A Glance at the Molecules That Regulate Oligodendrocyte Myelination
Abstract
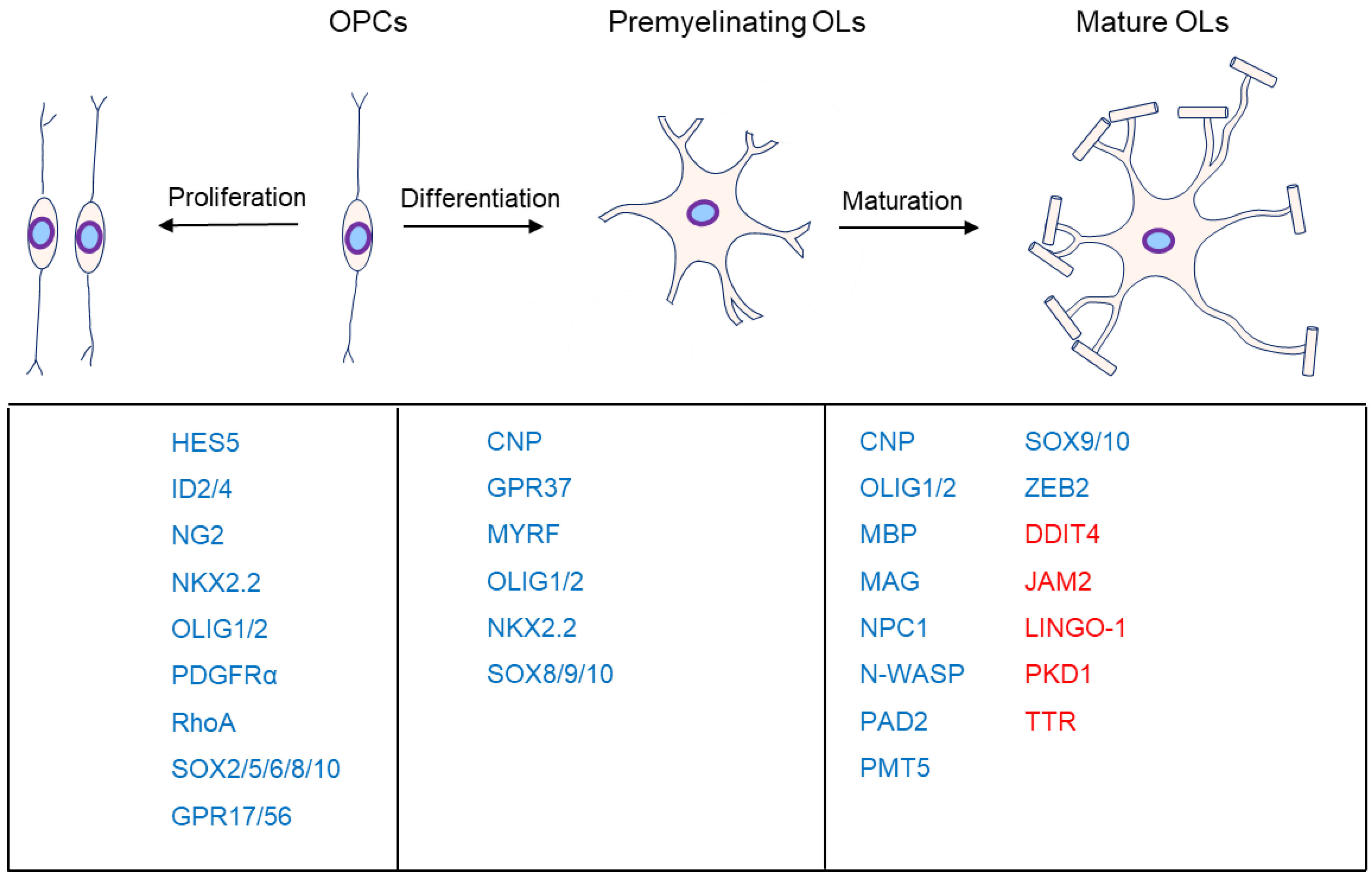
1. Introduction
2. OPC Proliferation
2.1. Wnt Signaling Pathway
2.2. FGF2/FGFR1
2.3. GPR56
2.4. GPR17
2.5. SOX2
2.6. NOTCH1
2.7. SHH
3. OL Differentiation
3.1. AKT-mTOR
3.2. ERK1/2
3.3. GPR37
3.4. SOX10-MYRF
3.5. NRG1
3.6. BDNF
3.7. GlcNAc
3.8. OLIG2
3.9. PDE
4. OL Maturation
4.1. DDIT4
4.2. JAM2
4.3. PKD1
4.4. TTR
4.5. LINGO-1
4.6. N-WASP
4.7. PRMT5
4.8. ZEB2
4.9. PAD2
4.10. NPC1
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil |
| AC | Adenylate cyclase |
| ADGRG1 | Adhesion G protein-coupled receptor G1 |
| AKT | AKT serine/threonine kinase 1 |
| BACE1 | Beta-site APP-cleaving enzyme 1 |
| Bdnf | Brain-derived neurotrophic factor |
| BFPP | Bilateral frontoparietal polymicrogyria |
| BMP | Bone morphogenetic protein |
| BOC | BOC cell adhesion associated, oncogene regulated |
| BPA | Bisphenol-A |
| cAMP | Cyclic adenosine monophosphate |
| CNP | 2,3-cyclic nucleotide 3-phosphodiesterase |
| CNS | Central nervous system |
| CREB | cAMP responsive element binding protein 1 |
| DDIT4 | DNA damage inducible transcript 4 |
| DLG1 | Discs large MAGUK scaffold protein 1 |
| ER | Endoplasmic reticulum |
| ErbB | Erb-b2 receptor tyrosine kinase |
| FGF2 | Fibroblast growth factor 2 |
| GAP | RAS p21 protein activator 1 |
| GlcNAc | N-acetylglucosamine |
| Gli1 | GLI family zinc finger 1 |
| GPR56 | G protein-coupled receptor 56 |
| HDAC | Histone deacetylase |
| HES5 | Hes family bHLH transcription factor 5 |
| ID2 | Inhibitor of DNA binding 2 |
| JAM2 | Junction adhesion molecule 2 |
| LEF | Lymphoid enhancer binding factor |
| LINGO-1 | Leucine-rich repeat and Ig-like domain-containing Nogo receptor interacting protein 1 |
| LPC | Lysophosphatidylcholine |
| MAG | Myelin-associated glycoprotein |
| MAPK | Mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| MEK1 | MAPK kinase 1 |
| Mib1 | MIB E3 ubiquitin protein ligase 1 |
| MTMR2 | Myotubularin-related protein 2 |
| mTOR | Mechanistic target of rapamycin |
| MYRF | Myelin regulatory factor |
| NG2 | Chondroitin sulfate proteoglycan neuron-glia antigen 2 |
| Nkx2.2 | NK2 homeobox 2 |
| NOTCH1 | Notch receptor 1 |
| NPC | Niemann–Pick Type C disease |
| NPC1 | NPC intracellular cholesterol transporter 1 |
| NRG1 | Neuregulin-1 |
| N-WASP | Neural Wiskott–Aldrich syndrome protein |
| p75NTR | p75 neurotrophin receptor |
| OL | Oligodendrocyte |
| OLIG2 | Oligodendrocyte transcription factor 2 |
| OPC | Oligodendrocyte progenitor cell |
| PAD2 | Peptidyl-arginine deiminase 2 |
| PAELR | Parkin-associated endothelin B-like receptor |
| PDEs | Phosphodiesterases |
| PDGFRα | Platelet-derived growth factor receptor α-subunit |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PKA | Protein kinase A |
| PKD1 | Protein kinase D1 |
| PPH | Primary palmar hyperhidrosis |
| PTEN | Phosphatase and tensin homolog |
| RAB35 | RAB35, member RAS oncogene family |
| RAPTOR | Regulatory-associated protein of MTOR complex 1 |
| RHEB | Ras homolog, mTORC1 binding |
| RhoA | Ras homolog family member A |
| RICTOR | RPTOR independent companion of MTOR complex 2 |
| SHH | Sonic hedgehog |
| Smo | Smoothened, frizzled class receptor |
| SOX2 | SRY (sex-determining region Y)-box transcription factor 2 |
| TCF7L2 | Transcription factor 7-like 2 |
| TG2 | Transglutaminase-2 |
| TMEM98 | Transmembrane protein 98 |
| TNF-α | Tumor necrosis factor-α |
| TRKB | Tropomyosin-related kinase receptor B |
| TSC1 | Tuberous sclerosis protein 1 |
| TTR | Transthyretin |
| WNT | Wingless-type MMTV integration site family |
| Xaf1 | XIAP-associated factor 1 |
| ZEB2 | Zinc finger E-box binding homeobox 2 |
References
- Jeffries, M.A.; Obr, A.E.; Urbanek, K.; Fyffe-Maricich, S.L.; Wood, T.L. Cnp Promoter-Driven Sustained ERK1/2 Activation Increases B-Cell Activation and Suppresses Experimental Autoimmune Encephalomyelitis. ASN Neuro 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Richter, N.; Fan, Z.; Siemonsmeier, G.; Pivneva, T.; Jordan, P.; Steinhauser, C.; Semtner, M.; Nolte, C.; Kettenmann, H. Oligodendrocytes in the Mouse Corpus Callosum Maintain Axonal Function by Delivery of Glucose. Cell Rep. 2018, 22, 2383–2394. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nave, K.A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2015, 8, a020479. [Google Scholar] [CrossRef] [PubMed]
- van Tilborg, E.; de Theije, C.G.M.; van Hal, M.; Wagenaar, N.; de Vries, L.S.; Benders, M.J.; Rowitch, D.H.; Nijboer, C.H. Origin and dynamics of oligodendrocytes in the developing brain: Implications for perinatal white matter injury. Glia 2018, 66, 221–238. [Google Scholar] [CrossRef]
- Emery, B. Regulation of oligodendrocyte differentiation and myelination. Science 2010, 330, 779–782. [Google Scholar] [CrossRef]
- Mi, S.; Miller, R.H.; Lee, X.; Scott, M.L.; Shulag-Morskaya, S.; Shao, Z.; Chang, J.; Thill, G.; Levesque, M.; Zhang, M.; et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 2005, 8, 745–751. [Google Scholar] [CrossRef]
- Tian, C.; Zou, S.; Hu, B. Extraocular Source of Oligodendrocytes Contribute to RetinalMyelination and Optokinetic Responses in Zebrafish. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2129–2138. [Google Scholar] [CrossRef][Green Version]
- Mitew, S.; Hay, C.M.; Peckham, H.; Xiao, J.; Koenning, M.; Emery, B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 2014, 276, 29–47. [Google Scholar] [CrossRef]
- Goldman, S.A.; Kuypers, N.J. How to make an oligodendrocyte. Development 2015, 142, 3983–3995. [Google Scholar] [CrossRef]
- Emery, B.; Lu, Q.R. Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb. Perspect. Biol. 2015, 7, e020461. [Google Scholar] [CrossRef]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Kessler, J.A. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development 2004, 131, 4131–4142. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, H.; Wang, S.; Koito, H.; Li, J.; Ye, F.; Hoang, J.; Escobar, S.S.; Gow, A.; Arnett, H.A.; et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 2009, 12, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sdrulla, A.; Johnson, J.E.; Yokota, Y.; Barres, B.A. A Role for the Helix-Loop-Helix Protein Id2 in the Control of Oligodendrocyte Development. Neuron 2001, 29, 603–614. [Google Scholar] [CrossRef]
- Guo, F.; Lang, J.; Sohn, J.; Hammond, E.; Chang, M.; Pleasure, D. Canonical Wnt signaling in the oligodendroglial lineage--puzzles remain. Glia 2015, 63, 1671–1693. [Google Scholar] [CrossRef]
- Ye, F.; Chen, Y.; Hoang, T.; Montgomery, R.L.; Zhao, X.H.; Bu, H.; Hu, T.; Taketo, M.M.; van Es, J.H.; Clevers, H.; et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 2009, 12, 829–838. [Google Scholar] [CrossRef]
- Fancy, S.P.; Baranzini, S.E.; Zhao, C.; Yuk, D.I.; Irvine, K.A.; Kaing, S.; Sanai, N.; Franklin, R.J.; Rowitch, D.H. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009, 23, 1571–1585. [Google Scholar] [CrossRef]
- Shimizu, T.; Kagawa, T.; Wada, T.; Muroyama, Y.; Takada, S.; Ikenaka, K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev. Biol. 2005, 282, 397–410. [Google Scholar] [CrossRef]
- Fu, H.; Kesari, S.; Cai, J. Tcf7l2 is tightly controlled during myelin formation. Cell. Mol. Neurobiol. 2012, 32, 345–352. [Google Scholar] [CrossRef]
- Huang, H.; Wu, H.; He, W.; Zhou, F.; Yu, X.; Yi, M.; Du, J.; Xie, B.; Qiu, M. Id2 and Id4 are not the major negative regulators of oligodendrocyte differentiation during early central nervous system development. Glia 2022, 70, 590–601. [Google Scholar] [CrossRef]
- Dai, J.; Bercury, K.K.; Macklin, W.B. Interaction of mTOR and Erk1/2 signaling to regulate oligodendrocyte differentiation. Glia 2014, 62, 2096–2109. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Tan, B.; Wang, J.; Wang, J.; Zhou, H.; Shi, J.; He, Q.; Yang, B. 5-Fluorouracil causes severe CNS demyelination by disruption of TCF7L2/HDAC1/HDAC2 complex in adolescent mice. Toxicology 2014, 325, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Flint, N.C.; Murtie, J.C.; Le, T.Q.; Armstrong, R.C. Retroviral lineage analysis of fibroblast growth factor receptor signaling in FGF2 inhibition of oligodendrocyte progenitor differentiation. Glia 2006, 54, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Magy, L.; Keita, M.; Richard, L.; Piaser, M.; Vallat, J.-M. Transient exposure to FGF2 enhances myelination in embryonic brain cell cocultures. Exp. Neurol. 2003, 181, 17–24. [Google Scholar] [CrossRef]
- Magy, L.; Mertens, C.; Avellana-Adalid, V.; Keita, M.; Lachapelle, F.; Nait-Oumesmar, B.; Fontaine, B.; Baron-Van Evercooren, A. Inducible expression of FGF2 by a rat oligodendrocyte precursor cell line promotes CNS myelination in vitro. Exp. Neurol. 2003, 184, 912–922. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Armstrong, R.C. Interaction of Fibroblast Growth Factor 2 (FGF2) and Notch Signaling Components in Inhibition of Oligodendrocyte Progenitor (OP) Differentiation. Neurosci. Lett. 2007, 421, 27–32. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Li, L.; Wang, X.; Wang, Q.; Jiang, S.; Tang, C.; Zhou, S.; Xu, N.; Cui, Y.; et al. Acute Kidney Injury Sensitizes the Brain Vasculature to Ang II (Angiotensin II) Constriction via FGFBP1 (Fibroblast Growth Factor Binding Protein 1). Hypertension 2020, 76, 1924–1934. [Google Scholar] [CrossRef]
- Murtie, J.C.; Zhou, Y.X.; Le, T.Q.; Armstrong, R.C. In vivo analysis of oligodendrocyte lineage development in postnatal FGF2 null mice. Glia 2005, 49, 542–554. [Google Scholar] [CrossRef]
- Mierzwa, A.J.; Zhou, Y.X.; Hibbits, N.; Vana, A.C.; Armstrong, R.C. FGF2 and FGFR1 signaling regulate functional recovery following cuprizone demyelination. Neurosci. Lett. 2013, 548, 280–285. [Google Scholar] [CrossRef]
- Furusho, M.; Dupree, J.L.; Nave, K.A.; Bansal, R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6631–6641. [Google Scholar] [CrossRef]
- Thummler, K.; Rom, E.; Zeis, T.; Lindner, M.; Brunner, S.; Cole, J.J.; Arseni, D.; Mucklisch, S.; Edgar, J.M.; Schaeren-Wiemers, N.; et al. Polarizing receptor activation dissociates fibroblast growth factor 2 mediated inhibition of myelination from its neuroprotective potential. Acta Neuropathol. Commun. 2019, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.A.; Ryu, J.K.; Chang, K.J.; Etxeberria, A.; Bardehle, S.; Mendiola, A.S.; Kamau-Devers, W.; Fancy, S.P.J.; Thor, A.; Bushong, E.A.; et al. Fibrinogen Activates BMP Signaling in Oligodendrocyte Progenitor Cells and Inhibits Remyelination after Vascular Damage. Neuron 2017, 96, 1003–1012.e1007. [Google Scholar] [CrossRef] [PubMed]
- Chiou, B.; Gao, C.; Giera, S.; Folts, C.J.; Kishore, P.; Yu, D.; Oak, H.C.; Jiang, R.; Piao, X. Cell type-specific evaluation of ADGRG1/GPR56 function in developmental central nervous system myelination. Glia 2021, 69, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Salzman, G.S.; Ackerman, S.D.; Ding, C.; Koide, A.; Leon, K.; Luo, R.; Stoveken, H.M.; Fernandez, C.G.; Tall, G.G.; Piao, X.; et al. Structural Basis for Regulation of GPR56/ADGRG1 by Its Alternatively Spliced Extracellular Domains. Neuron 2016, 91, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Vainshtein, A.; Maik-Rachline, G.; Peles, E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun. 2016, 7, 10884. [Google Scholar] [CrossRef]
- Piao, X.; Hill, R.S.; Bodell, A.; Chang, B.S.; Basel-Vanagaite, L.; Straussberg, R.; Dobyns, W.B.; Qasrawi, B.; Winter, R.M.; Innes, A.M.; et al. G protein-coupled receptor-dependent development of human frontal cortex. Science 2004, 303, 2033–2036. [Google Scholar] [CrossRef]
- Mehta, P.; Piao, X. Adhesion G-Protein Coupled Receptors and Extracellular Matrix Proteins: Roles in Myelination and Glial Cell Development. Dev. Dyn. 2017, 246, 275–384. [Google Scholar] [CrossRef]
- Ackerman, S.D.; Garcia, C.; Piao, X.; Gutmann, D.H.; Monk, K.R. The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Galpha12/13 and RhoA. Nat. Commun. 2015, 6, 6122. [Google Scholar] [CrossRef]
- Giera, S.; Deng, Y.; Luo, R.; Ackerman, S.D.; Mogha, A.; Monk, K.R.; Ying, Y.; Jeong, S.J.; Makinodan, M.; Bialas, A.R.; et al. The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat. Commun. 2015, 6, 6121. [Google Scholar] [CrossRef]
- Giera, S.; Luo, R.; Ying, Y.; Ackerman, S.D.; Jeong, S.-J.; Stoveken, H.M.; Folts, C.J.; Welsh, C.A.; Tall, G.G.; Stevens, B.; et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. eLife 2018, 29, e33385. [Google Scholar] [CrossRef]
- Lecca, D.; Raffaele, S.; Abbracchio, M.P.; Fumagalli, M. Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 2020, 68, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Daniele, S.; Lecca, D.; Lee, P.R.; Parravicini, C.; Fields, R.D.; Rosa, P.; Antonucci, F.; Verderio, C.; Trincavelli, M.L.; et al. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J. Biol. Chem. 2011, 286, 10593–10604. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, E.; Bonifacino, T.; Raffaele, S.; Milanese, M.; Morgante, E.; Bonanno, G.; Abbracchio, M.P.; Fumagalli, M. Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice. Int. J. Mol. Sci. 2020, 21, 2395. [Google Scholar] [CrossRef] [PubMed]
- Ciana, P.; Fumagalli, M.; Trincavelli, M.L.; Verderio, C.; Rosa, P.; Lecca, D.; Ferrario, S.; Parravicini, C.; Capra, V.r.; Gelosa, P.; et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006, 25, 4615–4627. [Google Scholar] [CrossRef] [PubMed]
- Benned-Jensen, T.; Rosenkilde, M.M. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br. J. Pharmacol. 2010, 159, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Hennen, S.; Wang, H.; Peters, L.; Merten, N.; Simon, K.; Spinrath, A.; Blattermann, S.; Akkari, R.; Schrage, R.; Schroder, R.; et al. Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. Sci. Signal. 2013, 6, ra93. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Trincavelli, M.L.; Fumagalli, M.; Zappelli, E.; Lecca, D.; Bonfanti, E.; Campiglia, P.; Abbracchio, M.P.; Martini, C. Does GRK-beta arrestin machinery work as a “switch on” for GPR17-mediated activation of intracellular signaling pathways? Cell. Signal. 2014, 26, 1310–1325. [Google Scholar] [CrossRef]
- Agier, J.; Różalska, S.; Wódz, K.; Brzezińska-Błaszczyk, E. Leukotriene receptor expression in mast cells is affected by their agonists. Cell. Immunol. 2017, 317, 37–47. [Google Scholar] [CrossRef]
- Lecca, D.; Trincavelli, M.L.; Gelosa, P.; Sironi, L.; Ciana, P.; Fumagalli, M.; Villa, G.; Verderio, C.; Grumelli, C.; Guerrini, U.; et al. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS ONE 2008, 3, e3579. [Google Scholar] [CrossRef]
- Fumagalli, M.; Bonfanti, E.; Daniele, S.; Zappelli, E.; Lecca, D.; Martini, C.; Trincavelli, M.L.; Abbracchio, M.P. The ubiquitin ligase Mdm2 controls oligodendrocyte maturation by intertwining mTOR with G protein-coupled receptor kinase 2 in the regulation of GPR17 receptor desensitization. Glia 2015, 63, 2327–2339. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; Meng, H.; Li, Y.; Dmitriev, P.; Tian, F.; Page, J.C.; Lu, Q.R.; He, Z. Robust Myelination of Regenerated Axons Induced by Combined Manipulations of GPR17 and Microglia. Neuron 2020, 108, 876–886.e874. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.T.; Marangon, D.; Negri, C.; Menichetti, G.; Fumagalli, M.; Gelosa, P.; Dimou, L.; Furlan, R.; Lecca, D.; Abbracchio, M.P. Differential local tissue permissiveness influences the final fate of GPR17-expressing oligodendrocyte precursors in two distinct models of demyelination. Glia 2018, 66, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Vigano, F.; Schneider, S.; Cimino, M.; Bonfanti, E.; Gelosa, P.; Sironi, L.; Abbracchio, M.P.; Dimou, L. GPR17 expressing NG2-Glia: Oligodendrocyte progenitors serving as a reserve pool after injury. Glia 2016, 64, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z.; Sun, Y.; Lin, L.; You, N.; Liu, X.; Li, H.; Ma, Y.; Cao, L.; Han, Y.; Liu, M.; et al. Olig2-Targeted G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Survival in Response to Lysolecithin-Induced Demyelination. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 10560–10573. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.; Hennen, S.; Merten, N.; Blattermann, S.; Gillard, M.; Kostenis, E.; Gomeza, J. The Orphan G Protein-coupled Receptor GPR17 Negatively Regulates Oligodendrocyte Differentiation via Galphai/o and Its Downstream Effector Molecules. J. Biol. Chem. 2016, 291, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Dong, L.; Zhou, H.; Li, Q.; Huang, G.; Bai, S.J.; Liao, L. G-Protein-Coupled Receptor Gpr17 Regulates Oligodendrocyte Differentiation in Response to Lysolecithin-Induced Demyelination. Sci. Rep. 2018, 8, 4502. [Google Scholar] [CrossRef]
- Roberts, S.L.; Dun, X.P.; Doddrell, R.D.S.; Mindos, T.; Drake, L.K.; Onaitis, M.W.; Florio, F.; Quattrini, A.; Lloyd, A.C.; D’Antonio, M.; et al. Sox2 expression in Schwann cells inhibits myelination in vivo and induces influx of macrophages to the nerve. Development 2017, 144, 3114–3125. [Google Scholar]
- Dai, J.; Bercury, K.K.; Ahrendsen, J.T.; Macklin, W.B. Olig1 function is required for oligodendrocyte differentiation in the mouse brain. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 4386–4402. [Google Scholar] [CrossRef]
- Shen, S.; Sandoval, J.; Swiss, V.A.; Li, J.; Dupree, J.; Franklin, R.J.; Casaccia-Bonnefil, P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 2008, 11, 1024–1034. [Google Scholar] [CrossRef]
- Pedre, X.; Mastronardi, F.; Bruck, W.; Lopez-Rodas, G.; Kuhlmann, T.; Casaccia, P. Changed histone acetylation patterns in normal-appearing white matter and early multiple sclerosis lesions. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 3435–3445. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Gui, X.; Croteau, C.; Song, L.; Xu, J.; Wang, A.; Bannerman, P.; Guo, F. Sox2 Is Essential for Oligodendroglial Proliferation and Differentiation during Postnatal Brain Myelination and CNS Remyelination. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 1802–1820. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.A.; Hos, D.; Kuspert, M.; Lang, R.A.; Lovell-Badge, R.; Wegner, M.; Reiprich, S. Stem cell factor Sox2 and its close relative Sox3 have differentiation functions in oligodendrocytes. Development 2014, 141, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Stidworthy, M.F. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain A J. Neurol. 2004, 127, 1928–1941. [Google Scholar] [CrossRef]
- Popko, B. Notch signaling: A rheostat regulating oligodendrocyte differentiation? Dev. Cell 2003, 5, 668–669. [Google Scholar] [CrossRef]
- Givogri, M.I.; Costa, R.M.; Schonmann, V.; Silva, A.J.; Campagnoni, A.T.; Bongarzone, E.R. Central nervous system myelination in mice with deficient expression of Notch1 receptor. J. Neurosci. Res. 2002, 67, 309–320. [Google Scholar] [CrossRef]
- Liu, D.; Liang, X.C.; Sun, Y.; Wu, Y.N.; Zhang, H. Combination of Quercetin, Hirudin and Cinnamaldehyde Promotes Schwann Cell Differentiation and Myelination against High Glucose by Inhibiting ERK Signaling Pathway. Chin. J. Integr. Med. 2020, 26, 591–598. [Google Scholar] [CrossRef]
- Tandon, A.; Singh, S.J.; Gupta, M.; Singh, N.; Shankar, J.; Arjaria, N.; Goyal, S.; Chaturvedi, R.K. Notch pathway up-regulation via curcumin mitigates bisphenol-A (BPA) induced alterations in hippocampal oligodendrogenesis. J. Hazard. Mater. 2020, 392, 122052. [Google Scholar] [CrossRef]
- Fang, M.; Yu, Q.; Ou, B.; Huang, H.; Yi, M.; Xie, B.; Yang, A.; Qiu, M.; Xu, X. Genetic Evidence that Dorsal Spinal Oligodendrocyte Progenitor Cells are Capable of Myelinating Ventral Axons Effectively in Mice. Neurosci. Bull. 2020, 36, 1474–1483. [Google Scholar] [CrossRef]
- Zakaria, M.; Ferent, J.; Hristovska, I.; Laouarem, Y.; Zahaf, A.; Kassoussi, A.; Mayeur, M.E.; Pascual, O.; Charron, F.; Traiffort, E. The Shh receptor Boc is important for myelin formation and repair. Development 2019, 146, 172502. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Armstrong, R.C. Postnatal Sonic hedgehog (Shh) responsive cells give rise to oligodendrocyte lineage cells during myelination and in adulthood contribute to remyelination. Exp. Neurol. 2018, 299, 122–136. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Sullivan, G.M.; Armstrong, R.C. Genetic detection of Sonic hedgehog (Shh) expression and cellular response in the progression of acute through chronic demyelination and remyelination. Neurobiol. Dis. 2018, 115, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Laouarem, Y.; Traiffort, E. Developmental and Repairing Production of Myelin: The Role of Hedgehog Signaling. Front. Cell. Neurosci. 2018, 12, 305. [Google Scholar] [CrossRef]
- Wang, L.C.; Almazan, G. Role of Sonic Hedgehog Signaling in Oligodendrocyte Differentiation. Neurochem. Res. 2016, 41, 3289–3299. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, S.R.; Etxeberria, A.; Shen, Y.-A.A.; Chan, J.R. Initiation of CNS Myelination in the Optic Nerve Is Dependent on Axon Caliber. Cell Rep. 2018, 25, 544–550.e543. [Google Scholar] [CrossRef] [PubMed]
- Sabatelli, M.; Mignogna, T.; Lippi, G.; Servidei, S.; Manfredi, G.; Ricci, E.; Bertini, E.; Monaco, M.L.; Tonali, P. Autosomal recessive hypermyelinating neuropathy. Acta Neuropathol. 1995, 87, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mitew, S.; Gobius, I.; Fenlon, L.R.; McDougall, S.J.; Hawkes, D.; Xing, Y.L.L.; Bujalka, H.; Gundlach, A.L.; Richards, L.J.; Kilpatrick, T.J.; et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 2018, 22, 306. [Google Scholar] [CrossRef] [PubMed]
- Redmond, S.A.; Mei, F.; Eshed-Eisenbach, Y.; Osso, L.A.; Leshkowitz, D.; Shen, Y.A.; Kay, J.N.; Aurrand-Lions, M.; Lyons, D.A.; Peles, E.; et al. Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination. Neuron 2016, 91, 824–836. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Flores, A.I.; Wang, F.; Macklin, W.B. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 6860–6870. [Google Scholar] [CrossRef]
- Flores, A.I.; Narayanan, S.P.; Morse, E.N.; Shick, H.E.; Yin, X.; Kidd, G.; Avila, R.L.; Kirschner, D.A.; Macklin, W.B. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 7174–7183. [Google Scholar] [CrossRef]
- Domenech-Estevez, E.; Baloui, H.; Meng, X.; Zhang, Y.; Deinhardt, K.; Dupree, J.L.; Einheber, S.; Chrast, R.; Salzer, J.L. Akt Regulates Axon Wrapping and Myelin Sheath Thickness in the PNS. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 4506–4521. [Google Scholar] [CrossRef]
- Maire, C.L.; Ramkissoon, S.; Hayashi, M.; Haidar, S.; Ramkissoon, L.; DiTomaso, E.; Ligon, K.L. Pten loss in Olig2 expressing neural progenitor cells and oligodendrocytes leads to interneuron dysplasia and leukodystrophy. Stem Cells 2014, 32, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, B.; Wong, K.M.; Babetto, E.; Milbrandt, J. mTORC1 promotes proliferation of immature Schwann cells and myelin growth of differentiated Schwann cells. Proc. Natl. Acad. Sci. USA 2017, 114, E4261–E4270. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Belin, S.; Piguet, F.; Vaccari, I.; Scarlino, S.; Brambilla, P.; Martinelli Boneschi, F.; Feltri, M.L.; Wrabetz, L.; Quattrini, A.; et al. DDIT4/REDD1/RTP801 is a novel negative regulator of Schwann cell myelination. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 15295–15305. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Figlia, G.; Norrmen, C.; Pereira, J.A.; Gerber, D.; Suter, U. Dual function of the PI3K-Akt-mTORC1 axis in myelination of the peripheral nervous system. eLife 2017, 6, e29241. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Figlia, G.; Gerber, D.; Suter, U. Myelination and mTOR. Glia 2018, 66, 693–707. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, L.; He, X.; Wang, H.; Lin, W.; Wang, H.; Yoon, S.O.; Wood, T.L.; Lu, Q.R. Regulation of PERK-eIF2alpha signalling by tuberous sclerosis complex-1 controls homoeostasis and survival of myelinating oligodendrocytes. Nat. Commun. 2016, 7, 12185. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Bachmann, L.; Norrmen, C.; Trotzmuller, M.; Kofeler, H.; Ruegg, M.A.; Hall, M.N.; Suter, U. Balanced mTORC1 activity in oligodendrocytes is required for accurate CNS myelination. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 8432–8448. [Google Scholar] [CrossRef]
- Sawade, L.; Grandi, F.; Mignanelli, M.; Patino-Lopez, G.; Klinkert, K.; Langa-Vives, F.; Di Guardo, R.; Echard, A.; Bolino, A.; Haucke, V. Rab35-regulated lipid turnover by myotubularins represses mTORC1 activity and controls myelin growth. Nat. Commun. 2020, 11, 2835. [Google Scholar] [CrossRef]
- Colognato, H.; Nishiyama, A. Introduction to the Special Issue on The oligodendrocyte niche in development and repair. Neurosci. Lett. 2020, 730, 134957. [Google Scholar] [CrossRef] [PubMed]
- Baron, W.; Colognato, H.; ffrench-Constant, C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia 2005, 49, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Michel, K.; Zhao, T.; Karl, M.; Lewis, K.; Fyffe-Maricich, S.L. Translational control of myelin basic protein expression by ERK2 MAP kinase regulates timely remyelination in the adult brain. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 7850–7865. [Google Scholar] [CrossRef] [PubMed]
- Ishii, A.; Furusho, M.; Bansal, R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 175–186. [Google Scholar] [CrossRef]
- Gaesser, J.M.; Fyffe-Maricich, S.L. Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp. Neurol. 2016, 283, 501–511. [Google Scholar] [CrossRef]
- Suo, N.; Guo, Y.E.; He, B.; Gu, H.; Xie, X. Inhibition of MAPK/ERK pathway promotes oligodendrocytes generation and recovery of demyelinating diseases. Glia 2019, 67, 1320–1332. [Google Scholar] [CrossRef]
- Furqan, M.; Akinleye, A.; Mukhi, N.; Mittal, V.; Chen, Y.; Liu, D. STAT inhibitors for cancer therapy. J. Hematol. Oncol. 2013, 6, 90. [Google Scholar] [CrossRef]
- Planz, O. Development of cellular signaling pathway inhibitors as new antivirals against influenza. Antivir. Res. 2013, 98, 457–468. [Google Scholar] [CrossRef]
- Imai, Y.; Soda, M.; Inoue, H.; Hattori, N.; Mizuno, Y.; Takahashi, R. An Unfolded Putative Transmembrane Polypeptide, which Can Lead to Endoplasmic Reticulum Stress, Is a Substrate of Parkin. Cell 2001, 105, 891–902. [Google Scholar] [CrossRef]
- Smith, B.M.; Giddens, M.M.; Neil, J.; Owino, S.; Nguyen, T.T.; Duong, D.; Li, F.; Hall, R.A. Mice lacking Gpr37 exhibit decreased expression of the myelin-associated glycoprotein MAG and increased susceptibility to demyelination. Neuroscience 2017, 358, 49–57. [Google Scholar] [CrossRef]
- Kim, D.; Choi, J.O.; Fan, C.; Shearer, R.S.; Sharif, M.; Busch, P.; Park, Y. Homo-trimerization is essential for the transcription factor function of Myrf for oligodendrocyte differentiation. Nucleic Acids Res. 2017, 45, 5112–5125. [Google Scholar] [CrossRef] [PubMed]
- ffrench-Constant, C.; Bujalka, H.; Koenning, M.; Jackson, S.; Perreau, V.M.; Pope, B.; Hay, C.M.; Mitew, S.; Hill, A.F.; Lu, Q.R.; et al. MYRF Is a Membrane-Associated Transcription Factor That Autoproteolytically Cleaves to Directly Activate Myelin Genes. PLoS Biol. 2013, 11, e1001625. [Google Scholar]
- Li, Z.; Park, Y.; Marcotte, E.M. A Bacteriophage tailspike domain promotes self-cleavage of a human membrane-bound transcription factor, the myelin regulatory factor MYRF. PLoS Biol. 2013, 11, e1001624. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhen, X.; Li, B.; Yu, Q.; Huang, X.; Shi, N. Crystal structure of the MyRF ICA domain with its upstream beta-helical stalk reveals the molecular mechanisms underlying its trimerization and self-cleavage. Int. J. Biol. Sci. 2021, 17, 2931–2943. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Teng, P.; Du, J.; Meng, J.; Hu, X.; Tang, T.; Zhang, Z.; Qi, Y.B.; Qiu, M. Interactive Repression of MYRF Self-Cleavage and Activity in Oligodendrocyte Differentiation by TMEM98 Protein. J. Neurosci. 2018, 38, 9829–9839. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.H.; McKie, L.; Hurd, T.W.; Riley, S.; Wills, J.; Barnard, A.R.; Young, F.; MacLaren, R.E.; Jackson, I.J. The nanophthalmos protein TMEM98 inhibits MYRF self-cleavage and is required for eye size specification. PLoS Genet. 2020, 16, e1008583. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Plemel, J.R.; Assinck, P.; Manesh, S.B.; Muir, F.G.W.; Hirata, R.; Berson, M.; Liu, J.; Wegner, M.; Emery, B.; et al. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 2017, 134, 403–422. [Google Scholar] [CrossRef]
- Zhen, X.; Li, B.; Hu, F.; Yan, S.; Meloni, G.; Li, H.; Shi, N. Crystal structure of the DNA-binding domain of Myelin-gene Regulatory Factor. Sci. Rep. 2017, 7, 3696. [Google Scholar] [CrossRef]
- Chen, B.; Zhu, Y.; Ye, S.; Zhang, R. Structure of the DNA-binding domain of human myelin-gene regulatory factor reveals its potential protein-DNA recognition mode. J. Struct. Biol. 2018, 203, 170–178. [Google Scholar] [CrossRef]
- Fan, C.; An, H.; Sharif, M.; Kim, D.; Park, Y. Functional mechanisms of MYRF DNA-binding domain mutations implicated in birth defects. J. Biol. Chem. 2021, 296, 100612. [Google Scholar] [CrossRef]
- Choi, J.O.; Fan, C.; Kim, D.; Sharif, M.; An, H.; Park, Y. Elucidating the transactivation domain of the pleiotropic transcription factor Myrf. Sci. Rep. 2018, 8, 13075. [Google Scholar] [CrossRef] [PubMed]
- Aprato, J.; Sock, E.; Weider, M.; Elsesser, O.; Frob, F.; Wegner, M. Myrf guides target gene selection of transcription factor Sox10 during oligodendroglial development. Nucleic Acids Res. 2020, 48, 1254–1270. [Google Scholar] [CrossRef] [PubMed]
- Koenning, M.; Jackson, S.; Hay, C.M.; Faux, C.; Kilpatrick, T.J.; Willingham, M.; Emery, B. Myelin gene regulatory factor is required for maintenance of myelin and mature oligodendrocyte identity in the adult CNS. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 12528–12542. [Google Scholar] [CrossRef] [PubMed]
- Emery, B.; Agalliu, D.; Cahoy, J.D.; Watkins, T.A.; Dugas, J.C.; Mulinyawe, S.B.; Ibrahim, A.; Ligon, K.L.; Rowitch, D.H.; Barres, B.A. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 2009, 138, 172–185. [Google Scholar] [CrossRef]
- Xin, M.; Yue, T.; Ma, Z.; Wu, F.F.; Gow, A.; Lu, Q.R. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1354–1365. [Google Scholar] [CrossRef]
- Takada, N.; Kucenas, S.; Appel, B. Sox10 is necessary for oligodendrocyte survival following axon wrapping. Glia 2010, 58, 996–1006. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Hornig, J.; Frob, F.; Vogl, M.R.; Hermans-Borgmeyer, I.; Tamm, E.R.; Wegner, M. The transcription factors Sox10 and Myrf define an essential regulatory network module in differentiating oligodendrocytes. PLoS Genet. 2013, 9, e1003907. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, Y.; Kim, B.; Wang, H.; Zhao, C.; He, X.; Liu, L.; Liu, W.; Wu, L.M.; Mao, M.; et al. Olig2 targets chromatin remodelers to enhancers to initiate oligodendrocyte differentiation. Cell 2013, 152, 248–261. [Google Scholar] [CrossRef]
- Kurahashi, H.; Azuma, Y.; Masuda, A.; Okuno, T.; Nakahara, E.; Imamura, T.; Saitoh, M.; Mizuguchi, M.; Shimizu, T.; Ohno, K.; et al. MYRF is associated with encephalopathy with reversible myelin vacuolization. Ann. Neurol. 2018, 83, 98–106. [Google Scholar] [CrossRef]
- Rossetti, L.Z.; Glinton, K.; Yuan, B.; Liu, P.; Pillai, N.; Mizerik, E.; Magoulas, P.; Rosenfeld, J.A.; Karaviti, L.; Sutton, V.R.; et al. Review of the phenotypic spectrum associated with haploinsufficiency of MYRF. Am. J. Med. Genetics. Part A 2019, 179, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Garnai, S.J.; Brinkmeier, M.L.; Emery, B.; Aleman, T.S.; Pyle, L.C.; Veleva-Rotse, B.; Sisk, R.A.; Rozsa, F.W.; Ozel, A.B.; Li, J.Z.; et al. Variants in myelin regulatory factor (MYRF) cause autosomal dominant and syndromic nanophthalmos in humans and retinal degeneration in mice. PLoS Genet. 2019, 15, e1008130. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, N.; Yang, X.; Zhao, Z.; Zhang, J.; Zhang, M.; Zhang, D.; Ge, J.; Fan, Z. Myelin regulatory factor deficiency is associated with the retinal photoreceptor defects in mice. Vis. Neurosci. 2021, 38, E005. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, F.; Zhou, S.; Qiu, M. MYRF: A Mysterious Membrane-Bound Transcription Factor Involved in Myelin Development and Human Diseases. Neurosci. Bull. 2021, 37, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.K.; Kosciuczyk, K.; Tapley, L.; Karimi-Abdolrezaee, S. Dysregulation of the neuregulin-1-ErbB network modulates endogenous oligodendrocyte differentiation and preservation after spinal cord injury. Eur. J. Neurosci. 2013, 38, 2693–2715. [Google Scholar] [CrossRef]
- Canoll, P.D.; Musacchio, J.M.; Hardy, R.; Reynolds, R.; Marchionni, M.A.; Salzer, J.L. GGF/Neuregulin Is a Neuronal Signal That Promotes the Proliferation and Survival and Inhibits the Differentiation of Oligodendrocyte Progenitors. Neuron 1996, 17, 229–243. [Google Scholar] [CrossRef]
- Ortega, M.C.; Bribian, A.; Peregrin, S.; Gil, M.T.; Marin, O.; de Castro, F. Neuregulin-1/ErbB4 signaling controls the migration of oligodendrocyte precursor cells during development. Exp. Neurol. 2012, 235, 610–620. [Google Scholar] [CrossRef]
- Kataria, H.; Alizadeh, A.; Shahriary, G.M.; Saboktakin Rizi, S.; Henrie, R.; Santhosh, K.T.; Thliveris, J.A.; Karimi-Abdolrezaee, S. Neuregulin-1 promotes remyelination and fosters a pro-regenerative inflammatory response in focal demyelinating lesions of the spinal cord. Glia 2018, 66, 538–561. [Google Scholar] [CrossRef]
- Hu, X.; Hicks, C.W.; He, W.; Wong, P.; Macklin, W.B.; Trapp, B.D.; Yan, R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006, 9, 1520–1525. [Google Scholar] [CrossRef]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef]
- Mei, L.; Xiong, W.-C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Prior, M.; He, W.; Hu, X.; Tang, X.; Shen, W.; Yadav, S.; Kiryu-Seo, S.; Miller, R.; Trapp, B.D.; et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J. Biol. Chem. 2011, 286, 23967–23974. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Nave, K.A. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron 2014, 83, 27–49. [Google Scholar] [CrossRef]
- Wang, Z.; Colognato, H.; Ffrench-Constant, C. Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia 2007, 55, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Kataria, H.; Karimi-Abdolrezaee, S. Neuregulin-1: A novel regulator of glial response in spinal cord injury. Neural Regen. Res. 2017, 12, 1616–1617. [Google Scholar] [PubMed]
- Goebbels, S.; Oltrogge, J.H.; Kemper, R.; Heilmann, I.; Bormuth, I.; Wolfer, S.; Wichert, S.P.; Mobius, W.; Liu, X.; Lappe-Siefke, C.; et al. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8953–8964. [Google Scholar] [CrossRef]
- Nave, K.A. Myelination and support of axonal integrity by glia. Nature 2010, 468, 244–252. [Google Scholar] [CrossRef]
- Ding, Z.; Dai, C.; Zhong, L.; Liu, R.; Gao, W.; Zhang, H.; Yin, Z. Neuregulin-1 converts reactive astrocytes toward oligodendrocyte lineage cells via upregulating the PI3K-AKT-mTOR pathway to repair spinal cord injury. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 134, 111168. [Google Scholar] [CrossRef]
- Hu, X.; Schlanger, R.; He, W.; Macklin, W.B.; Yan, R. Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1868–1873. [Google Scholar] [CrossRef]
- Wolpowitz, D.; Mason, T.B.A.; Dietrich, P.; Mendelsohn, M.; Talmage, D.A.; Role, L.W. Cysteine-Rich Domain Isoforms of the Neuregulin-1 Gene Are Required for Maintenance of Peripheral Synapses. Neuron 2000, 25, 79–91. [Google Scholar] [CrossRef]
- Adlkofer, K.; Lai, C. Role of Neuregulins in Glial Cell Development. Glia 2000, 29, 104–111. [Google Scholar] [CrossRef]
- Taveggia, C.; Thaker, P.; Petrylak, A.; Caporaso, G.L.; Toews, A.; Falls, D.L.; Einheber, S.; Salzer, J.L. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 2008, 56, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, B.G.; Agarwal, A.; Sereda, M.W.; Garratt, A.N.; Muller, T.; Wende, H.; Stassart, R.M.; Nawaz, S.; Humml, C.; Velanac, V.; et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 2008, 59, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Modol-Caballero, G.; Herrando-Grabulosa, M.; Verdes, S.; Garcia-Lareu, B.; Hernandez, N.; Francos-Quijorna, I.; Lopez-Vales, R.; Bosch, A.; Navarro, X. Gene Therapy Overexpressing Neuregulin 1 Type I in Combination With Neuregulin 1 Type III Promotes Functional Improvement in the SOD1(G93A) ALS Mice. Front. Neurol. 2021, 12, 693309. [Google Scholar] [CrossRef]
- Xiao, J.; Wong, A.W.; Willingham, M.M.; van den Buuse, M.; Kilpatrick, T.J.; Murray, S.S. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 2010, 18, 186–202. [Google Scholar] [CrossRef]
- Sy, M.; Brandt, A.U.; Lee, S.-U.; Newton, B.L.; Pawling, J.; Golzar, A.; Rahman, A.M.A.; Yu, Z.; Cooper, G.; Scheel, M.; et al. N-acetylglucosamine drives myelination by triggering oligodendrocyte precursor cell differentiation. J. Biol. Chem. 2020, 295, 17413–17424. [Google Scholar] [CrossRef]
- Sock, E.; Wegner, M. Using the lineage determinants Olig2 and Sox10 to explore transcriptional regulation of oligodendrocyte development. Dev. Neurobiol. 2021, 81, 892–901. [Google Scholar] [CrossRef]
- Kageyama, R.; Shimojo, H.; Ohtsuka, T. Dynamic control of neural stem cells by bHLH factors. Neurosci. Res. 2019, 138, 12–18. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Y.; Cai, W.H.; Hurlock, E.C.; Wu, H.; Kernie, S.G.; Parada, L.F.; Lu, Q.R. A crucial role for Olig2 in white matter astrocyte development. Development 2007, 134, 1887–1899. [Google Scholar] [CrossRef]
- Ju, J.; Liu, Q.; Zhang, Y.; Liu, Y.; Jiang, M.; Zhang, L.; He, X.; Peng, C.; Zheng, T.; Lu, Q.R.; et al. Olig2 regulates Purkinje cell generation in the early developing mouse cerebellum. Sci. Rep. 2016, 6, 30711. [Google Scholar] [CrossRef]
- Li, H.; Richardson, W.D. Evolution of the CNS myelin gene regulatory program. Brain Res. 2016, 1641, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Xian, K.; Hurlock, E.; Xin, M.; Kernie, S.G.; Parada, L.F.; Lu, Q.R. A critical role for dorsal progenitors in cortical myelination. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zuo, H.; Maher, B.J.; Serwanski, D.R.; LoTurco, J.J.; Lu, Q.R.; Nishiyama, A. Olig2-dependent developmental fate switch of NG2 cells. Development 2012, 139, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common Developmental Requirement for Olig Function Indicates a Motor Neuron/Oligodendrocyte Connection. Cell 2002, 109, 75–86. [Google Scholar] [CrossRef]
- Takebayashi, H.; Nabeshima, Y.; Yoshida, S.; Chisaka, O.; Ikenaka, K.; Nabeshima, Y. The Basic Helix-Loop-Helix Factor Olig2 Is Essential for the Development of Motoneuron and Oligodendrocyte Lineages. Curr. Biol. 2002, 12, 1157–1163. [Google Scholar] [CrossRef]
- Zhou, Q.; Anderson, D.J. The bHLH Transcription Factors OLIG2 and OLIG1 Couple Neuronal and Glial Subtype Specification. Cell 2002, 109, 61–73. [Google Scholar] [CrossRef]
- Ligon, K.L.; Kesari, S.; Kitada, M.; Sun, T.; Arnett, H.A.; Alberta, J.A.; Anderson, D.J.; Stiles, C.D.; Rowitch, D.H. Development of NG2 neural progenitor cells requires Olig gene function. Proc. Natl. Acad. Sci. USA 2006, 103, 7853–7858. [Google Scholar] [CrossRef]
- Mei, F.; Wang, H.; Liu, S.; Niu, J.; Wang, L.; He, Y.; Etxeberria, A.; Chan, J.R.; Xiao, L. Stage-specific deletion of Olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 8454–8462. [Google Scholar] [CrossRef]
- Li, C.; Xie, Z.; Xing, Z.; Zhu, H.; Zhou, W.; Xie, S.; Zhang, Z.; Li, M.H. The Notch Signaling Pathway Regulates Differentiation of NG2 Cells into Oligodendrocytes in Demyelinating Diseases. Cell. Mol. Neurobiol. 2021. [CrossRef]
- Tapson, V.F.; Torres, F.; Kermeen, F.; Keogh, A.M.; Allen, R.P.; Frantz, R.P.; Badesch, D.B.; Frost, A.E.; Shapiro, S.M.; Laliberte, K.; et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): A randomized controlled trial. Chest 2012, 142, 1383–1390. [Google Scholar] [CrossRef]
- Munoz-Esquivel, J.; Gottle, P.; Aguirre-Cruz, L.; Flores-Rivera, J.; Corona, T.; Reyes-Teran, G.; Kury, P.; Torres, K.J. Sildenafil Inhibits Myelin Expression and Myelination of Oligodendroglial Precursor Cells. ASN Neuro 2019, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Follis, R.M.; Carter, B.D. Myelin Avoids the JAM. Neuron 2016, 91, 713–716. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diez-Revuelta, N.; Higuero, A.M.; Velasco, S.; Penas-de-la-Iglesia, M.; Gabius, H.J.; Abad-Rodriguez, J. Neurons define non-myelinated axon segments by the regulation of galectin-4-containing axon membrane domains. Sci. Rep. 2017, 7, 12246. [Google Scholar] [CrossRef] [PubMed]
- Schottlaender, L.V.; Abeti, R.; Jaunmuktane, Z.; Macmillan, C.; Chelban, V.; O’Callaghan, B.; McKinley, J.; Maroofian, R.; Efthymiou, S.; Athanasiou-Fragkouli, A.; et al. Bi-allelic JAM2 Variants Lead to Early-Onset Recessive Primary Familial Brain Calcification. Am. J. Hum. Genet. 2020, 106, 412–421. [Google Scholar] [CrossRef]
- Marinho, W.; de Oliveira, J.R.M. JAM2: A New Culprit at the Pathophysiology of Primary Familial Brain Calcification. J. Mol. Neurosci. MN 2021, 71, 1723–1724. [Google Scholar] [CrossRef]
- Jaggi, M.; Du, C.; Zhang, W.; Balaji, K.C. Protein kinase D1: A protein of emerging translational interest. Front. Biosci. 2007, 12, 3757–3767. [Google Scholar] [CrossRef]
- Youssef, I.; Ricort, J.M. Deciphering the Role of Protein Kinase D1 (PKD1) in Cellular Proliferation. Mol. Cancer Res. MCR 2019, 17, 1961–1974. [Google Scholar] [CrossRef]
- Omer, S.; Jin, S.C.; Koumangoye, R.; Robert, S.M.; Duran, D.; Nelson-Williams, C.; Huttner, A.; DiLuna, M.; Kahle, K.T.; Delpire, E. Protein kinase D1 variant associated with human epilepsy and peripheral nerve hypermyelination. Clin. Genet. 2021, 100, 176–186. [Google Scholar] [CrossRef]
- Cen, C.; Luo, L.D.; Li, W.Q.; Li, G.; Tian, N.X.; Zheng, G.; Yin, D.M.; Zou, Y.; Wang, Y. PKD1 Promotes Functional Synapse Formation Coordinated with N-Cadherin in Hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 183–199. [Google Scholar] [CrossRef]
- Fleming, C.E.; Saraiva, M.J.; Sousa, M.M. Transthyretin enhances nerve regeneration. J. Neurochem. 2007, 103, 831–839. [Google Scholar] [CrossRef]
- Finsterer, J.; Wanschitz, J.; Quasthoff, S.; Iglseder, S.; Loscher, W.; Grisold, W. Causally treatable, hereditary neuropathies in Fabry’s disease, transthyretin-related familial amyloidosis, and Pompe’s disease. Acta Neurol. Scand. 2017, 136, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, B.; Pagnin, M.; Lee, J.Y.; Petratos, S.; Richardson, S.J. The Role of Transthyretin in Oligodendrocyte Development. Sci. Rep. 2020, 10, 4189. [Google Scholar] [CrossRef] [PubMed]
- Pagnin, M.; Dekiwadia, C.; Petratos, S.; Richardson, S.J. Enhanced re-myelination in transthyretin null mice following cuprizone mediated demyelination. Neurosci. Lett. 2022, 766, 136287. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhu, Z.; Wang, Y.; Zeng, L.; Wang, T.; Luo, J.; Zou, T.B.; Li, R.; Sun, X.; Zhou, G.; et al. LINGO-1 shRNA Loaded by Pluronic F-127 Promotes Functional Recovery After Ventral Root Avulsion. Tissue Engineerin. Part A 2019, 25, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.E.H.; Dief, A.E.; El Azhary, N.M.; Abdelmonsif, D.A.; El-Fetiany, O.S. LINGO-1 siRNA nanoparticles promote central remyelination in ethidium bromide-induced demyelination in rats. J. Physiol. Biochem. 2019, 75, 89–99. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Z.; Zheng, S.; Chen, L.; Wan, Y.; Deng, Y.; Yang, R. Lingo-1 shRNA and Notch signaling inhibitor DAPT promote differentiation of neural stem/progenitor cells into neurons. Brain Res. 2016, 1634, 34–44. [Google Scholar] [CrossRef]
- Mi, S.; Hu, B.; Hahm, K.; Luo, Y.; Kam Hui, E.S.; Yuan, Q.; Wong, W.M.; Wang, L.; Su, H.; Chu, T.H.; et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 2007, 13, 1228–1233. [Google Scholar] [CrossRef]
- Sun, J.J.; Ren, Q.G.; Xu, L.; Zhang, Z.J. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in experimental autoimmune encephalomyelitis mice. Sci. Rep. 2015, 5, 14235. [Google Scholar] [CrossRef]
- Shao, Z.; Lee, X.; Huang, G.; Sheng, G.; Henderson, C.E.; Louvard, D.; Sohn, J.; Pepinsky, B.; Mi, S. LINGO-1 Regulates Oligodendrocyte Differentiation through the Cytoplasmic Gelsolin Signaling Pathway. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 3127–3137. [Google Scholar] [CrossRef]
- Hanf, K.J.M.; Arndt, J.W.; Liu, Y.; Gong, B.J.; Rushe, M.; Sopko, R.; Massol, R.; Smith, B.; Gao, Y.; Dalkilic-Liddle, I.; et al. Functional activity of anti-LINGO-1 antibody opicinumab requires target engagement at a secondary binding site. mAbs 2020, 12, 1713648. [Google Scholar] [CrossRef]
- Yang, C.; Tang, J.; Liang, X.; Qi, Y.; Luo, Y.; Xie, Y.; Wang, J.; Jiang, L.; Zhou, C.; Huang, C.; et al. Anti-LINGO-1 antibody treatment improves chronic stress-induced spatial memory impairments and oligodendrocyte loss in the hippocampus. Behav. Brain Res. 2020, 393, 112765. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Li, M.; Wu, W.T.; Yick, L.W.; Lee, X.; Shao, Z.; Wang, J.; So, K.F.; McCoy, J.M.; Pepinsky, R.B.; et al. LINGO-1 antagonist promotes functional recovery and axonal sprouting after spinal cord injury. Mol. Cell. Neurosci. 2006, 33, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Jepson, S.; Vought, B.; Gross, C.H.; Gan, L.; Austen, D.; Frantz, J.D.; Zwahlen, J.; Lowe, D.; Markland, W.; Krauss, R. LINGO-1, a transmembrane signaling protein, inhibits oligodendrocyte differentiation and myelination through intercellular self-interactions. J. Biol. Chem. 2012, 287, 22184–22195. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Pepinsky, R.B.; Cadavid, D. Blocking LINGO-1 as a therapy to promote CNS repair: From concept to the clinic. CNS Drugs 2013, 27, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Katanov, C.; Novak, N.; Vainshtein, A.; Golani, O.; Dupree, J.L.; Peles, E. N-Wasp Regulates Oligodendrocyte Myelination. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 6103–6111. [Google Scholar] [CrossRef]
- Novak, N.; Bar, V.; Sabanay, H.; Frechter, S.; Jaegle, M.; Snapper, S.B.; Meijer, D.; Peles, E. N-WASP is required for membrane wrapping and myelination by Schwann cells. J. Cell Biol. 2011, 192, 243–250. [Google Scholar] [CrossRef]
- Jin, F.; Dong, B.; Georgiou, J.; Jiang, Q.; Zhang, J.; Bharioke, A.; Qiu, F.; Lommel, S.; Feltri, M.L.; Wrabetz, L.; et al. N-WASp is required for Schwann cell cytoskeletal dynamics, normal myelin gene expression and peripheral nerve myelination. Development 2011, 138, 1329–1337. [Google Scholar] [CrossRef]
- Bacon, C.; Lakics, V.; Machesky, L.; Rumsby, M. N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination. Glia 2007, 55, 844–858. [Google Scholar] [CrossRef]
- Scaglione, A.; Patzig, J.; Liang, J.; Frawley, R.; Bok, J.; Mela, A.; Yattah, C.; Zhang, J.; Teo, S.X.; Zhou, T.; et al. PRMT5-mediated regulation of developmental myelination. Nat. Commun. 2018, 9, 2840. [Google Scholar] [CrossRef]
- He, L.; Yu, K.; Lu, F.; Wang, J.; Wu, L.N.; Zhao, C.; Li, Q.; Zhou, X.; Liu, H.; Mu, D.; et al. Transcriptional Regulator ZEB2 Is Essential for Bergmann Glia Development. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 1575–1587. [Google Scholar] [CrossRef]
- Quintes, S.; Brinkmann, B.G. Transcriptional inhibition in Schwann cell development and nerve regeneration. Neural Regen. Res. 2017, 12, 1241–1246. [Google Scholar] [PubMed]
- Wu, L.M.; Wang, J.; Conidi, A.; Zhao, C.; Wang, H.; Ford, Z.; Zhang, L.; Zweier, C.; Ayee, B.G.; Maurel, P.; et al. Zeb2 recruits HDAC-NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat. Neurosci. 2016, 19, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, S.V.; Sullivan, A.M.; O’Keeffe, G.W. Zeb2: A multifunctional regulator of nervous system development. Prog. Neurobiol. 2015, 132, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Chen, Y.; Wang, H.; Xu, X.; Yang, B.; He, Q.; Shou, W.; Chen, Y.; Higashi, Y.; van den Berghe, V.; et al. Dual-mode modulation of Smad signaling by Smad-interacting protein Sip1 is required for myelination in the central nervous system. Neuron 2012, 73, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Quintes, S.; Brinkmann, B.G.; Ebert, M.; Frob, F.; Kungl, T.; Arlt, F.A.; Tarabykin, V.; Huylebroeck, D.; Meijer, D.; Suter, U.; et al. Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat. Neurosci. 2016, 19, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Falcao, A.M.; Meijer, M.; Scaglione, A.; Rinwa, P.; Agirre, E.; Liang, J.; Larsen, S.C.; Heskol, A.; Frawley, R.; Klingener, M.; et al. PAD2-Mediated Citrullination Contributes to Efficient Oligodendrocyte Differentiation and Myelination. Cell Rep. 2019, 27, 1090–1102.e1010. [Google Scholar] [CrossRef]
- Dreyton, C.J.; Knuckley, B.; Jones, J.E.; Lewallen, D.M.; Thompson, P.R. Mechanistic studies of protein arginine deiminase 2: Evidence for a substrate-assisted mechanism. Biochemistry 2014, 53, 4426–4433. [Google Scholar] [CrossRef]
- Liu, Y.; Lightfoot, Y.L.; Seto, N.; Carmona-Rivera, C.; Moore, E.; Goel, R.; O’Neil, L.; Mistry, P.; Hoffmann, V.; Mondal, S.; et al. Peptidylarginine deiminases 2 and 4 modulate innate and adaptive immune responses in TLR-7-dependent lupus. JCI Insight 2018, 3, e124729. [Google Scholar] [CrossRef]
- Musse, A.A.; Li, Z.; Ackerley, C.A.; Bienzle, D.; Lei, H.; Poma, R.; Harauz, G.; Moscarello, M.A.; Mastronardi, F.G. Peptidylarginine deiminase 2 (PAD2) overexpression in transgenic mice leads to myelin loss in the central nervous system. Dis. Models Mech. 2008, 1, 229–240. [Google Scholar] [CrossRef]
- Mastronardi, F.G.; Wood, D.D.; Mei, J.; Raijmakers, R.; Tseveleki, V.; Dosch, H.M.; Probert, L.; Casaccia-Bonnefil, P.; Moscarello, M.A. Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: A role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 11387–11396. [Google Scholar] [CrossRef]
- Beniac, D.R.; Wood, D.D.; Palaniyar, N.; Ottensmeyer, F.P.; Moscarello, M.A.; Harauz, G. Cryoelectron microscopy of protein-lipid complexes of human myelin basic protein charge isomers differing in degree of citrullination. J. Struct. Biol. 2000, 129, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guan, Y.; Feng, X.; Rolfs, A.; Schluter, H.; Luo, J. Proteomics of the corpus callosum to identify novel factors involved in hypomyelinated Niemann-Pick Type C disease mice. Mol. Brain 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Feng, X.; Rolfs, A.; Luo, J. Lovastatin promotes myelin formation in NPC1 mutant oligodendrocytes. J. Neurol. Sci. 2018, 386, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lieberman, A.P. Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin. PLoS Genet. 2013, 9, e1003462. [Google Scholar] [CrossRef]



| Factors | Potential Pathway | OPC Proliferation | OL Differentiation | OL Maturation | References |
|---|---|---|---|---|---|
| HDAC | HDAC-TCF7L2 | + | [16,19,22] | ||
| WNT | WNT-β-Catenin-TCF7L2 | + | − | [17,18,19] | |
| GPR17 | GPR17-ID2/ID4; GPR17-OLIG2; GPR17-ERK1/2 | +/− | − | − | [13,42,43,50,51,52,54,55,56] |
| GPR56 | GPR56/Gα12/13-RhoA | + | − | − | [33,34,36,37,38,39] |
| LINGO-1 | LINGO-1-RhoA | − | − | [6,174,175,176,177,178,179,180,181,182,183] | |
| TG2 | TG2-Laminin-GPR56 | + | [40] | ||
| ERK1/2 | MEK-ERK1/2 | + | + | [93,94,95,96,97,98] | |
| GPR37 | GPR37-cAMP-Raf-MAPK-ERK1/2 | − | +/− | [35,100] | |
| OLIG2 | OLIG2-NKX2.2; OLIG2-SOX8/10 | + | + | +/− | [152,158] |
| ZEB2 | OLIG2-ZEB2 | + | [194,195] | ||
| SOX10-MYRF | SOX10-MYRF | + | + | [102,103,107,109,110,111,112,113,114,117,118] | |
| AKT-mTOR | PI3K-AKT-mTOR | + | + | [78,79,80,81,90] | |
| DDIT4 | DDIT4-mTOR | − | [83] | ||
| NRG1 | NRG1/ErbB- PI3K-AKT-mTOR | + | + | +/− | [125,126,127,128,129,130,134,135,136,137,138,142,143] |
| TTR | TTR-pAKT | − | [172,173] | ||
| BDNF | BDNF/TrkB | + | + | [145] | |
| FGF2 | FGF2/FGFR1 | + | − | +/− | [23,24,25,28,29,31] |
| GlcNAc | GlcNAc-PDGF-α | + | + | [146] | |
| JAM2 | \ | − | [77,162] | ||
| NG2 | \ | + | − | [9,10,11] | |
| NOTCH1 | NOTCH1-HES5 | − | − | − | [63,64,65,66,67] |
| NPC1 | \ | + | [202,203,204] | ||
| N-WASP | \ | + | [185,186,187] | ||
| PAD2 | PAD2-TNF-α; PAD2-MBP | − | − | [196,198,199,200,201] | |
| PDE | \ | + | + | [160,161] | |
| PKD1 | \ | − | [168] | ||
| PRMT5 | \ | + | + | [189] | |
| SOX2 | \ | + | +/− | [59,60,61,62] | |
| SHH | SHH-BOC | + | + | [69,70,71,72,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, Y.; Zou, S. A Glance at the Molecules That Regulate Oligodendrocyte Myelination. Curr. Issues Mol. Biol. 2022, 44, 2194-2216. https://doi.org/10.3390/cimb44050149
Wang S, Wang Y, Zou S. A Glance at the Molecules That Regulate Oligodendrocyte Myelination. Current Issues in Molecular Biology. 2022; 44(5):2194-2216. https://doi.org/10.3390/cimb44050149
Chicago/Turabian StyleWang, Shunqi, Yingxing Wang, and Suqi Zou. 2022. "A Glance at the Molecules That Regulate Oligodendrocyte Myelination" Current Issues in Molecular Biology 44, no. 5: 2194-2216. https://doi.org/10.3390/cimb44050149
APA StyleWang, S., Wang, Y., & Zou, S. (2022). A Glance at the Molecules That Regulate Oligodendrocyte Myelination. Current Issues in Molecular Biology, 44(5), 2194-2216. https://doi.org/10.3390/cimb44050149







