Comparison of Retinal Metabolic Activity and Structural Development between rd10 Mice and Normal Mice Using Multiphoton Fluorescence Lifetime Imaging Microscopy
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vis. Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hawes, N.L.; Pardue, M.T.; German, A.M.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Rengarajan, K.; Boyd, A.P.; Sidney, S.S.; et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vis. Res. 2007, 47, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Gargini, C.; Terzibasi, E.; Mazzoni, F.; Strettoi, E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: A morphological and ERG study. J. Comp. Neurol. 2007, 500, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Samardzija, M.; Wariwoda, H.; Imsand, C.; Huber, P.; Heynen, S.R.; Gubler, A.; Grimm, C. Activation of survival pathways in the degenerating retina of rd10 mice. Exp. Eye Res. 2012, 99, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.R.; Osawa, S.; Xiong, Y.; Dhungana, S.; Carlson, J.; McRitchie, S.; Fennell, T.R. Broad spectrum metabolomics for detection of abnormal metabolic pathways in a mouse model for retinitis pigmentosa. Exp. Eye Res. 2019, 184, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef]
- Centonze, V.E.; White, J.G. Multiphoton Excitation Provides Optical Sections from Deeper within Scattering Specimens than Confocal Imaging. Biophys. J. 1998, 75, 2015–2024. [Google Scholar] [CrossRef]
- Chance, B.; Baltscheffsky, H. Respiratory Enzymes in Oxidative Phosphorylation. J. Biol. Chem. 1958, 233, 736–739. [Google Scholar] [CrossRef]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef]
- Browne, A.W.; Arnesano, C.; Harutyunyan, N.; Khuu, T.; Martinez, J.C.; Pollack, H.A.; Koos, D.S.; Lee, T.C.; Fraser, S.E.; Moats, R.A.; et al. Structural and Functional Characterization of Human Stem-Cell-Derived Retinal Organoids by Live Imaging. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3311–3318. [Google Scholar]
- Sameni, S.; Syed, A.; Marsh, J.L.; Digman, M.A. The phasor-FLIM fingerprints reveal shifts from OXPHOS to enhanced glycolysis in Huntington Disease. Sci. Rep. 2016, 6, 34755. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y. Two-Photon Microscopy (TPM) and Fluorescence Lifetime Imaging Microscopy (FLIM) of Retinal Pigment Epithelium (RPE) of Mice In Vivo. In Mouse Retinal Phenotyping; Methods in Molecular Biology; Tanimoto, N., Ed.; Springer: New York, NY, USA, 2018; Volume 1753, pp. 73–88. [Google Scholar] [CrossRef]
- Sanchez, T.; Wang, T.; Pedro, M.V.; Zhang, M.; Esencan, E.; Sakkas, D.; Needleman, D.; Seli, E. Metabolic imaging with the use of fluorescence lifetime imaging microscopy (FLIM) accurately detects mitochondrial dysfunction in mouse oocytes. Fertil. Steril. 2018, 110, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Kesavamoorthy, N.; Junge, J.A.; Fraser, S.E.; Ameri, H. Insights into Metabolic Activity and Structure of the Retina through Multiphoton Fluorescence Lifetime Imaging Microscopy in Mice. Cells 2022, 11, 2265. [Google Scholar] [CrossRef]
- Ranjit, S.; Dvornikov, A.; Levi, M.; Furgeson, S.; Gratton, E. Characterizing fibrosis in UUO mice model using multiparametric analysis of phasor distribution from FLIM images. Biomed. Opt. Express 2016, 7, 3519. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.; Gamage, K.C.; Messinger, J.D.; Curcio, C.A.; Hammer, M. Fluorescence lifetimes and spectra of RPE and sub-RPE deposits in histology of control and AMD eyes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef]
- Solberg, Y.; Dysli, C.; Möller, B.; Wolf, S.; Zinkernagel, M.S. Fluorescence lifetimes in patients with hydroxychloroquine retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2165–2172. [Google Scholar] [CrossRef]
- Dysli, C.; Wolf, S.; Berezin, M.Y.; Sauer, L.; Hammer, M.; Zinkernagel, M.S. Fluorescence lifetime imaging opthalmoscopy. Prog. Retin. Eye Res. 2017, 60, 120–143. [Google Scholar] [CrossRef]
- Hayaran, A.; Bijlani, V. Polyacrylamide as an infiltrating and embedding medium for vibratome sectioning of human fetal cerebellum containing DiI-filled axons. J. Neurosci. Methods 1992, 42, 65–68. [Google Scholar] [CrossRef]
- Wang, P.; Hecht, F.; Ossato, G.; Tille, S.; Fraser, S.E.; Junge, J.A. Complex wavelet filter improves FLIM phasors for photon starved imaging experiments. Biomed. Opt. Express 2021, 12, 3463–3473. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Christie, R.; Nikitin, A.Y.; Hyman, B.T.; Webb, W.W. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 2003, 100, 7075–7080. [Google Scholar] [CrossRef]
- Ranjit, S.; Dvornikov, A.; Stakic, M.; Hong, S.-H.; Levi, M.; Evans, R.M.; Gratton, E. Imaging Fibrosis and Separating Collagens using Second Harmonic Generation and Phasor Approach to Fluorescence Lifetime Imaging. Sci. Rep. 2015, 5, 13378. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lan, X.; Liu, Y.; Shen, Z.; Lu, J.; Ni, X. Characteristics of blood fluorescence spectra using low-level, 457.9-nm excitation from Ar+ laser. Chin. Opt. Lett. 2004, 2, 160–161. [Google Scholar]
- Kooragayala, K.; Gotoh, N.; Li, W.; Nellissery, J.; Kaden, T.R.; Covian-Garcia, R.; Balaban, R.; Cogliati, T.; Swaroop, A. Changes in mitochondria respiration in degenerating mouse retina identified by a novel ex vivo assay. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4667. [Google Scholar]
- Agathocleous, M.; Love, N.K.; Randlett, O.; Harris, J.J.; Liu, J.; Murray, A.J.; Harris, W.A. Metabolic differentiation in the embryonic retina. Nat. Cell Biol. 2012, 14, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Fiske, B.P.; Vander Heiden, M.G. Seeing the Warburg effect in the developing retina. Nat. Cell Biol. 2012, 14, 790–791. [Google Scholar] [CrossRef] [PubMed]
- Joyal, J.S.; Gantner, M.L.; Smith, L.E.H. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Barhoum, R.; Martínez-Navarrete, G.; Corrochano, S.; Germain, F.; Fernandez-Sanchez, L.; de la Rosa, E.; de la Villa, P.; Cuenca, N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience 2008, 155, 698–713. [Google Scholar] [CrossRef]
- Chacko, J.V.; Eliceiri, K.W. NAD(P)H fluorescence lifetime measurements in fixed biological tissues. Methods Appl. Fluoresc. 2019, 7, 044005. [Google Scholar] [CrossRef]
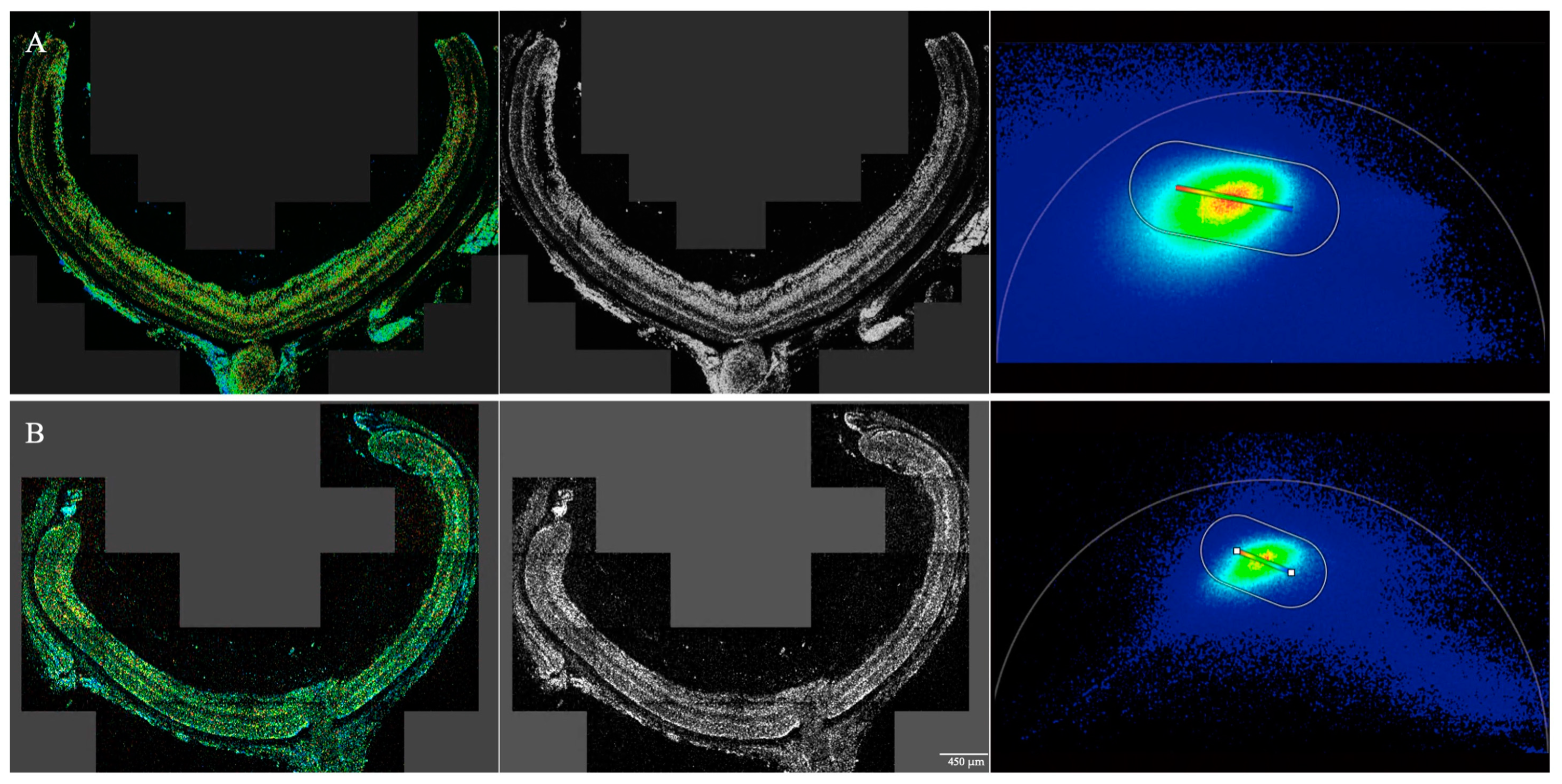

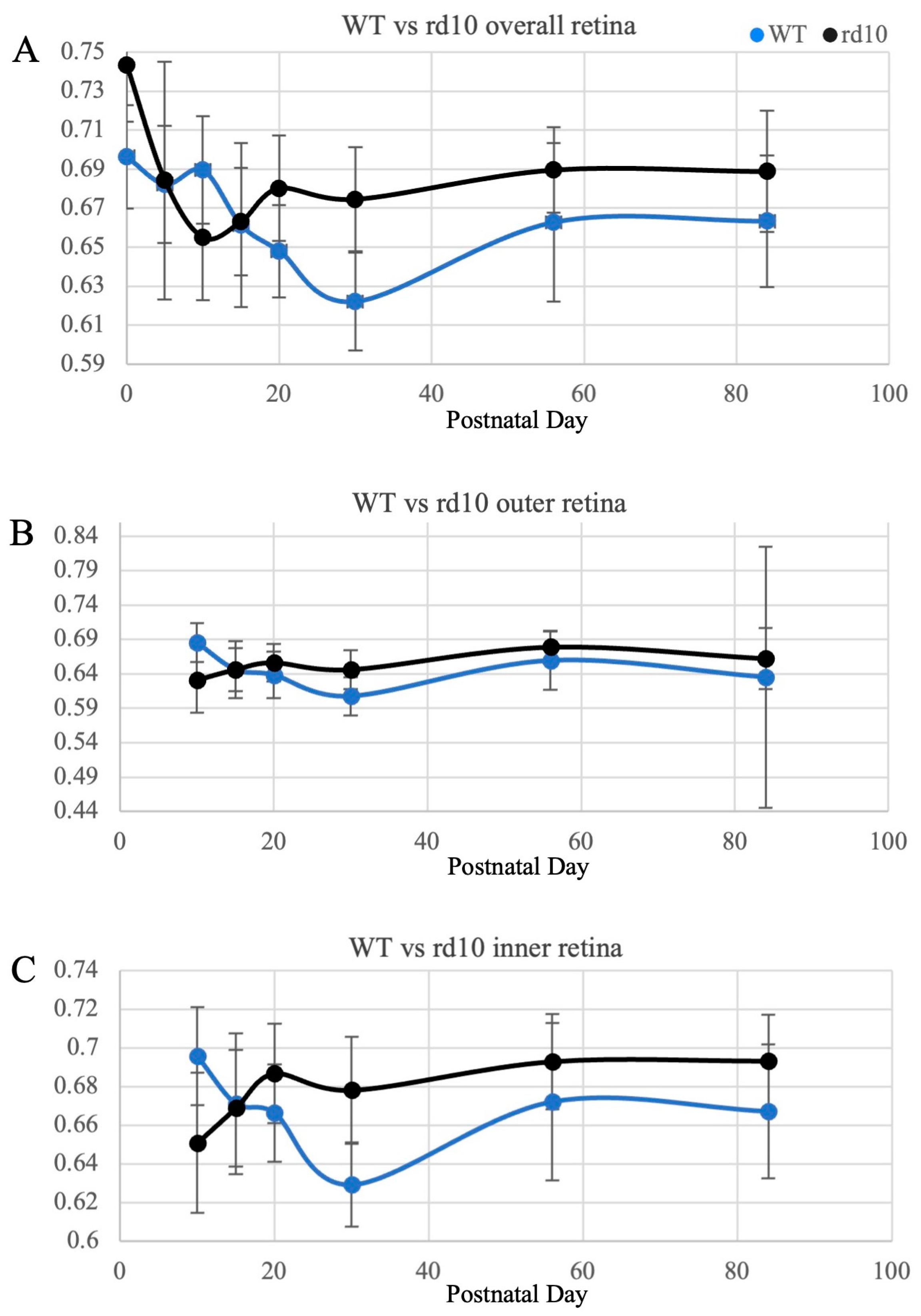

| Retinal Region | Postnatal Day | WT | rd10 | p-Value |
|---|---|---|---|---|
| Overall retina | 0 | 69.6 | 74.3 | <0.0001 |
| 5 | 68.2 | 68.4 | 0.8417 | |
| 10 | 69.0 | 65.5 | 0.0001 | |
| 15 | 66.1 | 66.3 | 0.6294 | |
| 20 | 64.8 | 68.0 | <0.0001 | |
| 30 | 62.2 | 67.5 | <0.0001 | |
| 56 | 66.3 | 69.0 | 0.0002 | |
| 84 | 66.3 | 68.9 | <0.0001 | |
| Outer retina | 10 | 68.5 | 63.1 | <0.0001 |
| 15 | 64.6 | 64.7 | 0.5458 | |
| 20 | 63.8 | 65.6 | 0.0032 | |
| 30 | 60.7 | 64.6 | 0.0006 | |
| 56 | 65.9 | 67.9 | 0.0214 | |
| 84 | 63.5 | 66.2 | 0.3601 | |
| Inner retina | 10 | 69.6 | 65.1 | <0.0001 |
| 15 | 67.1 | 67.0 | 0.9240 | |
| 20 | 66.6 | 68.6 | 0.0002 | |
| 30 | 62.9 | 67.8 | <0.0001 | |
| 56 | 67.2 | 69.3 | 0.0061 | |
| 84 | 66.7 | 69.3 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, E.; Kesavamoorthy, N.; Junge, J.A.; Zheng, M.; Craft, C.M.; Ameri, H. Comparison of Retinal Metabolic Activity and Structural Development between rd10 Mice and Normal Mice Using Multiphoton Fluorescence Lifetime Imaging Microscopy. Curr. Issues Mol. Biol. 2024, 46, 612-620. https://doi.org/10.3390/cimb46010039
Su E, Kesavamoorthy N, Junge JA, Zheng M, Craft CM, Ameri H. Comparison of Retinal Metabolic Activity and Structural Development between rd10 Mice and Normal Mice Using Multiphoton Fluorescence Lifetime Imaging Microscopy. Current Issues in Molecular Biology. 2024; 46(1):612-620. https://doi.org/10.3390/cimb46010039
Chicago/Turabian StyleSu, Erin, Niranjana Kesavamoorthy, Jason A. Junge, Mengmei Zheng, Cheryl Mae Craft, and Hossein Ameri. 2024. "Comparison of Retinal Metabolic Activity and Structural Development between rd10 Mice and Normal Mice Using Multiphoton Fluorescence Lifetime Imaging Microscopy" Current Issues in Molecular Biology 46, no. 1: 612-620. https://doi.org/10.3390/cimb46010039
APA StyleSu, E., Kesavamoorthy, N., Junge, J. A., Zheng, M., Craft, C. M., & Ameri, H. (2024). Comparison of Retinal Metabolic Activity and Structural Development between rd10 Mice and Normal Mice Using Multiphoton Fluorescence Lifetime Imaging Microscopy. Current Issues in Molecular Biology, 46(1), 612-620. https://doi.org/10.3390/cimb46010039






