-AMPK Signaling in C2C12 Myotubes. J. Nutr. Metab. 2014, 2014, 239750. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise training-induced regulation of mitochondrial quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Xiang, L.; Jia, G.; Liu, G.; Zhao, H.; Huang, Z. Leucine regulates slow-twitch muscle fibers expression and mitochondrial function by Sirt1/AMPK signaling in porcine skeletal muscle satellite cells. Anim. Sci. J. 2019, 90, 255–263. [Google Scholar] [CrossRef]
- Hwang, C.E.; Kim, S.C.; Kim, D.H.; Lee, H.Y.; Suh, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef]
- Jung, K.; Kim, I.H.; Han, D. Effect of medicinal plant extracts on forced swimming capacity in mice. J. Ethnopharmacol. 2004, 93, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Luo, C.; Huang, Y.; Zhan, J.; Lei, J.; Li, N.; Huang, X.; Luo, H. Evaluation of antifatigue and antioxidant activities of the marine microalgae. Food Sci. Biotechnol. 2020, 29, 549–557. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Luo, Y.; Yan, H.; Yu, J. Lycopene increases the proportion of slow-twitch muscle fiber by AMPK signaling to improve muscle anti-fatigue ability. J. Nutr. Biochem. 2021, 94, 108750. [Google Scholar] [CrossRef]
- Umek, N.; Horvat, S.; Cvetko, E. Skeletal muscle and fiber type-specific intramyocellular lipid accumulation in obese mice. Bosn J. Basic Med. Sci. 2021, 21, 730–738. [Google Scholar] [CrossRef] [PubMed]


| Group | Lactate (mg/dL) | BUN (mg/dL) | CK (U/L) |
|---|---|---|---|
| Control | 1.30 ± 0.12 | 22.5 ± 2.4 | 1186 ± 299 |
| PWBPH (250 mg/kg) | 1.07 ± 0.13 * | 19.4 ± 1.1 * | 802 ± 129 * |
| PWBPH (500 mg/kg) | 1.12 ± 0.07 * | 19.6 ± 1.1 * | 966 ± 103 |
| Leu (500 mg/kg) | 1.14 ± 0.07 * | 20.3 ± 0.6 * | 945 ± 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.W.; Lee, G.H.; Kim, J.Y.; Kim, C.Y.; Choo, Y.M.; Cho, W.; Han, E.H.; Hwang, Y.P.; Kim, Y.A.; Jeong, H.G. Effect of Porcine Whole Blood Protein Hydrolysate on Slow-Twitch Muscle Fiber Expression and Mitochondrial Biogenesis via the AMPK/SIRT1 Pathway. Int. J. Mol. Sci. 2022, 23, 1229. https://doi.org/10.3390/ijms23031229
Jin SW, Lee GH, Kim JY, Kim CY, Choo YM, Cho W, Han EH, Hwang YP, Kim YA, Jeong HG. Effect of Porcine Whole Blood Protein Hydrolysate on Slow-Twitch Muscle Fiber Expression and Mitochondrial Biogenesis via the AMPK/SIRT1 Pathway. International Journal of Molecular Sciences. 2022; 23(3):1229. https://doi.org/10.3390/ijms23031229
Chicago/Turabian StyleJin, Sun Woo, Gi Ho Lee, Ji Yeon Kim, Chae Yeon Kim, Young Moo Choo, Whajung Cho, Eun Hee Han, Yong Pil Hwang, Yong An Kim, and Hye Gwang Jeong. 2022. "Effect of Porcine Whole Blood Protein Hydrolysate on Slow-Twitch Muscle Fiber Expression and Mitochondrial Biogenesis via the AMPK/SIRT1 Pathway" International Journal of Molecular Sciences 23, no. 3: 1229. https://doi.org/10.3390/ijms23031229
APA StyleJin, S. W., Lee, G. H., Kim, J. Y., Kim, C. Y., Choo, Y. M., Cho, W., Han, E. H., Hwang, Y. P., Kim, Y. A., & Jeong, H. G. (2022). Effect of Porcine Whole Blood Protein Hydrolysate on Slow-Twitch Muscle Fiber Expression and Mitochondrial Biogenesis via the AMPK/SIRT1 Pathway. International Journal of Molecular Sciences, 23(3), 1229. https://doi.org/10.3390/ijms23031229







