Insights into the Molecular Mechanism of Endothelial Glycocalyx Dysfunction during Heart Surgery
Abstract
1. Introduction
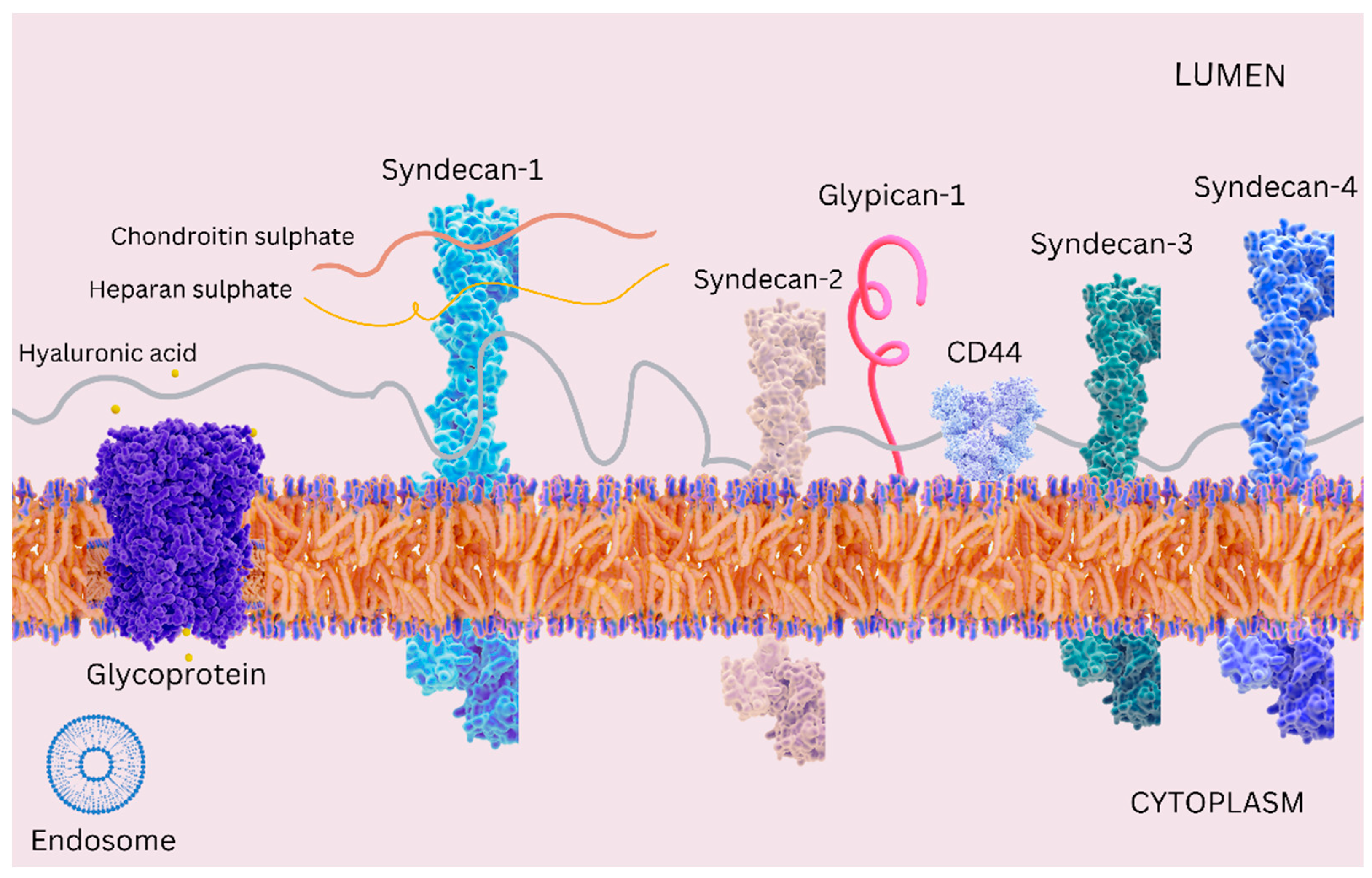
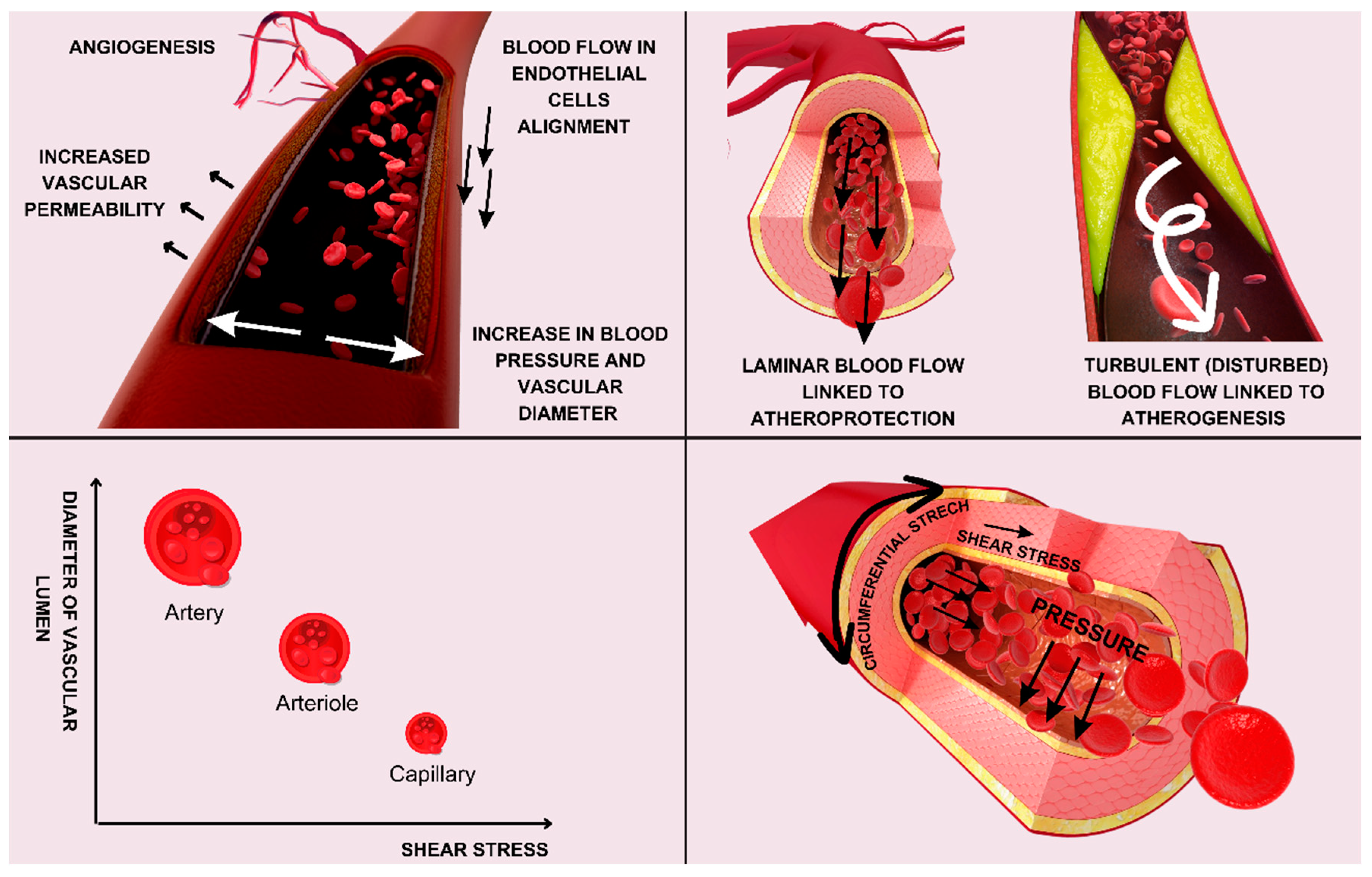
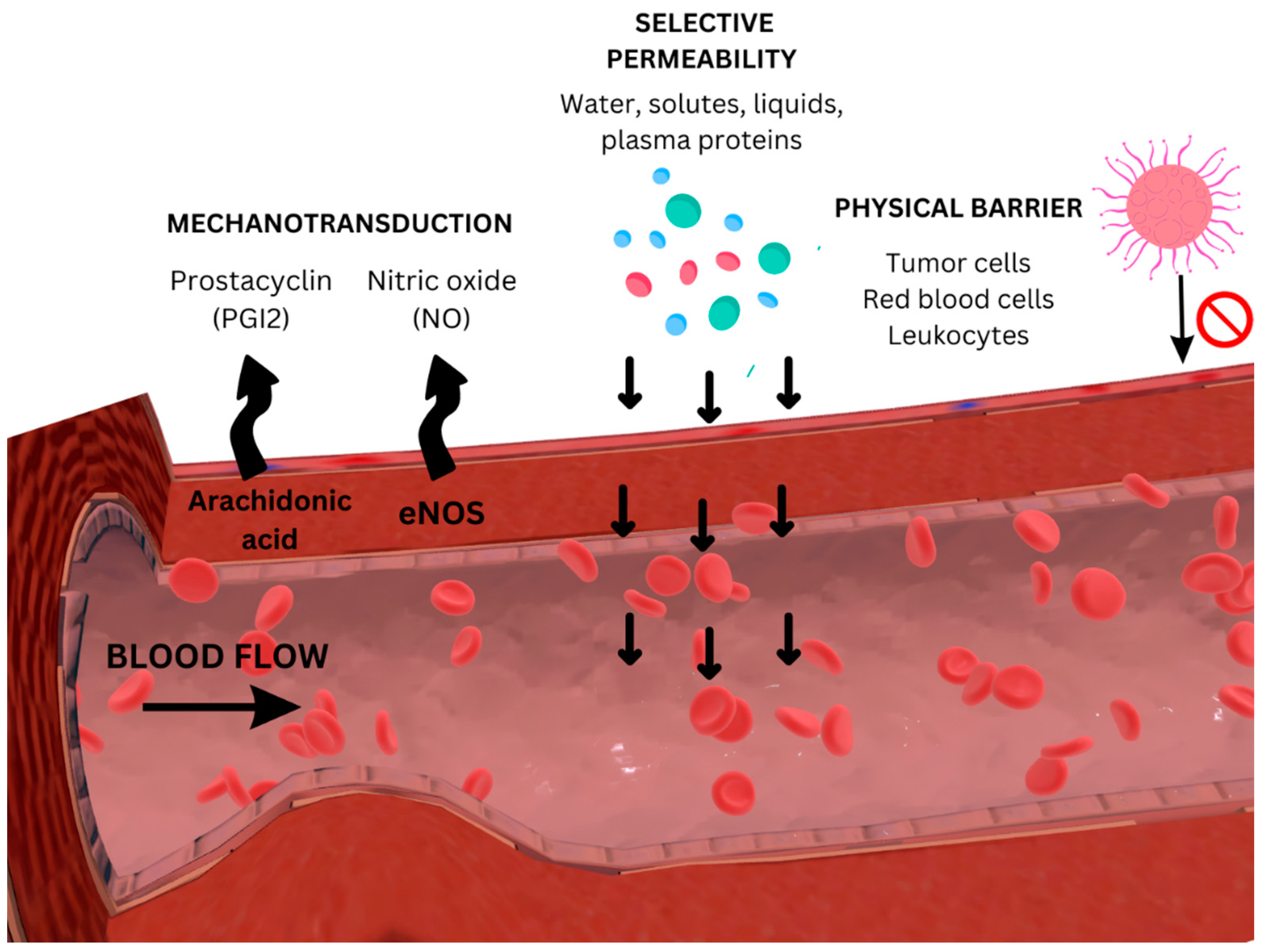
2. Emerging Insights into the Endothelial Glycocalyx in Cardiovascular Diseases
2.1. Atherosclerosis
2.2. Hypertension and Aging
3. Understanding the Effects of Heart Surgery on Vascular Health
4. Emerging Insights into the Endothelial Glycocalyx during Heart Surgery
5. Estimation of Endothelial Glycocalyx Damage in Heart Surgical Settings
6. Multifaceted Approaches for Preserving Endothelial Glycocalyx Integrity in Heart Surgery
6.1. Management of Fluid Dynamics and Protein-Based Therapeutic Approaches
6.2. Sustaining Normal Blood-Glucose Levels
6.3. Stabilizers of Atherosclerotic Plaques
6.4. Anti-Inflammatory Treatment
6.5. Anticoagulants
6.6. Anesthetics and Anesthesia Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; Oude Brink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Starling, E.H. On the Absorption of Fluids from the Connective Tissue Spaces. J. Physiol. 1896, 19, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Afsar, B.; Ortiz, A.; Kanbay, M. The role of endothelial glycocalyx in health and disease. Clin. Kidney. J. 2019, 12, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An. Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [PubMed]
- Pillinger, N.L.; Kam, P. Endothelial glycocalyx: Basic science and clinical implications. Anesth. Intensive Care 2017, 45, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.H.; Murphy, H.A.; George, E.M. The glycocalyx: A central regulator of vascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R508–R518. [Google Scholar] [CrossRef] [PubMed]
- Jedlicka, J.; Becker, B.F.; Chappell, D. Endothelial glycocalyx. Crit. Care Clin. 2020, 36, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tiemeier, G.L.; van der Berg, B.M.; Rabelink, T.J. Endothelial glycocalyx Hyaluronan: Rendothelial glycocalyx ulation and Role in Prevention of Diabetic Complications. Am. J. Pathol. 2020, 190, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Cosgun, Z.C.; Fels, B.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx: From structure to function. Am. J. Pathol. 2020, 190, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Stachtea, X.N.; Tykesson, E.; van Kuppevelt, T.H.; Feinstein, R.; Malmström, A.; Reijmers, R.M.; Maccarana, M. Dermatan Sulphate-Free Mice Display Embryological Defects and Are Neonatal Lethal Despite Normal Lymphoid and Non-Lymphoid Organogenesis. PLoS ONE 2015, 10, e0140279. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Lamanna, W.C.; Esko, J.D. Heparan sulphate proteoglycans. Cold Spring Harb Perspect Biol. 2011, 3, a004952. [Google Scholar] [CrossRef] [PubMed]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef] [PubMed]
- Magoon, R.; Shri, I.; Das, D. Outcomes Following On-Pump Versus Of-Pump CABG: Apprising the “Bypassed”. Braz. J. Cardiovasc. Surg. 2023, 38, 318–319. [Google Scholar] [CrossRef]
- Mahmoud, M.; Mayer, M.; Cancel, L.M.; Bartosch, A.M.; Mathews, R.; Tarbell, J.M. The glycocalyx core protein Glypican 1 protects vessel wall endothelial cells from stiffness-mediated dysfunction and disease. Cardiovasc. Res. 2021, 117, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2021, 12, 37–71. [Google Scholar] [CrossRef] [PubMed]
- Pahakis, M.Y.; Kosky, J.R.; Dull, R.O.; Tarbell, J.M. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 2007, 355, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, X.F.; Fu, B.M.; Tarbell, J.M. The Role of Endothelial Surface Glycocalyx in Mechanosensing and Transduction. Adv. Exp. Med. Biol. 2018, 1097, 1–27. [Google Scholar] [PubMed]
- Tan, A.; Newey, C.; Falter, F. Pulsatile Perfusion during Cardiopulmonary Bypass: A Literature Review. J. Extra. Corpor. Technol. 2022, 54, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 711003. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Masuda, M.; Harada, N.; Lopes, R.B.; Fujiwara, K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 1997, 72, 229–237. [Google Scholar] [PubMed]
- Masuda, M.; Osawa, M.; Shigematsu, H.; Harada, N.; Fujiwara, K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett. 1997, 408, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.S. Morphological aspects of extracellular polysaccharides. J. Histochem. Cytochem. 1963, 11, 14–23. [Google Scholar] [CrossRef]
- Brouns, S.L.N.; Provenzale, I.; van Geffen, J.P.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Localized endothelial-based control of platelet aggrendothelial glycocalyx ation and coagulation under flow: A proof-of-principle vessel-on-a-chip study. J. Thromb. Haemost. 2020, 18, 931–941. [Google Scholar] [CrossRef]
- Kincses, A.; Santa-Maria, A.R.; Walter, F.R.; Dér, L.; Horányi, N.; Lipka, D.V.; Valkai, S.; Deli, M.A.; Dér, A. A chip device to determine surface charge properties of confluent cell monolayers by measuring streaming potential. Lab. Chip. 2020, 20, 3792–3805. [Google Scholar] [CrossRef] [PubMed]
- Klitzman, B.; Dulingg, B.R. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am. J. Physiol. Heart Circ. Physiol. 1979, 237, H481–H490. [Google Scholar] [CrossRef] [PubMed]
- Schierke, F.; Wyrwoll, M.J.; Wisdorf, M.; Niedzielski, L.; Maase, M.; Ruck, T.; Meuth, S.G.; Kusche-Vihrog, K. Nanomechanics of the endothelial glycocalyx contribute to Na+-induced vascular inflammation. Sci. Rep. 2017, 7, 46476. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.R.; Zamani, A.; Osborne, J.I.A.; Zamani, R.; Akrami, M. Critical evaluation of stents in coronary angioplasty: A systematic review. Biomed. Eng. Online. 2021, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Kei, C.Y.; Singh, K.; Dautov, R.F.; Nguyen, T.H.; Chirkov, Y.Y.; Horowitz, J.D. Coronary “Microvascular Dysfunction”: Evolving Understanding of Pathophysiology, Clinical Implications, and Potential Therapeutics. Int. J. Mol. Sci. 2023, 24, 11287. [Google Scholar] [CrossRef] [PubMed]
- Sukudom, S.; Smart, L.; Macdonald, S. Association between intravenous fluid administration and endothelial glycocalyx shedding in humans: A systematic review. Intensive Care Med. Exp. 2024, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Bruegger, D.; Potzel, J.; Jacob, M.; Brettner, F.; Vogeser, M.; Conzen, P.; Becker, B.F.; Rehm, M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care 2014, 18, 538. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiu, Y.; Bai, B. The Expression, Rendothelial glycocalyx ulation, and Biomarker Potential of Glypican-1 in Cancer. Front. Oncol. 2019, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.C.; Taylor, R.G.; Jones, N.D.; St Clair, R.W.; Cornhill, J.F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab. Investig. 1982, 46, 123–138. [Google Scholar] [PubMed]
- van den Berg, B.M.; Spaan, J.A.; Rolf, T.M.; Vink, H. Atherogenic rendothelial glycocalyx ion and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am. J. Physiol. Heart. Circ. Physiol. 2006, 290, H915–H920. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, B.M.; Spaan, J.A.; Vink, H. Impaired glycocalyx barrier properties contribute to enhanced intimal low-density lipoprotein accumulation at the carotid artery bifurcation in mice. Pflugers. Arch. 2009, 457, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Cancel, L.M.; Ebong, E.E.; Mensah, S.; Hirschberg, C.; Tarbell, J.M. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 2016, 252, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Freudenberger, T.; Melchior-Becker, A.; Röck, K.; Ter Braak, M.; Jastrow, H.; Kinzig, M.; Lucke, S.; Suvorava, T.; Kojda, G.; et al. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: Novel insights into the role of hyaluronan synthesis. Circulation 2010, 122, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Barash, U.; Nguyen, H.M.; Yang, S.M.; Ilan, N. Biology of the Heparanase-Heparan Sulphate Axis and Its Role in Disease Pathogenesis. Semin. Thromb. Hemost. 2021, 47, 240–253. [Google Scholar] [PubMed]
- Sneath, R.J.; Mangham, D.C. The normal structure and function of CD44 and its role in neoplasia. Mol. Pathol. 1998, 51, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Nijst, P.; Kiefer, K.; Tang, W.H. Endothelial glycocalyx as biomarker for cardiovascular diseases: Mechanistic and clinical implications. Curr. Heart Fail. Rep. 2017, 14, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Son, D.J.; Kumar, S.; Takabe, W.; Kim, C.W.; Ni, C.W.; Alberts-Grill, N.; Jang, I.H.; Kim, S.; Kim, W.; Won Kang, S.; et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat. Commun. 2013, 4, 3000. [Google Scholar] [CrossRef] [PubMed]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Vink, H.; Hiramatsu, O.; Kajita, T.; Shigeto, F.; Spaan, J.A.; Kajiya, F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H722–H726. [Google Scholar] [CrossRef]
- Pahakis, M.; Kosky, J.; Tarbell, J. Sialic acids And Heparan Sulphate Proteoglycans Are Mechanosensory Components of the Endothelial Cell Glycocalyx. In Proceedings of the 2005 Summer Bioengineering Conference, Vail, CO, USA, 22 June 2005; pp. 137–138. [Google Scholar]
- Gopal, S. Syndecans in Inflammation at a Glance. Front. Immunol. 2020, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Nijst, P.; Cops, J.; Martens, P.; Swennen, Q.; Dupont, M.; Tang, W.H.W.; Mullens, W. Endovascular shedding markers in patients with heart failure with reduced ejection fraction: Results from a single-center exploratory study. Microcirculation 2018, 25, 2. [Google Scholar] [CrossRef] [PubMed]
- Kusche-Vihrog, K.; Oberleithner, H. An emerging concept of vascular salt sensitivity. Biol. Rep. 2012, 4, 20. [Google Scholar] [CrossRef]
- Oberleithner, H. Vascular endothelium: A vulnerable transit zone for merciless sodium. Nephrol. Dial. Transplant. 2014, 29, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, Z.C.; Liu, Y. Attenuating Pulmonary Hypertension by Protecting the Intendothelial glycocalyx rity of Glycocalyx in Rats Model of Pulmonary Artery Hypertension. Inflammation 2019, 42, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Voumvourakis, A.; Makavos, G.; Triantafyllidi, H.; Pavlidis, G.; Katogiannis, K.; Benas, D.; Vlastos, D.; Trivilou, P.; Varoudi, M.; et al. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J. Clin. Hypertens. 2018, 20, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Garcia-Valencia, O.; Milic, N.M.; Codsi, E.; Cubro, H.; Nath, M.C.; White, W.M.; Nath, K.A.; Garovic, V.D. Early Onset Preeclampsia Is Associated with Glycocalyx Dendothelial glycocalyx radation and Reduced Microvascular Perfusion. J. Am. Heart Assoc. 2019, 8, e010647. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.R.; Bloom, S.I.; Campbell, R.A.; Phuong, T.T.T.; Gates, P.E.; Lesniewski, L.A.; Rondina, M.; Donato, A.J. Advanced age results in a diminished endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H531–H539. [Google Scholar] [CrossRef] [PubMed]
- Verrier, E.D. Cardiac surgery. J. Am. Coll. Surg. 1999, 188, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, D.; Meuris, B.; Meyns, B.; Verbrugghe, P. Global cardiac surgery: Access to cardiac surgical care around the world. J. Thorac. Cardiovasc. Surg. 2020, 159, 987–996.e6. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.; Milojevic, M.; Boer, C.; De Somer, F.M.J.J.; Gudbjartsson, T.; van den Goor, J.; Jones, T.J.; Lomivorotov, V.; Merkle, F.; Ranucci, M. EACTS/EACTA/EBCP Committee Reviewers. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 210–251. [Google Scholar] [PubMed]
- Sirendothelial Glycocalyxar, S.; Groenwold, R.H.; de Heer, F.; Bots, M.L.; van der Graaf, Y.; van Herwerden, L.A. Performance of the original EuroSCORE. Eur. J. Cardiothorac. Surg. 2012, 41, 746–754. [Google Scholar]
- Knežević, D.; Ćurko-Cofek, B.; Batinac, T.; Laškarin, G.; Rakić, M.; Šoštarič, M.; Zdravković, M.; Šustić, A.; Sotošek, V.; Batičić, L. Endothelial Dysfunction in Patients Undergoing Cardiac Surgery: A Narrative Review and Clinical Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.P.M.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.Y.; Degenhardt, R.; Pleva, L.; et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients with Coronary Stent Restenosis. J. Am. Coll Cardiol. 2020, 75, 2664–2678. [Google Scholar]
- Institute of Medicine (US) Committee on Social Security Cardiovascular Disability Criteria. Cardiovascular Disability: Updating the Social Security Listings; National Academies Press US: Washington, DC, USA, 2010. [Google Scholar]
- Conrad, C.; Eltzschig, H.K. Disease Mechanisms of Perioperative Organ Injury. Anesth. Analg. 2020, 131, 1730–1750. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, E.; Passov, A.; Andersson, S.; Suojaranta, R.; Niemi, T.; Raivio, P.; Salmenperä, M.; Schramko, A. Glycocalyx Dendothelial glycocalyx radation and Inflammation in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Goligorsky, M.S. Perioperative implication of the endothelial glycocalyx. Korean J. Anesthesiol. 2018, 71, 92–102. [Google Scholar]
- Dogné, S.; Flamion, B. Endothelial glycocalyx Impairment in Disease: Focus on Hyaluronan Shedding. Am. J. Pathol. 2020, 190, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.S.; Auf dem Keller, U. Matrix Metalloproteinases: How Much Can They Do? Int. J. Mol. Sci. 2020, 21, 2678. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, E.J.; Banda, M.J.; Leppert, D. Matrix metalloproteinases in immunity. J. Immunol. 1996, 156, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rabia, B.; Thanigaimani, S.; Golledge, J. The Potential Involvement of Glycocalyx Disruption in Abdominal Aortic Aneurysm Pathogenesis. Cardiovasc. Pathol. 2024, 9, 107629. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cano, I.; D‘Amore, P.A. Update on the Role of the Endothelial Glycocalyx in Angiogenesis and Vascular Inflammation. Front. Cell Dev. Biol. 2021, 9, 734276. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Granato, D.C.; Yokoo, S.; Domingues, R.R.; Trindade, D.M.; Paes Leme, A.F. Mass spectrometry-based proteomics revealed Glypican-1 as a novel ADAM17 substrate. J. Proteomics. 2017, 151, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; LeBlanc, M.E.; Cano, I.; Saez-Torres, K.L.; Saint-Geniez, M.; Ng, Y.S.; D‘Amore, P.A. ADAM10 and ADAM17 proteases mediate proinflammatory cytokine-induced and constitutive cleavage of endomucin from the endothelial surface. J. Biol. Chem. 2020, 295, 6641–6651. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Nallasamy, P.; Jialal, I. Toll-like receptors 2 and 4 mediate hyperglycemia induced macrovascular aortic endothelial cell inflammation and perturbation of the endothelial glycocalyx. J. Diabetes Complicat. 2016, 30, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.P.; Cao, Y.; Zhao, S.L.; Huang, Y.X.; Yang, K.; Huang, W. Memory T cells delay the progression of atherosclerosis via AMPK signaling pathway. Ann. Transl. Med. 2019, 7, 782. [Google Scholar] [CrossRef] [PubMed]
- Abassi, Z.; Armaly, Z.; Heyman, S.N. Glycocalyx Dendothelial glycocalyx radation in Ischemia-Reperfusion Injury. Am. J. Pathol. 2020, 190, 752–767. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.C.; Masopust, D. Understanding Subset Diversity in T Cell Memory. Immunity. 2018, 48, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Mahmoud, A.M.; Le Master, E.; Levitan, I.; Phillips, S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced dendothelial glycocalyx radation of endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H647–H663. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Meendothelial Glycocalyxan, J.E.; Jannaway, M.; Coleman, D.C.; SYuan, S.Y. A disintendothelial glycocalyx rin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc. Res. 2018, 13, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.; McDonald, K.; Burkat, D.; Leask, R.L. Stenosis Hemodynamics Disrupt the Endothelial Cell Glycocalyx by MMP Activity Creating a Proinflammatory Environment. Ann. Biomed. Eng. 2017, 45, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, R.D.; Butler, M.J.; Newman, G.; Desideri, S.; Russell, A.; Lay, A.C.; Neal, C.R.; Qiu, Y.; Fawaz, S.; Onions, K.L.; et al. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2020, 7, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Milusev, A.; Rieben, R.; Sorvillo, N. The Endothelial glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders. Front. Cardiovasc. Med. 2022, 9, 897087. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Zheng, Y.; Shang, S. Expressions and clinical significance of factors related to acute coronary syndrome. J. Biol. Rendothelial Glycocalyx Homeost. Agents. 2018, 38, 299–305. [Google Scholar]
- Reine, T.M.; Lanzalaco, F.; Kristiansen, O.; Enget, A.R.; Satchell, S.; Jenssen, T.G.; Kolset, S.O. Matrix metalloproteinase-9 mediated shedding of syndecan-4 in glomerular endothelial cells. Microcirculation 2019, 3, e12534. [Google Scholar] [CrossRef]
- Sieve, I.; Münster-Kühnel, A.K.; Hilfiker-Kleiner, D. Rendothelial glycocalyx ulation and function of endothelial glycocalyx layer in vascular diseases. Vascul. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ibrahimi, L.; Azar, D.T.; Han, K.Y. The Role of Membrane-Type 1 Matrix Metalloproteinase-Substrate Interactions in Pathogenesis. Int. J. Mol. Sci. 2023, 24, 2183. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.G.; Patel, V.; Dull, R.O. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol. Scand. 2021, 65, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Weaver, O.; Friedman, J.K.; Rodriguez, L.A.; Hoof, M.A.; Drury, R.H.; Packer, J.T.; Smith, A.; Guidry, C.; Duchesne, J.C. Hypoxia/reoxygenation decreases endothelial glycocalyx via reactive oxygen species and calcium signaling in a cellular model for shock. J. Trauma Acute Care Surg. 2019, 87, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Koning, N.J.; Vonk, A.B.A.; Vink, H.; Boer, C. Side-by-Side Alterations in Glycocalyx Thickness and Perfused Microvascular Density During Acute Microcirculatory Alterations in Cardiac Surgery. Microcirculation 2016, 23, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, W.; Zhou, J.; He, G.; Ye, J.; Fang, F.; Luo, J.; Wang, M.; Xu, H.; Wang, W. Correlation between acute dendothelial glycocalyx radation of the endothelial glycocalyx and microcirculation dysfunction during cardiopulmonary bypass in cardiac surgery. Microvasc. Res. 2019, 124, 37–42. [Google Scholar] [CrossRef]
- Bol, M.E.; Huckriede, J.B.; van de Pas, K.G.H.; Delhaas, T.; Lorusso, R.; Nicolaes, G.A.F.; Sels, J.E.M.; van de Poll, M.C.G. Multimodal measurement of glycocalyx dendothelial glycocalyx radation during coronary artery bypass grafting. Front. Med. 2022, 9, 1045728. [Google Scholar] [CrossRef] [PubMed]
- Squiccimarro, E.; Stasi, A.; Lorusso, R.; Paparella, D. Narrative review of the systemic inflammatory reaction to cardiac surgery and cardiopulmonary bypass. Artif. Organs. 2022, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, A.; Ushiyama, A.; Iijima, T. Time-Dependent Dynamics Required for the Degradation and Restoration of the Vascular Endothelial Glycocalyx Layer in Lipopolysaccharide-Treated Septic Mice. Front. Cardiovasc. Med. 2021, 8, 730298. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef] [PubMed]
- Passov, A.; Schramko, A.; Salminen, U.S.; Aittomäki, J.; Andersson, S.; Pesonen, E. Endothelial glycocalyx during early reperfusion in patients undergoing cardiac surgery. PLoS ONE 2021, 16, e0251747. [Google Scholar] [CrossRef] [PubMed]
- Dekker, N.A.M.; Veerhoek, D.; Koning, N.J.; van Leeuwen, A.L.I.; Elbers, P.W.G.; van den Brom, C.E.; Vonk, A.B.A.; Boer, C. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 2019, 74, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Hadem, J.; Rossnick, R.; Hesse, B.; Herr, M.; Hansen, M.; Bergmann, A.; Kensah, G.; Maess, C.; Baraki, H.; Kümpers, P.; et al. Endothelial dysfunction following coronary artery bypass grafting: Influence of patient and procedural factors. Herz 2020, 45, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Girão-Silva, T.; Fonseca-Alaniz, M.H.; Ribeiro-Silva, J.C.; Lee, J.; Patil, N.P.; Dallan, L.A.; Baker, A.B.; Harmsen, M.C.; Kriendothelial Glycocalyxer, J.E.; Miyakawa, A.A. High stretch induces endothelial dysfunction accompanied by oxidative stress and actin remodeling in human saphenous vein endothelial cells. Sci. Rep. 2021, 11, 13493. [Google Scholar] [CrossRef] [PubMed]
- Abou-Arab, O.; Kamel, S.; Beyls, C.; Huette, P.; Bar, S.; Lorne, E.; Galmiche, A.; Guinot, P.G. Vasoplendothelial glycocalyx ia After Cardiac Surgery Is Associated with Endothelial glycocalyx Alterations. J. Cardiothorac. Vasc. Anesth. 2020, 34, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Brettner, F.; Chappell, D.; Nebelsiek, T.; Hauer, D.; Schelling, G.; Becker, B.F.; Rehm, M.; Weis, F. Preinterventional hydrocortisone sustains the endothelial glycocalyx in cardiac surgery. Clin. Hemorheol. Microcirc. 2019, 71, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Baumann, A.; Pietroschinsky, E.; Franklin, J.; Illerhaus, A.; Buchwald, D.; Hinkelbein, J.; Zahn, P.K.; Annecke, T. Hemoadsorption: Effective in reducing circulating fragments of the endothelial glycocalyx during cardiopulmonary bypass in patients undergoing on-pump cardiac surgery? Minerva. Anestesiologica 2021, 87, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Robich, M.; Ryzhov, S.; Kacer, D.; Palmeri, M.; Peterson, S.M.; Quinn, R.D.; Carter, D.; Sheppard, F.; Hayes, T.; Sawyer, D.B.; et al. Prolonged Cardiopulmonary Bypass is Associated with Endothelial glycocalyx Dendothelial glycocalyx radation. J. Surg. Res. 2020, 251, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Rovas, A.; Sackarnd, J.; Rossaint, J.; Kampmeier, S.; Pavenstädt, H.; Vink, H.; Kümpers, P. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: The NOSTRADAMUS study. Crit. Care 2021, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Rovas, A.; Seidel, L.M.; Vink, H.; Pohlkötter, T.; Pavenstädt, H.; Ertmer, C.; Hessler, M.; Kümpers, P. Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit. Care 2019, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Thymis, J.; Simitsis, P.; Koliou, G.A.; Katsanos, S.; Triantafyllou, C.; Kousathana, F.; Pavlidis, G.; Kountouri, A.; Polyzogopoulou, E.; et al. Impaired Endothelial glycocalyx Predicts Adverse Outcome in Subjects Without Overt Cardiovascular Disease: A 6-Year Follow-up Study. J. Cardiovasc. Transl. Res. 2022, 15, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T. Biomarkers of Endothelial glycocalyx Intendothelial glycocalyx rity for Cardiovascular Events in Individuals Without Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2023, 16, 971–972. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kitai, T.; Morales, R.; Kiefer, K.; Chaikijurajai, T.; Tang, W.H.W. Usefulness of Serum Biomarkers of Endothelial glycocalyx Damage in Prognosis of Decompensated Patients with Heart Failure with Reduced Ejection Fraction. Am. J. Cardiol. 2022, 176, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Kawamura, I.; Suzuki, K.; Okada, H.; Ishihara, T.; Tomita, H.; Suzuki, K.; Takada, C.; Sampei, S.; Kano, S.; et al. Serum syndecan-1 concentration in hospitalized patients with heart failure may predict readmission-free survival. PLoS ONE 2021, 16, e0260350. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Vink, H. (Microvascular Health Solutions LLC). Synergistic Glycocalyx Treatment Compositions and Methods. U.S. Patent No. US20160296603A1, 8 April 2016. [Google Scholar]
- Hippensteel, J.A.; Uchimido, R.; Tyler, P.D.; Burke, R.C.; Han, X.; Zhang, F.; McMurtry, S.A.; Colbert, J.F.; Lindsell, C.J.; Angus, D.C.; et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx dendothelial glycocalyx radation. Crit. Care 2019, 23, 259. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mwangi, J.G.; Stanley, T.K.; Mitra, R.; Ebong, E.E. Rendothelial glycocalyx eneration and Assessment of the Endothelial glycocalyx To Address Cardiovascular Disease. Ind. Eng. Chem. Res. 2021, 60, 17328–17347. [Google Scholar] [CrossRef]
- Barelli, S.; Alberio, L. The role of plasma transfusion in massive bleeding: Protecting the endothelial glycocalyx? Front. Med. 2018, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of albumin in the preservation of endothelial glycocalyx intendothelial glycocalyx rity and the microcirculation: A review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Harris, N.R. Endothelial glycocalyx in retina, hyperglycemia, and diabetic retinopathy. Am. J. Physiol. Cell Physiol. 2023, 324, C1061–C1077. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.; Teoh, H.; Campeau, M.A.; Verma, S.; Leask, R.L. Empagliflozin restores the intendothelial glycocalyx rity of the endothelial glycocalyx in vitro. Mol. Cell Biochem. 2019, 459, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients with Prediabetes with Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef]
- Targosz-Korecka, M.; Malek-Zietek, K.E.; Kloska, D.; Rajfur, Z.; Stepien, E.Ł.; Grochot-Przeczek, A.; Szymonski, M. Metformin attenuates adhesion between cancer and endothelial cells in chronic hyperglycemia by recovery of the endothelial glycocalyx barrier. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129533. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Du, X.; Wu, Y.; Hua, L.; Wan, L.; Yan, N. Simvastatin promotes endothelial dysfunction by activating the Wnt/betacatenin pathway under oxidative stress. Int. J. Mol. Med. 2019, 44, 1289–1298. [Google Scholar] [PubMed]
- Song, J.W.; Zullo, J.A.; Liveris, D.; Dragovich, M.; Zhang, X.F.; Goligorsky, M.S. Therapeutic Restoration of Endothelial glycocalyx in Sepsis. J. Pharmacol. Exp. Ther. 2017, 361, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Uchimido, R.; Schmidt, E.P.; Shapiro, N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care 2019, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, C.; Nikolaou, M.; Ikonomidis, I.; Bamias, G.; Kouretas, D.; Andreadou, I.; Tsoumani, M.; Thymis, J.; Papaconstantinou, I. Effects of Anti-Inflammatory Treatment and Surgical Intervention on Endothelial glycocalyx, Peripheral and Coronary Microcirculatory Function and Myocardial Deformation in Inflammatory Bowel Disease Patients: A Two-Arms Two-Stage Clinical Trial. Diagnostics 2021, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Groß, S.; Neumann, T.; Illerhaus, A.; Vink, H.; Klasen, G.; Gathof, B.; Annecke, T. Immediate effects of whole blood donation on the endothelial surface layer and glycocalyx shedding. Blood Transfus. 2021, 19, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef] [PubMed]
- Ashry, N.A.; Abdelaziz, R.R.; Suddek, G.M. The potential effect of imatinib against hypercholesterolemia induced atherosclerosis, endothelial dysfunction and hepatic injury in rabbits. Life Sci. 2020, 243, 117275. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Kudo, D.; Saito, S.; Uchino, S.; Yamakawa, K.; Iizuka, Y.; Sanui, M.; Takimoto, K.; Mayumi, T.; Ono, K.; et al. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: A multicenter retrospective observational study. Shock 2016, 46, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Bruegger, D.; Rehm, M.; Conzen, P.; Welsch, U.; Becker, B.F. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007, 107, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Chappell, D.; Jacob, M.; Hofmann-Kiefer, K.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc. Res. 2009, 83, 388–396. [Google Scholar] [CrossRef] [PubMed]
- ElSaadani, M.; Ahmed, S.M.; Jacovides, C.; Lopez, A.; Johnson, V.E.; Kaplan, L.J.; Schwab, C.W.; Smith, D.H.; Pascual, J.L. Antithrombin III ameliorates post-traumatic brain injury cerebral leukocyte mobilization enhancing recovery of blood brain barrier intendothelial glycocalyx rity. J. Trauma Acute Care Surg. 2021, 90, 274–280. [Google Scholar] [CrossRef]
- Yini, S.; Heng, Z.; Xin, A.; Xiaochun, M. Effect of unfractionated heparin on endothelial glycocalyx in a septic shock model. Acta Anaesthesiol. Scand. 2015, 59, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Cheng, Y.; Wu, W.; Yuan, L.; Liu, X. Glycocalyx Impairment in Vascular Disease: Focus on Inflammation. Front. Cell Dev. Biol. 2021, 9, 730621. [Google Scholar] [CrossRef] [PubMed]
- Oduah, E.I.; Linhardt, R.J.; Sharfstein, S.T. Heparin: Past, Present, and Future. Pharmaceuticals 2016, 9, 38. [Google Scholar] [CrossRef]
- Karlsson, K.; Marklund, S.L. Heparin-induced release of extracellular superoxide dismutase to human blood plasma. Biochem. J. 1987, 242, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, E.; Baek, S.-H.; Kim, H.Y.; Kim, J.-Y.; Park, J.; Choi, E.-J. Sevoflurane did not show better protective effect on endothelial glycocalyx layer compared to propofol during lung resection surgery with one lung ventilation. J. Thorac. Dis. 2018, 10, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Morales, D.; Gutierrez, R.; Barahona, M.; Cerda, O.; Caceres, M. Effect of sevoflurane and propofol on tourniquet-induced endothelial damage: A pilot randomized controlled trial for knee-ligament surgery. BMC Anesthesiol. 2020, 20, 121. [Google Scholar] [CrossRef]
- Kim, N.Y.; Kim, K.J.; Lee, K.Y.; Shin, H.J.; Cho, J.; Nam, D.J.; Kim, S.Y. Effect of volatile and total intravenous anesthesia on syndecan-1 shedding after minimally invasive gastrectomy: A randomized trial. Sci. Rep. 2021, 11, 1511. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.Q.; Sun, J.H.; Wu, Q.L.; Feng, L.Y.; Fan, Y.X.; Ye, J.X.; Gao, W.; He, G.L.; Wang, W.J. Protective effect of sevoflurane on vascular endothelial glycocalyx in patients undergoing heart valve surgery: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 477–486. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kršek, A.; Batičić, L.; Ćurko-Cofek, B.; Batinac, T.; Laškarin, G.; Miletić-Gršković, S.; Sotošek, V. Insights into the Molecular Mechanism of Endothelial Glycocalyx Dysfunction during Heart Surgery. Curr. Issues Mol. Biol. 2024, 46, 3794-3809. https://doi.org/10.3390/cimb46050236
Kršek A, Batičić L, Ćurko-Cofek B, Batinac T, Laškarin G, Miletić-Gršković S, Sotošek V. Insights into the Molecular Mechanism of Endothelial Glycocalyx Dysfunction during Heart Surgery. Current Issues in Molecular Biology. 2024; 46(5):3794-3809. https://doi.org/10.3390/cimb46050236
Chicago/Turabian StyleKršek, Antea, Lara Batičić, Božena Ćurko-Cofek, Tanja Batinac, Gordana Laškarin, Silvija Miletić-Gršković, and Vlatka Sotošek. 2024. "Insights into the Molecular Mechanism of Endothelial Glycocalyx Dysfunction during Heart Surgery" Current Issues in Molecular Biology 46, no. 5: 3794-3809. https://doi.org/10.3390/cimb46050236
APA StyleKršek, A., Batičić, L., Ćurko-Cofek, B., Batinac, T., Laškarin, G., Miletić-Gršković, S., & Sotošek, V. (2024). Insights into the Molecular Mechanism of Endothelial Glycocalyx Dysfunction during Heart Surgery. Current Issues in Molecular Biology, 46(5), 3794-3809. https://doi.org/10.3390/cimb46050236





