Investigation of Genetic Factors and Clinical Data in Breast Cancer Highlights the Importance of Breastfeeding and Cancer History
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. DNA Extraction and Quantification
2.3. Genotyping
2.4. Statistical Analysis
3. Results
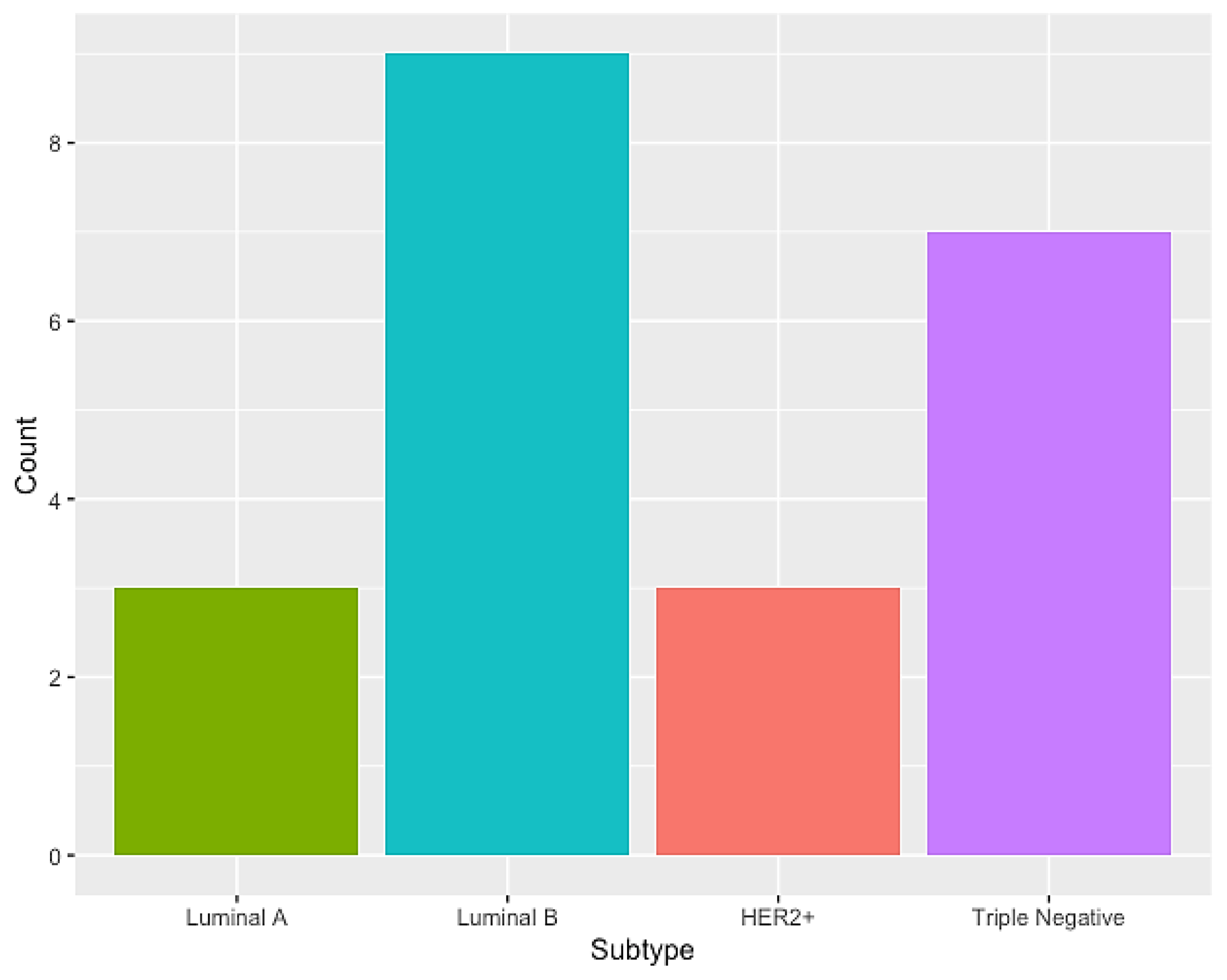
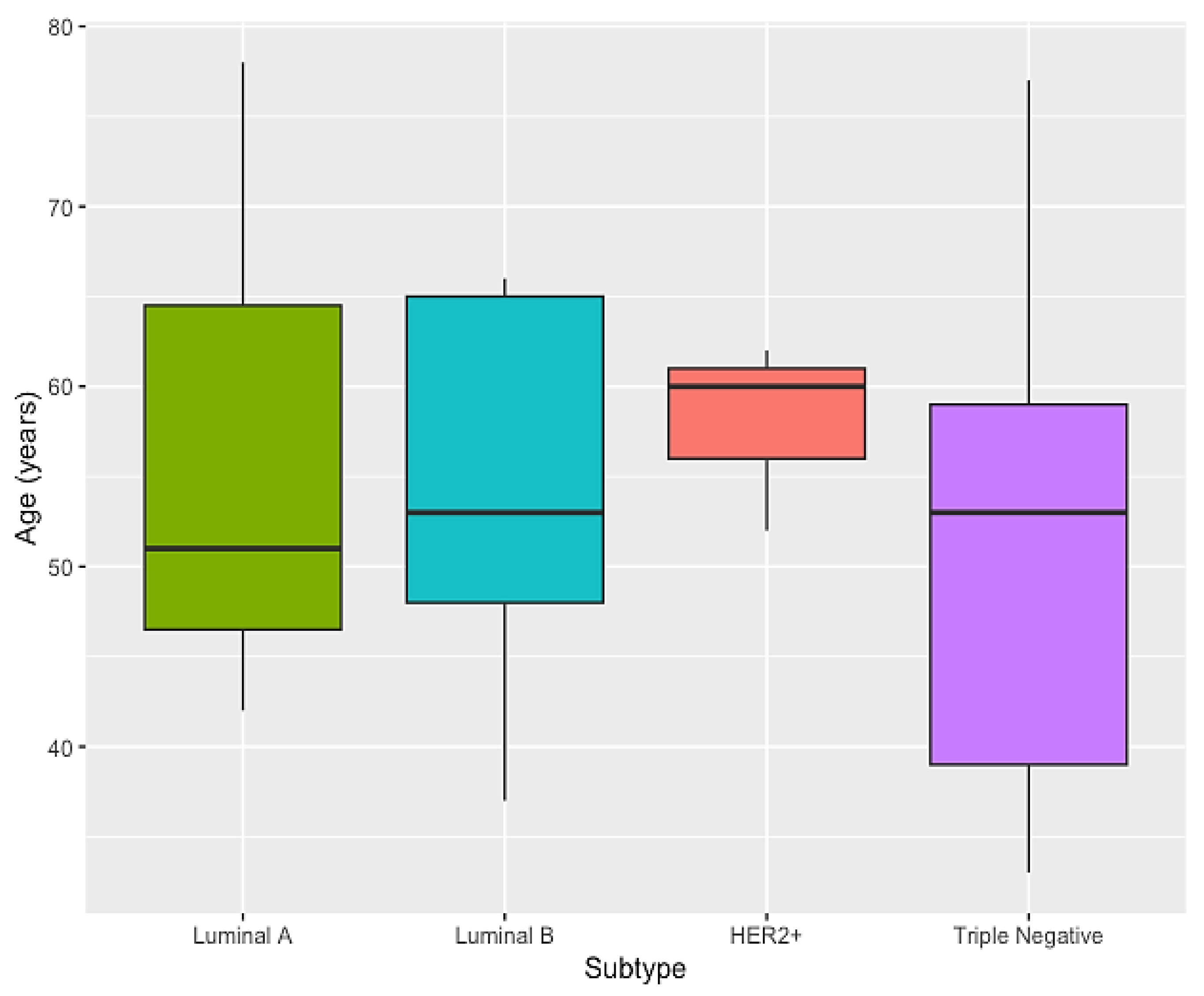
3.1. Characterization of the Sample Population
3.2. Selection of Investigated Polymorphisms
3.3. Analysis of Genomic Ancestry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC—International Agency for Research on Cancer. Available online: https://www.iarc.who.int/ (accessed on 4 March 2023).
- INCA—Instituto Nacional de Câncer. Available online: https://www.inca.gov.br/ (accessed on 4 March 2023).
- Russo, J.; Moral, R.; Balogh, G.A.; Mailo, D.; Russo, I.H. The Protective Role of Pregnancy in Breast Cancer. Breast Cancer Res. BCR 2005, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Beaber, E.F.; Holt, V.L.; Malone, K.E.; Porter, P.L.; Daling, J.R.; Li, C.I. Reproductive Factors, Age at Maximum Height, and Risk of Three Histologic Types of Breast Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 3427–3434. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, V.; Iorio, E.; Miodini, P.; Silvestri, M.; Dugo, M.; Daidone, M.G. Metabolic Footprints and Molecular Subtypes in Breast Cancer. Dis. Markers 2017, 2017, 7687851. [Google Scholar] [CrossRef] [PubMed]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- de Souza Barreto-Neto, N.J.; Pinheiro, A.B.; Oliveira, J.F.; Crusoé, N.S.D.R.; Bertrand, S.A.B.; Machado, M.C.M.; Pinto, R.M.O.; Carvalho-Junior, J.D.; Machado, C.A.C. Perfil epidemiológico dos subtipos moleculares de carcinoma ductal da mama em população de pacientes em Salvador, Bahia. Rev. Bras. Mastol. 2014, 24, 98–102. [Google Scholar] [CrossRef]
- TNM Classification of Malignant Tumours|UICC. Available online: https://www.uicc.org/what-we-do/sharing-knowledge/tnm (accessed on 4 March 2023).
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.-Y.; Lin, L.-T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad Targeting of Resistance to Apoptosis in Cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Goldar, S.; Khaniani, M.S.; Derakhshan, S.M.; Baradaran, B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 2129–2144. [Google Scholar] [CrossRef]
- Sambrook, J.; Rusell, D.W. Molecular Cloning, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Cavalcante, G.C.; de Moraes, M.R.; Valente, C.M.D.; Silva, C.S.; Modesto, A.A.C.; de Assumpção, P.B.; de Assumpção, P.P.; Santos, S.; Ribeiro-Dos-Santos, Â. Investigation of INDEL Variants in Apoptosis: The Relevance to Gastric Cancer. BMC Med. Genet. 2020, 21, 207. [Google Scholar] [CrossRef]
- Resque, R.L.; Freitas, N.D.S.D.C.; Rodrigues, E.M.R.; Guerreiro, J.F.; Santos, N.P.C.D.; Ribeiro dos Santos, A.; Zago, M.A.; Santos, S. Estimates of Interethnic Admixture in the Brazilian Population Using a Panel of 24 X-Linked Insertion/Deletion Markers. Am. J. Hum. Biol. Off. J. Hum. Biol. Counc. 2010, 22, 849–852. [Google Scholar] [CrossRef]
- Andrade, R.B.; Amador, M.A.T.; Cavalcante, G.C.; Leitão, L.P.C.; Fernandes, M.R.; Modesto, A.A.C.; Moreira, F.C.; Khayat, A.S.; Assumpção, P.P.; Ribeiro-Dos-Santos, Â.; et al. Estimating Asian Contribution to the Brazilian Population: A New Application of a Validated Set of 61 Ancestry Informative Markers. G3 2018, 8, 3577–3582. [Google Scholar] [CrossRef] [PubMed]
- Região Norte—Estimativa dos Casos Novos. Available online: https://www.gov.br/inca/pt-br/assuntos/cancer/numeros/estimativa/regiao/norte/norte (accessed on 28 February 2023).
- Brewer, H.R.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. Family History and Risk of Breast Cancer: An Analysis Accounting for Family Structure. Breast Cancer Res. Treat. 2017, 165, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.; Baumgartner, K.B.; Byers, T.; Giuliano, A.R.; Herrick, J.S.; Murtaugh, M.A.; Slattery, M.L. Reproductive History in Relation to Breast Cancer Risk among Hispanic and Non-Hispanic White Women. Cancer Causes Control 2008, 19, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shantakumar, S.; Terry, M.B.; Teitelbaum, S.L.; Britton, J.A.; Millikan, R.C.; Moorman, P.G.; Neugut, A.I.; Gammon, M.D. Reproductive Factors and Breast Cancer Risk among Older Women. Breast Cancer Res. Treat. 2007, 102, 365–374. [Google Scholar] [CrossRef]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between Luminal A and Luminal B Subtypes According to Ki-67, Tumor Size, and Progesterone Receptor Negativity Providing Prognostic Information. Clin. Med. Insights Oncol. 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Frankel, S.R.; Chi, D.-C. Anti-Apoptotic Bcl-2. Cancer Ther. Targets 2017, 833–850. [Google Scholar] [CrossRef]
- Javid, J.; Mir, R.; Mirza, M.; Imtiyaz, A.; Prasant, Y.; Mariyam, Z.; Julka, P.K.; Mohan, A.; Lone, M.; Ray, P.C.; et al. CC Genotype of Anti-Apoptotic Gene BCL-2 (-938 C/A) Is an Independent Prognostic Marker of Unfavorable Clinical Outcome in Patients with Non-Small-Cell Lung Cancer. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2015, 17, 289–295. [Google Scholar] [CrossRef]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Park, W.S.; Nam, S.W.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Somatic Mutations of CASP3 Gene in Human Cancers. Hum. Genet. 2004, 115, 112–115. [Google Scholar] [CrossRef]
- Sargazi, S.; Abghari, A.Z.; Sarani, H.; Sheervalilou, R.; Mirinejad, S.; Saravani, R.; Eskandari, E. Relationship Between CASP9 and CASP10 Gene Polymorphisms and Cancer Susceptibility: Evidence from an Updated Meta-Analysis. Appl. Biochem. Biotechnol. 2021, 193, 4172–4196. [Google Scholar] [CrossRef]
- Cingeetham, A.; Vuree, S.; Dunna, N.R.; Gorre, M.; Nanchari, S.R.; Edathara, P.M.; Mekkaw, P.; Annamaneni, S.; Digumarthi, R.R.; Sinha, S.; et al. Association of Caspase9 Promoter Polymorphisms with the Susceptibility of AML in South Indian Subjects. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 8813–8822. [Google Scholar] [CrossRef]
- Sagne, C.; Marcel, V.; Bota, M.; Martel-Planche, G.; Nobrega, A.; Palmero, E.I.; Perriaud, L.; Boniol, M.; Vagner, S.; Cox, D.G.; et al. Age at Cancer Onset in Germline TP53 Mutation Carriers: Association with Polymorphisms in Predicted G-Quadruplex Structures. Carcinogenesis 2014, 35, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.-K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.; Anderson, M.; Soussi, T. TP53 Mutations in Human Cancer: Database Reassessment and Prospects for the next Decade. Hum. Mutat. 2014, 35, 672–688. [Google Scholar] [CrossRef] [PubMed]
| Clinical Data | % | p-Value | |
|---|---|---|---|
| Age | 30–55 | 54.4 | |
| >55 | 45.4 | ||
| Mean | 54.3 | 0.862 1 | |
| Cancer history | Yes | 22.7 | 0.337 |
| No | 77.3 | ||
| Breastfeeding | Yes | 22.7 | 0.337 |
| No | 77.3 | ||
| Number of births | 1–2 | 54.5 | 0.901 |
| 3–5 | 45.4 | ||
| Age at menarche | 10–13 | 59 | 0.151 |
| 14–17 | 41 | ||
| Menopause | Yes | 50 | 0.193 |
| No | 50 | ||
| Smoking | Yes | 18 | 0.729 |
| No | 82 | ||
| Alcohol Consumption | Yes | 9 | 0.365 |
| No | 91 | ||
| Variables | Sample Number | % | p-Value 1 | |
|---|---|---|---|---|
| Cancer history | Yes | 5 | 22.7 | 0.022 |
| No | 17 | 77.3 | ||
| Breastfeeding | Yes | 5 | 22.7 | 0.022 |
| No | 17 | 77.3 | ||
| Number of births | 1–2 | 12 | 54.5 | 0.128 |
| 3–5 | 10 | 45.4 | ||
| Age at menarche | 10–13 | 13 | 59 | 0.375 |
| 14–17 | 9 | 41 | ||
| Menopause | Yes | 11 | 50 | 0.261 |
| Smoking | Yes | 4 | 18 | 0.736 |
| Alcohol Consumption | Yes | 2 | 9 | 0.843 |
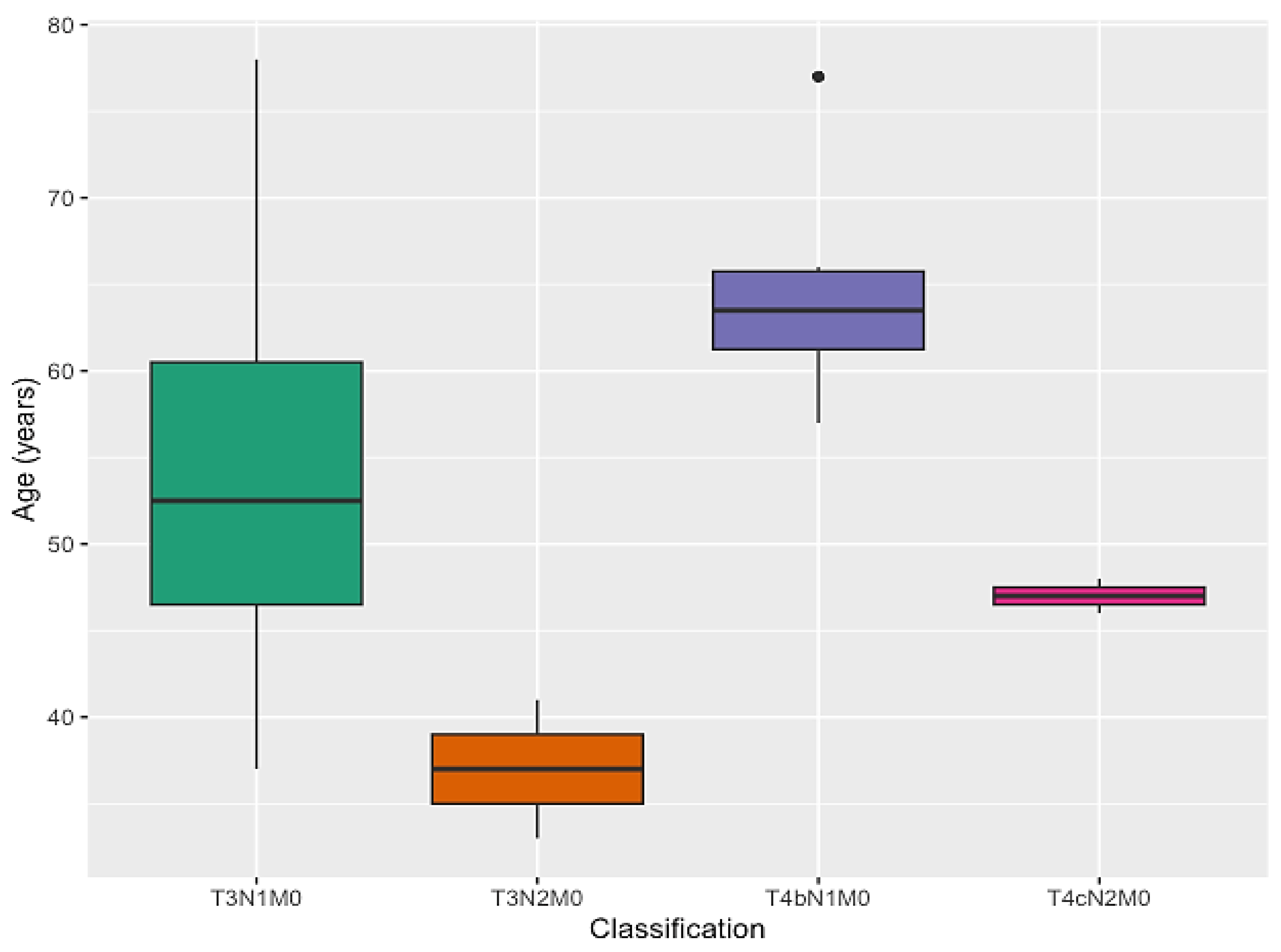
| T3N1M0 | 12 | 54.5 | 0.288 2 | |
| T3N2M0 | 2 | 9 | ||
| T4N1M0 | 6 | 27.5 | ||
| T4N2M0 | 2 | 9 | ||
| Gene | ID | Region | Alleles |
|---|---|---|---|
| BCL2 | rs11269260 | INTRON | TCTATCACCGATCATT/− |
| CASP3 | rs4647655 | INTRON | −/AAATCCTGAA |
| CASP9 | rs4645982 | INTRON | −/TCCCCGCACTGACCTCCACG |
| CASP9 | rs61079693 | INTRON | AAAA/− |
| TP53 | rs17880560 | 3′ UTR | −/GCCGTG |
| Genes | Genotype | Case (68.75%) | Control (31.25%) | p-Value | OR (CI 95%) |
|---|---|---|---|---|---|
| BCL2 | rs11269260 | ||||
| DEL/DEL | 6 (30%) | 4 (40%) | 0.455 | 0.444 (0.053–3.738) | |
| INS/DEL | 12 (60%) | 4 (40%) | 0.305 | 2.250 (0.478–10.595) | |
| INS/INS | 2 (10%) | 2 (20%) | 0.585 | 0.643 (0.132–3.140) | |
| Deletion allele | 0.6 (60%) | 0.6 (60%) | |||
| Insertion allele | 0.4 (40%) | 0.4 (40%) | |||
| CASP3 | rs4647655 | ||||
| DEL/DEL | 10 (53%) | 4 (40%) | 0.433 | 1.875 (0.390–9.013) | |
| INS/DEL | 7 (37%) | 5 (50%) | 0.354 | 0.467 (0.093–2.339) | |
| INS/INS | 2 (10%) | 1 (10%) | 0.965 | 1.059 (0.084–13.329) | |
| Deletion allele | 0.72 (72%) | 0.50 (50%) | |||
| Insertion allele | 0.28 (28%) | 0.50 (50%) | |||
| CASP9 | rs4645982 | ||||
| DEL/DEL | 3 (23%) | 3 (37.5%) | 0.481 | 0.500 (0.073–3.435) | |
| INS/DEL | 6 (46.2%) | 3 (37.5%) | 0.698 | 1.429 (0.236–8.637) | |
| INS/INS | 4 (30.8%) | 2 (25%) | 0.777 | 1.333 (0.183–9.725) | |
| Deletion allele | 0.46 (46%) | 0.625 (62.5%) | |||
| Insertion allele | 0.54 (54%) | 0.375 (37.5%) | |||
| rs61079693 | |||||
| DEL/DEL | 4 (25%) | 1 (16.7%) | 0.926 | 0.909 (0.123–6.715) | |
| INS/DEL | 10 (62.5%) | 3 (50%) | 0.481 | 2 (0.291–13.738) | |
| INS/INS | 2 (12.5%) | 2 (33.3%) | 0.306 | 0.308 (0.032–2.942) | |
| Deletion allele | 0.625 (62.5%) | 0.5 (50%) | |||
| Insertion allele | 0.375 (37.5%) | 0.5 (50%) | |||
| TP53 | rs17880560 | ||||
| DEL/DEL | 9 (90%) | 8 (47.1%) | 0.172 | 0.281 (0.046–1.734) | |
| INS/DEL | 1 (10%) | 8 (47.1%) | 0.073 | 8.000 (0.822–77.814) | |
| INS/INS | 0 | 1 (5.8%) | 0.994 | 3.383 × 10−8 (0.000–∞) | |
| Deletion allele | 0.9 (90%) | 0.53 (53%) | |||
| Insertion allele | 0.1 (10%) | 0.47 (47%) |
| Genomic Ancestry | Case (N = 22) | Control (N = 10) | p-Value |
|---|---|---|---|
| European | 0.643 ± 0.272 | 0.657 ± 0.313 | 0.730 |
| African | 0.211 ± 0.235 | 0.081 ± 0.132 | 0.122 |
| Native American | 0.147 ± 0.115 | 0.262 ± 0.221 | 0.247 |
| Genomic Ancestry | Luminal A (N = 3) | Luminal B (N = 9) | HER2+ (N = 3) | Triple Negative (N = 7) | p-Value | p-Value (EU + AF + NA) |
|---|---|---|---|---|---|---|
| European | 0.350 ± 0.473 | 0.762 ± 0.193 | 0.525 ± 0.120 | 0.665 ± 0.243 | 0.3845 | 0.1668 |
| African | 0.450 ± 0.382 | 0.117 ± 0.150 | 0.267 ± 0.226 | 0.205 ± 0.230 | 0.2408 | |
| Native American | 0.200 ± 0.095 | 0.122 ± 0.088 | 0.209 ± 0.215 | 0.130 ± 0.114 | 0.7590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercês, A.; da-Silva-Cruz, R.; Silva, C.S.; Burbano, R.; Ribeiro-dos-Santos, Â.; Cavalcante, G.C. Investigation of Genetic Factors and Clinical Data in Breast Cancer Highlights the Importance of Breastfeeding and Cancer History. Curr. Issues Mol. Biol. 2023, 45, 7933-7943. https://doi.org/10.3390/cimb45100501
Mercês A, da-Silva-Cruz R, Silva CS, Burbano R, Ribeiro-dos-Santos Â, Cavalcante GC. Investigation of Genetic Factors and Clinical Data in Breast Cancer Highlights the Importance of Breastfeeding and Cancer History. Current Issues in Molecular Biology. 2023; 45(10):7933-7943. https://doi.org/10.3390/cimb45100501
Chicago/Turabian StyleMercês, Amanda, Rebecca da-Silva-Cruz, Caio S. Silva, Rommel Burbano, Ândrea Ribeiro-dos-Santos, and Giovanna C. Cavalcante. 2023. "Investigation of Genetic Factors and Clinical Data in Breast Cancer Highlights the Importance of Breastfeeding and Cancer History" Current Issues in Molecular Biology 45, no. 10: 7933-7943. https://doi.org/10.3390/cimb45100501
APA StyleMercês, A., da-Silva-Cruz, R., Silva, C. S., Burbano, R., Ribeiro-dos-Santos, Â., & Cavalcante, G. C. (2023). Investigation of Genetic Factors and Clinical Data in Breast Cancer Highlights the Importance of Breastfeeding and Cancer History. Current Issues in Molecular Biology, 45(10), 7933-7943. https://doi.org/10.3390/cimb45100501







