The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients
Abstract
:1. Introduction
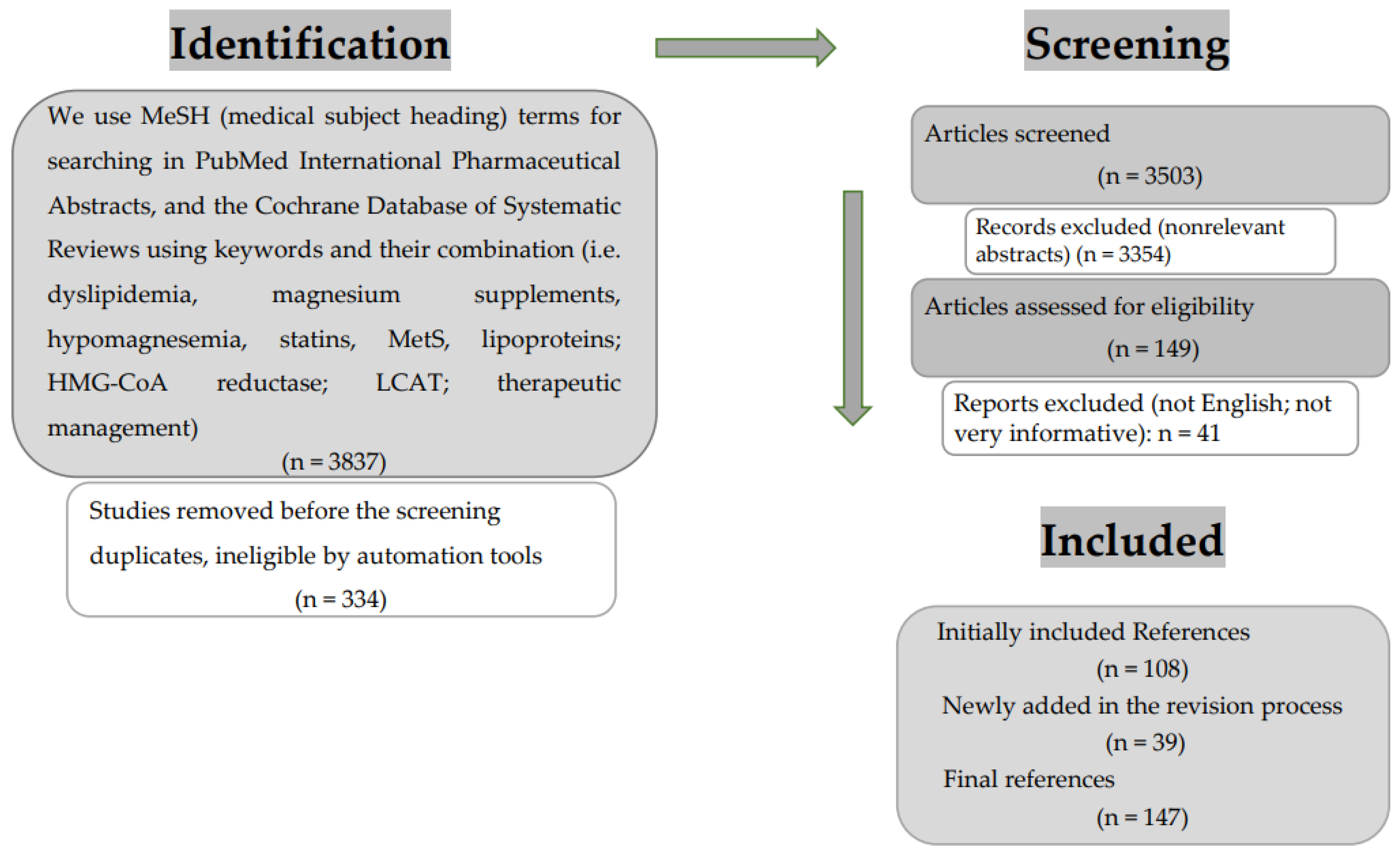
2. Literature Selection
3. General Considerations about the Metabolism of Plasmatic Lipoproteins
4. Role of Statins in Managing Dyslipidemia
4.1. Classification of Statins
4.2. Action of Statins on HMG-CoA
4.3. Action of Statin over Lecithin–Cholesterol Acyltransferase (LCAT)
4.4. Administration of Statins
5. Magnesium and Dyslipidemia
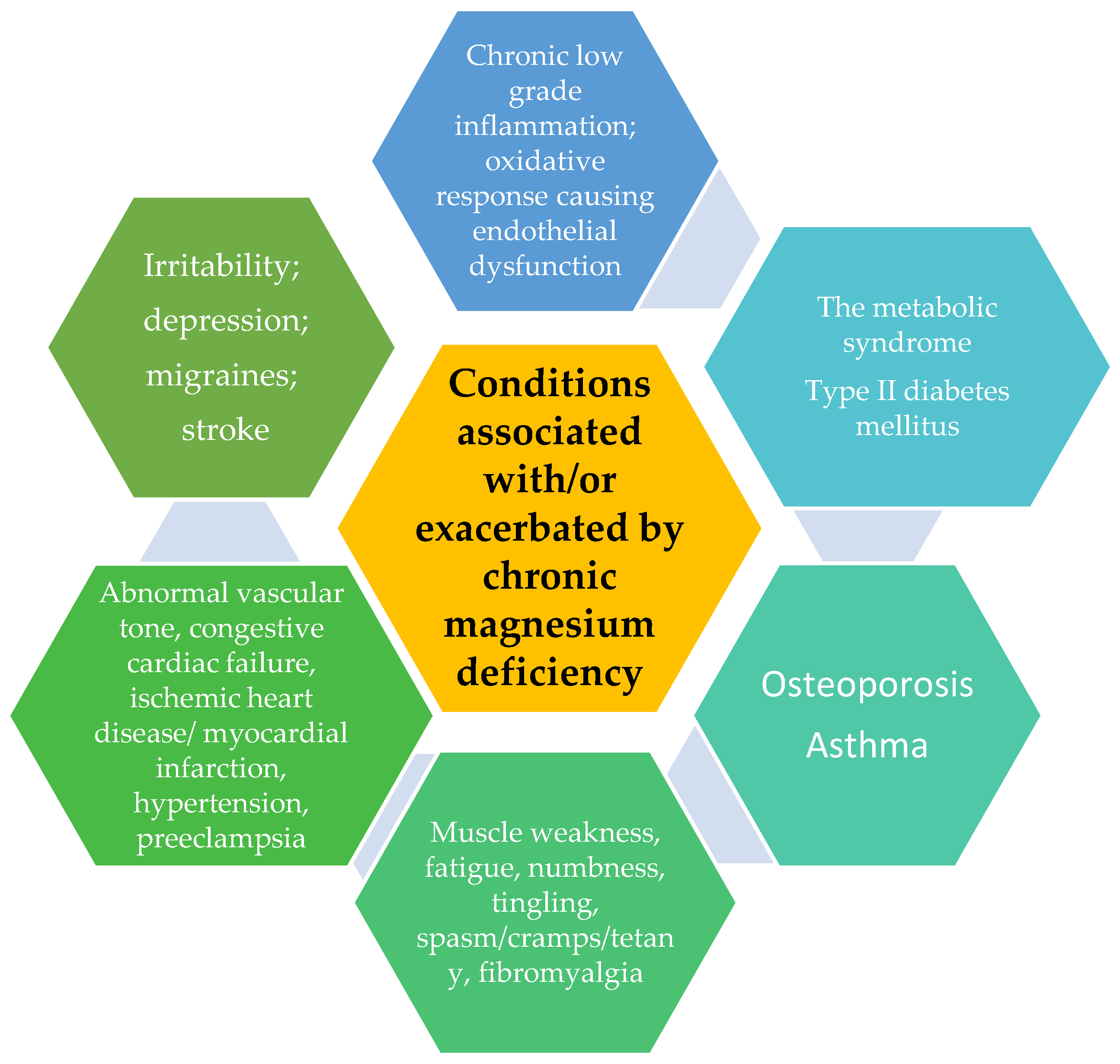
5.1. Magnesium Deficiency
5.2. Magnesium and MetS
5.3. Magnesium–Statin-like Effect
6. Statin vs. Magnesium Comparison for Cholesterol Control
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029. [Google Scholar] [CrossRef]
- Mir, R.; Elfaki, I.; Javid, J.; Barnawi, J.; Altayar, M.A.; Albalawi, S.O.; Jalal, M.M.; Tayeb, F.J.; Yousif, A.; Ullah, M.F.; et al. Genetic Determinants of Cardiovascular Disease: The Endothelial Nitric Oxide Synthase 3 (eNOS3), Krüppel-Like Factor-14 (KLF-14), Methylenetetrahydrofolate Reductase (MTHFR), MiRNAs27a and Their Association with the Predisposition and Susceptibility to Coronary Artery Disease. Life 2022, 12, 1905. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Elfaki, I.; Mir, R.; Abu-Duhier, F.; Alotaibi, M.; Alalawy, A.; Barnawi, J.; Babakr, A.; Mir, M.; Mirghani, H. Clinical Implications of MiR128, Angiotensin I Converting Enzyme and Vascular Endothelial Growth Factor Gene Abnormalities and Their Association with T2D. Curr. Issues Mol. Biol. 2021, 43, 1859–1875. [Google Scholar] [CrossRef]
- Padam, P.; Barton, L.; Wilson, S.; David, A.; Walji, S.; de Lorenzo, F.; Ray, K.K.; Jones, B.; Cegla, J. Lipid lowering with inclisiran: A real-world single-center experience. Open Heart 2022, 9, e002184. [Google Scholar] [CrossRef]
- Aygun, S.; Tokgozoglu, L. Comparison of Current International Guidelines for the Management of Dyslipidemia. J. Clin. Med. 2022, 11, 7249. [Google Scholar] [CrossRef]
- Otelea, M.R.; Nartea, R.; Popescu, F.G.; Covaleov, A.; Mitoiu, B.I.; Nica, A.S. The Pathological Links between Adiposity and the Carpal Tunnel Syndrome. Curr. Issues Mol. Biol. 2022, 44, 2646–2663. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Chen, W.; Srinivasan, S.R.; Bond, M.G.; Tang, R.; Urbina, E.M.; Berenson, G.S. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA 2003, 290, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Morais, J.B.S.; Severo, J.S.; Santos, L.R.D.; de Sousa Melo, S.R.; de Oliveira Santos, R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. Role of Magnesium in Oxidative Stress in Individuals with Obesity. Biol. Trace Elem. Res. 2017, 176, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The Role of Magnesium in the Pathogenesis of Metabolic Disorders. Nutrients 2022, 14, 1714. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gutiérrez, A.; Sánchez-Pimienta, T.G.; Carriquiry, A.; da Costa, T.M.; Ariza, A.C. Higher dietary magnesium intake is associated with lower body mass index, waist circumference and serum glucose in Mexican adults. Nutr. J. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R.; Woo, J.G.; Sinaiko, A.R.; Daniels, S.R.; Ikonen, J.; Juonala, M.; Kartiosuo, N.; Lehtimäki, T.; Magnussen, C.G.; Viikari, J.S.A.; et al. Childhood cardiovascular risk factors and adult cardiovascular events. N. Engl. J. Med. 2022, 386, 1877–1888. [Google Scholar] [CrossRef]
- Gordon, S.M. Chapter 12—Proteomic Diversity in HDL: A Driving Force for Particle Function and Target for Therapeutic Intervention. In The HDL Handbook, 2nd ed.; Tsugikazu, K., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 293–322. ISBN 9780124078673. [Google Scholar] [CrossRef]
- .Yeang, C.; Witztum, J.L.; Tsimikas, S. Novel method for quantification of lipoprotein (a)-cholesterol: Implications for improving accuracy of LDL-C measurements. J. Lipid Res. 2021, 62, 100053. [Google Scholar] [CrossRef]
- Flores-Dorantes, M.T.; Díaz-López, Y.E.; Gutiérrez-Aguilar, R. Environment and Gene Association with Obesity and Their Impact on Neurodegenerative and Neurodevelopmental Diseases. Front. Neurosci. 2020, 14, 863. [Google Scholar] [CrossRef]
- Gillard, B.K.; Lin, H.Y.; Massey, J.B.; Pownall, H.J. Apolipoproteins A-I, A-II, and E are independently distributed among intracellular and newly secreted HDL of human hepatoma cells. Biochim. Biophys. Acta 2009, 1791, 1125–1132. [Google Scholar] [CrossRef] [Green Version]
- Pirahanchi, Y.; Sinawe, H.; Dimri, M. Biochemistry, LDL Cholesterol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, X.; Su, T.; Xiao, H.; Gao, P.; Xiong, C.; Liu, J.; Zou, H. Association of the HDL-c Level with HsCRP, IL-6, U-NAG, RBP and Cys-C in Type 2 Diabetes Mellitus, Hypertension, and Chronic Kidney Disease: An Epidemiological Survey. Diabetes Metab. Syndr. Obes. 2020, 13, 3645–3654. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Davidson, M.H.; Le, N.A.; Burkle, J.; Pourfarzib, R. Underappreciated opportunities for high-density lipoprotein particles in risk stratification and potential targets of therapy. Cardiovasc. Drug Ther. 2015, 29, 41–50. [Google Scholar] [CrossRef]
- Kontush, A.; Chapman, M.J. Antiatherogenic small, dense HDL—Guardian angel of the arterial wall? Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 144–153. [Google Scholar] [CrossRef]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, V.; Gennaro, M.L. Foam Cells: One Size Doesn’t Fit All. Trends Immunol. 2019, 40, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Kuklenyik, Z.; Jones, J.; Gardner, M.; Schieltz, D.; Parks, B.; Toth, C.; Rees, J.; Andrews, M.; Carter, K.; Lehtikoski, A.; et al. Core lipid, surface lipid and apolipoprotein composition analysis of lipoprotein particles as a function of particle size in one workflow integrating asymmetric flow field-flow fractionation and liquid chromatography-tandem mass spectrometry. PLoS ONE. 2018, 13, e0194797. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, function, and genetics of lipoprotein (a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef] [Green Version]
- Tsimikas, S.; Fazio, S.; Ferdinand, K.C.; Ginsberg, H.N.; Koschinsky, M.L.; Marcovina, S.M.; Moriarty, P.M.; Rader, D.J.; Remaley, A.T.; Reyes-Soffer, G.; et al. NHLBI working group recommendations to reduce lipoprotein(a)-the mediated risk of cardiovascular disease and aortic stenosis. J. Am. Coll. Cardiol. 2018, 71, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Kunnen, S.; Van Eck, M. Lecithin:cholesterol acyltransferase: Old friend or foe in atherosclerosis? J. Lipid Res. 2012, 53, 1783–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein (a) as a cause of cardiovascular disease: Insights from epidemiology, genetics, and biology. J. Lipid Res. 2016, 57, 1953–1975. [Google Scholar] [CrossRef] [Green Version]
- Viney, N.J.; Yeang, C.; Yang, X.; Xia, S.; Witztum, J.L.; Tsimikas, S. Relationship between “LDL-C”, estimated true LDL-C, apolipoprotein B-100, and PCSK9 levels following lipoprotein(a) lowering with an antisense oligonucleotide. J. Clin. Lipidol. 2018, 12, 702–710. [Google Scholar] [CrossRef]
- Raitakari, O.; Kartiosuo, N.; Pahkala, K.; Hutri-Kähönen, N.; Bazzano, L.A.; Chen, W.; Urbina, E.M.; Jacobs, D.R.; Sinaiko, A.; Steinberger, J.; et al. Lipoprotein(a) in Youth and Prediction of Major Cardiovascular Outcomes in Adulthood. Circulation 2023, 147, 23–31. [Google Scholar] [CrossRef]
- Shen, W.J.; Azhar, S.; Kraemer, F.B. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu. Rev. Physiol. 2018, 80, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.P.; Koschinsky, M.L.; Moriarty, P.M. Expert position statements: Comparison of recommendations for the care of adults and youth with elevated lipoprotein(a). Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.; Yao, Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr. Metab. 2010, 7, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feingold, K.R. Triglyceride Lowering Drugs. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Hofland, J., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. [Google Scholar]
- Botham, K.M.; Mayes, P.A. Lipid Transport & Storage. In Harper’s Illustrated Biochemistry; Rodwell, V.W., Bender, D.A., Botham, K.M., Kennelly, P.J., Weil, P., Eds.; McGraw Hill: New York, NY, USA, 2016; Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=1366§ionid=73244168 (accessed on 11 February 2023).
- Kumari, A.; Kristensen, K.K.; Ploug, M.; Winther, A.-M.L. The Importance of Lipoprotein Lipase Regulation in Atherosclerosis. Biomedicines 2021, 9, 782. [Google Scholar] [CrossRef]
- Leth-Espensen, K.Z.; Kristensen, K.K.; Kumari, A.; Winther, A.-M.L.; Young, S.G.; Jørgensen, T.J.D.; Ploug, M. The intrinsic instability of the hydrolase domain of lipoprotein lipase facilitates its inactivation by ANGPTL4-catalyzed unfolding. Proc. Natl. Acad. Sci. USA 2021, 118, e2026650118. [Google Scholar] [CrossRef] [PubMed]
- Birrane, G.; Beigneux, A.P.; Dwyer, B.; Strack-Logue, B.; Kristensen, K.K.; Francone, O.L.; Fong, L.G.; Mertens, H.D.T.; Pan, C.Q.; Ploug, M.; et al. Structure of the lipoprotein lipase–GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc. Natl. Acad. Sci. USA 2019, 116, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, K.K.; Leth-Espensen, K.Z.; Mertens, H.D.T.; Birrane, G.; Meiyappan, M.; Olivecrona, G.; Jørgensen, T.J.D.; Young, S.G.; Ploug, M. Unfolding of monomeric lipoprotein lipase by ANGPTL4: Insight into the regulation of plasma triglyceride metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 4337–4346. [Google Scholar] [CrossRef]
- He, C.; Weston, T.A.; Jung, R.S.; Fong, L.G.; Young, S.G.; Jiang, H.; He, C.; Weston, T.A.; Jung, R.S.; Heizer, P.; et al. NanoSIMS Analysis of Intravascular Lipolysis and Lipid Movement across Capillaries and into Article NanoSIMS Analysis of Intravascular Lipolysis and Lipid Movement across Capillaries and into Cardiomyocytes. Cell Metab. 2018, 27, 1055.e3–1066.e3. [Google Scholar] [CrossRef] [Green Version]
- Grosskopf, I.; Baroukh, N.; Lee, S.-J.; Kamari, Y.; Harats, D.; Rubin, E.M.; Pennacchio, L.A.; Cooper, A.D. Apolipoprotein A-V Deficiency Results in Marked Hypertriglyceridemia Attributable to Decreased Lipolysis of Triglyceride-Rich Lipoproteins and Removal of Their Remnants. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2573–2579. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, P.J.; Alborn, W.E.; Sloan, J.H.; Ulmer, M.; Boodhoo, A.; Knierman, M.D.; Schultze, A.E.; Konrad, R.J. The Novel Apolipoprotein A5 Is Present in Human Serum, Is Associated with VLDL, HDL, and Chylomicrons, and Circulates at Very Low Concentrations Compared with Other Apolipoproteins. Clin. Chem. 2005, 51, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Lookene, A.; Beckstead, J.A.; Nilsson, S.; Olivecrona, G.; Ryan, R.O. Apolipoprotein A-V-heparin interactions: Implications for plasma lipoprotein metabolism. J. Biol. Chem. 2005, 280, 25383–25387. [Google Scholar] [CrossRef] [Green Version]
- Skoczyńska, A.; Wojakowska, A.; Turczyn, B.; Zatońska, K.; Wołyniec, M.; Szuba, A. Serum CETP and PLTP activity in middle-aged men living in urban or rural area of the Lower Silesia region. PURE Poland sub-study. Arch. Med. Sci. 2016, 12, 704–714. [Google Scholar] [CrossRef]
- Williams, K.J.; Chen, K. Recent insights into factors affecting remnant lipoprotein uptake. Curr. Opin. Lipidol. 2010, 21, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.; Karpe, F.; Milne, R.W.; Hamsten, A. Differences in apolipoprotein and lipid composition between human chylomicron remnants and very low-density lipoproteins isolated from fasting and postprandial plasma. J. Lipid Res. 1998, 39, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Altabas, V.; Biloš, L.S.K. The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. Int. J. Mol. Sci. 2022, 23, 2663. [Google Scholar] [CrossRef]
- Dzobo, K.E.; Hanford, K.M.L.; Kroon, J. Vascular Metabolism as Driver of Atherosclerosis: Linking Endothelial Metabolism to Inflammation. Immunometabolism 2021, 3, e210020. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications. National Library of Medicine. 29 November 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430940/ (accessed on 19 February 2023).
- Bansal, A.B.; Cassagnol, M. HMG-CoA Reductase Inhibitors. National Library of Medicine. 4 July 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542212/ (accessed on 18 February 2023).
- Singh, S.; Zahoor, I.; Sharma, N.; Behl, T.; Kanojia, N.; Sehgal, A.; Mohan, S.; Almoshari, Y.; Salawi, A.; Aleya, L.; et al. Insights into the pivotal role of statins and its nanoformulations in hyperlipidemia. Environ. Sci. Pollut. Res. Int. 2022, 29, 76514–76531. [Google Scholar] [CrossRef]
- Thongtang, N.; Sitthananun, C.; Sriussadaporn, S.; Nitiyanant, W. Efficacy of low- and moderate-intensity statins for achieving low-density lipoprotein cholesterol targets in Thai type 2 diabetic patients. J. Diabetes Metab. Disord. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, L.; Jayyousi, A.; Bener, A.; Zuby, B.; Zirie, M. Comparison of Efficacy and Safety of Rosuvastatin, Atorvastatin and Pravastatin among Dyslipidemic Diabetic Patients. ISRN Pharmacol. 2013, 2013, 146579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galicia-Garcia, U.; Jebari, S.; Larrea-Sebal, A.; Uribe, K.B.; Siddiqi, H.; Ostolaza, H.; Benito-Vicente, A.; Martin, C. Statin Treatment-Induced Development of Type 2 Diabetes: From Clinical Evidence to Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 4725. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins? Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Ward, N.C.; Watts, G.F.; Ecke, R.H. Statin Toxicity. Mechanistic Insights and Clinical Implications. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, C.W. Statins in therapy: Understanding their hydrophilicity, lipophilicity, binding to 3-hydroxy-3-methylglutaryl-CoA reductase, ability to cross the blood brain barrier and metabolic stability based on electrostatic molecular orbital studies. Eur. J. Med. Chem. 2014, 85, 661–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.; Baek, A.; Sakkiah, S.; Park, C.; John, S.; Lee, K.W. Exploration of Virtual Candidates for Human HMG-CoA Reductase Inhibitors Using Pharmacophore Modeling and Molecular Dynamics Simulations. PLoS ONE 2013, 8, e83496. [Google Scholar] [CrossRef] [Green Version]
- Das, K.C.; Hossain, M.U.; Moniruzzaman, M.; Salimullah, M.; Akhteruzzaman, S. High-Risk Polymorphisms Associated with the Molecular Function of Human HMGCR Gene Infer the Inhibition of Cholesterol Biosynthesis. BioMed Res. Int. 2022, 2022, 4558867. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Wu, J.; Zhang, T.; Ma, X.; Du, Z.; Xu, J.; You, J.; Wang, L.; Chen, N.; Luo, M.; et al. Statin-Induced Geranylgeranyl Pyrophosphate Depletion Promotes Ferroptosis-Related Senescence in Adipose Tissue. Nutrients 2022, 14, 4365. [Google Scholar] [CrossRef]
- Formanowicz, D.; Radom, M.; Rybarczyk, A.; Tanas, K.; Formanowicz, P. Control of Cholesterol Metabolism Using a Systems Approach. Biology 2022, 11, 430. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Koushki, K.; Shahbaz, S.K.; Mashayekhi, K.; Sadeghi, M.; Zayeri, Z.D.; Taba, M.Y.; Banach, M.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Anti-inflammatory Action of Statins in Cardiovascular Disease: The Role of Inflammasome and Toll-Like Receptor Pathways. Clin. Rev. Allergy Immunol. 2021, 60, 175–199. [Google Scholar] [CrossRef]
- Wan Ahmad, W.N.; Sakri, F.; Mokhsin, A.; Rahman, T.; Mohd Nasir, N.; Abdul-Razak, S.; Md Yasin, M.; Mohd Ismail, A.; Ismail, Z.; Nawawi, H. Low serum high density lipoprotein cholesterol concentration is an independent predictor for enhanced inflammation and endothelial activation. PLoS ONE 2015, 10, e0116867, Erratum in PLoS ONE 2015, 10, e0142245. [Google Scholar] [CrossRef] [Green Version]
- Saeedi, R.; Li, M.; Frohlich, J. A review on lecithin cholesterol acyltransferase deficiency. Clin. Biochem. 2015, 48, 472–475. [Google Scholar] [CrossRef]
- Ossoli, A.; Pavanello, C.; Calabresi, L. High-Density Lipoprotein, Lecithin:Cholesterol Acyltransferase, and Atherosclerosis. Endocrinol. Metab. 2016, 31, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Elias-Lopez, D.; Martagon, A.J.; Perez-Mwndez, O.A.; Odonez Sanchez, M.L.; Segura, Y.; Tusie, M.T.; Aguilar-Salinas, C.A. LCAT deficiency: A systematic review with the clinical and genetic description of Mexican kindred. Lipids Health Dis. 2021, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Ossoli, A.; Simonelli, S.; Varrenti, M.; Morici, N.; Oliva, F.; Stucchi, M.; Gomaraschi, M.; Strazzella, A.; Arnaboldi, L.; Thomas, M.J.; et al. Recombinant LCAT (lecithin:cholesterol acyltransferase) rescues defective HDL (high-density lipoprotein)-mediated endothelial protection in acute coronary syndrome. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 915–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldoni, F.; Baldassarre, D.; Castelnuovo, S.; Ossoli, A.; Amato, M.; van Capelleveen, J.; Hovingh, G.K.; De Groot, E.; Bochem, A.; Simonelli, S.; et al. Complete and Partial Lecithin:Cholesterol Acyltransferase Deficiency Is Differentially Associated with Atherosclerosis. Circulation 2018, 138, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.A.; Karathanasis, S.; Remaley, A.T. Novel LCAT-based Therapeutic Approaches. Curr. Opin. Lipidol. 2020, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Pedrelli, M.; Parini, P.; Kindberg, J.; Arnemo, J.M.; Bjorkhem, I.; Aasa, U.; Westerståhl, M.; Walentinsson, A.; Pavanello, C.; Turri, M.; et al. Vasculoprotective properties of plasma lipoproteins from brown bears (Ursus arctos). J Lipid Res 2021, 62, 100065. [Google Scholar] [CrossRef]
- George, R.T.; Abuhatzira, L.; Stoughton, S.M.; Karathanasis, S.K.; She, D.; Jin, C.; Buss, N.; Bakker Arkema, B.; Ongstad, E.L.; Koren, M.; et al. MEDI6012: Recombinant human lecithin cholesterol acyltransferase, high-density lipoprotein, and low-density lipoprotein receptor-mediated reverse cholesterol transport. J. Am. Heart Assoc. 2021, 10, e01457. [Google Scholar] [CrossRef]
- Kamal, S.M. Effects of single-dose morning and evening administration of pravastatin on antioxidant markers in cholesterol-fed rabbits. J. Exp. Pharmacol. 2011, 3, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L.; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghe, G.; Toth, P.P.; Bungau, S.; Behl, T.; Ilie, M.; Pantea Stoian, A.; Bratu, O.G.; Bacalbasa, N.; Rus, M.; Diaconu, C.C. Cardiovascular Risk and Statin Therapy Consideration in Women. Diagnostics 2020, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.E.; Colantonio, L.D.; Zhao, H.; Bittner, V.; Dai, Y.; Farkouh, M.E.; Monda, K.L.; Safford, M.M.; Muntner, P.; Woodward, M. Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J. Am. Coll. Cardiol. 2018, 71, 1729–1737. [Google Scholar] [CrossRef]
- Nanna, M.G.; Wang, T.Y.; Xiang, Q.; Goldberg, A.C.; Robinson, J.G.; Roger, V.L.; Virani, S.S.; Wilson, P.W.F.; Louie, M.J.; Koren, A.; et al. Sex Differences in the Use of Statins in Community Practice. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005562. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.J.; Liao, C.X.; Liu, Q.; Guo, Y.; Yang, T.; Chen, J.Y.; Wang, Y.T.; Hu, J.H.; Xu, D.Y. Efficacy and Safety of Statins and Exercise Combination Therapy Compared to Statin Monotherapy in Patients with Dyslipidaemia: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2017, 24, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Lee, O.S.; Zhang, J.; Jung, S.H.; Kim, H.S.; Lee, M.K.; Lee, H.Y. High-Intensity Statin Therapy Is “Too Much,” Thus Not Indicated for Very Elderly Patients. Pulse 2018, 6, 19–31. [Google Scholar] [CrossRef]
- Lee, K.; Lee, M.; Kim, D.W.; Kim, J.; Lim, S.; Choo, E.H.; Kim, C.; Park, C.S.; Kim, H.Y.; Yoo, K.D.; et al. Clinical impact of statin intensity according to age in patients with acute myocardial infarction. PLoS ONE 2022, 17, e0269301. [Google Scholar] [CrossRef]
- Karie, S.; Launay-Vacher, V.; Deray, G.; Isnard-Bagnis, C. Statins in patients with kidney failure: Efficacy, tolerance, and prescription guidelines in patients with chronic kidney disease and renal transplant. Presse Med. 2006, 35 Pt 1, 219–229. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Gao, Y. Efficacy of statins on renal function in patients with chronic kidney disease: A systematic review and meta-analysis. Ren Fail. 2021, 43, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Bangalore, S.; Fayyad, R.; Melamed, S.; Hovingh, K.; DeMicco, D.; Waters, D.D. Atorvastatin Has a Dose-Dependent Beneficial Effect on Kidney Function and Associated Cardiovascular Outcomes: Post Hoc Analysis of 6 Double-Blind Randomized Controlled Trials. J. Am. Heart Assoc. 2019, 8, e010827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, R.D.P.; Degobi, N.A.H.; Bertoluci, M.C. Choosing statins: A review to guide clinical practice. Arch. Endocrinol. Metab. 2020, 64, 639–653. [Google Scholar] [CrossRef]
- Sung, S.; Al-Karaghouli, M.; Kalainy, S.; Cabrera Garcia, L.; Abraldes, J.G. A systematic review on pharmacokinetics, cardiovascular outcomes and safety profiles of statins in cirrhosis. BMC Gastroenterol. 2021, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Biscetii, F.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Nicolazzi, M.A.; Covino, M.; Gasbarrini, A.; Massetti, M.; Flex, A. Statins in Hikh Cardiovascular Risk Patients: Do Comorbidities and Characteristics Matter? Int. J. of Mol. Sci. 2022, 23, 9326. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Alvarado-Cardenas, M.; Pinal-Fernandez, I.; Trallero-Araguas, E.; Milisenda, J.C.; Angeles Martinez, M.; Marin, A.; Labrador-Horrillo, M.; Juarez, C.; Grau-Junyent, J.M. Statin-induced myalgia and myositis: An update on pathogenesis and clinical recommendations. Expert Rev. Clin. Immunol. 2018, 14, 215–224. [Google Scholar] [CrossRef]
- Torres, P.A.; Helmstetter, J.A.; Kaye, A.M.; Kaye, A.D. Rhabdomyolysis: Pathogenesis, diagnosis, and treatment. Ochsner J. 2015, 15, 58–69. [Google Scholar] [PubMed]
- Buettner, C.; Greenman, R.L.; Ngo, L.H.; Wu, J.S. Effects of Coenzyme Q10 on Skeletal Muscle Oxidative Metabolism in Statin Users Assessed Using 31P Magnetic Resonance Spectroscopy: A Randomized Controlled Study. J. Nat. Sci. 2016, 2, e212. [Google Scholar]
- Attardo, S.; Musumeci, O.; Velardo, D.; Toscano, A. Statins Neuromuscular Adverse Effects. Int. J. Mol. Sci. 2022, 23, 8364. [Google Scholar] [CrossRef]
- Cheon, D.Y.; Jo, S.-H. Adverse effects of statin therapy and their treatment. Cardiovasc. Prev. Pharmacother. 2022, 4, 1–6. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol metabolism, pancreatic β-cell function and diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol Sin. 2016, 32, 631–639. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sahebkar, A.; Rodríguez-Morán, M.; Guerrero-Romero, F. Effect of Magnesium Supplementation on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Clin. Pharmacol. 2017, 73, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The Case for an Evidence-Based Reference Interval for Serum Magnesium: The Time Has Come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Chronic Magnesium Deficiency and Human Disease; Time for Reappraisal? QJM Int. J. Med. 2018, 111, 759–763. [Google Scholar] [CrossRef] [Green Version]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi Mousavi, S.; Ghoreishy, S.M.; Hemmati, A.; Mohammadi, H. Association between Magnesium Concentrations and Prediabetes: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 24388. [Google Scholar] [CrossRef] [PubMed]
- Alkazemi, D.; Alsouri, N.; Zafar, T.; Kubow, S. Hypomagnesemia and the Metabolic Syndrome among Apparently Healthy Kuwaiti Adults: A Cross-Sectional Study. Nutrients 2022, 14, 5257. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Melo, S.R.D.S.; Severo, J.S.; Morais, J.B.S.; da Silva, L.D.; de Paiva Sousa, M.; de Sousa, T.G.V.; Henriques, G.S.; do Nascimento Marreiro, D. Cardiovascular Diseases in Obesity: What Is the Role of Magnesium? Biol. Trace Elem. Res. 2021, 199, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Simental-Mendía, L.E.; Gamboa-Gómez, C.I.; Guerrero-Romero, F. Oral Magnesium Supplementation and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Adv. Chronic Kidney Dis. 2018, 25, 261–266. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamedifard, Z.; Farrokhian, A.; Reiner, Ž.; Bahmani, F.; Asemi, Z.; Ghotbi, M.; Taghizadeh, M. The Effects of Combined Magnesium and Zinc Supplementation on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Lipids Health Dis. 2020, 19, 112. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral Magnesium Supplementation Improves Endothelial Function and Attenuates Subclinical Atherosclerosis in Thiazide-Treated Hypertensive Women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Verma, H.; Garg, R. Effect of Magnesium Supplementation on Type 2 Diabetes Associated Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Low Serum Magnesium Levels and Metabolic Syndrome. Acta Diabetol. 2002, 39, 209–213. [Google Scholar] [CrossRef]
- Rasheed, H.; Elahi, S.; Ajaz, H. Serum Magnesium and Atherogenic Lipid Fractions in Type II Diabetic Patients of Lahore, Pakistan. Biol. Trace Elem. Res. 2012, 148, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Toprak, O.; Sarı, Y.; Koç, A.; Sarı, E.; Kırık, A. The Impact of Hypomagnesemia on Erectile Dysfunction in Elderly, Non-Diabetic, Stage 3 and 4 Chronic Kidney Disease Patients: A Prospective Cross-Sectional Study. Clin. Interv. Aging 2017, 12, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Dey, R.; Rajappa, M.; Parameswaran, S.; Revathy, G. Hypomagnesemia and Atherogenic Dyslipidemia in Chronic Kidney Disease: Surrogate Markers for Increased Cardiovascular Risk. Clin. Exp. Nephrol. 2015, 19, 1054–1061. [Google Scholar] [CrossRef]
- Bain, L.K.; Myint, P.K.; Jennings, A.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Welch, A.A. The relationship between dietary magnesium intake, stroke and its major risk factors, blood pressure and cholesterol, in the EPIC-Norfolk cohort. Int. J. Cardiol. 2015, 196, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Liu, C.; Tian, Y.; Zhang, X.; Ye, H.; Jin, L.; Ruan, L.; Sun, Z.; Zhu, Y. Higher Levels of Magnesium and Lower Levels of Calcium in Whole Blood Are Positively Correlated with the Metabolic Syndrome in a Chinese Population: A Case-Control Study. Ann. Nutr. Metab. 2016, 69, 125–134. [Google Scholar] [CrossRef]
- Rosanoff, A.; Costello, R.B.; Johnson, G.H. Effectively Prescribing Oral Magnesium Therapy for Hypertension: A Categorized Systematic Review of 49 Clinical Trials. Nutrients 2021, 13, 195. [Google Scholar] [CrossRef]
- Jin, H.; Nicodemus-Johnson, J. Gender and Age Stratified Analyses of Nutrient and Dietary Pattern Associations with Circulating Lipid Levels Identify Novel Gender and Age-Specific Correlations. Nutrients 2018, 10, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; Van Horn, L.; Jacobs, D.R.; Savage, P.J. Magnesium Intake and Incidence of Metabolic Syndrome Among Young Adults. Circulation 2006, 113, 1675–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, S.; Kushner, H.; Falkner, B. Low Dietary Magnesium Is Associated with Insulin Resistance in a Sample of Young, Nondiabetic Black Americans. Am. J. Hypertens. 1999, 12, 747–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Kawasaka, T.; Nakamura, M. The effects of high oral magnesium supplementation on blood pressure, serum lipids and related variables in apparently healthy Japanese subjects. Br. J. Nutr. 1997, 78, 737–750. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between Dietary Magnesium Intake and Metabolic Syndrome. Nutrients 2022, 14, 2013. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2021, 13, 139. [Google Scholar] [CrossRef]
- Rashvand, S.; Mobasseri, M.; Tarighat-Esfanjani, A. Effects of Choline and Magnesium Concurrent Supplementation on Coagulation and Lipid Profile in Patients with Type 2 Diabetes Mellitus: A Pilot Clinical Trial. Biol. Trace Elem. Res. 2020, 194, 328–335. [Google Scholar] [CrossRef]
- Rotter, I.; Kosik-Bogacka, D.; Dolegowska, B.; Safranow, K.; Karakiewicz, B.; Laszczynska, M. Relationship between Serum Magnesium Concentration and Metabolic and Hormonal Disorders in Middle-Aged and Older Men. Magnes. Res. 2015, 28, 99–107. [Google Scholar] [CrossRef]
- Suliburska, J.; Cofta, S.; Gajewska, E.; Kalmus, G.; Sobieska, M.; Samborski, W.; Krejpcio, Z.; Drzymala-Czyz, S.; Bogdanski, P. The Evaluation of Selected Serum Mineral Concentrations and Their Association with Insulin Resistance in Obese Adolescents. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2396–2400. [Google Scholar] [PubMed]
- Dai, B.; Li, X.; Xu, J.; Zhu, Y.; Huang, L.; Tong, W.; Yao, H.; Chow, D.H.; Qin, L. Synergistic effects of magnesium ions and simvastatin on attenuation of high-fat diet-induced bone loss. Bioact Mater. 2021, 6, 2511–2522. [Google Scholar] [CrossRef]
- Cambray, S.; Ibarz, M.; Bermudez-Lopez, M.; Marti-Antonio, M.; Bozic, M.; Fernandez, E.; Valdivielso, J.M. Magnesium Levels Modify the Effect of Lipid Parameters on Carotid Intima Media Thickness. Nutrients 2020, 12, 2631. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, Z.-Y.; Zhou, P.-H.; Zhou, X.; Zhang, D.-L.; He, N.; Zhang, J.; Liu, Y.-H.; Gu, Q. Effects of Oral Selenium and Magnesium Co-Supplementation on Lipid Metabolism, Antioxidative Status, Histopathological Lesions, and Related Gene Expression in Rats Fed a High-Fat Diet. Lipids Health Dis. 2018, 17, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayón-Orea, C.; Fresán, U.; Barbagallo, M.; Ruiz-Canela, M.; Martínez-González, M.A. Low Dietary Magnesium and Overweight/Obesity in a Mediterranean Population: A Detrimental Synergy for the Development of Hypertension. The SUN Project. Nutrients 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. [Google Scholar]
- Song, Y.; Ridker, P.M.; Manson, J.E.; Cook, N.R.; Buring, J.E.; Liu, S. Magnesium Intake, C-Reactive Protein, and the Prevalence of Metabolic Syndrome in Middle-Aged and Older U.S. Women. Diabetes Care 2005, 28, 1438–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbagallo, M. Role of Magnesium in Insulin Action, Diabetes and Cardio-Metabolic Syndrome X. Mol. Aspects Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Akyüz, O.; Gücün, M.; Demirci, R.; Celik, M. Relationship Between Serum Magnesium Level and Insulin Resistance in Turkey Non-Obese Adult Population. Biol. Trace Elem. Res. 2022, 200, 3070–3077. [Google Scholar] [CrossRef]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.-F.; Vesa, C.M.; Behl, T.; Bungau, A.F.; et al. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Jaquez-Chairez, F.O.; Rodríguez-Morán, M. Magnesium in Metabolic Syndrome: A Review Based on Randomized, Double-Blind Clinical Trials. Magnes. Res. 2016, 29, 146–153. [Google Scholar] [CrossRef]
- Lopez-Ridaura, R.; Willett, W.C.; Rimm, E.B.; Liu, S.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Magnesium Intake and Risk of Type 2 Diabetes in Men and Women. Diabetes Care 2004, 27, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. Dietary Magnesium Intake in Relation to Plasma Insulin Levels and Risk of Type 2 Diabetes in Women. Diabetes Care 2004, 27, 59–65. [Google Scholar] [CrossRef] [Green Version]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical Magnesium Deficiency: A Principal Driver of Cardiovascular Disease and a Public Health Crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J. Magnesium Metabolism in Type 2 Diabetes Mellitus, Metabolic Syndrome and Insulin Resistance. Highlight Issue Cell. Regul. Magnes. 2007, 458, 40–47. [Google Scholar] [CrossRef]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef]
- Ridker, P.; Buring, J.; Cook, N.; Rifai, N. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events. An 8-year Follow-Up of 14,719 Initially Healthy American Women. Circulation 2003, 12, 391–397. [Google Scholar] [CrossRef]
- Cruz, K.J.C.; de Oliveira, A.R.S.; Pinto, D.P.; Morais, J.B.S.; da Silva Lima, F.; Colli, C.; Torres-Leal, F.L.; do Nascimento Marreiro, D. Influence of Magnesium on Insulin Resistance in Obese Women. Biol. Trace Elem. Res. 2014, 160, 305–310. [Google Scholar] [CrossRef]
- Farshidi, H.; Sobhani, A.R.; Eslami, M.; Azarkish, F.; Eftekhar, E.; Keshavarz, M.; Soltani, N. Magnesium Sulfate Administration in Moderate Coronary Artery Disease Patients Improves Atherosclerotic Risk Factors: A Double-Blind Clinical Trial Study. J. Cardiovasc. Pharmacol. 2020, 76, 321–328. [Google Scholar] [CrossRef]
- Fung, T.T.; Manson, J.E.; Solomon, C.G.; Liu, S.; Willett, W.C.; Hu, F.B. The Association between Magnesium Intake and Fasting Insulin Concentration in Healthy Middle-Aged Women. J. Am. Coll. Nutr. 2003, 22, 533–538. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Smith, L.; Dominguez, L.J.; Barbagallo, M. Effect of Magnesium Supplementation on Inflammatory Parameters: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 679. [Google Scholar] [CrossRef] [PubMed]
- Zheltova, A.A.; Kharitonova, M.V.; Iezhitsa, I.N.; Spasov, A.A. Magnesium Deficiency and Oxidative Stress: An Update. BioMedicine 2016, 6, 20. [Google Scholar] [CrossRef]
- Larsson, S.C.; Burgess, S.; Michaëlsson, K. Serum Magnesium Levels and Risk of Coronary Artery Disease: Mendelian Randomisation Study. BMC Med. 2018, 16, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joris, P.; Plat, J.; Bakker, S.; Mensink, R. Long-Term Magnesium Supplementation Improves Arterial Stiffness in Overweight and Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Intervention Study. Atherosclerosis 2016, 252, e90. [Google Scholar] [CrossRef]
- Song, Y.; He, K.; Levitan, E.B.; Manson, J.E.; Liu, S. Effects of Oral Magnesium Supplementation on Glycaemic Control in Type 2 Diabetes: A Meta-Analysis of Randomized Double-Blind Controlled Trials. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.; Sahebkar, A.; Rodríguez-Morán, M.; Guerrero-Romero, F. A Systematic Review and Meta-Analysis of Randomized Controlled Trials on the Effects of Magnesium Supplementation on Insulin Sensitivity and Glucose Control. Pharmacol. Res. 2016, 111, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ghimire, S.; Kim, E. Magnesium Supplementation Reduces Postoperative Arrhythmias After Cardiopulmonary Bypass in Pediatrics: A Metaanalysis of Randomized Controlled Trials. Pediatr. Cardiol. 2013, 34, 1396–1403. [Google Scholar] [CrossRef]
- Rosanoff, A.; Seelig, M. Comparison of Mechanism and Functional Effects of Magnesium and Statin Pharmaceuticals. J. Am. Coll. Nutr. 2004, 23, 501S–505S. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Cruz, K.; Severo, J.; Morais, J.; Freitas, T.; Araújo, R.; Marreiro, D. Hypomagnesemia and Its Relation with Chronic Low-Grade Inflammation in Obesity. Rev. Assoc. Médica Bras. 2017, 63, 156–163. [Google Scholar] [CrossRef]
- Randell, E.W.; Mathews, M.; Gadag, V.; Zhang, H.; Sun, G. Relationship between Serum Magnesium Values, Lipids and Anthropometric Risk Factors. Atherosclerosis 2008, 196, 413–419. [Google Scholar] [CrossRef]
- Jamilian, M.; Samimi, M.; Faraneh, A.E.; Aghadavod, E.; Shahrzad, H.D.; Chamani, M.; Mafi, A.; Asemi, Z. Magnesium Supplementation Affects Gene Expression Related to Insulin and Lipid in Patients with Gestational Diabetes. Magnes. Res. 2017, 30, 71–79. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.; Downes, M.; Evans, R. PPARγ Signaling and Metabolism: The Good, the Bad and the Future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Altura, B.; Shah, N.; Li, Z.; Jiang, X.-C.; Perez-Albela, J.; Altura, B.T. Magnesium Deficiency Upregulates Serine Palmitoyl Transferase (SPT 1 and SPT 2) in Cardiovascular Tissues: Relationship to Serum Ionized Mg and Cytochrome c. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H932–H938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehidoost, R.; Taghipour Boroujeni, G.; Feizi, A.; Aminorroaya, A.; Amini, M. Effect of Oral Magnesium Supplement on Cardiometabolic Markers in People with Prediabetes: A Double Blind Randomized Controlled Clinical Trial. Sci. Rep. 2022, 12, 18209. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.Y.; Shon, Y.H. Natural Magnesium-Enriched Deep-Sea Water Improves Insulin Resistance and the Lipid Profile of Prediabetic Adults: A Randomized, Double-Blinded Crossover Trial. Nutrients 2020, 12, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Romero, F.; Rodríguez-Morán, M. Hypomagnesemia Is Linked to Low Serum HDL-Cholesterol Irrespective of Serum Glucose Values. J. Diabetes Complicat. 2000, 14, 272–276. [Google Scholar] [CrossRef]
- Shahbah, D.; Naga, A.; Hassan, T.; Zakaria, M.; Beshir, M.; El-Morshedy, S.E.; Abdalhady, M.; Kamel, E.; Rahman, D.; Kamel, L.; et al. Status of Serum Magnesium in Egyptian Children with Type 1 Diabetes and Its Correlation to Glycemic Control and Lipid Profile. Medicine 2016, 95, e5166. [Google Scholar] [CrossRef]
- Mishra, S.; Padmanaban, P.; Deepti, G.; Sarkar, G.; Sumathi, S.; Toora, B. Serum Magnesium and Dyslipidemia in Type-2 Diabetes Mellitus. Biomed. Res. 2011, 23, 295–300. [Google Scholar]
- Mahalle, N.; Kulkarni, M.; Naik, S. Is Hypomagnesaemia a Coronary Risk Factor among Indians with Coronary Artery Disease? J. Cardiovasc. Dis. Res. 2012, 3, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Asbaghi, O.; Moradi, S.; Nezamoleslami, S.; Moosavian, S.P.; ali Hojjati Kermani, M.; Lazaridi, A.V.; Miraghajani, M. The Effects of Magnesium Supplementation on Lipid Profile Among Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Trace Elem. Res. 2021, 199, 861–873. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Wang, L.; Li, S.; Zhang, Q.; Xiao, P.; Wang, K.; Zhuang, M.; Jiang, Y. Association between Serum Magnesium and Blood Lipids: Influence of Type 2 Diabetes and Central Obesity. Br. J. Nutr. 2018, 120, 250–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyun, M.H.; Lee, Y.; Choi, B.G.; Na, J.O.; Choi, C.U.; Kim, J.W.; Kim, E.J.; Rha, S.W.; Park, C.G.; Lee, E.; et al. Roles of Achieved Levels of Low-Density Lipoprotein Cholesterol and High-Sensitivity C-Reactive Protein on Cardiovascular Outcome in Statin Therapy. Cardiovasc. Ther. 2019, 2019, 3824823. [Google Scholar] [CrossRef] [Green Version]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clin. 2018, 150, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Udupi, A.; Rebeiro, C.; Bolar Suryakanth, V.; Kamath, A.; Panduranga Shenoy, R. Association of Low-Density Lipoprotein-Cholesterol and Its Small, Dense Phenotype with Six-Month Cardiovascular Morbidity. Rep. Biochem. Mol. Biol. 2022, 11, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rosanoff, A. Magnesium and hypertension. Clin. Calcium 2005, 15, 255–260. [Google Scholar]
- El-Haggar Sahar, M.; Mostafa Tarek, M. Role of Magnesium Supplement on Hyperlipidemia and L-CAT Level in Patient on Atorvastatin Therapy. Br. J. Pharm. Res. 2014, 4, 1521–1534. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W. Beyond low-density lipoprotein: Addressing the atherogenic lipid triad in type 2 diabetes mellitus and the metabolic syndrome. Am. J. Cardiovasc. Drugs 2005, 5, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Liu, J.; Jia, Y.; Han, T.; Zhao, X.; Sun, C.; Na, L. The association of dietary flavonoids, magnesium and their interactions with the metabolic syndrome in Chinese adults: A prospective cohort study. Br. J. Nutr. 2021, 126, 892–902. [Google Scholar] [CrossRef]
- Yang, N.; He, L.; Li, Y.; Xu, L.; Ping, F.; Li, W.; Zhang, H. Reduced Insulin Resistance Partly Mediated the Association of High Dietary Magnesium Intake with Less Metabolic Syndrome in a Large Chinese Population. Diabetes Metab. Syndr. Obes. 2020, 13, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Yamori, Y.; Sagara, M.; Mizushima, S.; Liu, L.; Ikeda, K.; Nara, Y. An inverse association between magnesium in 24-h urine and cardiovascular risk factors in middle-aged subjects in 50 CARDIAC Study populations. Hypertens. Res. 2015, 38, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Dibaba, D.T.; Chen, C.; Lu, L.; Bidulescu, A.; Fly, A.D.; Xun, P.; Judd, S.E.; Cushman, M.; Kahe, K. Magnesium intake is inversely associated with the risk of metabolic syndrome in the Reasons for geographic and racial differences in stroke (REGARDS) cohort study. Clin. Nutr. 2021, 40, 2337–2342. [Google Scholar] [CrossRef] [PubMed]

| Apolipoprotein | Lipoprotein Association | Function | References |
|---|---|---|---|
| ApoA-I | HDL | Triggers LCAT; controls ABC transporter | [13,14,15,16,17] |
| ApoA-II | HDL | Structural protein for HDL, Activates hepatic lipase | [14,18,19] |
| ApoA-IV | Chylomicrons, HDL | Unknown | [14,19,20,21,22] |
| ApoB-48 | Chylomicrons | Structural protein for chylomicrons | [13,14,15,16,17] |
| ApoB-100 | VLDL, LDL | Binds to LDL receptor | [17,18] |
| ApoC-I | VLDL, LDL | Activates LCAT | [14] |
| ApoC-II | Chylomicrons, VLDL, LDL | Activates lipoprotein lipase | [13,14,15] |
| Apo C-III | Chylomicrons, VLDL, LDL | Inhibits lipoprotein lipase | [13,15,18] |
| ApoD | HDL | Unknown (has been proposed to play a role in stabilizing LCAT and increasing cholesterol esterification) | [14] |
| ApoE | Chylomicrons, VLDL, LDL | Triggers clearance of VLDL and chylomicron remnants | [13,14,15,22] |
| Causes of Magnesium Deficiency | |
|---|---|
| Insufficient intake A diet low in Magnesium [102,105] Slimming cures [102,106] | Intensification of losses At the gastrointestinal level: vomiting; purgatives/laxatives; trailing diarrhea [103,107,108] By the renal route: nephropathies; diuretics; chronic alcoholism; diabetes [104,109,110,111,112,113,114,115] |
| Decreased intestinal absorption Conditions after intestinal resection [103,116] Diarrhea [103,107,108] Malabsorption [103,107,108,117] Chron’s disease [103,108,117] Ulcerative colitis [103,117] Coeliac disease [103,107] | Stressing Factors Pregnancy [32,34,102,118,119] Lactation [32,34,102,118,119] The growing period [102,120] Sport performance [102,121,122,123] Old age [103,107,109,119,124,125] Convalescence [86,87,102] |
| Endocrine disorders Hyperthyroidism [104,126,127] Aldosteronism [104,126,127] Hyperparathyroidism [104,126,127,128] Poorly controlled diabetes [104,109,129] | Interference of absorption Increased calcium intake [102,117,128] Hyper protein diet [102,108] Lipid foods [102,128,130,131] Excessive alcohol consumption [102,119,132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nartea, R.; Mitoiu, B.I.; Ghiorghiu, I. The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients. Curr. Issues Mol. Biol. 2023, 45, 3146-3167. https://doi.org/10.3390/cimb45040205
Nartea R, Mitoiu BI, Ghiorghiu I. The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients. Current Issues in Molecular Biology. 2023; 45(4):3146-3167. https://doi.org/10.3390/cimb45040205
Chicago/Turabian StyleNartea, Roxana, Brindusa Ilinca Mitoiu, and Ioana Ghiorghiu. 2023. "The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients" Current Issues in Molecular Biology 45, no. 4: 3146-3167. https://doi.org/10.3390/cimb45040205
APA StyleNartea, R., Mitoiu, B. I., & Ghiorghiu, I. (2023). The Link between Magnesium Supplements and Statin Medication in Dyslipidemic Patients. Current Issues in Molecular Biology, 45(4), 3146-3167. https://doi.org/10.3390/cimb45040205






