Plasticity of Response Properties of Mouse Visual Cortex Neurons Induced by Optogenetic Tetanization In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
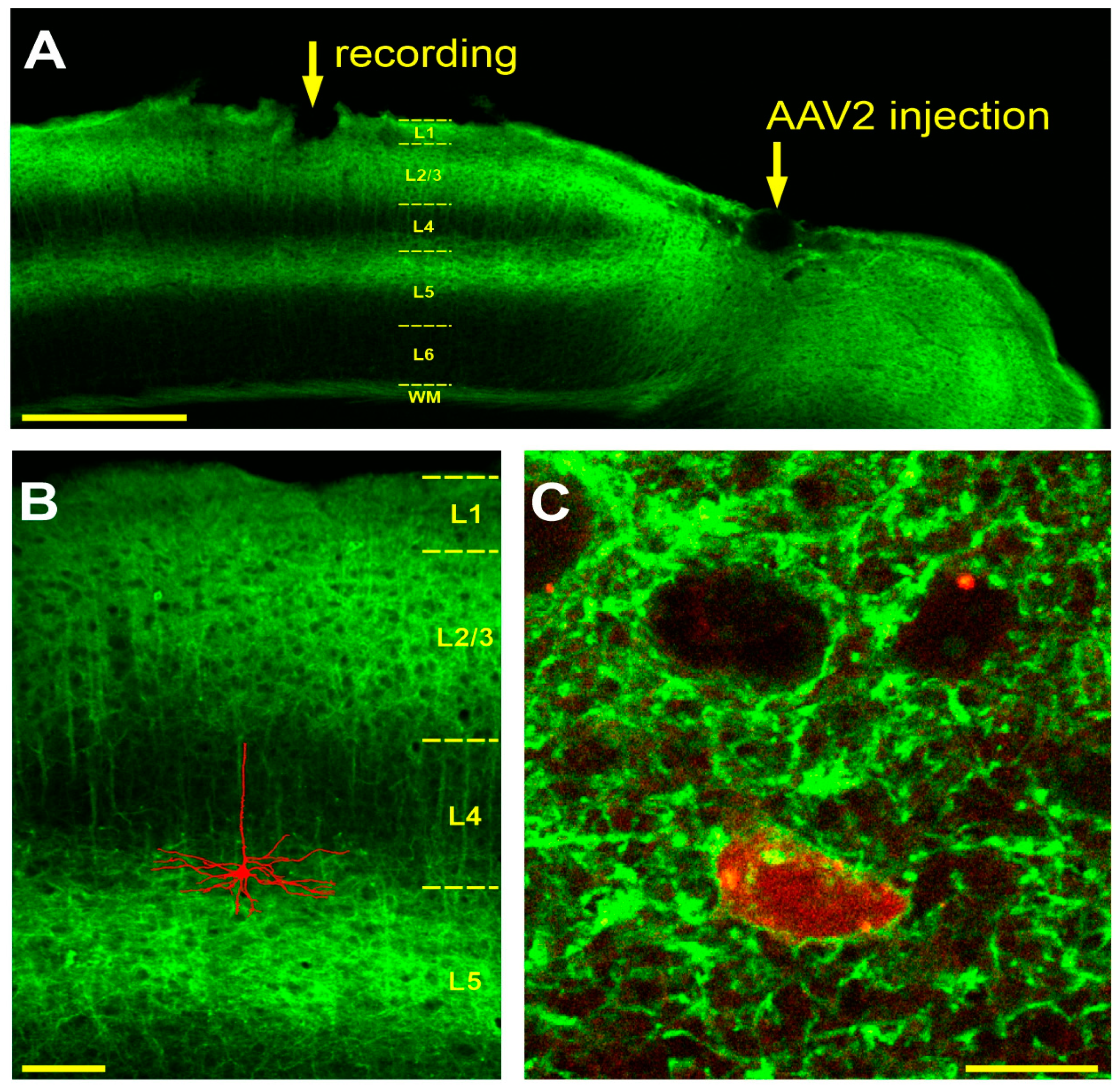
2.2. AAV Virus Production and Injection
2.3. Surgery
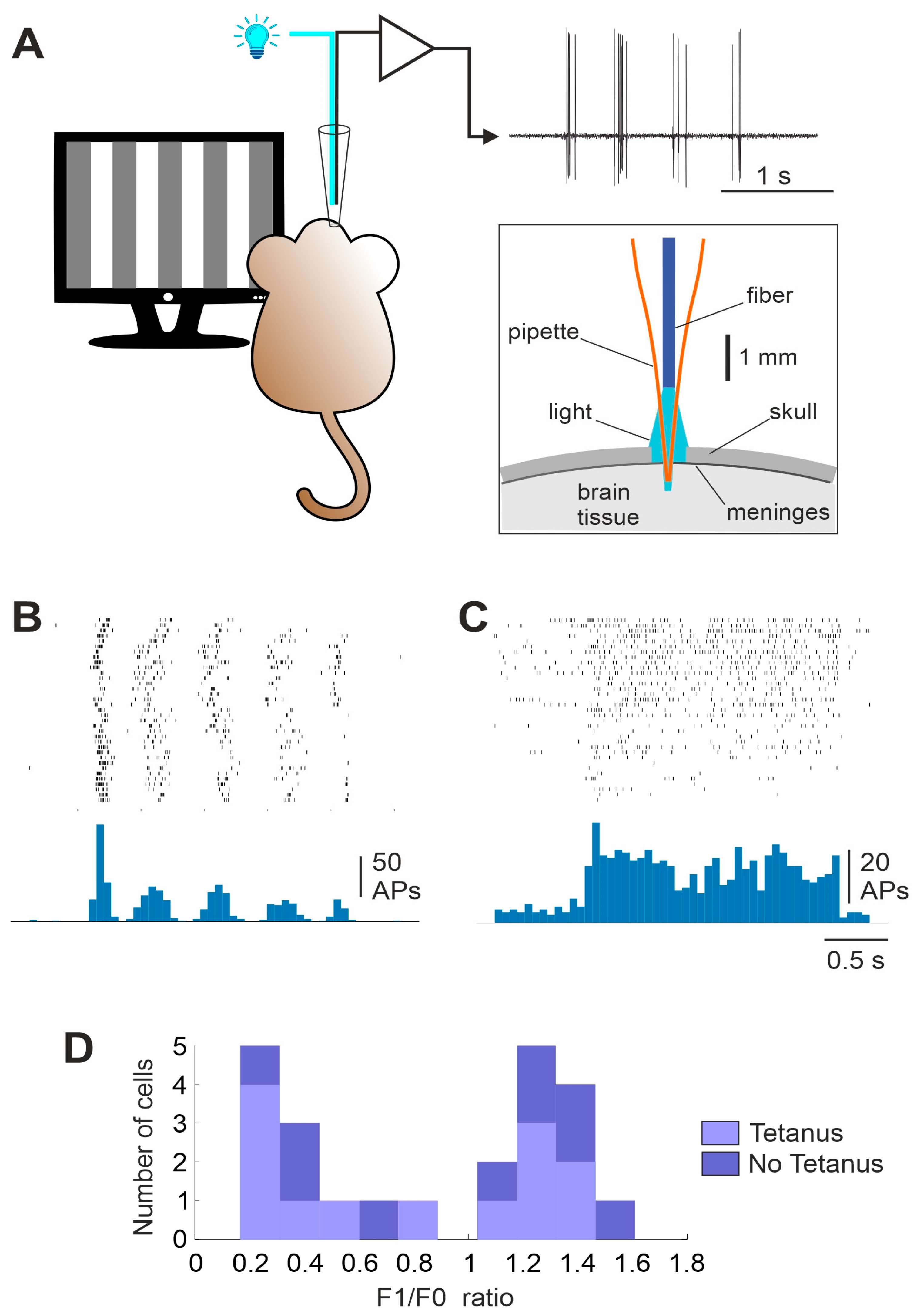
2.4. Visual Stimulation
2.5. Intrinsic Optical Imaging
2.6. Electrophysiological Recording
2.7. Labeling
2.8. Data Analysis
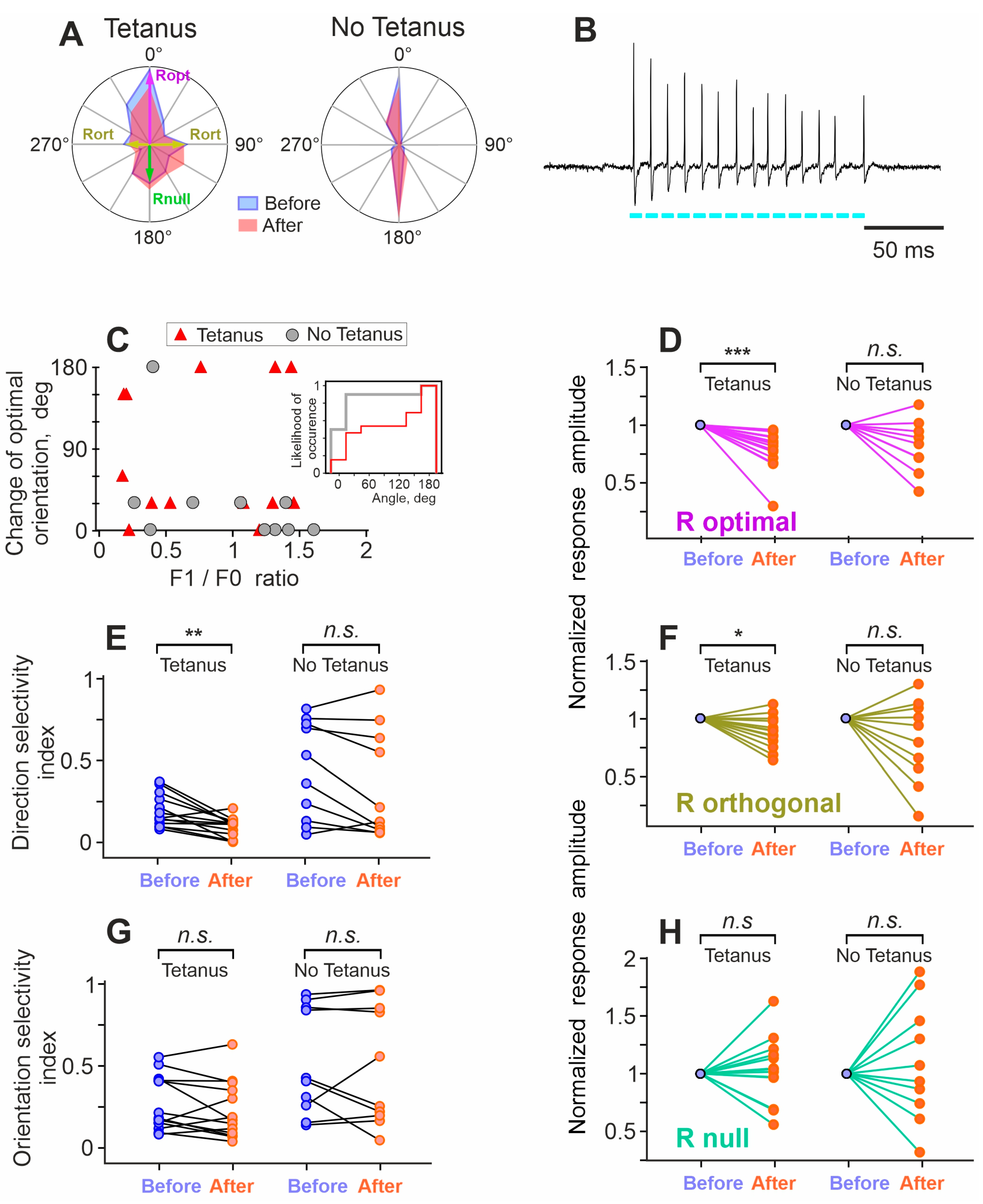
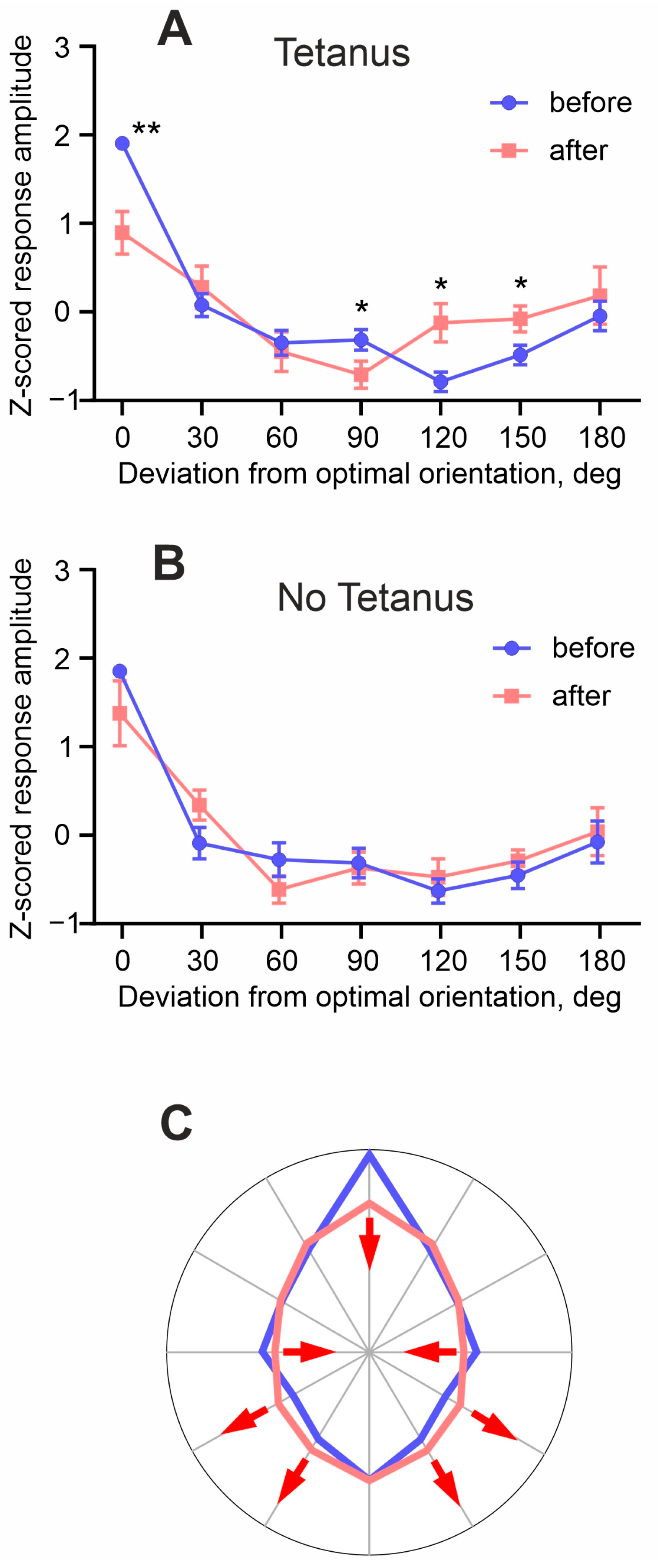
3. Results
4. Discussion
4.1. Homosynaptic and Heterosynaptic Plasticity—Terminology
4.2. Plasticity of Responses and Receptive Fields in Visual Cortex Neurons
4.3. Paradigm of Optogenetic Tetanization to Study Heterosynaptic Plasticity
4.4. Limitations of the Present Study
4.5. Is Optogenetic Tetanization a Purely Postsynaptic Induction Protocol?
4.6. Possible Mechanisms and Functional Consequences of Plasticity Induced by Optogenetic Tetanization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malenka, R.C.; Bear, M.F. LTP and LTD: An Embarrassment of Riches. Neuron 2004, 44, 5–21. [Google Scholar] [CrossRef]
- Caporale, N.; Dan, Y. Spike Timing-Dependent Plasticity: A Hebbian Learning Rule. Annu. Rev. Neurosci. 2008, 31, 25–46. [Google Scholar] [CrossRef]
- Bliss, T.V.P.; Collingridge, G.L. A Synaptic Model of Memory: Long-Term Potentiation in the Hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef]
- Zalutsky, R.A.; Nicoll, R.A. Comparison of Two Forms of Long-Term Potentiation in Single Hippocampal Neurons. Science 1990, 248, 1619–1624. [Google Scholar] [CrossRef]
- Chistiakova, M.; Bannon, N.M.; Chen, J.-Y.; Bazhenov, M.; Volgushev, M. Homeostatic Role of Heterosynaptic Plasticity: Models and Experiments. Front. Comput. Neurosci. 2015, 9, 89. [Google Scholar] [CrossRef]
- Lynch, G.S.; Dunwiddie, T.; Gribkoff, V. Heterosynaptic Depression: A Postsynaptic Correlate of Long-Term Potentiation. Nature 1977, 266, 737–739. [Google Scholar] [CrossRef]
- Kandel, E.R.; Tauc, L. Heterosynaptic Facilitation in Neurones of the Abdominal Ganglion of Aplysia Depilans. J. Physiol. 1965, 181, 1–27. [Google Scholar] [CrossRef]
- Kandel, E.R.; Tauc, L. Mechanism of Heterosynaptic Facilitation in the Giant Cell of the Abdominal Ganglion of Aplysia Depilans. J. Physiol. 1965, 181, 28–47. [Google Scholar] [CrossRef]
- Bailey, C.H.; Giustetto, M.; Huang, Y.Y.; Hawkins, R.D.; Kandel, E.R. Is Heterosynaptic Modulation Essential for Stabilizing Hebbian Plasticity and Memory? Nat. Rev. Neurosci. 2000, 1, 11–20. [Google Scholar] [CrossRef]
- Eyding, D.; Schweigart, G.; Eysel, U.T. Spatio-Temporal Plasticity of Cortical Receptive Fields in Response to Repetitive Visual Stimulation in the Adult Cat. Neuroscience 2002, 112, 195–215. [Google Scholar] [CrossRef]
- Meliza, C.D.; Dan, Y. Receptive-Field Modification in Rat Visual Cortex Induced by Paired Visual Stimulation and Single-Cell Spiking. Neuron 2006, 49, 183–189. [Google Scholar] [CrossRef]
- Smirnov, I.V.; Malyshev, A.Y. Paired Optogenetic and Visual Stimulation Can Change the Orientation Selectivity of Visual Cortex Neurons. Biochem. Biophys. Res. Commun. 2023, 646, 63–69. [Google Scholar] [CrossRef]
- Miller, K.D. Synaptic Economics: Competition and Cooperation in Synaptic Plasticity. Neuron 1996, 17, 371–374. [Google Scholar] [CrossRef]
- Zenke, F.; Hennequin, G.; Gerstner, W. Synaptic Plasticity in Neural Networks Needs Homeostasis with a Fast Rate Detector. PLoS Comput. Biol. 2013, 9, e1003330. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Lonjers, P.; Lee, C.; Chistiakova, M.; Volgushev, M.; Bazhenov, M. Heterosynaptic Plasticity Prevents Runaway Synaptic Dynamics. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 15915–15929. [Google Scholar] [CrossRef]
- Christofi, G.; Nowicky, A.V.; Bolsover, S.R.; Bindman, L.J. The Postsynaptic Induction of Nonassociative Long-Term Depression of Excitatory Synaptic Transmission in Rat Hippocampal Slices. J. Neurophysiol. 1993, 69, 219–229. [Google Scholar] [CrossRef]
- Kuhnt, U.; Kleschevnikov, A.M.; Voronin, L.L. Long Term Enhancement of Synaptic Transmission in the Hippocampus after Tetanization of Single Neurons by Short Intracellular Current Pulses. Neurosci. Res. Commun. 1994, 14, 115–123. [Google Scholar]
- Tsien, J.Z. Linking Hebb’s Coincidence-Detection to Memory Formation. Curr. Opin. Neurobiol. 2000, 10, 266–273. [Google Scholar] [CrossRef]
- White, G.; Levy, W.B.; Steward, O. Spatial Overlap between Populations of Synapses Determines the Extent of Their Associative Interaction during the Induction of Long-Term Potentiation and Depression. J. Neurophysiol. 1990, 64, 1186–1198. [Google Scholar] [CrossRef]
- Royer, S.; Paré, D. Conservation of Total Synaptic Weight through Balanced Synaptic Depression and Potentiation. Nature 2003, 422, 518–522. [Google Scholar] [CrossRef]
- Oh, W.C.; Parajuli, L.K.; Zito, K. Heterosynaptic Structural Plasticity on Local Dendritic Segments of Hippocampal CA1 Neurons. Cell Rep. 2015, 10, 162–169. [Google Scholar] [CrossRef]
- Engert, F.; Bonhoeffer, T. Synapse Specificity of Long-Term Potentiation Breaks down at Short Distances. Nature 1997, 388, 279–284. [Google Scholar] [CrossRef]
- Schuman, E.M.; Madison, D. V Locally Distributed Synaptic Potentiation in the Hippocampus. Science 1994, 263, 532–536. [Google Scholar] [CrossRef]
- Simonova, N.A.; Volgushev, M.A.; Malyshev, A.Y. Enhanced Non-Associative Long-Term Potentiation in Immature Granule Cells in the Dentate Gyrus of Adult Rats. Front. Synaptic Neurosci. 2022, 14, 889947. [Google Scholar] [CrossRef]
- Volgushev, M.; Voronin, L.L.; Chistiakova, M.; Singer, W. Relations between Long-Term Synaptic Modifications and Paired-Pulse Interactions in the Rat Neocortex. Eur. J. Neurosci. 1997, 9, 1656–1665. [Google Scholar] [CrossRef]
- Espadas-Alvarez, A.J.; Bannon, M.J.; Orozco-Barrios, C.E.; Escobedo-Sanchez, L.; Ayala-Davila, J.; Reyes-Corona, D.; Soto-Rodriguez, G.; Escamilla-Rivera, V.; De Vizcaya-Ruiz, A.; Eugenia Gutierrez-Castillo, M.; et al. Regulation of Human GDNF Gene Expression in Nigral Dopaminergic Neurons Using a New Doxycycline-Regulated NTS-Polyplex Nanoparticle System. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1363–1375. [Google Scholar] [CrossRef]
- Chistiakova, M.; Ilin, V.; Roshchin, M.; Bannon, N.; Malyshev, A.; Kisvárday, Z.; Volgushev, M. Distinct Heterosynaptic Plasticity in Fast Spiking and Non-Fast-Spiking Inhibitory Neurons in Rat Visual Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 6865–6878. [Google Scholar] [CrossRef]
- Bravarenko, N.I.; Gusev, P.V.; Balaban, P.M.; Voronin, L.L. Postsynaptic Induction of Long-Term Synaptic Facilitation in Snail Central Neurones. Neuroreport 1995, 6, 1182–1186. [Google Scholar] [CrossRef]
- El-Boustani, S.; Ip, J.P.K.; Breton-Provencher, V.; Knott, G.W.; Okuno, H.; Bito, H.; Sur, M. Locally Coordinated Synaptic Plasticity of Visual Cortex Neurons in vivo. Science 2018, 360, 1349–1354. [Google Scholar] [CrossRef]
- Peirce, J.; Gray, J.R.; Simpson, S.; MacAskill, M.; Höchenberger, R.; Sogo, H.; Kastman, E.; Lindeløv, J.K. PsychoPy2: Experiments in Behavior Made Easy. Behav. Res. Methods 2019, 51, 195–203. [Google Scholar] [CrossRef]
- Idzhilova, O.S.; Smirnova, G.R.; Petrovskaya, L.E.; Kolotova, D.A.; Ostrovsky, M.A.; Malyshev, A.Y. Cationic Channelrhodopsin from the Alga Platymonas Subcordiformis as a Promising Optogenetic Tool. Biochemistry 2022, 87, 1327–1334. [Google Scholar] [CrossRef]
- Jeong, Y.; Cho, H.-Y.; Kim, M.; Oh, J.-P.; Kang, M.S.; Yoo, M.; Lee, H.-S.; Han, J.-H. Synaptic Plasticity-Dependent Competition Rule Influences Memory Formation. Nat. Commun. 2021, 12, 3915. [Google Scholar] [CrossRef]
- Dean, A.F.; Tolhurst, D.J. On the Distinctness of Simple and Complex Cells in the Visual Cortex of the Cat. J. Physiol. 1983, 344, 305–325. [Google Scholar] [CrossRef]
- Skottun, B.C.; De Valois, R.L.; Grosof, D.H.; Movshon, J.A.; Albrecht, D.G.; Bonds, A.B. Classifying Simple and Complex Cells on the Basis of Response Modulation. Vision Res. 1991, 31, 1079–1086. [Google Scholar] [CrossRef]
- Volgushev, M.; Pernberg, J.; Eysel, U.T. γ-Frequency Fluctuations of the Membrane Potential and Response Selectivity in Visual Cortical Neurons. Eur. J. Neurosci. 2003, 17, 1768–1776. [Google Scholar] [CrossRef]
- Zhuang, J.; Stoelzel, C.R.; Bereshpolova, Y.; Huff, J.M.; Hei, X.; Alonso, J.-M.; Swadlow, H.A. Layer 4 in Primary Visual Cortex of the Awake Rabbit: Contrasting Properties of Simple Cells and Putative Feedforward Inhibitory Interneurons. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 11372–11389. [Google Scholar] [CrossRef]
- Levy, W.B.; Steward, O. Temporal Contiguity Requirements for Long-Term Associative Potentiation/Depression in the Hippocampus. Neuroscience 1983, 8, 791–797. [Google Scholar] [CrossRef]
- Lourenço, J.; Pacioni, S.; Rebola, N.; van Woerden, G.M.; Marinelli, S.; DiGregorio, D.; Bacci, A. Non-Associative Potentiation of Perisomatic Inhibition Alters the Temporal Coding of Neocortical Layer 5 Pyramidal Neurons. PLoS Biol. 2014, 12, e1001903. [Google Scholar] [CrossRef]
- Sjöström, P.J.; Häusser, M. A Cooperative Switch Determines the Sign of Synaptic Plasticity in Distal Dendrites of Neocortical Pyramidal Neurons. Neuron 2006, 51, 227–238. [Google Scholar] [CrossRef]
- Glanzman, D.L.; Mackey, S.L.; Hawkins, R.D.; Dyke, A.M.; Lloyd, P.E.; Kandel, E.R. Depletion of Serotonin in the Nervous System of Aplysia Reduces the Behavioral Enhancement of Gill Withdrawal as Well as the Heterosynaptic Facilitation Produced by Tail Shock. J. Neurosci. Off. J. Soc. Neurosci. 1989, 9, 4200–4213. [Google Scholar] [CrossRef]
- Yao, H.; Dan, Y. Stimulus Timing-Dependent Plasticity in Cortical Processing of Orientation. Neuron 2001, 32, 315–323. [Google Scholar] [CrossRef]
- Kowalski, J.; Gan, J.; Jonas, P.; Pernía-Andrade, A.J. Intrinsic Membrane Properties Determine Hippocampal Differential Firing Pattern in vivo in Anesthetized Rats. Hippocampus 2016, 26, 668–682. [Google Scholar] [CrossRef]
- Steriade, M.; Timofeev, I.; Grenier, F. Natural Waking and Sleep States: A View from inside Neocortical Neurons. J. Neurophysiol. 2001, 85, 1969–1985. [Google Scholar] [CrossRef]
- Timofeev, I.; Grenier, F.; Steriade, M. Disfacilitation and Active Inhibition in the Neocortex during the Natural Sleep-Wake Cycle: An Intracellular Study. Proc. Natl. Acad. Sci. USA 2001, 98, 1924–1929. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Olcese, U.; Lazimy, Y.M.; Faraguna, U.; Esser, S.K.; Williams, J.C.; Cirelli, C.; Tononi, G. Cortical Firing and Sleep Homeostasis. Neuron 2009, 63, 865–878. [Google Scholar] [CrossRef]
- Timofeev, I.; Bonjean, M.E.; Bazhenov, M. Cellular Mechanisms of Thalamocortical Oscillations in the Sleeping Brain. In Neuronal Oscillations of Wakefulness and Sleep: Windows on Spontaneous Activity of the Brain; Dang-Vu, T.T., Courtemanche, R., Eds.; Springer: New York, NY, USA, 2020; pp. 119–170. ISBN 978-1-0716-0653-7. [Google Scholar]
- Peixoto, H.M.; Cruz, R.M.S.; Moulin, T.C.; Leão, R.N. Modeling the Effect of Temperature on Membrane Response of Light Stimulation in Optogenetically-Targeted Neurons. Front. Comput. Neurosci. 2020, 14, 5. [Google Scholar] [CrossRef]
- Sjöström, P.J.; Turrigiano, G.G.; Nelson, S.B. Rate, Timing, and Cooperativity Jointly Determine Cortical Synaptic Plasticity. Neuron 2001, 32, 1149–1164. [Google Scholar] [CrossRef]
- Feldman, D.E. Synaptic Mechanisms for Plasticity in Neocortex. Annu. Rev. Neurosci. 2009, 32, 33–55. [Google Scholar] [CrossRef]
- Stark, E.; Koos, T.; Buzsáki, G. Diode Probes for Spatiotemporal Optical Control of Multiple Neurons in Freely Moving Animals. J. Neurophysiol. 2012, 108, 349–363. [Google Scholar] [CrossRef]
- Wang, H.; Peca, J.; Matsuzaki, M.; Matsuzaki, K.; Noguchi, J.; Qiu, L.; Wang, D.; Zhang, F.; Boyden, E.; Deisseroth, K.; et al. High-Speed Mapping of Synaptic Connectivity Using Photostimulation in Channelrhodopsin-2 Transgenic Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 8143–8148. [Google Scholar] [CrossRef]
- Thomson, A.M.; West, D.C.; Wang, Y.; Bannister, A.P. Synaptic Connections and Small Circuits Involving Excitatory and Inhibitory Neurons in Layers 2-5 of Adult Rat and Cat Neocortex: Triple Intracellular Recordings and Biocytin Labelling in vitro. Cereb. Cortex 2002, 12, 936–953. [Google Scholar] [CrossRef]
- Petreanu, L.; Mao, T.; Sternson, S.M.; Svoboda, K. The Subcellular Organization of Neocortical Excitatory Connections. Nature 2009, 457, 1142–1145. [Google Scholar] [CrossRef]
- Hammond, P.; Kim, J.N. Role of Suppression in Shaping Orientation and Direction Selectivity of Complex Neurons in Cat Striate Cortex. J. Neurophysiol. 1996, 75, 1163–1176. [Google Scholar] [CrossRef]
- Priebe, N.J.; Ferster, D. Direction Selectivity of Excitation and Inhibition in Simple Cells of the Cat Primary Visual Cortex. Neuron 2005, 45, 133–145. [Google Scholar] [CrossRef]
- Rossi, L.F.; Harris, K.D.; Carandini, M. Spatial Connectivity Matches Direction Selectivity in Visual Cortex. Nature 2020, 588, 648–652. [Google Scholar] [CrossRef]
- Volgushev, M.; Chen, J.-Y.; Ilin, V.; Goz, R.; Chistiakova, M.; Bazhenov, M. Partial Breakdown of Input Specificity of STDP at Individual Synapses Promotes New Learning. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 8842–8855. [Google Scholar] [CrossRef]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.-C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid Regulation of Depression-Related Behaviours by Control of Midbrain Dopamine Neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef]
- Brown, W.M.; Bäcker, A. Optimal Neuronal Tuning for Finite Stimulus Spaces. Neural Comput. 2006, 18, 1511–1526. [Google Scholar] [CrossRef]
- Montemurro, M.A.; Panzeri, S. Optimal Tuning Widths in Population Coding of Periodic Variables. Neural Comput. 2006, 18, 1555–1576. [Google Scholar] [CrossRef]
- Ghose, G.M.; Yang, T.; Maunsell, J.H.R. Physiological Correlates of Perceptual Learning in Monkey V1 and V2. J. Neurophysiol. 2002, 87, 1867–1888. [Google Scholar] [CrossRef]
- Seitz, A.R. Perceptual Learning. Curr. Biol. 2017, 27, R631–R636. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bear, M.F. Visual Experience Induces Long-Term Potentiation in the Primary Visual Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 16304–16313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, I.V.; Osipova, A.A.; Smirnova, M.P.; Borodinova, A.A.; Volgushev, M.A.; Malyshev, A.Y. Plasticity of Response Properties of Mouse Visual Cortex Neurons Induced by Optogenetic Tetanization In Vivo. Curr. Issues Mol. Biol. 2024, 46, 3294-3312. https://doi.org/10.3390/cimb46040206
Smirnov IV, Osipova AA, Smirnova MP, Borodinova AA, Volgushev MA, Malyshev AY. Plasticity of Response Properties of Mouse Visual Cortex Neurons Induced by Optogenetic Tetanization In Vivo. Current Issues in Molecular Biology. 2024; 46(4):3294-3312. https://doi.org/10.3390/cimb46040206
Chicago/Turabian StyleSmirnov, Ivan V., Aksiniya A. Osipova, Maria P. Smirnova, Anastasia A. Borodinova, Maxim A. Volgushev, and Alexey Y. Malyshev. 2024. "Plasticity of Response Properties of Mouse Visual Cortex Neurons Induced by Optogenetic Tetanization In Vivo" Current Issues in Molecular Biology 46, no. 4: 3294-3312. https://doi.org/10.3390/cimb46040206
APA StyleSmirnov, I. V., Osipova, A. A., Smirnova, M. P., Borodinova, A. A., Volgushev, M. A., & Malyshev, A. Y. (2024). Plasticity of Response Properties of Mouse Visual Cortex Neurons Induced by Optogenetic Tetanization In Vivo. Current Issues in Molecular Biology, 46(4), 3294-3312. https://doi.org/10.3390/cimb46040206







