Open-Label Clinical Trial on the Impact of Autologous Dendritic Cell Therapy on Albuminuria and Inflammatory Biomarkers (Interleukin-6, Interleukin-10, Tumor Necrosis Factor α) in Diabetic Kidney Disease (DKD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Study Procedure
2.4. DC Preparation
2.5. Safety Evaluation
2.6. Laboratory Testing
2.7. Statistics
3. Results
3.1. Subject Characteristics
3.2. Changes in Albuminuria and eGFR
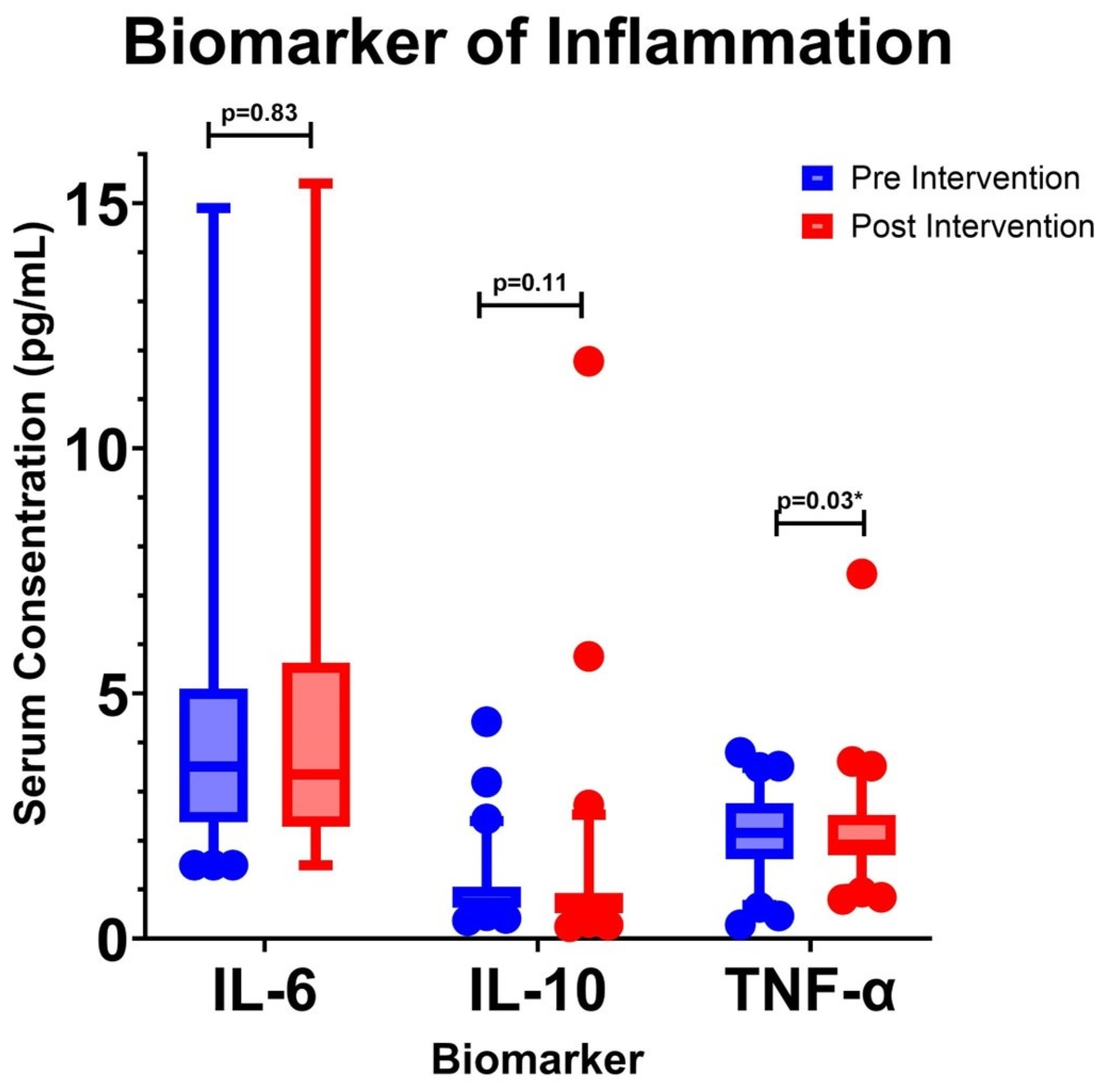
3.3. Changes in Inflammatory Biomarkers
3.4. Changes in Ratio of Pro-Inflammatory and Anti-Inflammatory Cytokines
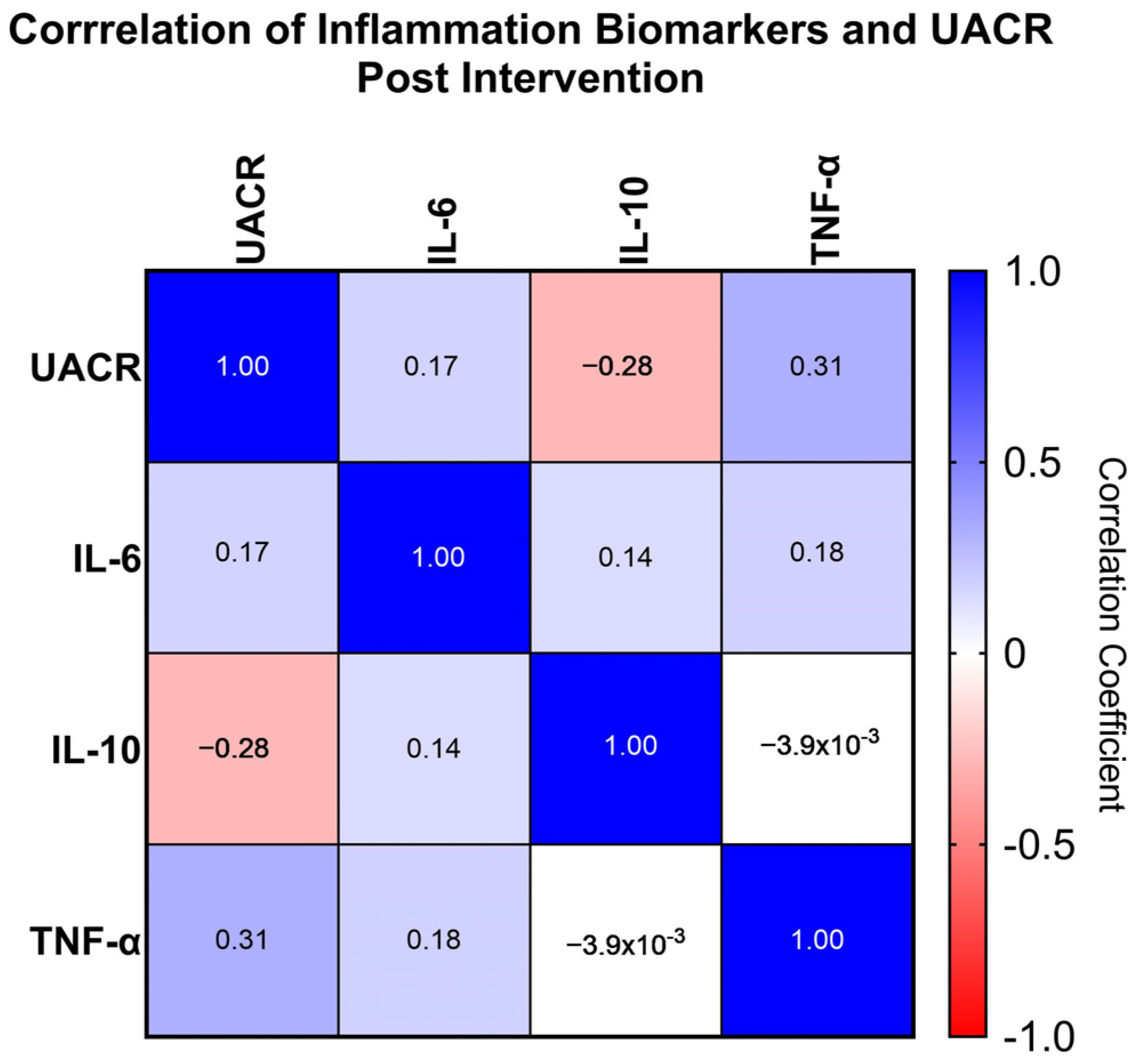
3.5. Correlation of UACR and Inflammation Biomarkers
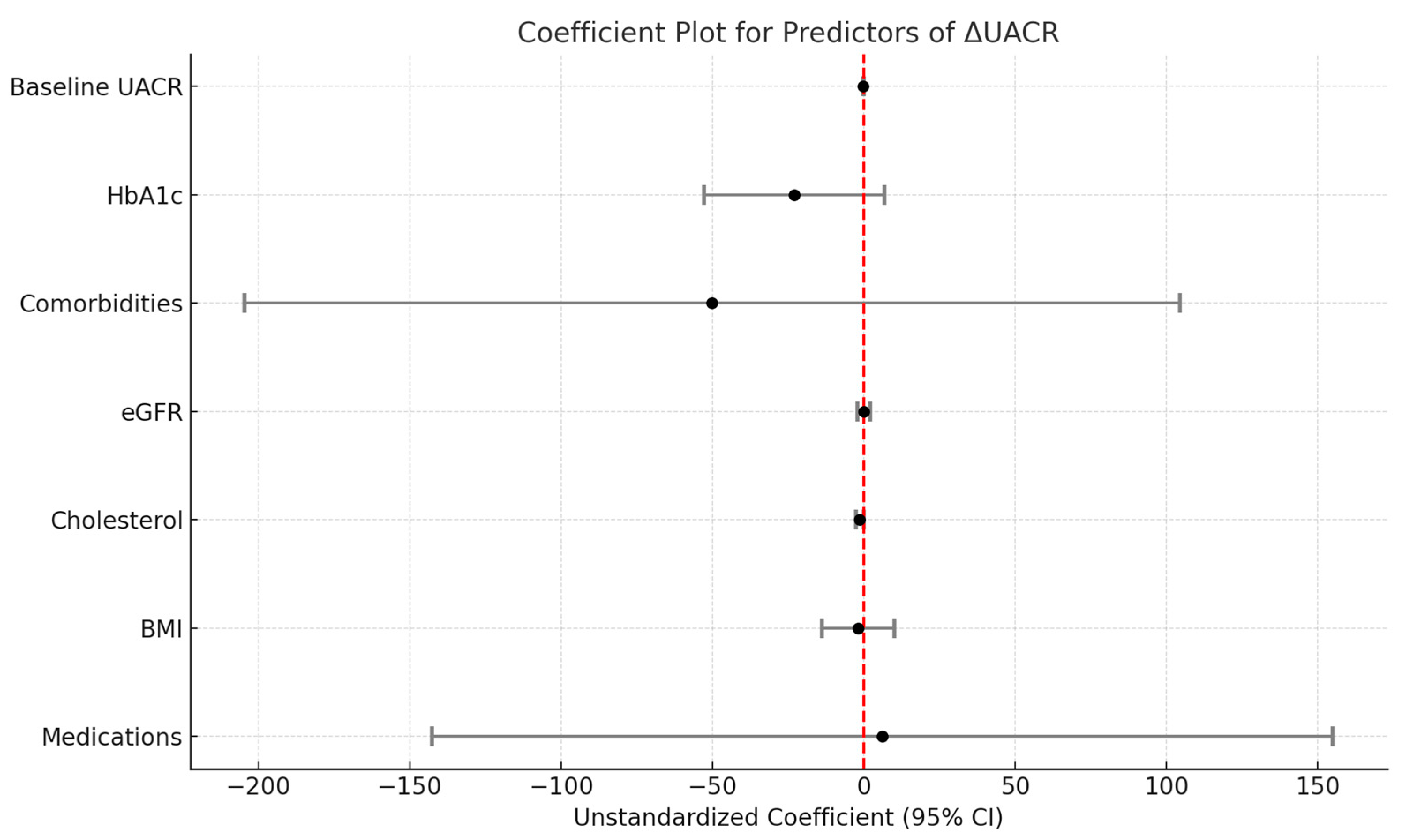
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Webber, S. International Diabetes Federation. Diabetes Res. Clin. Pract. 2013, 102, 147–148. [Google Scholar]
- Kementerian Kesehatan Republik Indonesia. Laporan Riskesdas 2018 Nasional.pdf; Lembaga Penerbit Balitbangkes: Jakarta, Indonesia, 2018. [Google Scholar]
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front. Endocrinol. 2021, 12, 672350. [Google Scholar] [CrossRef]
- D’Marco, L.; de Valencia, V.H.G.U.; Guerra-Torres, X.; de Asturias, M.H.U.P.; Viejo, I.; Lopez-Romero, L.; Yugueros, A.; Bermúdez, V.; Bolívar, F.d.C.d.l.S.U.S. Non-albuminuric Diabetic Kidney Disease Phenotype: Beyond Albuminuria. Eur. Endocrinol. 2022, 18, 102. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal Structure in Normoalbuminuric and Albuminuric Patients With Type 2 Diabetes and Impaired Renal Function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef]
- Bhalla, V.; Zhao, B.; Azar, K.M.; Wang, E.J.; Choi, S.; Wong, E.C.; Fortmann, S.P.; Palaniappan, L.P. Racial/Ethnic Differences in the Prevalence of Proteinuric and Nonproteinuric Diabetic Kidney Disease. Diabetes Care 2013, 36, 1215–1221. [Google Scholar] [CrossRef]
- Hou, J.; Xiong, W.; Cao, L.; Wen, X.; Li, A. Spironolactone Add-on for Preventing or Slowing the Progression of Diabetic Nephropathy: A Meta-analysis. Clin. Ther. 2015, 37, 2086–2103.e10. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, X.; Jin, X.; Wang, S.; Liang, W.; Cong, X. Efficacy and safety of eplerenone treatment for patients with diabetic nephropathy: A meta-analysis. PLoS ONE 2022, 17, e0265642. [Google Scholar] [CrossRef]
- Jonny, J.; Putranto, T.A.; Sitepu, E.C.; Irfon, R. Dendritic cell vaccine as a potential strategy to end the COVID-19 pandemic. Why should it be Ex Vivo? Expert Rev. Vaccines 2022, 21, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Abedalqader, T.; Ben-Bernard, G.; Mondy, J.M.; Bian, X.; Conley, S.M.; Zhu, X.; Herrmann, S.M.; Kukla, A.; Lorenz, E.C.; et al. A Systematic Review and Meta-Analysis of Cell-Based Interventions in Experimental Diabetic Kidney Disease. Stem Cells Transl. Med. 2021, 10, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Packham, D.K.; Fraser, I.R.; Kerr, P.G.; Segal, K.R. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 2016, 12, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sávio-Silva, C.; Beyerstedt, S.; Soinski-Sousa, P.E.; Casaro, E.B.; Balby-Rocha, M.T.A.; Simplício-Filho, A.; Alves-Silva, J.; Rangel, B. Mesenchymal Stem Cell Therapy for Diabetic Kidney Disease: A Review of the Studies Using Syngeneic, Autologous, Allogeneic, and Xenogeneic Cells. Stem Cells Int. 2020, 2020, 8833725. [Google Scholar] [CrossRef]
- Jonny, J.; Sitepu, E.C.; Lister, I.N.E.; Chiuman, L.; Putranto, T.A. The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines 2024, 12, 972. [Google Scholar] [CrossRef]
- Perkeni. Pedoman Pengelolaan dan Pencegahan Diabetes Melitus di Indonesia; Perhimpunan Endokrinologi Indonesia: Jakarta, Indonesia, 2021. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Soeatmadji, D.W.; Rosandi, R.; Saraswati, M.R.; Sibarani, R.P.; Tarigan, W.O. Clinicodemographic Profile and Outcomes of Type 2 Diabetes Mellitus in the Indonesian Cohort of DISCOVER: A 3-Year Prospective Cohort Study. J. ASEAN Fed. Endocr. Soc. 2023, 38, 68–74. [Google Scholar] [CrossRef]
- Ismail, L.; Materwala, H.; Al Kaabi, J. Association of risk factors with type 2 diabetes: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 1759–1785. [Google Scholar] [CrossRef]
- Srinivas, R.; Vishwanath, S. Pathophysiology of Hypertension in Chronic Kidney Disease. Hypertens. J. 2018, 4, 166–169. [Google Scholar] [CrossRef]
- Ohishi, M. Hypertension with diabetes mellitus: Physiology and pathology. Hypertens. Res. 2018, 41, 389–393. [Google Scholar] [CrossRef]
- Curovic, V.R.; Jongs, N.; Kroonen, M.Y.; Zobel, E.H.; Hansen, T.W.; Sen, T.; Laverman, G.D.; Kooy, A.; Persson, F.; Rossing, P.; et al. Optimization of Albuminuria-Lowering Treatment in Diabetes by Crossover Rotation to Four Different Drug Classes: A Randomized Crossover Trial. Diabetes Care 2023, 46, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Mathu Selvarajah, M.; Mendis, S. Randomized Controlled Trial of Treatment of Chronic Kidney Disease of Uncertain Aetiolgy with Enalapril. J. Clin. Toxicol. 2016, 6, 281. [Google Scholar] [CrossRef]
- Yarlagadda, C.; Reddy, A.J.; Landau, A.B.; Travis, L.M.; Perrone, C.G.; Idriss, A.; Patel, R.; Abutineh, M.A. An Investigation on the Efficacy of Glucagon-Like Peptide 1 Receptor Agonists Drugs in Reducing Urine Albumin-to-Creatinine Ratio in Patients With Type 2 Diabetes: A Potential Treatment for Diabetic Nephropathy. Cureus 2023, 15, e36438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, X.; Xia, S.; Xiao, X.; Chen, J.; Li, L. Relationship between dapagliflozin and urinary albumin-to-creatinine ratio in patients with diabetes mellitus and cardiovascular disease: An observational study. Cardiol. Plus 2023, 8, 263–268. [Google Scholar] [CrossRef]
- Carlini, V.; Noonan, D.M.; Abdalalem, E.; Goletti, D.; Sansone, C.; Calabrone, L.; Albini, A. The multifaceted nature of IL-10: Regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023, 14, 1161067. [Google Scholar] [CrossRef]
- Naz, S.; Shafique, N.; Sharif, S.; Manzoor, F.; Saifi, S.Z.; Firasat, S.; Kaul, H. Association of Interleukin 10 (IL-10) Gene with Type 2 Diabetes Mellitus by Single Nucleotide Polymorphism of Its Promotor Region G/A 1082. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 285–289. [Google Scholar] [CrossRef]
- Barry, J.C.; Shakibakho, S.; Durrer, C.; Simtchouk, S.; Jawanda, K.K.; Cheung, S.T.; Mui, A.L.; Little, J.P. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci. Rep. 2016, 6, 21244. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Y.; Zhang, Y.; Jin, H.; Shou, S. The role of IL-10 in kidney disease. Int. Immunopharmacol. 2022, 108, 108917. [Google Scholar] [CrossRef]
- Genheimer, C.W.; Ilagan, R.M.; Spencer, T.; Kelley, R.W.; Werdin, E.; Choudhury, S.; Jain, D.; Ludlow, J.W.; Basu, J. Molecular Characterization of the Regenerative Response Induced by Intrarenal Transplantation of Selected Renal Cells in a Rodent Model of Chronic Kidney Disease. Cells Tissues Organs 2012, 196, 374–384. [Google Scholar] [CrossRef]
- Niewczas, M.A.; Pavkov, M.E.; Skupien, J.; Smiles, A.; Dom, Z.I.M.; Wilson, J.M.; Park, J.; Nair, V.; Schlafly, A.; Saulnier, P.-J.; et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Lin, J.; Hu, F.B.; Mantzoros, C.; Curhan, G.C. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia 2010, 53, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wu, C.; Shao, Y.; Liu, S.; Zhou, Y.; Wang, Q. Correlation between serum miR-154-5p and urinary albumin excretion rates in patients with type 2 diabetes mellitus: A cross-sectional cohort study. Front. Med. 2020, 14, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Aly, R.H.; Ahmed, A.E.; Hozayen, W.G.; Rabea, A.M.; Ali, T.M.; El Askary, A.; Ahmed, O.M. Patterns of Toll-Like Receptor Expressions and Inflammatory Cytokine Levels and Their Implications in the Progress of Insulin Resistance and Diabetic Nephropathy in Type 2 Diabetic Patients. Front. Physiol. 2020, 11, 609223. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Fei, Y.; Saulnier, P.-J.; Wang, N. Circulating TNF receptors and risk of renal disease progression, cardiovascular disease events and mortality in patients with diabetes: A systematic review and meta-analysis. Endocrine 2020, 68, 32–43. [Google Scholar] [CrossRef]
- Waijer, S.W.; Sen, T.; Arnott, C.; Neal, B.; Kosterink, J.G.; Mahaffey, K.W.; Parikh, C.R.; de Zeeuw, D.; Perkovic, V.; Neuen, B.L.; et al. Association between TNF Receptors and KIM-1 with Kidney Outcomes in Early-Stage Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 251–259. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Evans, S.; Hult, C.; Joslyn, L.R.; Wessler, T.; Millar, J.A.; Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; et al. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018, 285, 147–167. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; He, J.; Li, Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front. Immunol. 2022, 13, 958790. [Google Scholar] [CrossRef]
- Sangoi, M.; Carvalho, J.; Guarda, N.; Duarte, T.; Duarte, M.; Premaor, M.; Comim, F.; Moretto, M.; Moresco, R. Association between Urinary Levels of Interleukin-6, Interleukin-10 and Tumor Necrosis Factor-Alpha with Glomerular and Tubular Damage Indicators in Patients with Type 2 Diabetes. Clin. Lab. 2019, 65, 2193–2197. [Google Scholar] [CrossRef]
- Li, Y.; Mu, Y.; Ji, Q.; Huang, Q.; Kuang, H.; Ji, L.; Yang, X. Hypoglycaemia, Abnormal Lipids, and Cardiovascular Disease among Chinese with Type 2 Diabetes. BioMed Res. Int. 2015, 2015, 862896. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Takahashi, H.; Ishikawa, H.; Yoshida, S.; Kazuta, K.; Utsuno, A.; Ueyama, E. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: Results of the Long-Term ASP1941 Safety Evaluation in Patients with Type 2 Dia. Diabetes Obes. Metab. 2015, 17, 152–160. [Google Scholar] [CrossRef]
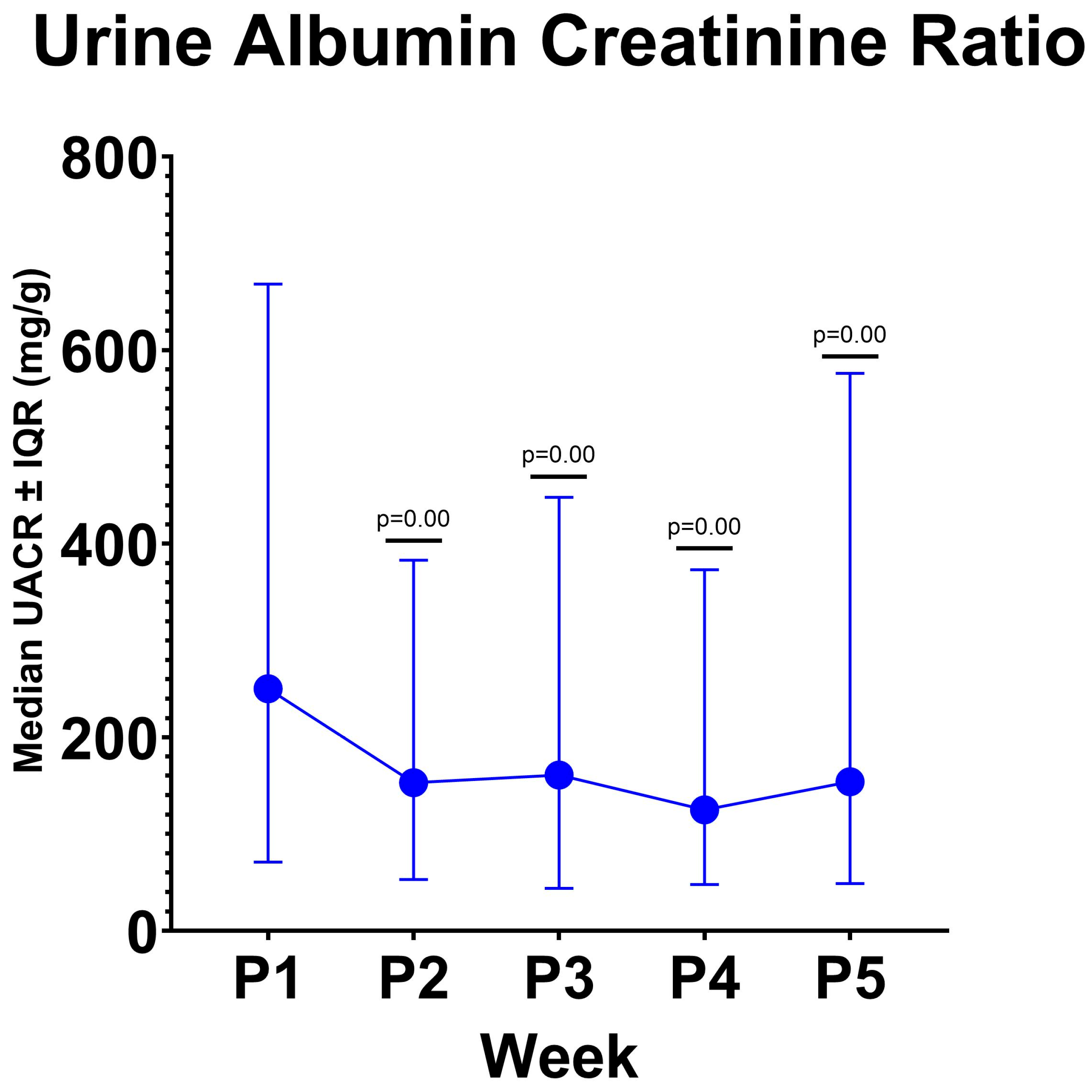

| Visit | UACR Median (25th–75th Percentile) (mg/g) | p-Value Hypothesis Test |
|---|---|---|
| Baseline (P1) | 250 (71–668) | |
| Week 1 (P2) | 153 (53–383) | 0.00 * |
| Week 2 (P3) | 161 (44–448) | 0.00 * |
| Week 3 (P4) | 125 (48–373) | 0.00 * |
| Week 4 (P5) | 164 (49–576) | 0.00 * |
| Median (25th–75th Percentile) Pre Intervention/P1 (pg/mL) | Median (25th–75th Percentile) Post Intervention/P5 (pg/mL) | p-Value | |
|---|---|---|---|
| IL-6 | 3.51 (2.38–5.06) | 3.35 (2.34–5.27) | 0.83 |
| IL-10 | 0.74 (0.63–1.05) | 0.63 (0.53–0.91) | 0.11 |
| TNF-α | 2.16 (1.62–2.74) | 1.92 (1.71–2.52) | 0.03 * |
| Median (25th–75th Percentile) Pre Intervention (pg/mL) | Median (25th–75th Percentile) Post Intervention (pg/mL) | p-Value | |
|---|---|---|---|
| Ratio IL-6/IL-10 | 4.68 (2.66–7.04) | 4.79 (3.03–7.58) | 0.063 |
| Ratio TNF-α/IL-10 | 2.94(1.77–3.85) | 3.16(2.21–4.12) | 0.115 |
| Correlation Coefficient (p Value) | IL-6 | IL-10 | TNF |
|---|---|---|---|
| UACR | 0.172 (0.157) | −0.281 (0.019) * | 0.312 (0.009) * |
| IL-6 | - | 0.141 (0.249) | 0.179 (0.141) |
| IL-10 | - | - | −0.004 (0.975) |
| Predictor 1 | Unstandardized Coefficient (B) | Std. Error | p-Value |
|---|---|---|---|
| (Constant) | 508.293 | 218.750 | 0.023 |
| Medications | 6.154 | 75.899 | 0.936 |
| BMI | −1.868 | 6.116 | 0.761 |
| Cholesterol | −1.375 | 0.654 | 0.040 * |
| eGFR | −0.069 | −0.069 | 0.950 |
| Comorbidities | −50.136 | 78.775 | 0.527 |
| HbA1c | −22.984 | 15.177 | 0.135 |
| Baseline UACR | −0.159 | 0.028 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonny; Sitepu, E.C.; Hernowo, B.A.; Chiuman, L.; Lister, I.N.E.; Putranto, T.A. Open-Label Clinical Trial on the Impact of Autologous Dendritic Cell Therapy on Albuminuria and Inflammatory Biomarkers (Interleukin-6, Interleukin-10, Tumor Necrosis Factor α) in Diabetic Kidney Disease (DKD). Curr. Issues Mol. Biol. 2024, 46, 13662-13674. https://doi.org/10.3390/cimb46120816
Jonny, Sitepu EC, Hernowo BA, Chiuman L, Lister INE, Putranto TA. Open-Label Clinical Trial on the Impact of Autologous Dendritic Cell Therapy on Albuminuria and Inflammatory Biomarkers (Interleukin-6, Interleukin-10, Tumor Necrosis Factor α) in Diabetic Kidney Disease (DKD). Current Issues in Molecular Biology. 2024; 46(12):13662-13674. https://doi.org/10.3390/cimb46120816
Chicago/Turabian StyleJonny, Enda Cindylosa Sitepu, Bhimo Aji Hernowo, Linda Chiuman, I Nyoman Ehrich Lister, and Terawan Agus Putranto. 2024. "Open-Label Clinical Trial on the Impact of Autologous Dendritic Cell Therapy on Albuminuria and Inflammatory Biomarkers (Interleukin-6, Interleukin-10, Tumor Necrosis Factor α) in Diabetic Kidney Disease (DKD)" Current Issues in Molecular Biology 46, no. 12: 13662-13674. https://doi.org/10.3390/cimb46120816
APA StyleJonny, Sitepu, E. C., Hernowo, B. A., Chiuman, L., Lister, I. N. E., & Putranto, T. A. (2024). Open-Label Clinical Trial on the Impact of Autologous Dendritic Cell Therapy on Albuminuria and Inflammatory Biomarkers (Interleukin-6, Interleukin-10, Tumor Necrosis Factor α) in Diabetic Kidney Disease (DKD). Current Issues in Molecular Biology, 46(12), 13662-13674. https://doi.org/10.3390/cimb46120816


_Kim.png)




