Off the Beaten Path in Oncology: Active Brown Adipose Tissue by Virtue of Molecular Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 18F-FDG PET/CT Scanning Protocol
2.3. Image Processing and Interpretation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Santhanam, P.; Solnes, L.; Hannukainen, J.C.; Taïeb, D. Adiposity-related cancer and functional imaging of brown adipose tissue. Endocr. Pract. 2015, 21, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Huang, S.; Fletcher, L.A.; O’Mara, A.E.; Tal, I.; Brychta, R.J.; Cypess, A.M.; Chen, K.Y.; Leitner, B.P. Whole Body and Regional Quantification of Active Human Brown Adipose Tissue Using 18F-FDG PET/CT. J. Vis. Exp. 2019, 146, e58469. [Google Scholar] [CrossRef]
- Sampath, S.C.; Sampath, S.C.; Bredella, M.A.; Cypess, A.M.; Torriani, M. Imaging of Brown Adipose Tissue: State of the Art. Radiology 2016, 280, 4–19. [Google Scholar] [CrossRef]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, M.; Engles, J.M.; Ishimori, T.; Nicely, O.; Cohade, C.; Wahl, R.L. Intense (18)F-FDG Uptake in Brown Fat Can Be Reduced Pharmacologically. J. Nucl. Med. 2004, 45, 1189–1193. [Google Scholar]
- Celi, F.S. Brown Adipose Tissue--When It Pays to Be Inefficient. N. Engl. J. Med. 2009, 360, 1553–1556. [Google Scholar] [CrossRef]
- Nishio, M.; Saeki, K. The Remaining Mysteries about Brown Adipose Tissues. Cells 2020, 9, 2449. [Google Scholar] [CrossRef]
- Oka, M.; Kobayashi, N.; Matsumura, K.; Nishio, M.; Nakano, K.; Okamura, T.; Okochi, H.; Minamisawa, T.; Shiba, K.; Saeki, K. New Role for Growth/Differentiation Factor 15 in the Survival of Transplanted Brown Adipose Tissues in Cooperation with Interleukin-6. Cells 2020, 9, 1365. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Peijs, L.; Beaudry, J.L.; Jespersen, N.Z.; Nielsen, C.H.; Ma, T.; Brunner, A.D.; Larsen, T.J.; Bayarri-Olmos, R.; Prabhakar, B.S.; et al. Proteomics-Based Comparative Mapping of the Secretomes of Human Brown and White Adipocytes Reveals EPDR1 as a Novel Batokine. Cell Metab. 2019, 30, 963–975.e7. [Google Scholar] [CrossRef]
- Sponton, C.H.; Hosono, T.; Taura, J.; Jedrychowski, M.P.; Yoneshiro, T.; Wang, Q.; Takahashi, M.; Matsui, Y.; Ikeda, K.; Oguri, Y.; et al. The Regulation of Glucose and Lipid Homeostasis via PLTP as a Mediator of BAT-Liver Communication. EMBO Rep. 2020, 21, e49828. [Google Scholar] [CrossRef] [PubMed]
- Brendle, C.; Stefan, N.; Grams, E.; Soekler, M.; la Fougère, C.; Pfannenberg, C. Determinants of Activity of Brown Adipose Tissue in Lymphoma Patients. Sci. Rep. 2020, 10, 21802. [Google Scholar] [CrossRef] [PubMed]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The Different Shades of Fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Carey, A.L.; Kingwell, B.A. Brown Adipose Tissue in Humans: Therapeutic Potential to Combat Obesity. Pharmacol. Ther. 2013, 140, 26–33. [Google Scholar] [CrossRef]
- Boss, O.; Farmer, S.R. Recruitment of Brown Adipose Tissue as a Therapy for Obesity-Associated Diseases. Front. Endocrinol. 2012, 3, 14. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Hu, S.; Scotti, A.; Cai, K.; Wang, J.; Zhou, X.; Yang, D.; Figini, M.; Pan, L.; et al. Non-Invasive Imaging Methods for Brown Adipose Tissue Detection and Function Evaluation. Intern. Med. Open Access 2018, 8, 299. [Google Scholar] [CrossRef]
- Brendle, C.; Stefan, N.; Stef, I.; Ripkens, S.; Soekler, M.; la Fougère, C.; Nikolaou, K.; Pfannenberg, C. Impact of Diverse Chemotherapeutic Agents and External Factors on Activation of Brown Adipose Tissue in a Large Patient Collective. Sci. Rep. 2019, 9, 1901. [Google Scholar] [CrossRef]
- Puar, T.; van Berkel, A.; Gotthardt, M.; Havekes, B.; Hermus, A.R.M.M.; Lenders, J.W.M.; van Marken Lichtenbelt, W.D.; Xu, Y.; Brans, B.; Timmers, H.J.L.M. Genotype-Dependent Brown Adipose Tissue Activation in Patients with Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2016, 101, 224–232. [Google Scholar] [CrossRef]
- Cohade, C.; Osman, M.; Pannu, H.K.; Wahl, R.L. Uptake in Supraclavicular Area Fat (“USA-Fat”): Description on 18F-FDG PET/CT. J. Nucl. Med. 2003, 44, 170–176. [Google Scholar]
- Chen, K.Y.; Cypess, A.M.; Laughlin, M.R.; Haft, C.R.; Hu, H.H.; Bredella, M.A.; Enerbäck, S.; Kinahan, P.E.; Lichtenbelt, W.v.M.; Lin, F.I.; et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metab. 2016, 24, 210–222. [Google Scholar] [CrossRef]
- Upadhye, T.; Gandhi, A.; Basu, S. Evaluation of (18)F-FDG Uptake Pattern in Brown Adipose Tissue Over Extended Time Period as Assessed by Multiple Time Point (18)F-FDG-PET. Nucl. Med. Mol. Imaging 2013, 47, 89–97. [Google Scholar] [CrossRef]
- Basu, S.; Alavi, A. Optimizing Interventions for Preventing Uptake in the Brown Adipose Tissue in FDG-PET. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1421–1423. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected Evidence for Active Brown Adipose Tissue in Adult Humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Tapia, P.; Fernández-Galilea, M.; Robledo, F.; Mardones, P.; Galgani, J.E.; Cortés, V.A. Biology and Pathological Implications of Brown Adipose Tissue: Promises and Caveats for the Control of Obesity and Its Associated Complications. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1145–1164. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.-J.; Enerbäck, S.; et al. Functional Brown Adipose Tissue in Healthy Adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Ma, J.; Huang, M.; Wang, L.; Ye, W.; Tong, Y.; Wang, H. Obesity and Risk of Thyroid Cancer: Evidence from a Meta-Analysis of 21 Observational Studies. Med. Sci. Monit. 2015, 21, 283–291. [Google Scholar] [CrossRef]
- Beijer, E.; Schoenmakers, J.; Vijgen, G.; Kessels, F.; Dingemans, A.-M.; Schrauwen, P.; Wouters, M.; van Marken Lichtenbelt, W.; Teule, J.; Brans, B. A Role of Active Brown Adipose Tissue in Cancer Cachexia? Oncol. Rev. 2012, 6, e11. [Google Scholar] [CrossRef]
- Dong, M.; Lin, J.; Lim, W.; Jin, W.; Lee, H.J. Role of Brown Adipose Tissue in Metabolic Syndrome, Aging, and Cancer Cachexia. Front. Med. 2018, 12, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Verras, G.-I.; Tchabashvili, L.; Chlorogiannis, D.-D.; Mulita, F.; Argentou, M.-I. Updated Clinical Evidence on the Role of Adipokines and Breast Cancer: A Review. Cancers 2023, 15, 1572. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.A.; Gill, C.M.; Martinez-Salazar, E.L.; Torriani, M.; Bredella, M.A. Preliminary Investigation of Brown Adipose Tissue Assessed by PET/CT and Cancer Activity. Skelet. Radiol. 2019, 48, 413–419. [Google Scholar] [CrossRef]
- Chu, K.; Bos, S.A.; Gill, C.M.; Torriani, M.; Bredella, M.A. Brown Adipose Tissue and Cancer Progression. Skeletal Radiol. 2020, 49, 635–639. [Google Scholar] [CrossRef]
- Gilsanz, V.; Hu, H.H.; Smith, M.L.; Goodarzian, F.; Carcich, S.L.; Warburton, N.M.; Malogolowkin, M. The Depiction of Brown Adipose Tissue Is Related to Disease Status in Pediatric Patients with Lymphoma. AJR Am. J. Roentgenol. 2012, 198, 909–913. [Google Scholar] [CrossRef]
- Steinberg, J.D.; Vogel, W.; Vegt, E. Factors Influencing Brown Fat Activation in FDG PET/CT: A Retrospective Analysis of 15,000+ Cases. Br. J. Radiol. 2017, 90, 20170093. [Google Scholar] [CrossRef]
- Ginzac, A.; Barres, B.; Chanchou, M.; Gadéa, E.; Molnar, I.; Merlin, C.; Coudert, B.; Thivat, E.; Durando, X. A Decrease in Brown Adipose Tissue Activity Is Associated with Weight Gain during Chemotherapy in Early Breast Cancer Patients. BMC Cancer 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Gnaneswaran, S.; Tandy, B.; Fulham, M.J. Brown Fat FDG Uptake Abolished by Radiotherapy. Clin. Nucl. Med. 2015, 40, 437–438. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-Fat-Mediated Tumour Suppression by Cold-Altered Global Metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef]
- Bauwens, M.; Wierts, R.; van Royen, B.; Bucerius, J.; Backes, W.; Mottaghy, F.; Brans, B. Molecular Imaging of Brown Adipose Tissue in Health and Disease. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 776–791. [Google Scholar] [CrossRef]
- Jalloul, W.; Moscalu, M.; Grierosu, I.; Ionescu, T.; Stolniceanu, C.R.; Gutu, M.; Ghizdovat, V.; Mocanu, V.; Azoicai, D.; Iliescu, R.; et al. Brown Adipose Tissue Biodistribution and Correlations Particularities in Parathyroid Pathology Personalized Diagnosis. Diagnostics 2022, 12, 3182. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metabolism 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
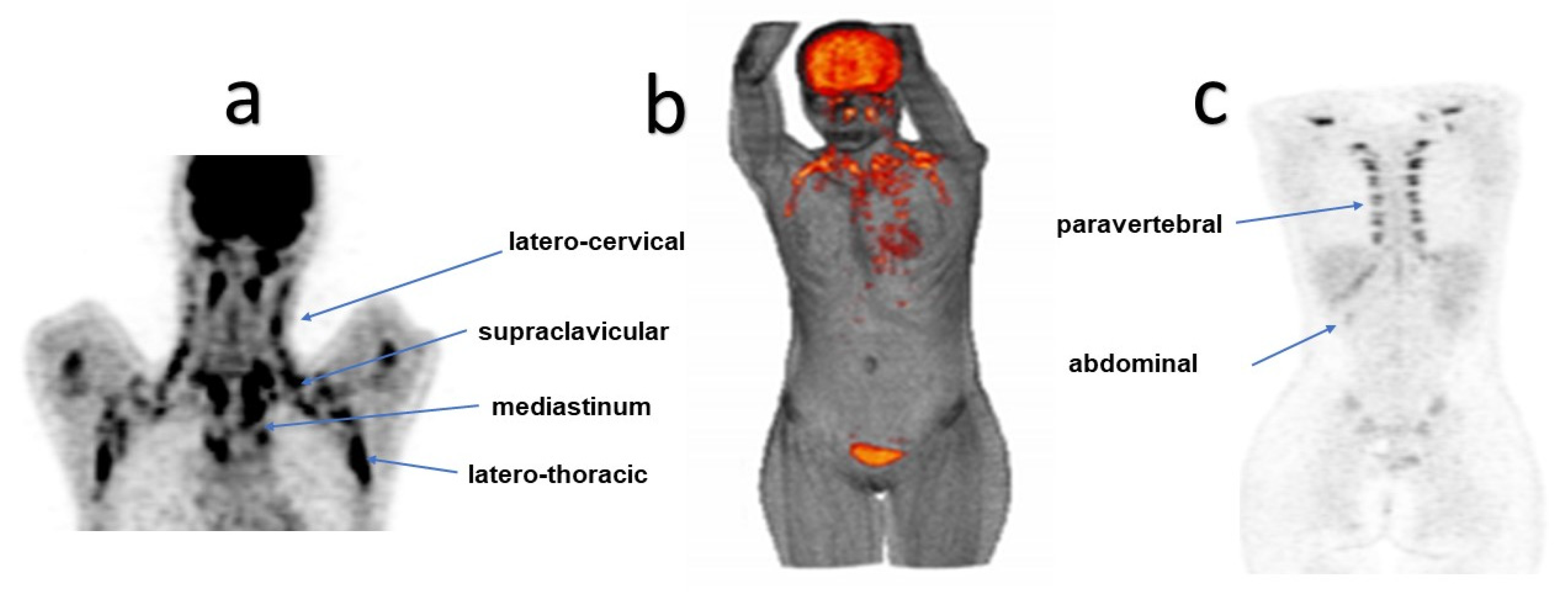














| Clinical Characteristics | Total Patients N = 82 | Nonobese Patients (NO) (BMI ≤ 25 kg/m2) N = 52 | Overweight and Obese (OOB) (BMI > 25 kg/m2) N = 30 | p-Value |
|---|---|---|---|---|
| Age, median (IQR), year Age, mean (SD), year | 33 (24–41) 33.8 (12.5) | 30.5 (23–40) 32.8 (11.8) | 33 (24–41) 35.4 (13.7) | 0.375 * |
| Gender, female/male, n (%) | 48/34 (58.5/41.5) | 30/22 (62.5/64.7) | 18/12 (37.5/35.3) | 0.837 ^ |
| Season, n (%) spring summer autumn winter | 18 (22) 10 (12.2) 30 (36.6) 24 (29.3) | 12 (66.7) 6 (60) 24 (80) 10 (41.7) | 6 (33.3) 4 (40) 6 (20) 14 (58.3) | 0.0335 ^ |
| Body weight, median (IQR), kg Body weight, mean (SD), kg | 70 (62–78) 71.5 (15.82) | 63 (58–70) 63.5 (8.72) | 80 (74–95) 85.3 (15.9) | <0.001 * |
| BMI, median (IQR), kg/m2 BMI, mean (SD), kg/m2 | 24.16 (21.68–27.06) 24.75 (5.02) | 22.05 (20.06–23.67) 21.90 (2.29) | 27.72 (26.06–32.47) 29.69 (4.62) | <0.001 * |
| Blood glucose level, median (IQR), (mg/dL) Blood glucose level, mean (SD), (mg/dL) | 91 (82–95) 90.83 (10.93) | 91 (84–95) 91.58 (10.89) | 91 (79–101) 89.53 (11.07) | 0.418 * |
| Diagnostic, n (%) Cervical Cancer Hodgkin’s Lymphoma Non-Hodgkin’s Lymphoma Breast Cancer Lung Cancer Gastrointestinal cancers | 11 (13.4) 26 (31.7) 12 (14.6) 10 (12.2) 12 (14.6) 11 (13.4) | 8 (72.7) 16 (61.5) 8 (66.7) 7 (70) 7 (58.3) 6 (54.5) | 3 (27.3) 10 (38.5) 4 (33.3) 3 (30) 5 (41.7) 5 (45.5) | 0.952 ^ |
| Treatment, n(%) with surgical treatment chemotherapy chemotherapy + radiotherapy without chemotherapy/radiotherapy without surgical treatment chemotherapy radiotherapy chemotherapy + radiotherapy | 58 (70.7) 40 (48.8) 16 (19.5) 2 (2.4) 24 (29.3) 14 (17.1) 2 (2.4) 8 (9.8) | 32 (55.2) 24 (60) 8 (50) 0 (0) 20 (83.3) 10 (71.4) 2 (100) 8 (100) | 26 (44.8) 16 (40) 8 (50) 2 (100) 4 (16.7) 4 (28.6) 0 (0) 0 (0) | 0.012 ^ |
| Total Patients N = 82 | Nonobese Patients (BMI ≤ 25 kg/m2) N = 52 | Overweight and Obese Patients (BMI > 25 kg/m2) N = 30 | p-Value | |
|---|---|---|---|---|
| BAT localisation, n (%) Unique location Multiple locations | 10 (12.2) 72 (87.8) | 8 (15.4) 44 (84.6) | 2 (6.67) 28 (93.3) | 0.245 ^ |
| BAT, n (%) homogeneous non-homogeneous | 36 (43.9) 46 (56.1) | 24 (46.2) 28 (53.9) | 12 (40) 18 (60) | 0.587 ^ |
| BAT, n (%) symmetric asymmetric | 58 (70.7) 24 (29.3) | 38 (73.1) 14 (26.9) | 20 (66.7) 10 (33.3) | 0.541 ^ |
| SUVmax(LBM) latero-cervical (g/mL) median (IQR) mean (SD) | 3.40(2.06–5.53) 3.66 (2.63) | 2.90 (0–4.72) 3.17 (2.59) | 4.29 (3.25–6.39) 4.50 (2.51) | 0.026 * |
| SUVmax(LBM) supraclavicular (g/mL) median (IQR) mean (SD) | 5.14 (3.45–6.77) 5.63 (3.25) | 4.79 (2.84–7.45) 5.20 (3.11) | 5.35 (4.12–6.77) 6.37 (3.40) | 0.115 * |
| SUVmax(LBM) paravertebral (g/mL) median (IQR) mean (SD) | 0 (0–4.90) 2.90 (4.02) | 0 (0–4.16) 2.72 (4.02) | 0 (0–5.79) 3.26 (4.07) | 0.426 * |
| SUVmax(LBM) latero-thoracic (g/mL) median (IQR) mean (SD) | 0 (0–3.21) 1.76 (2.90) | 0 (0–3.40) 2.27 (3.24) | 0 (0–5.11) 0.84 (1.85) | 0.014 * |
| SUVmax(LBM) mediastinal (g/mL) median (IQR) mean (SD) | 0 (0–1.84) 2.06 (4) | 0 (0–5.04) 2.52 (4.40) | 0 (0–5.60) 1.23 (3.05) | 0.164 * |
| SUVmax(LBM) abdominal (g/mL) median (IQR) mean (SD) | 0 (0–0) 0.21 (0.8) | 0 (0–0) 0.32 (0.98) | 0 (0–0) 0 (0) | 0.434 * |
| Multiple Linear Regression | Unstandardized Coefficients | Standardized Coefficients | t | p-Value | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| SUVmax(LBM) latero-cervical (g/mL) | |||||
| (Constant) | 1.532 | 2.226 | 0.688 | 0.493 | |
| Age | −0.061 | 0.024 | −0.288 | −2.518 | 0.014 |
| Gender | 0.636 | 0.582 | 0.120 | 1.092 | 0.278 |
| BMI | 0.149 | 0.059 | 0.285 | 2.544 | 0.013 * |
| Treatment strategy | −0.135 | 0.212 | −0.076 | −0.636 | 0.526 |
| Diagnostic | −0.034 | 0.175 | −0.022 | −0.196 | 0.845 |
| Model verification: ANOVA: F = 3.181, p = 0.012, (*) Marked effects are significant at p < 0.05. | |||||
| SUVmax(LBM) supraclavicular (g/mL) | |||||
| (Constant) | 3.138 | 2.936 | 1.069 | 0.289 | |
| Age | −0.019 | 0.032 | −0.074 | −0.608 | 0.545 |
| Gender | −0.113 | 0.767 | −0.017 | −0.148 | 0.883 |
| BMI | 0.141 | 0.077 | 0.217 | 1.817 | 0.073 |
| Treatment strategy | −0.099 | 0.279 | −0.045 | −0.355 | 0.724 |
| Diagnostic | 0.046 | 0.231 | 0.024 | 0.200 | 0.842 |
| Model verification: ANOVA: F = 0.958, p = 0.449, (*) Marked effects are significant at p < 0.05. | |||||
| SUVmax(LBM) paravertebral (g/mL) | |||||
| (Constant) | 5.297 | 3.249 | 1.630 | 0.107 | |
| Age | −0.100 | 0.037 | −0.292 | −2.675 | 0.009 * |
| Gender | 2.111 | 0.881 | 0.250 | 2.396 | 0.019 * |
| BMI | −0.016 | 0.083 | −0.021 | −0.195 | 0.846 |
| Treatment strategy | −0.510 | 0.317 | −0.186 | −1.609 | 0.111 |
| Diagnostic | −0.065 | 0.267 | −0.025 | −0.242 | 0.809 |
| Model verification: ANOVA: F = 2.410, p = 0.042, (*) Marked effects are significant at p < 0.05. | |||||
| SUVmax(LBM) latero-thoracic (g/mL) | |||||
| (Constant) | 6.417 | 2.217 | 2.895 | 0.005 * | |
| Age | −0.030 | 0.025 | −0.125 | −1.170 | 0.245 |
| Gender | 0.380 | 0.601 | 0.064 | 0.632 | 0.529 |
| BMI | −0.180 | 0.057 | −0.337 | −3.178 | 0.002 * |
| Treatment strategy | 0.090 | 0.216 | 0.047 | 0.418 | 0.677 |
| Diagnostic | −0.049 | 0.182 | −0.027 | −0.268 | 0.789 |
| Model verification: ANOVA: F = 3.286, p = 0.009, (*) Marked effects are significant at p < 0.05. | |||||
| SUVmax(LBM) mediastinal (g/mL) | |||||
| (Constant) | 4.962 | 3.299 | 1.504 | 0.136 | |
| Age | −0.031 | 0.038 | −0.095 | −0.830 | 0.409 |
| Gender | 0.240 | 0.895 | 0.029 | 0.268 | 0.789 |
| BMI | −0.081 | 0.084 | −0.110 | −0.967 | 0.336 |
| Treatment strategy | −0.204 | 0.322 | −0.077 | −0.634 | 0.528 |
| Diagnostic | 0.136 | 0.271 | 0.055 | 0.501 | 0.618 |
| Model verification: ANOVA: F = 0.338, p = 0.888, (*) Marked effects are significant at p < 0.05. | |||||
| SUVmax(LBM) abdominal (g/mL) | |||||
| (Constant) | 1.873 | 0.618 | 3.029 | 0.003 * | |
| Age | −0.015 | 0.007 | −0.221 | −2.057 | 0.043 |
| Gender | 0.103 | 0.168 | 0.063 | 0.615 | 0.540 |
| BMI | −0.042 | 0.016 | −0.287 | −2.690 | 0.009 * |
| Treatment strategy | −0.037 | 0.060 | −0.070 | −0.616 | 0.539 |
| Diagnostic | −0.061 | 0.051 | −0.124 | −1.201 | 0.233 |
| Model verification: ANOVA: F = 2.956, p = 0.016, (*) Marked effects are significant at p < 0.05. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalloul, W.; Moscalu, M.; Moscalu, R.; Jalloul, D.; Grierosu, I.C.; Ionescu, T.; Stolniceanu, C.R.; Ghizdovat, V.; Mocanu, V.; Iliescu, R.; et al. Off the Beaten Path in Oncology: Active Brown Adipose Tissue by Virtue of Molecular Imaging. Curr. Issues Mol. Biol. 2023, 45, 7891-7914. https://doi.org/10.3390/cimb45100499
Jalloul W, Moscalu M, Moscalu R, Jalloul D, Grierosu IC, Ionescu T, Stolniceanu CR, Ghizdovat V, Mocanu V, Iliescu R, et al. Off the Beaten Path in Oncology: Active Brown Adipose Tissue by Virtue of Molecular Imaging. Current Issues in Molecular Biology. 2023; 45(10):7891-7914. https://doi.org/10.3390/cimb45100499
Chicago/Turabian StyleJalloul, Wael, Mihaela Moscalu, Roxana Moscalu, Despina Jalloul, Irena Cristina Grierosu, Teodor Ionescu, Cati Raluca Stolniceanu, Vlad Ghizdovat, Veronica Mocanu, Radu Iliescu, and et al. 2023. "Off the Beaten Path in Oncology: Active Brown Adipose Tissue by Virtue of Molecular Imaging" Current Issues in Molecular Biology 45, no. 10: 7891-7914. https://doi.org/10.3390/cimb45100499
APA StyleJalloul, W., Moscalu, M., Moscalu, R., Jalloul, D., Grierosu, I. C., Ionescu, T., Stolniceanu, C. R., Ghizdovat, V., Mocanu, V., Iliescu, R., Pavaleanu, I., & Stefanescu, C. (2023). Off the Beaten Path in Oncology: Active Brown Adipose Tissue by Virtue of Molecular Imaging. Current Issues in Molecular Biology, 45(10), 7891-7914. https://doi.org/10.3390/cimb45100499






