Role of Filamin A in Growth and Migration of Breast Cancer—Review
Abstract
:1. Introduction
2. Breast Cancer
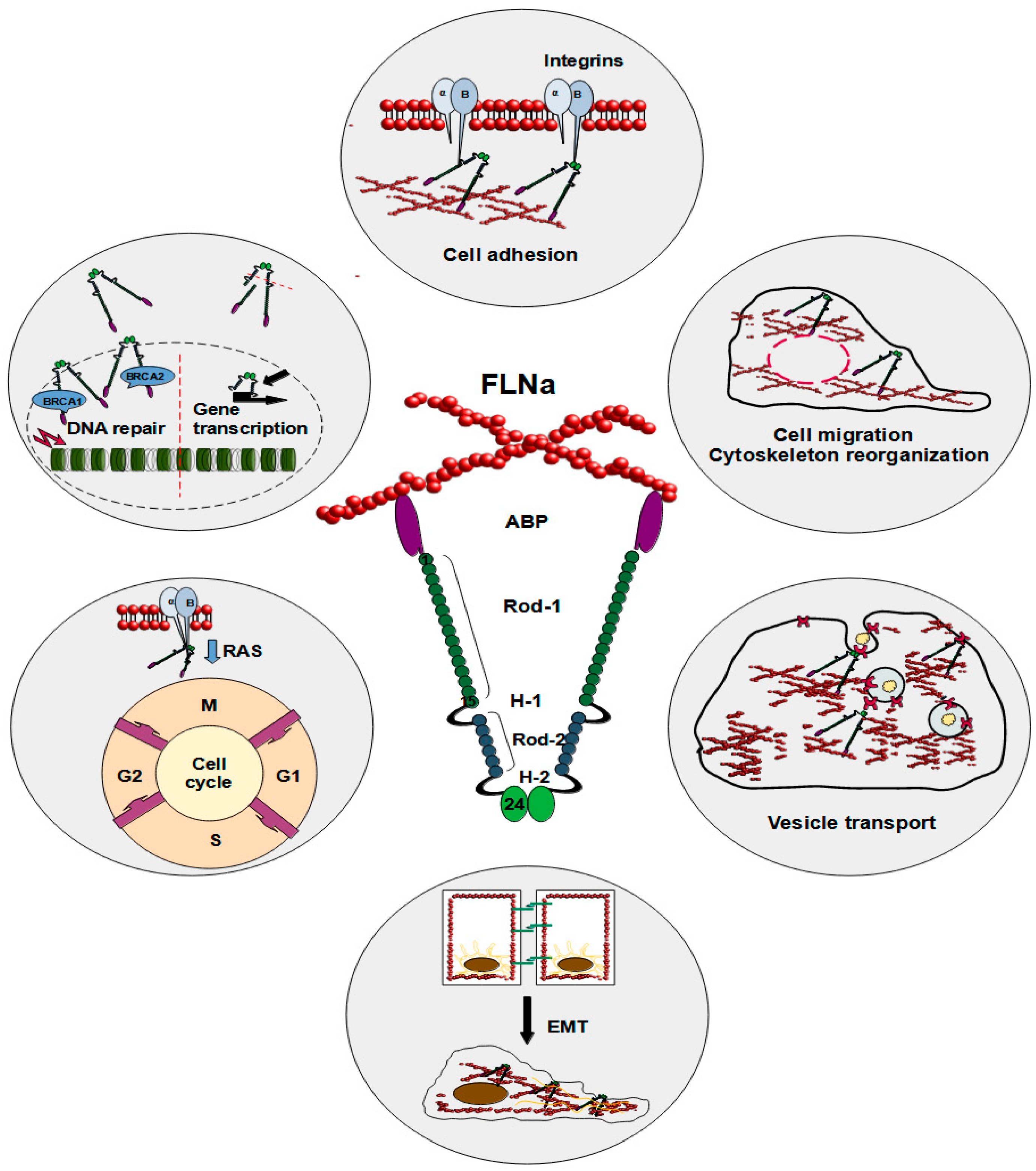
3. Filamin A and Its Functions
4. Role of Filamin A in Breast Cancer
4.1. The Expression of Filamin A in Breast Cancer
4.2. Role of Filamin A in Cell Migration and Invasiveness of Breast Cancer Cells
4.3. Role of Filamin A in DNA Repair and Therapy Outcome in Breast Cancer
4.4. Role of Filamin A in the Regulation of Gene Expression in Breast Cancer
4.5. Role of Filamin A in the Angiogenesis of Breast Cancer
4.6. Clinical Relevance of Filamin A in Breast Cancer and Future Perspectives
5. Conclusions
Funding
Conflicts of Interest
References
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast Cancer in Young Women: An Overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and Secondary Prevention of Breast Cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, S.; Ala, C.; Zhou, A.-X.; Akyürek, L.M. Filamin A Regulates Cardiovascular Remodeling. Int. J. Mol. Sci. 2021, 22, 6555. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.P.; Raslova, H.; Bryckaert, M. Filamin A: Key Actor in Platelet Biology. Blood 2019, 134, 1279–1288. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, X.; An, H.; Lv, Y.; Liu, X. The Function and Pathogenic Mechanism of Filamin A. Gene 2021, 784, 145575. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.Q.; Zhang, T.P.; Zhao, W.J.; Liu, Z.W.; You, L.; Zhou, L.; Guo, J.C.; Zhao, Y.P. Filamin A: Insights into Its Exact Role in Cancers. Pathol. Oncol. Res. POR 2016, 22, 245–252. [Google Scholar] [CrossRef]
- Xu, Y.; Bismar, T.A.; Su, J.; Xu, B.; Kristiansen, G.; Varga, Z.; Teng, L.; Ingber, D.E.; Mammoto, A.; Kumar, R.; et al. Filamin A Regulates Focal Adhesion Disassembly and Suppresses Breast Cancer Cell Migration and Invasion. J. Exp. Med. 2010, 207, 2421–2437. [Google Scholar] [CrossRef]
- Zhu, T.N.; He, H.J.; Kole, S.; D’Souza, T.; Agarwal, R.; Morin, P.J.; Bernier, M. Filamin A-Mediated down-Regulation of the Exchange Factor Ras-GRF1 Correlates with Decreased Matrix Metalloproteinase-9 Expression in Human Melanoma Cells. J. Biol. Chem. 2007, 282, 14816–14826. [Google Scholar] [CrossRef]
- Savoy, R.M.; Ghosh, P.M. The Dual Role of Filamin A in Cancer: Can’t Live with (Too Much of) It, Can’t Live without It. Endocr. Relat. Cancer 2013, 20, R341–R356. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Yeow, W.S.; Zou, C.; Wassell, R.; Wang, C.; Pestell, R.G.; Quong, J.N.; Quong, A.A. Cyclin D1/Cyclin-Dependent Kinase 4 Interacts with Filamin A and Affects the Migration and Invasion Potential of Breast Cancer Cells. Cancer Res. 2010, 70, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Weisman, P.S.; Ng, C.K.Y.; Brogi, E.; Eisenberg, R.E.; Won, H.H.; Piscuoglio, S.; De Filippo, M.R.; Ioris, R.; Akram, M.; Norton, L.; et al. Genetic Alterations of Triple Negative Breast Cancer by Targeted Next-Generation Sequencing and Correlation with Tumor Morphology. Mod. Pathol. 2016, 29, 476–488. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Vynios, D.; Brézillon, S. Epithelial-to-Mesenchymal Transition and Invadopodia Markers in Breast Cancer: Lumican a Key Regulator. Semin. Cancer Biol. 2020, 62, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.M.; Dunworth, M.; Sharma, G.; Loth, M.; Tandurella, J.; Cimino-Mathews, A.; Gentz, M.; Bracht, S.; Haynes, M.; Fertig, E.J.; et al. Triple Negative Breast Cancer Metastasis Involves Complex EMT Dynamics and Requires Vimentin HHS Public Access. Sci. Transl. Med. 2022, 14, 7571. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Borén, J.; Akyürek, L.M. Filamins in Cardiovascular Development. Trends Cardiovasc. Med. 2007, 17, 222–229. [Google Scholar] [CrossRef]
- Mao, Z.; Nakamura, F. Interaction of LARP4 to Filamin A Mechanosensing Domain Regulates Cell Migrations. Front. Cell Dev. Biol. 2023, 11, 1152109. [Google Scholar] [CrossRef] [PubMed]
- Lamsoul, I.; Dupré, L.; Lutz, P.G. Molecular Tuning of Filamin A Activities in the Context of Adhesion and Migration. Front. Cell Dev. Biol. 2020, 8, 591323. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, F.; Ithychanda, S.S.; Apostol, M.; Das, M.; Deshpande, G.; Plow, E.F.; Qin, J. A Mechanism of Platelet Integrin αIIbβ3 Outside-in Signaling through a Novel Integrin αIIb Subunit–Filamin–Actin Linkage. Blood 2023, 141, 2629–2641. [Google Scholar] [CrossRef]
- Kiema, T.; Lad, Y.; Jiang, P.; Oxley, C.L.; Baldassarre, M.; Wegener, K.L.; Campbell, I.D.; Ylänne, J.; Calderwood, D.A. The Molecular Basis of Filamin Binding to Integrins and Competition with Talin. Mol. Cell 2006, 21, 337–347. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Huttenlocher, A.; Kiosses, W.B.; Rose, D.M.; Woodside, D.G.; Schwartz, M.A.; Ginsberg, M.H. Increased Filamin Binding to β-Integrin Cytoplasmic Domains Inhibits Cell Migration. Nat. Cell Biol. 2001, 3, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.C.; Gorlin, J.B.; Kwiatkowski, D.J.; Hartwig, J.H.; Janmey, P.A.; Byers, H.R.; Stossel, T.P. Actin-Binding Protein Requirement for Cortical Stability and Efficient Locomotion. Science 1992, 255, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Caznok Silveira, A.C.; Antunes, A.S.L.M.; Athié, M.C.P.; da Silva, B.F.; Ribeiro Dos Santos, J.V.; Canateli, C.; Fontoura, M.A.; Pinto, A.; Pimentel-Silva, L.R.; Avansini, S.H.; et al. Between Neurons and Networks: Investigating Mesoscale Brain Connectivity in Neurological and Psychiatric Disorders. Front. Neurosci. 2024, 18, 1340345. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, Y.; Azuma, Y.; Suzuki, Y.; Hoshina, M.; Uchiyama, Y.; Mitsuhashi, S.; Miyatake, S.; Mizuguchi, T.; Takata, A.; Miyake, N.; et al. Hemizygous FLNA Variant in West Syndrome without Periventricular Nodular Heterotopia. Hum. Genome Var. 2020, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, X.; Zhang, Z.; He, Y.; Huo, B.; Guo, X.; Feng, X.; Fang, Z.-M.; Jiang, D.-S.; Zhu, X.-H. Downregulation of Filamin a Expression in the Aorta Is Correlated with Aortic Dissection. Front. Cardiovasc. Med. 2021, 8, 690846. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Weber, A.; Maly, K.; Manjaly, G.; Deek, J.; Tsvyetkova, O.; Stulić, M.; Toca-Herrera, J.L.; Jantsch, M.F. A-to-I RNA Editing of Filamin A Regulates Cellular Adhesion, Migration and Mechanical Properties. FEBS J. 2022, 289, 4580–4601. [Google Scholar] [CrossRef] [PubMed]
- Cukier, I.H.; Li, Y.; Lee, J.M. Cyclin B1/Cdk1 Binds and Phosphorylates Filamin A and Regulates Its Ability to Cross-Link Actin. FEBS Lett. 2007, 581, 1661–1672. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, A.M.; Ladha, F.A.; Hinson, J.T. The Cardiac Sarcomere and Cell Cycle. Curr. Cardiol. Rep. 2022, 24, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, M.; Zielińska, W.; Hałas-Wiśniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef]
- Hammer, A.; Rider, L.; Oladimeji, P.; Cook, L.; Li, Q.; Mattingly, R.R.; Diakonova, M. Tyrosyl Phosphorylated PAK1 Regulates Breast Cancer Cell Motility in Response to Prolactin through Filamin A. Mol. Endocrinol. 2013, 27, 455–465. [Google Scholar] [CrossRef]
- Carrasco-Ceballos, J.M.; Barrera-Hernández, D.; Locia-Espinosa, J.; Sampieri, C.L.; Lara-Reyes, J.A.; Hernández-Aguilar, M.E.; Aranda-Abreu, G.E.; Toledo-Cárdenas, M.R.; Chi-Castañeda, L.D.; Pérez-Estudillo, C.A.; et al. Involvement of the PRL-PAK1 Pathway in Cancer Cell Migration. Cancer Diagn. Progn. 2023, 3, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Fang, Y.; Chen, X.; Chen, X.; Xia, Z.; Huang, H.; Xia, Y.; Liu, P.; Tian, X.; Cai, Q. Targeting P21-Activated Kinase Suppresses Proliferation and Enhances Chemosensitivity in T-Cell Lymphoblastic Lymphoma. Blood Sci. 2023, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Diakonova, M. Tyrosyl Phosphorylated Serine-Threonine Kinase PAK1 Is a Novel Regulator of Prolactin-Dependent Breast Cancer Cell Motility and Invasion. Adv. Exp. Med. Biol. 2015, 846, 97–137. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, P.; Di Donato, M.; Licitra, F.; Sabbatino, E.; Tutino, V.; Castoria, G.; Migliaccio, A. Filamin A in Triple Negative Breast Cancer. Steroids 2024, 205, 109380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Jiang, Y.; Yang, H.; Zhao, D.; Li, L.; Liu, X. FLNA Promotes Chemoresistance of Colorectal Cancer through Inducing Epithelial-Mesenchymal Transition and Smad2 Signaling Pathway. Am. J. Cancer Res. 2020, 10, 403. [Google Scholar] [PubMed]
- Wieczorek, K.; Wiktorska, M.; Sacewicz-Hofman, I.; Boncela, J.; Lewiński, A.; Kowalska, M.A.; Niewiarowska, J. Filamin A Upregulation Correlates with Snail-Induced Epithelial to Mesenchymal Transition (EMT) and Cell Adhesion but Its Inhibition Increases the Migration of Colon Adenocarcinoma HT29 Cells. Exp. Cell Res. 2017, 359, 163–170. [Google Scholar] [CrossRef]
- Nakamura, F. The Role of Mechanotransduction in Contact Inhibition of Locomotion and Proliferation. Int. J. Mol. Sci. 2024, 25, 2135. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Moretti, A.; Sorrentino, C.; Toro, G.; Gentile, G.; Iolascon, G.; Castoria, G.; Migliaccio, A. Filamin A Cooperates with the Androgen Receptor in Preventing Skeletal Muscle Senescence. Cell Death Discov. 2023, 9, 437. [Google Scholar] [CrossRef]
- Joshua, L.M.; Huda, F.; Rao, S.; Ravi, B. Clinicopathological Significance of Immunohistochemical Expression of Filamin A in Breast Cancer. J. Carcinog. 2020, 19, 13. [Google Scholar] [CrossRef]
- Tian, H.M.; Liu, X.H.; Han, W.; Zhao, L.L.; Yuan, B.; Yuan, C.J. Differential Expression of Filamin A and Its Clinical Significance in Breast Cancer. Oncol. Lett. 2013, 6, 681–686. [Google Scholar] [CrossRef]
- Guo, Y.; Li, M.; Bai, G.; Li, X.; Sun, Z.; Yang, J.; Wang, L.; Sun, J. Filamin A Inhibits Tumor Progression through Regulating BRCA1 Expression in Human Breast Cancer. Oncol. Lett. 2018, 16, 6261–6266. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yue, J.; Lu, H.; Campbell, N.; Yang, Q.; Lan, S.; Haffty, B.G.; Yuan, C.; Shen, Z. Inhibition of Filamin-A Reduces Cancer Metastatic Potential. Int. J. Biol. Sci. 2013, 9, 67. [Google Scholar] [CrossRef]
- Ljepoja, B.; Schreiber, C.; Gegenfurtner, F.A.; García-Roman, J.; Köhler, B.; Zahler, S.; Rädler, J.O.; Wagner, E.; Roidl, A. Inducible microRNA-200c Decreases Motility of Breast Cancer Cells and Reduces Filamin A. PLoS ONE 2019, 14, e0224314. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, S.J.; Reddy, S.; Rybak, Y.; Adem, A.; Libutti, S.K. Down-Regulation of Filamin Ainteracting Protein 1-like Is Associated with Promoter Methylation and an Invasive Phenotype in Breast, Colon, Lung and Pancreatic Cancers. PLoS ONE 2013, 8, e82620. [Google Scholar] [CrossRef]
- Grande, V.; Schuld, J.; van der Ven, P.F.M.; Gruss, O.J.; Fürst, D.O. Filamin-A-Interacting Protein 1 (FILIP1) Is a Dual Compartment Protein Linking Myofibrils and Microtubules during Myogenic Differentiation and upon Mechanical Stress. Cell Tissue Res. 2023, 393, 133–147. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, L.; Xu, P.; Li, Y.; Ji, Z.; Kang, X. Filamin A Is a Potential Driver of Breast Cancer Metastasis via Regulation of MMP-1. Front. Oncol. 2022, 12, 836126. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.M.; Yang, L.L.; Ni, J.; Xu, S.P.; Yang, C.; Duan, P.; Lou, L.P.; Ruan, Q.R. Silencing Filamin A Inhibits the Invasion and Migration of Breast Cancer Cells by Up-Regulating 14-3-3σ. Curr. Med. Sci. 2018, 38, 461–466. [Google Scholar] [CrossRef]
- Ravid, D.; Chuderland, D.; Landsman, L.; Lavie, Y.; Reich, R.; Liscovitch, M. Filamin A Is a Novel Caveolin-1-Dependent Target in IGF-I-Stimulated Cancer Cell Migration. Exp. Cell Res. 2008, 314, 2762–2773. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yuan, Y.; Maestas, A.; Shen, Z. Recovery from DNA Damage-Induced G2 Arrest Requires Actin-Binding Protein Filamin-A/Actin-Binding Protein 280*. J. Biol. Chem. 2004, 279, 6098–6105. [Google Scholar] [CrossRef]
- Velkova, A.; Carvalho, M.A.; Johnson, J.O.; Tavtigian, S.V.; Monteiro, A.N.A. Identification of Filamin A as a BRCA1-Interacting Protein Required for Efficient DNA Repair. Cell Cycle 2010, 9, 1421–1433. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Sun, H.; Zhang, Z.; Gu, J. LncRNA MCM3AP-AS1 Promotes Chemoresistance in Triple-Negative Breast Cancer through the miR-524-5p/RBM39 Axis. Mol. Cell. Biochem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Alipournazari, P.; Pourmadadi, M.; Abdouss, M.; Rahdar, A.; Pandey, S. Enhanced Delivery of Doxorubicin for Breast Cancer Treatment Using pH-Sensitive Starch/PVA/g-C3N4 Hydrogel. Int. J. Biol. Macromol. 2024, 265, 130901. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Kobayashi, A.; Kawashima, M.; Kawaguchi-Sakita, N.; Nakakura, A.; Kataoka, Y.; Shide, K.; Mori, Y.; Yamazaki, K.; Toi, M.; et al. Quantitative Analysis of the Effect of Docetaxel-Induced Edema on Quality of Life in Patients with Breast Cancer and Related Factors: A Prospective Cohort Study. BMC Womens Health 2024, 24, 165. [Google Scholar] [CrossRef] [PubMed]
- Gwin, W.R.; Disis, M.L. Chapter 13—Harnessing the Immune System in HER2+ Disease. In Her2-Positive Breast Cancer; Hurvitz, S., McCann, K., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 213–230. ISBN 978-0-323-58122-6. [Google Scholar]
- Zhao, P.; Ma, W.; Hu, Z.; Zang, L.; Tian, Z.; Zhang, K. Filamin A (FLNA) Modulates Chemosensitivity to Docetaxel in Triple-Negative Breast Cancer through the MAPK/ERK Pathway. Tumor Biol. 2016, 37, 5107–5115. [Google Scholar] [CrossRef] [PubMed]
- Mouron, S.; Bueno, M.J.; Lluch, A.; Manso, L.; Calvo, I.; Cortes, J.; Garcia-Saenz, J.A.; Gil-Gil, M.; Martinez-Janez, N.; Apala, J.V.; et al. Phosphoproteomic Analysis of Neoadjuvant Breast Cancer Suggests That Increased Sensitivity to Paclitaxel Is Driven by CDK4 and Filamin A. Nat. Commun. 2022, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Manjaly, G.; Maly, K.; de Vries, M.R.; Janisiw, M.; König, L.; Nossent, A.Y.; Jantsch, M.F. Filamin A Pre-mRNA Editing Modulates Vascularization and Tumor Growth. Mol. Ther. Nucleic Acids 2022, 30, 522–534. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, A.-X.; Rouhi, P.; Uramoto, H.; Borén, J.; Cao, Y.; Pereira, T.; Akyürek, L.M.; Poellinger, L. Hypoxia-Induced and Calpain-Dependent Cleavage of Filamin A Regulates the Hypoxic Response. Proc. Natl. Acad. Sci. USA 2014, 111, 2560–2565. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, J.; He, Y.; Liu, X.; Mao, G.; Wei, B.; Liao, S.; Zhang, Q.; Li, J.; Zheng, L.; et al. R5, a Neutralizing Antibody to Robo1, Suppresses Breast Cancer Growth and Metastasis by Inhibiting Angiogenesis via down-Regulating Filamin A. Exp. Cell Res. 2020, 387, 111756. [Google Scholar] [CrossRef]
- Fredolini, C.; Pathak, K.V.; Paris, L.; Chapple, K.M.; Tsantilas, K.A.; Rosenow, M.; Tegeler, T.J.; Garcia-Mansfield, K.; Tamburro, D.; Zhou, W.; et al. Shotgun Proteomics Coupled to Nanoparticle-Based Biomarker Enrichment Reveals a Novel Panel of Extracellular Matrix Proteins as Candidate Serum Protein Biomarkers for Early-Stage Breast Cancer Detection. Breast Cancer Res. 2020, 22, 135. [Google Scholar] [CrossRef]
- Alper, Ö.; Stetler-Stevenson, W.G.; Harris, L.N.; Leitner, W.W.; Özdemirli, M.; Hartmann, D.; Raffeld, M.; Abu-Asab, M.; Byers, S.; Zhuang, H.; et al. Novel Anti-Filamin-A Antibody Detects a Secreted Variant of Filamin-A in Plasma from Patients with Breast Carcinoma and High-Grade Astrocytoma. Cancer Sci. 2009, 100, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Di Tommaso, L.; Soldani, C.; Franceschini, B.; Terrone, A.; Mimmo, A.; Vitali, E.; Roncalli, M.; Lania, A.; Torzilli, G. Filamin A Expression Predicts Early Recurrence of Hepatocellular Carcinoma after Hepatectomy. Liver Int. 2018, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Salimi, R.; Bandaru, S.; Devarakonda, S.; Gökalp, S.; Ala, C.; Alvandian, A.; Yener, N.; Akyürek, L.M. Blocking the Cleavage of Filamin A by Calpain Inhibitor Decreases Tumor Cell Growth. Anticancer Res. 2018, 38, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Mataga, M.A.; Rosenthal, S.; Heerboth, S.; Devalapalli, A.; Kokolus, S.; Evans, L.R.; Longacre, M.; Housman, G.; Sarkar, S. Anti-Breast Cancer Effects of Histone Deacetylase Inhibitors and Calpain Inhibitor. Anticancer Res. 2012, 32, 2523–2529. [Google Scholar] [PubMed]
| Role | Material | Conclusions | References |
|---|---|---|---|
| Expression | Breast cancer tissue | FLNa present in both normal tissue and non-malignant carcinoma | [39] |
| Exclusively cytoplasmic expression in all breast cancer samples Primarily membranous with some cytoplasmic presence in normal tissues | |||
| No significant relationship between FLNa levels and clinicopathological parameters | |||
| Breast cancer tissue | FLNa mostly undetectable in normal breast tissue and benign tumor samples | [40] | |
| Increase in the percentage of FLNa-positive samples with cancer’s progression | |||
| FLNa mostly located in cytoplasm, especially at the cell edges, present in basal cells and the intercellular substance | |||
| Correlation between FLNa levels and TNM stage, presence of metastases, vascular or neural invasion, and menstrual status | |||
| Breast cancer tissue | FLNa hardly noticeable in normal breast tissue and benign tumors | [41] | |
| Primarily found in the cytoplasm of myoepithelial and basal cells, or between cells in breast cancer tissue | |||
| Negative correlation between FLNa expression and tumor size Correlation between FLNa and increased expression of progesterone receptors | |||
| Breast cancer tissue and MDA-MB-231 cells | FLNa protein expression in small number of samples | [42] | |
| Primarily found in the cytoplasm, for MDA-MB-231 cells also at the cell membrane | |||
| MDA-MB-231 cells | Lower FLNa expression in cells transduced with a TET-off construct containing mir-200c | [43] | |
| MCF-7 cells | Increase of FLNa expression after knocking out mir-200c | ||
| MCF-7 cells | Lowering FILIP1L levels increases FLNa expression | [44] | |
| Migration and invasion | MCF-10A and MDA-MB-231 cells | While not fatal, knocking out FLNa lowers cancer cells’ ability to migrate | [46] |
| Mice xenograft | Mice with FLNa knocked out showed no metastasis | ||
| MDA-MB-231 cells | Silencing FLNa decreases the rate of migration and invasion of breast cancer cells | [47] | |
| MDA-MB-231 and MDA-MB-436 cells | Inhibition of FLNa hinders cell migration and invasion | [42] | |
| Mice xenograft | Mice injected with FLNa-deficient breast cancer cells showed less frequent metastasis | ||
| MCF-7 cells | Expression of caveolin 1 mediated by its filamin A phosphorylation enhancing properties increased cells motility | [48] | |
| MDA-MB-231, MCF-7, BT-549, Hs 578T, MDA-MB-468, BT-474, ZR-75-1, and HMECs | FILIP1 mRNA expression shows negative correlation with invasiveness of the cells | [44] | |
| DNA repair and therapy outcome | MDA-MB-231, HCC38, Htb126 and HCC19337 cells | Knocking down FLNa increases sensitization to docetaxel but not doxorubicin | [55] |
| Mouse xenograft | Mice with FLNa knocked down were more sensitive to docetaxel compared to control group | ||
| MDA-MB-231 cells | Elevated levels of FLNa increase sensitivity to paclitaxel | [56] | |
| Regulation of gene expression | MCF-7 and MDA-MB-231 cells | siRNA knockdown of JUN in wild type MDA-MB-231 and MCF-7 cells carried out FLNa mRNA decrease | [43] |
| MCF-7 cells | Increased levels of caveolin-1 increase FLNa expression Caveolin-1 and IGF-I-dependent phosphorylation of FLNa happens via the PI3K/Akt pathway | [48] | |
| Filamin A as a breast cancer marker | Serum samples | Using markers with combinations of proteins that contained FLNa carried high sensitivity and specificity against breast cancer | [60] |
| MCF-7, SK-BR-3, BT-474, BT-20, BT-549, and ZR-75-1 MCF-10A and HMECs | FLNa expression increased in media of breast cancer cells’ media, while not found in normal HMECs or MCF-10A cells | [61] | |
| Breast cancer tissues | Lack of FLNa could be used as a prognostic marker for cancer metastasis | [42] | |
| Angiogenesis | Mouse xenograft | Under hypoxic conditions calpain fragmentates FLNa, which interacts with HIF-1α facilitating its nuclear accumulation | [58] |
| Mouse xenograft 4T1, HUVEC cells | R5 downregulates FLNa which in turn inhibits breast cancer progression and metastasis by suppressing angiogenesis | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zawadka, P.; Zielińska, W.; Gagat, M.; Izdebska, M. Role of Filamin A in Growth and Migration of Breast Cancer—Review. Curr. Issues Mol. Biol. 2024, 46, 3408-3423. https://doi.org/10.3390/cimb46040214
Zawadka P, Zielińska W, Gagat M, Izdebska M. Role of Filamin A in Growth and Migration of Breast Cancer—Review. Current Issues in Molecular Biology. 2024; 46(4):3408-3423. https://doi.org/10.3390/cimb46040214
Chicago/Turabian StyleZawadka, Patryk, Wioletta Zielińska, Maciej Gagat, and Magdalena Izdebska. 2024. "Role of Filamin A in Growth and Migration of Breast Cancer—Review" Current Issues in Molecular Biology 46, no. 4: 3408-3423. https://doi.org/10.3390/cimb46040214





