Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants
Abstract
1. Introduction
2. Iron in Plant Health
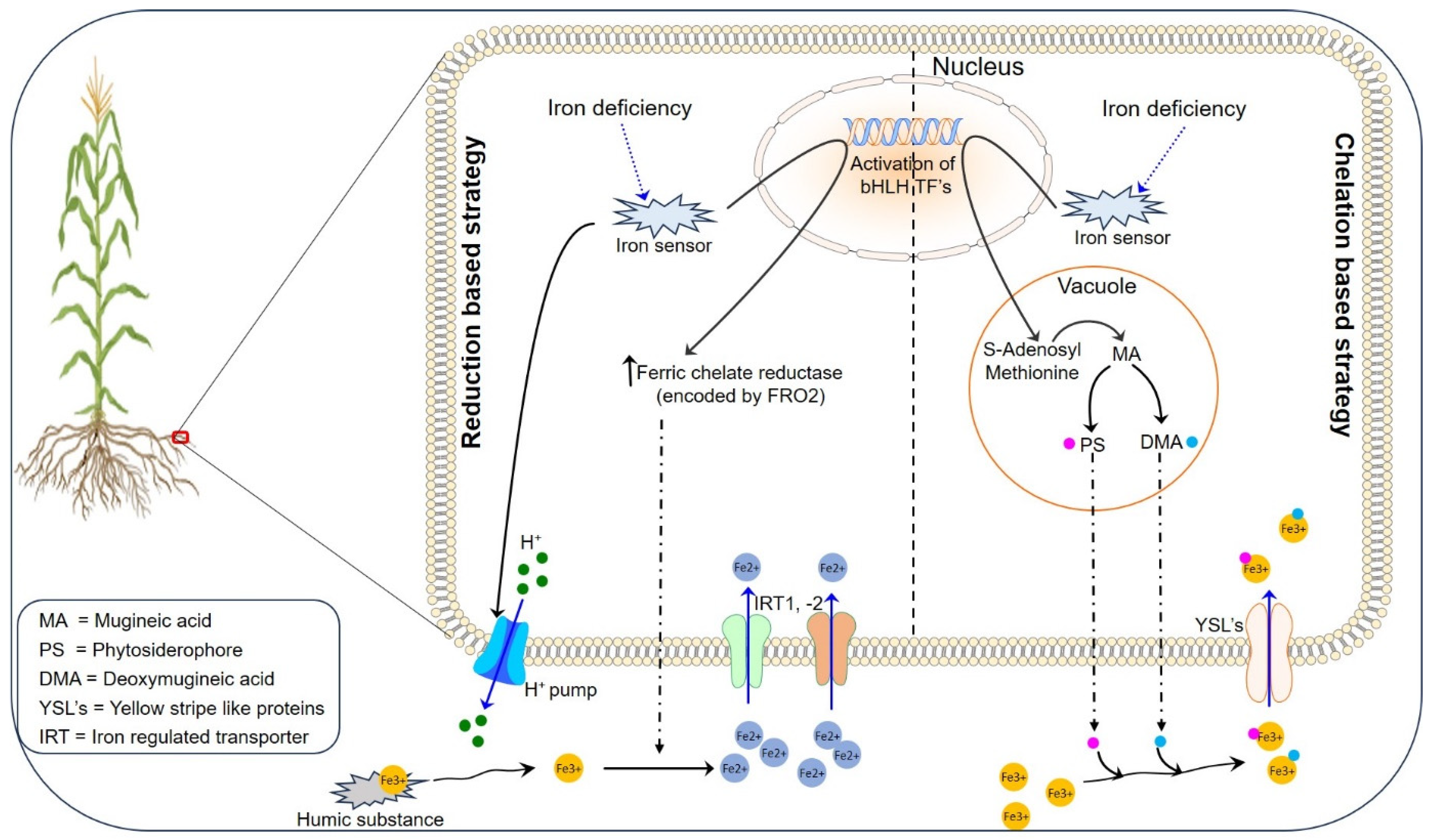
2.1. Iron Uptake, Translocation, and Storage in Plants
2.1.1. Reduction-Based Strategy for Iron Uptake
2.1.2. Chelation-Based Strategy for Iron Uptake
2.1.3. Iron Translocation and Storage
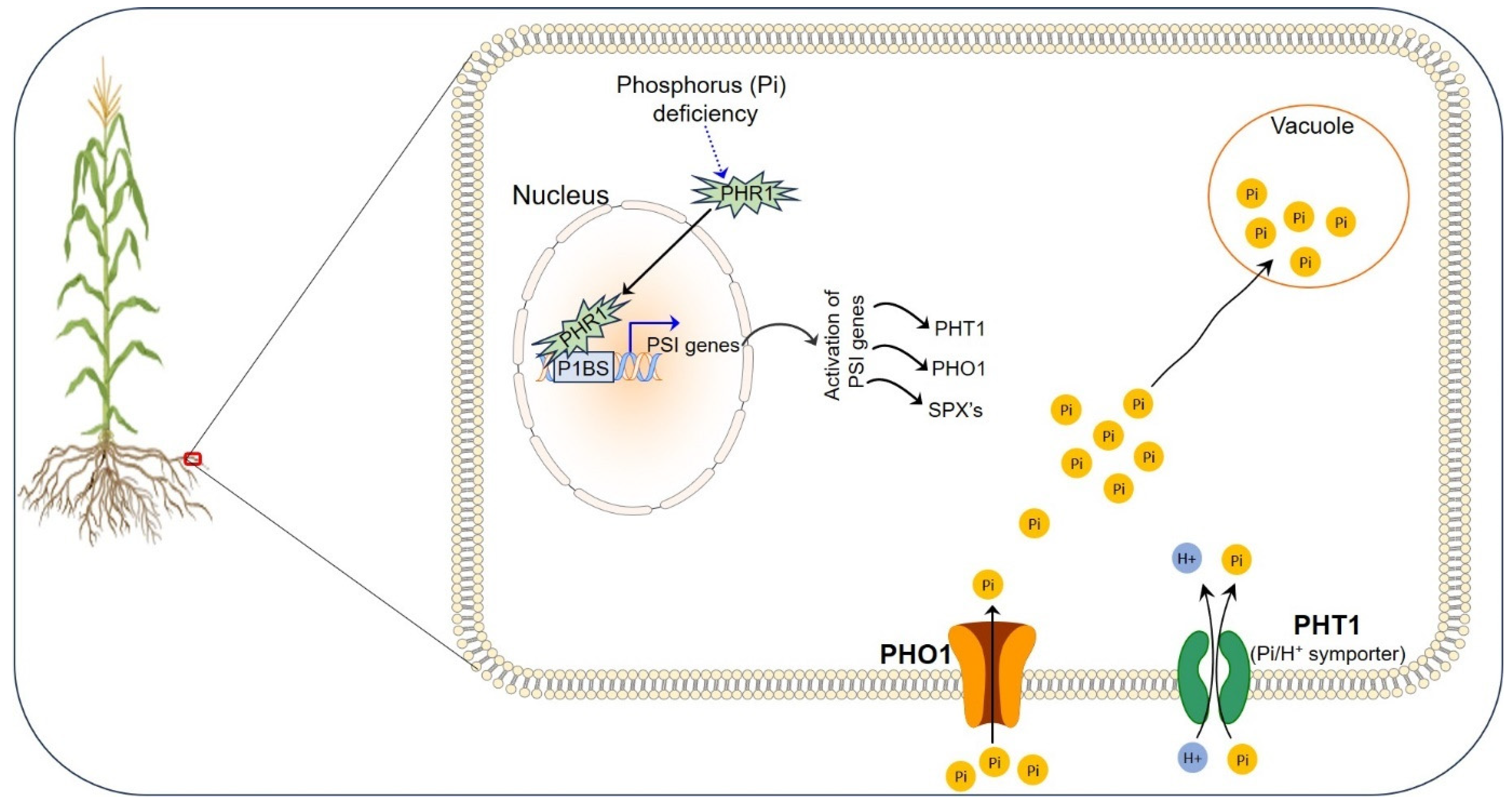
3. Phosphorus in Plant Health
Phosphorus Uptake, Translocation, and Storage in Plants
4. Roles of Iron and Phosphorus in Plants
4.1. Photosynthesis
4.2. Enzyme Activity
4.3. Metabolism
5. Deficiencies of Iron and Phosphorus in Plants
6. Bacteria-Mediated Uptake of Fe and P
6.1. Siderophores in Fe Uptake
6.2. Phosphate Solubilization
7. Toxicity Effects of Excess Fe and P on Plants
7.1. Germination and Plant Growth
7.2. Plant–Water Relation and Yield
7.3. Nutrient Uptake
8. Iron–Phosphorus Homeostasis in Plants
8.1. Fe-P Cross-Talk
8.2. Hormones concerning Fe and P in Plants
9. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Diao, X.; Silver, J.; Takeshima, H. Agricultural Mechanization and Agricultural Transformation; International Food Policy Research Institute: Washington, DC, USA, 2016; Volume 1527. [Google Scholar]
- Vanlauwe, B.; Wendt, J.; Giller, K.; Corbeels, M.; Gerard, B.; Nolte, C. A fourth principle is required to define Conservation Agriculture in sub-Saharan Africa: The appropriate use of fertilizer to enhance crop productivity. Field Crops Res. 2014, 155, 10–13. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) with Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, S.C.; ALal, M.; Kathpalia, R.; Bhatla, S.C. Plant mineral nutrition. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2018; pp. 37–81. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Pilon, M. Ancient and essential: The assembly of iron–sulfur clusters in plants. Trends Plant Sci. 2011, 16, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Kobayashi, T.; Itai, R.N.; Nishizawa, N.K. Iron deficiency responses in rice roots. Rice 2014, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nozoye, T.; Nishizawa, N.K. Iron transport and its regulation in plants. Free Radic. Biol. Med. 2019, 133, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Panda, S.K. Iron homeostasis in rice: Deficit and excess. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 227–235. [Google Scholar] [CrossRef]
- Helliwell, C.A.; Poole, A.; Peacock, W.J.; Dennis, E.S. Arabidopsis ent-Kaurene Oxidase Catalyzes Three Steps of Gibberellin Biosynthesis. Plant Physiol. 1999, 119, 507–510. [Google Scholar] [CrossRef]
- Kim, T.-W.; Hwang, J.-Y.; Kim, Y.-S.; Joo, S.-H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.-K. Arabidopsis CYP85A2, a Cytochrome P450, Mediates the Baeyer-Villiger Oxidation of Castasterone to Brassinolide in Brassinosteroid Biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arab. Book/Am. Soc. Plant Biol. 2011, 9, e0152. [Google Scholar] [CrossRef]
- Murphy, M.J.; Siegel, L.M.; Tove, S.R.; Kamin, H. Siroheme: A New Prosthetic Group Participating in Six-Electron Reduction Reactions Catalyzed by Both Sulfite and Nitrite Reductases. Proc. Natl. Acad. Sci. USA 1974, 71, 612–616. [Google Scholar] [CrossRef]
- Lancaster, J.; Vega, J.; Kamin, H.; Orme-Johnson, N.; Orme-Johnson, W.; Krueger, R.; Siegel, L. Identification of the iron-sulfur center of spinach ferredoxin-nitrite reductase as a tetranuclear center, and preliminary EPR studies of mechanism. J. Biol. Chem. 1979, 254, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Loe, R.; Navasero, S.A. Some mechanisms involved in the development of iron toxicity symptoms in the rice plant. Soil Sci. Plant Nutr. 1966, 12, 32–38. [Google Scholar] [CrossRef]
- Yamauchi, M. Rice bronzing in Nigeria caused by nutrient imbalances and its control by potassium sulfate application. Plant Soil 1989, 117, 275–286. [Google Scholar] [CrossRef]
- Asch, F.; Becker, M.; Kpongor, D.S. A quick and efficient screen for resistance to iron toxicity in lowland rice. J. Plant Nutr. Soil Sci. 2005, 168, 764–773. [Google Scholar] [CrossRef]
- Dorlodot, S.D.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2006, 1763, 595–608. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Hindt, M.N.; Guerinot, M.L. Getting a sense for signals: Regulation of the plant iron deficiency response. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 1521–1530. [Google Scholar] [CrossRef]
- Grillet, L.; Ouerdane, L.; Flis, P.; Hoang, M.T.T.; Isaure, M.-P.; Lobinski, R.; Curie, C.; Mari, S. Ascorbate Efflux as a New Strategy for Iron Reduction and Transport in Plants. J. Biol. Chem. 2014, 289, 2515–2525. [Google Scholar] [CrossRef]
- Gao, F.; Dubos, C. Transcriptional integration of plant responses to iron availability. J. Exp. Bot. 2021, 72, 2056–2070. [Google Scholar] [CrossRef] [PubMed]
- Varotto, C.; Maiwald, D.; Pesaresi, P.; Jahns, P.; Salamini, F.; Leister, D. The metal ion transporter IRT1 is necessary for iron homeostasis and efficient photosynthesis in Arabidopsis thaliana. Plant J. 2002, 31, 589–599. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef]
- Li, M.; Watanabe, S.; Gao, F.; Dubos, C. Iron Nutrition in Plants: Towards a New Paradigm? Plants 2023, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Bernal, M.; Scholle, M.; Holländer-Czytko, H.; Nguyen, N.T.; Piotrowski, M.; Krämer, U. Root-to-shoot iron partitioning in Arabidopsis requires IRON-REGULATED TRANSPORTER1 (IRT1) protein but not its iron (II) transport function. Plant J. 2022, 109, 992–1013. [Google Scholar] [CrossRef]
- Castaings, L.; Caquot, A.; Loubet, S.; Curie, C. The high-affinity metal Transporters NRAMP1 and IRT1 Team up to Take up Iron under Sufficient Metal Provision. Sci. Rep. 2016, 6, 37222. [Google Scholar] [CrossRef] [PubMed]
- Tergemina, E.; Elfarargi, A.F.; Flis, P.; Fulgione, A.; Göktay, M.; Neto, C.; Scholle, M.; Flood, P.J.; Xerri, S.-A.; Zicola, J.; et al. A two-step adaptive walk rewires nutrient transport in a challenging edaphic environment. Sci. Adv. 2022, 8, eabm9385. [Google Scholar] [CrossRef]
- Martin-Barranco, A.; Spielmann, J.; Dubeaux, G.; Vert, G.; Zelazny, E. Dynamic Control of the High-Affinity Iron Uptake Complex in Root Epidermal Cells. Plant Physiol. 2020, 184, 1236–1250. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Bashir, K.; Nakanishi, H.; Nishizawa, N.K. The role of rice phenolics efflux transporter in solubilizing apoplasmic iron. Plant Signal. Behav. 2011, 6, 1624–1626. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M.; et al. Acquisition and Homeostasis of Iron in Higher Plants and Their Probable Role in Abiotic Stress Tolerance. Front. Environ. Sci. 2018, 5, 86. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, Y.; Zhong, M.; Zhang, L.; Chai, X.; Jiang, X.; Yang, X. Effects of Iron Deficiency Stress on Plant Growth and Quality in Flowering Chinese Cabbage and Its Adaptive Response. Agronomy 2022, 12, 875. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.-F.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef]
- Lee, S.; Chiecko, J.C.; Kim, S.A.; Walker, E.L.; Lee, Y.; Guerinot, M.L.; An, G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 2009, 150, 786–800. [Google Scholar] [CrossRef]
- Inoue, H.; Kobayashi, T.; Nozoye, T.; Takahashi, M.; Kakei, Y.; Suzuki, K.; Nakazono, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Rice OsYSL15 Is an Iron-regulated Iron(III)-Deoxymugineic Acid Transporter Expressed in the Roots and Is Essential for Iron Uptake in Early Growth of the Seedlings. J. Biol. Chem. 2009, 284, 3470–3479. [Google Scholar] [CrossRef]
- Nozoye, T.; Nagasaka, S.; Kobayashi, T.; Takahashi, M.; Sato, Y.; Sato, Y.; Uozumi, N.; Nakanishi, H.; Nishizawa, N.K. Phytosiderophore Efflux Transporters Are Crucial for Iron Acquisition in Graminaceous Plants. J. Biol. Chem. 2011, 286, 5446–5454. [Google Scholar] [CrossRef]
- Haydon, M.J.; Kawachi, M.; Wirtz, M.; Hillmer, S.; Hell, R.; Krämer, U. Vacuolar Nicotianamine Has Critical and Distinct Roles under Iron Deficiency and for Zinc Sequestration in Arabidopsis. Plant Cell 2012, 24, 724–737. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 2006, 45, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, F.; Shou, H.; Huang, F.; Zheng, L.; He, F.; Li, J.; Zhao, F.-J.; Ueno, D.; Ma, J.F.; et al. Mutation in Nicotianamine Aminotransferase Stimulated the Fe(II) Acquisition System and Led to Iron Accumulation in Rice. Plant Physiol. 2007, 145, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Physiological and cellular aspects of phytotoxicity tolerance in plants: The role of membrane transporters and implications for crop breeding for water logging tolerance. New Phytol. 2011, 190, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Santos, C.; Gomes, A.; Vasconcelos, M.W. Cultivar variability of iron uptake mechanisms in rice (Oryza sativa L.). Plant Physiol. Biochem. 2014, 85, 21–30. [Google Scholar] [CrossRef]
- Yokosho, K.; Yamaji, N.; Feng Ma, J. OsFRDL1 expressed in nodes is required for distribution of iron into grains in rice. J. Exp. Bot. 2016, 67, 5485–5494. [Google Scholar] [CrossRef] [PubMed]
- Mori, S. Iron transport in graminaceous plants. Met. Ions Biol. Syst. 1998, 35, 215–238. [Google Scholar]
- Tsukamoto, T.; Nakanishi, H.; Uchida, H.; Watanabe, S.; Matsuhashi, S.; Mori, S.; Nishizawa, N.K. 52Fe Translocation in Barley as Monitored by a Positron-Emitting Tracer Imaging System (PETIS): Evidence for the Direct Translocation of Fe from Roots to Young Leaves via Phloem. Plant Cell Physiol. 2009, 50, 48–57. [Google Scholar] [CrossRef]
- Takahashi, M.; Terada, Y.; Nakai, I.; Nakanishi, H.; Yoshimura, E.; Mori, S.; Nishizawa, N.K. Role of Nicotianamine in the Intracellular Delivery of Metals and Plant Reproductive Development. Plant Cell 2003, 15, 1263–1280. [Google Scholar] [CrossRef]
- Hell, R.; Stephan, U.W. Iron uptake, trafficking and homeostasis in plants. Planta 2003, 216, 541–551. [Google Scholar] [CrossRef]
- Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. A rice FRD3-like (OsFRDL1) gene is expressed in the cells involved in long-distance transport. Soil Sci. Plant Nutr. 2004, 50, 1133–1140. [Google Scholar] [CrossRef]
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-Mediated Efflux of Citrate into the Root Vasculature Is Necessary for Efficient Iron Translocation. Plant Physiol. 2007, 144, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rellán-Álvarez, R.; Giner-Martínez-Sierra, J.; Orduna, J.; Orera, I.; Rodríguez-Castrillón, J.; García-Alonso, J.I.; Abadía, J.; Álvarez-Fernández, A. Identification of a Tri-Iron(III), Tri-Citrate Complex in the Xylem Sap of Iron-Deficient Tomato Resupplied with Iron: New Insights into Plant Iron Long-Distance Transport. Plant Cell Physiol. 2010, 51, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, R.; Kato, M.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Identification of Zn–nicotianamine and Fe–2′-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol. 2012, 53, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yamaji, N.; Ma, J.F. Further characterization of ferric--phytosiderophore transporters ZmYS1 and HvYS1 in maize and barley. J. Exp. Bot. 2009, 60, 3513–3520. [Google Scholar] [CrossRef]
- Aoyama, T.; Kobayashi, T.; Takahashi, M.; Nagasaka, S.; Usuda, K.; Kakei, Y.; Ishimaru, Y.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL18 is a rice iron(III)–deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of Iron in Arabidopsis Seed Requires the Vacuolar Membrane Transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Yi, H.; Gong, J. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Darbani, B.; Briat, J.-F.; Holm, P.B.; Husted, S.; Noeparvar, S.; Borg, S. Dissecting plant iron homeostasis under short and long-term iron fluctuations. Biotechnol. Adv. 2013, 31, 1292–1307. [Google Scholar] [CrossRef]
- Belouchi, A.; Kwan, T.; Gros, P. Cloning and characterization of the OsNramp family from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions. Plant Mol. Biol. 1997, 33, 1085–1092. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelièvre, F.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Krämer, U.; Thomine, S. Export of Vacuolar Manganese by AtNRAMP3 and AtNRAMP4 Is Required for Optimal Photosynthesis and Growth under Manganese Deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Poirier, Y.; Bucher, M. Phosphate transport and homeostasis in Arabidopsis. Arab. Book/Am. Soc. Plant Biol. 2002, 1, e0024. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Functions of mineral nutrients, macronutrients. In Mineral Nutrition of Higher Plants; Academic Press: London, UK, 1997. [Google Scholar]
- Kaur, G.; Shukla, V.; Meena, V.; Kumar, A.; Tyagi, D.; Singh, J.; Kandoth, P.K.; Mantri, S.; Rouached, H.; Pandey, A.K. Physiological and molecular responses to combinatorial iron and phosphate deficiencies in hexaploid wheat seedlings. Genomics 2021, 113, 3935–3950. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Syvertsen, J.P. Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytol. 1985, 101, 667–676. [Google Scholar] [CrossRef]
- López-Arredondo, D.L.; Leyva-González, M.A.; González-Morales, S.I.; López-Bucio, J.; Herrera-Estrella, L. Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops. Annu. Rev. Plant Biol. 2014, 65, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Liu, F.; Xu, Y.; Jiang, H.; Jiang, C.; Du, Y.; Gong, C.; Wang, W.; Zhu, S.; Han, G.; Cheng, B. Systematic identification, evolution and expression analysis of the Zea mays PHT1 gene family reveals several new members involved in root colonization by arbuscular mycorrhizal fungi. Int. J. Mol. Sci. 2016, 17, 930. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Huang, T.-K.; Yang, S.-Y.; Hong, Y.-T.; Huang, S.-M.; Wang, F.-N.; Chiang, S.-F.; Tsai, S.-Y.; Lu, W.-C.; Chiou, T.-J. Identification of plant vacuolar transporters mediating phosphate storage. Nat. Commun. 2016, 7, 11095. [Google Scholar] [CrossRef]
- Versaw, W.K.; Garcia, L.R. Intracellular transport and compartmentation of phosphate in plants. Curr. Opin. Plant Biol. 2017, 39, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 2002, 31, 341–353. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Guo, Y.; Chen, L.; Liang, R.; Gu, M.; Xu, G.; Zhao, J.; Walk, T.; Liao, H. Functional characterization of 14 Pht1 family genes in yeast and their expressions in response to nutrient starvation in soybean. PLoS ONE 2012, 7, e47726. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, X.; Hu, R.; Wang, Y.; Xiao, C.; Jiang, Y.; Zhang, X.; Zheng, C.; Fu, Y.-F. The pattern of Phosphate transporter 1 genes evolutionary divergence in Glycine max L. BMC Plant Biol. 2013, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chang, X.-J.; Ye, Y.; Xie, W.-B.; Wu, P.; Lian, X.-M. Comprehensive Sequence and Whole-Life-Cycle Expression Profile Analysis of the Phosphate Transporter Gene Family in Rice. Mol. Plant 2011, 4, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.-F.; Chen, Y.; Sun, M.-M.; Wang, Y.; Chen, Y.-F. The Transcription Factor NIGT1.2 Modulates Both Phosphate Uptake and Nitrate Influx during Phosphate Starvation in Arabidopsis and Maize. Plant Cell 2020, 32, 3519–3534. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, X.; Wang, H.; Liao, D.; Gu, M.; Qu, H.; Sun, S.; Xu, G. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant Biol. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Ullrich-Eberius, C.I.; Novacky, A.; van Bel, A.J.E. Phosphate uptake inLemna gibba G1: Energetics and kinetics. Planta 1984, 161, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sakano, K. Proton/Phosphate Stoichiometry in Uptake of Inorganic Phosphate by Cultured Cells of Catharanthus roseus (L.) G. Don. Plant Physiol. 1990, 93, 479–483. [Google Scholar] [CrossRef]
- Karlsson, P.M.; Herdean, A.; Adolfsson, L.; Beebo, A.; Nziengui, H.; Irigoyen, S.; Ünnep, R.; Zsiros, O.; Nagy, G.; Garab, G.; et al. The Arabidopsis thylakoid transporter PHT4;1 influences phosphate availability for ATP synthesis and plant growth. Plant J. 2015, 84, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, L.; Luan, M.; Wang, Y.; Zhang, C.; Zhang, B.; Shi, J.; Zhao, F.-G.; Lan, W.; Luan, S. A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E6571–E6578. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Wang, C.; Arpat, B.A.; Wang, Z.; Poirier, Y.; Tyerman, S.D.; Wu, P.; Shou, H.; Whelan, J. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 2012, 193, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jia, X.; Zhang, Y.; Xu, L.; Menand, B.; Zhao, H.; Zeng, H.; Dolan, L.; Zhu, Y.; Yi, K. Loss of two families of SPX domain-containing proteins required for vacuolar polyphosphate accumulation coincides with the transition to phosphate storage in green plants. Mol. Plant 2021, 14, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kuo, H.-F.; Chiou, T.-J. Intracellular phosphate sensing and regulation of phosphate transport systems in plants. Plant Physiol. 2021, 187, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Secco, D.; Baumann, A.; Poirier, Y. Characterization of the Rice PHO1 Gene Family Reveals a Key Role for OsPHO1;2 in Phosphate Homeostasis and the Evolution of a Distinct Clade in Dicotyledons. Plant Physiol. 2010, 152, 1693–1704. [Google Scholar] [CrossRef]
- Wang, Y.; Ribot, C.; Rezzonico, E.; Poirier, Y. Structure and Expression Profile of the Arabidopsis PHO1 Gene Family Indicates a Broad Role in Inorganic Phosphate Homeostasis. Plant Physiol. 2004, 135, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Frank, T.; Tan, Y.; Zhou, C.; Jabnoune, M.; Arpat, A.B.; Cui, H.; Huang, J.; He, Z.; Poirier, Y.; et al. Disruption of OsSULTR3;3 reduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains. New Phytol. 2016, 211, 926–939. [Google Scholar] [CrossRef]
- Yamaji, N.; Takemoto, Y.; Miyaji, T.; Mitani-Ueno, N.; Yoshida, K.T.; Ma, J.F. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature 2017, 541, 92–95. [Google Scholar] [CrossRef]
- Arpat, A.B.; Magliano, P.; Wege, S.; Rouached, H.; Stefanovic, A.; Poirier, Y. Functional expression of PHO1 to the Golgi and trans-Golgi network and its role in export of inorganic phosphate. Plant J. 2012, 71, 479–491. [Google Scholar] [CrossRef]
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Pumplin, N.; Harrison, M.J. Phosphate in the arbuscular mycorrhizal symbiosis: Transport properties and regulatory roles. Plant Cell Environ. 2007, 30, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Wang, F.; Yang, J.; Gao, M.; Li, C.; Liu, Y.; Liu, Y.; Yamaji, N.; Ma, J.F.; et al. The Rice CK2 Kinase Regulates Trafficking of Phosphate Transporters in Response to Phosphate Levels. Plant Cell 2015, 27, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Deng, M.; Xu, J.; Zhu, X.; Mao, C. Molecular mechanisms of phosphate transport and signaling in higher plants. Semin. Cell Dev. Biol. 2018, 74, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, J.; Wang, Y.; Wang, F.; Mao, W.; He, Q.; Xu, J.; Wu, Z.; Mao, C. PROTEIN PHOSPHATASE95 Regulates Phosphate Homeostasis by Affecting Phosphate Transporter Trafficking in Rice. Plant Cell 2020, 32, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Raghothama, K.G. Phosphorus and plant nutrition: An overview. Phosphorus Agric. Environ. 2005, 46, 353–378. [Google Scholar]
- Myouga, F.; Akiyama, K.; Motohashi, R.; Kuromori, T.; Ito, T.; Iizumi, H.; Ryusui, R.; Sakurai, T.; Shinozaki, K. The Chloroplast Function Database: A large-scale collection of Arabidopsis Ds/Spm-or T-DNA-tagged homozygous lines for nuclear-encoded chloroplast proteins, and their systematic phenotype analysis. Plant J. 2010, 61, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Barak, P. Iron nutrition of plants in calcareous soils. Adv. Agron. 1982, 35, 217–240. [Google Scholar]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Briat, J.-F.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe, and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of PHOSPHATE STARVATION RESPONSE 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Institute of Plant Nutrition University of Hohenheim: Stuttgart, Germany, 1995. [Google Scholar]
- Dekock, P.C.; Hall, A.; Inkson, R.H.E. Active Iron in Plant Leaves. Ann. Bot. 1979, 43, 737–740. [Google Scholar] [CrossRef]
- Saenchai, C.; Bouain, N.; Kisko, M.; Prom-U-Thai, C.; Doumas, P.; Rouached, H. The Involvement of OsPHO1;1 in the Regulation of Iron Transport Through Integration of Phosphate and Zinc Deficiency Signaling. Front. Plant Sci. 2016, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-I.; Shahzad, Z.; Dorone, Y.; Clowez, S.; Zhao, K.; Bouain, N.; Lay-Pruitt, K.S.; Cho, H.; Rhee, S.Y.; Rouached, H. Interdependent iron and phosphorus availability controls photosynthesis through retrograde signaling. Nat. Commun. 2021, 12, 7211. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, H.P.; Winterbourn, C.C. The superoxide-dependent transfer of iron from ferritin to transferrin and lactoferrin. Biochem. J. 1988, 256, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Arunachala, R.; Paulkumar, K.; Ranjitsin, A.; Annadurai, G. Environmental Assessment due to Air Pollution near Iron Smelting Industry. J. Environ. Sci. Technol. 2009, 2, 179–186. [Google Scholar] [CrossRef]
- Wilkinson, R.E.; Ohki, K. Influence of Manganese Deficiency and Toxicity on Isoprenoid Syntheses. Plant Physiol. 1988, 87, 841–846. [Google Scholar] [CrossRef]
- Asad, A.; Rafique, R. Effect of Zinc, Copper, Iron, Manganese and Boron on the Yield and Yield Components of Wheat Crop in Tehsil Peshawar. Pak. J. Biol. Sci. 2000, 3, 1615–1620. [Google Scholar] [CrossRef]
- Kerkeb, L.; Connolly, E.L. Iron transport and metabolism in plants. In Genetic Engineering: Principles and Methods; Springer: Boston, MA, USA, 2006; pp. 119–140. [Google Scholar]
- Hofner, W. Iron and manganese compounds in the Blutungssaft of Helianthus annuus. Physiol. Plant 1970, 23, 673–677. [Google Scholar] [CrossRef]
- Temple, S.J.; Vance, C.P.; Gantt, J.S. Glutamate synthase and nitrogen assimilation. Trends Plant Sci. 1998, 3, 51–56. [Google Scholar] [CrossRef]
- Hemalatha, K.; Venkatesan, S. Impact of Iron Toxicity on Certain Enzymes and Biochemical Parameters of Tea. Asian J. Biochem. 2011, 6, 384–394. [Google Scholar] [CrossRef]
- Bashir, K.; Nagasaka, S.; Itai, R.N.; Kobayashi, T.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Expression and enzyme activity of glutathione reductase is upregulated by Fe-deficiency in graminaceous plants. Plant Mol. Biol. 2007, 65, 277–284. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Corpas, F.J.; Sandalio, L.M.; Leterrier, M.; Rodríguez-Serrano, M.; Del Río, L.A.; Palma, J.M. Glutathione reductase from pea leaves: Response to abiotic stress and characterization of the peroxisomal isozyme. New Phytol. 2006, 170, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kaminaka, H.; Morita, S.; Nakajima, M.; Masumura, T.; Tanaka, K. Gene cloning and expression of cytosolic glutathione reductase in rice (Oryza sativa L.). Plant Cell Physiol. 1998, 39, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Maffi, D.; Zocchi, G. Iron availability affects the function of mitochondria in cucumber roots. New Phytol. 2009, 182, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Rosato, A. From Genes to Metalloproteins: A Bioinformatic Approach. Eur. J. Inorg. Chem. 2007, 2007, 2546–2555. [Google Scholar] [CrossRef]
- Hammond, J.P.; White, P.J. Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. J. Exp. Bot. 2008, 59, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, F.; Wang, Y.; Lin, R.; Wang, Z.; Mao, C. Molecular mechanisms and genetic improvement of low-phosphorus tolerance in rice. Plant Cell Environ. 2023, 46, 1104–1119. [Google Scholar] [CrossRef] [PubMed]
- Eckerson, S.H. Influence of phosphorus deficiency on the metabolism of tomato plants. Am. J. Bot. 1929, 16, 852. [Google Scholar]
- Levene, P.A.; Rolf, I.P. Structure and significance of the phosphatides. Physiol. Rev. 1921, 1, 327–393. [Google Scholar] [CrossRef]
- Kayoumu, M.; Iqbal, A.; Muhammad, N.; Li, X.; Li, L.; Wang, X.; Gui, H.; Qi, Q.; Ruan, S.; Guo, R.; et al. Phosphorus Availability Affects the Photosynthesis and Antioxidant System of Contrasting Low-P-Tolerant Cotton Genotypes. Antioxidants 2023, 12, 466. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, X.-X.; Liu, Y.-M.; Liu, D.-Y.; Du, Y.-F.; Chen, X.-P.; Zou, C.-Q. The role of phosphorus supply in maximizing the leaf area, photosynthetic rate, coordinated to grain yield of summer maize. Field Crops Res. 2018, 219, 113–119. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.-W.; Wang, Y.-Y.; Huang, Z.-R.; Ye, X.; Chen, L.-S.; Yang, L.-T. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, H.; Peng, L.; Ren, W.; Gong, J.; Liu, P.; Wu, X.; Huang, F. Comparative Study Reveals Insights of Sheep grass (Leymus chinensis) Coping with Phosphate-Deprived Stress Condition. Front. Plant Sci. 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Alotaibi, M.A.; Alhammad, B.A.; Alharbi, B.M.; Refay, Y.; Badawy, S.A. Effects of ZnO Nanoparticles and Biochar of Rice Straw and Cow Manure on Characteristics of Contaminated Soil and Sunflower Productivity, Oil Quality, and Heavy Metals Uptake. Agronomy 2020, 10, 790. [Google Scholar] [CrossRef]
- Geng, G.; Wang, G.; Stevanato, P.; Lv, C.; Wang, Q.; Yu, L.; Wang, Y. Physiological and Proteomic Analysis of Different Molecular Mechanisms of Sugar Beet Response to Acidic and Alkaline pH Environment. Front. Plant Sci. 2021, 12, 682799. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, V.; Zhang, J.; Li, J.; You, Q.; Zhao, P.; Wang, P.; Lei, M. High transcriptome plasticity drives phosphate starvation responses in tomato. Stress Biol. 2022, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Karim, M.R.; Hu, Y.G.; Shen, R.; Lan, P. Greater morphological and primary metabolic adaptations in roots contribute to phosphate-deficiency tolerance in the bread wheat cultivar Kenong199. BMC Plant Biol. 2021, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, W.; Zhao, H.; Liang, Q.; Zhang, G.; Cai, S. Integrated Transcriptome and Metabolome Analysis Reveals the Divergent Evolution of Low-P Tolerance in Cultivated and Tibetan Wild Barley. Environ. Exp. Bot. 2024, 219, 105641. [Google Scholar] [CrossRef]
- Soumya, P.R.; Sharma, S.; Meena, M.K.; Pandey, R. Response of diverse bread wheat genotypes in terms of root architectural traits at seedling stage in response to low phosphorus stress. Plant Physiol. Rep. 2021, 26, 152–161. [Google Scholar] [CrossRef]
- Naz, M.; Hussain, S.; Ashraf, I.; Farooq, M. Exogenous application of proline and phosphorus help improving maize performance under salt stress. J. Plant Nutr. 2023, 46, 2342–2350. [Google Scholar] [CrossRef]
- Glanz-Idan, N.; Wolf, S. Upregulation of photosynthesis in mineral nutrition-deficient tomato plants by reduced source-to-sink ratio. Plant Signal. Behav. 2020, 15, 1712543. [Google Scholar] [CrossRef] [PubMed]
- Nadira, U.A.; Ahmed, I.M.; Zeng, J.; Bibi, N.; Cai, S.; Wu, F.; Zhang, G. The changes in physiological and biochemical traits of Tibetan wild and cultivated barley in response to low phosphorus stress. Soil Sci. Plant Nutr. 2014, 60, 832–842. [Google Scholar] [CrossRef]
- Zou, X.; Wei, D.; Wu, P.; Zhang, Y.; Hu, Y.; Chen, S.; Ma, X. Strategies of organic acid production and exudation in response to low-phosphorus stress in Chinese fir genotypes differing in phosphorus-use efficiencies. Trees 2018, 32, 897–912. [Google Scholar] [CrossRef]
- Lin, Z.-H.; Chen, L.-S.; Chen, R.-B.; Zhang, F.-Z.; Jiang, H.-X.; Tang, N. CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 2009, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Q.; Yuan, W.; Xu, F.; Aslam, M.M.; Miao, R.; Li, Y.; Wang, Q.; Li, X.; Zhang, X.; et al. The genome evolution and low-phosphorus adaptation in white lupin. Nat. Commun. 2020, 11, 1069. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Li, Y.; Wan, X.; Ma, Z.; Cao, J.; Li, Z.; He, F.; Wang, Y.; Wan, L.; et al. Analysis of physiological and miRNA responses to Pi deficiency in alfalfa (Medicago sativa L.). Plant Mol. Biol. 2018, 96, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-T.; Zhou, Y.-F.; Wang, Y.-Y.; Wu, Y.-M.; Ye, X.; Guo, J.-X.; Chen, L.-S. Magnesium Deficiency Induced Global Transcriptome Change in Citrus sinensis Leaves Revealed by RNA-Seq. Int. J. Mol. Sci. 2019, 20, 3129. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers. Soil Sci. 1966, 101, 346. [Google Scholar] [CrossRef]
- Denison, R.F.; Kiers, E.T. 11 Sustainable crop nutrition: Constraints and opportunities. In Plant Nutritional Genomics; Wiley-Blackwell: Oxford, UK, 2009; p. 242. [Google Scholar]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Kobayashi, T.; Maeda, K.; Suzuki, Y.; Nishizawa, N.K. Simultaneous Enhancement of iron Deficiency Tolerance and Iron Accumulation in Rice by Combining the Knockdown of OsHRZ Ubiquitin Ligases with the Introduction of Engineered Ferric-chelate Reductase. Rice 2022, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Chowardhara, B.; Regon, P.; Kar, S.; Saha, B.; Panda, S.K. Iron deficiency in blackgram (Vigna mungo L.): Redox status and antioxidant activity. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 156, 411–426. [Google Scholar]
- Riaz, N.; Guerinot, M.L. All together now: Regulation of the iron deficiency response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Cho, S.W.; Kwon, T.H.; Yang, M.S. Purification and characterization of phytoferritin. BMB Rep. 1996, 29, 540–544. [Google Scholar]
- Ghazy, N.; El-Nahrawy, S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 2021, 203, 1195–1209. [Google Scholar] [CrossRef]
- Sinha, A.K.; Venkateswaran, B.P.; Tripathy, S.C.; Sarkar, A.; Prabhakaran, S. Effects of growth conditions on siderophore producing bacteria and siderophore production from Indian Ocean sector of Southern Ocean. J. Basic Microbiol. 2019, 59, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Subramanium, N.; Sundaram, L. Siderophore producing Pseudomonas spp. isolated from rhizospheric soil and enhancing iron content in Arachis hypogaea L. plant. J. Agric. Technol 2020, 16, 429–442. [Google Scholar]
- Singh, P.; Singh, R.K.; Guo, D.-J.; Sharma, A.; Singh, R.N.; Li, D.-P.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; Yang, L.-T.; et al. Whole Genome Analysis of Sugarcane Root-Associated Endophyte Pseudomonas aeruginosa B18-A Plant Growth-Promoting Bacterium with Antagonistic Potential Against Sporisorium scitamineum. Front. Microbiol. 2021, 12, 628376. [Google Scholar] [CrossRef]
- Sah, S.; Singh, R. Siderophore: Structural and functional characterization-a comprehensive review. Agriculture 2015, 6, 97–114. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Shen, N.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: A review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, P.; Guo, D.-J.; Sharma, A.; Li, D.-P.; Li, X.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; et al. Root-Derived Endophytic Diazotrophic Bacteria Pantoea cypripedii AF1 and Kosakonia arachidis EF1 Promote Nitrogen Assimilation and Growth in Sugarcane. Front. Microbiol. 2021, 12, 774707. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.K.; Li, H.-B.; Guo, D.-J.; Sharma, A.; Lakshmanan, P.; Malviya, M.K.; Song, X.-P.; Solanki, M.K.; Verma, K.K.; et al. Diazotrophic Bacteria Pantoea dispersa and Enterobacter asburiae Promote Sugarcane Growth by Inducing Nitrogen Uptake and Defense-Related Gene Expression. Front. Microbiol. 2021, 11, 600417. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, P.; Li, H.B.; Song, Q.Q.; Guo, D.J.; Solanki, M.K.; Verma, K.K.; Malviya, M.K.; Song, X.P.; Lakshmanan, P.; et al. Diversity of nitrogen-fixing rhizobacteria associated with sugarcane, a comprehensive study of plant-microbe interactions for growth enhancement in Saccharum spp. BMC Plant Biol. 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, P.; Li, H.-B.; Guo, D.-J.; Song, Q.-Q.; Yang, L.-T.; Malviya, M.K.; Song, X.-P.; Li, Y.-R. Plant-PGPR interaction study of plant growth-promoting diazotrophs Kosakonia radicincitans BA1 and Stenotrophomonas maltophilia COA2 to enhance growth and stress-related gene expression in Saccharum spp. J. Plant Interact. 2020, 15, 427–445. [Google Scholar] [CrossRef]
- Aznar, A.; Dellagi, A. New insights into the role of siderophores as triggers of plant immunity: What can we learn from animals? J. Exp. Bot. 2015, 66, 3001–3010. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Ustiatik, R.; Nuraini, Y.; Suharjono, S.; Handayanto, E. Siderophore Production of the Hg-Resistant Endophytic Bacteria Isolated from Local Grass in the Hg-Contaminated Soil. J. Ecol. Eng. 2021, 22, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.P.; Xu, J.U. A simple double layered chrome azurol S agar (SD CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. Afr. J. Microbiol. Res. 2011, 5, 4321–4327. [Google Scholar]
- Singh, R.; Pandey, D.K.; Kumar, A. PGPR isolates from the rhizosphere of vegetable crop Momordica charantia: Characterization and application as biofertilizer. Int. J. Curr. Microbiol. Appl Sci. 2017, 6, 1789–1802. [Google Scholar]
- Ahmed, E.; Holmstrom, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Schütze, E.; Ahmed, E.; Voit, A.; Klose, M.; Greyer, M.; Svatoš, A.; Merten, D.; Roth, M.; Holmström, S.J.M.; Kothe, E. Siderophore production by streptomycetes—Stability and alteration of ferrihydroxamates in heavy metal-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 19376–19383. [Google Scholar] [CrossRef] [PubMed]
- Lurthy, T.; Cantat, C.; Jeudy, C.; Declerck, P.; Gallardo, K.; Barraud, C.; Leroy, F.; Ourry, A.; Lemanceau, P.; Salon, C.; et al. Impact of Bacterial Siderophores on Iron Status and Ionome in Pea. Front. Plant Sci. 2020, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, J.; Wang, W.; Chen, B.; Li, E.; Chen, S. Siderophore and indolic acid production by Paenibacillus triticisoli BJ-18 and their plant growth-promoting and antimicrobe abilities. PeerJ 2020, 8, e9403. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Brandt, N.; Van Antwerpen, P.; Wintjens, R.; Matthijs, S. Two new siderophores produced by Pseudomonas sp. NCIMB 10586: The anti-oomycete non-ribosomal peptide synthetase-dependent mupirochelin and the NRPS-independent triabactin. Front. Microbiol. 2023, 14, 1143861. [Google Scholar] [CrossRef] [PubMed]
- Saharan, B.S.; Chaudhary, T.; Mandal, B.S.; Kumar, D.; Kumar, R.; Sadh, P.K.; Duhan, J.S. Microbe-Plant Interactions Targeting Metal Stress: New Dimensions for Bioremediation Applications. J. Xenobiotics 2023, 13, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-solubilizing bacteria: Their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.I.; Pereira, M.C.; de Carvalho, A.M.X.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-Solubilizing Microorganisms: A Key to Sustainable Agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Kouas, S.; Labidi, N.; Debez, A.; Abdelly, C. Effect of P on nodule formation and N fixation in bean. Agron. Sustain. Dev. 2005, 25, 389–393. [Google Scholar] [CrossRef]
- Rajguru, B.R.; Bhatt, V.D. Review on Mechanism of Mineral Phosphate Solubilization in Fast-Growing Rhizobia Based on Sugar Utilization. Plant Breed. Biotechnol. 2022, 10, 203–211. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, Y.; Cao, H.; Zhao, Y.; Li, Z.; Wang, H.; Chen, M.; Tang, Q. Simultaneous Determination of 13 Organic Acids in Liquid Culture Media of Edible Fungi Using High-Performance Liquid Chromatography. BioMed. Res. Int. 2020, 2020, 2817979. [Google Scholar] [CrossRef] [PubMed]
- Djuuna, I.A.F.; Prabawardani, S.; Massora, M. Population Distribution of Phosphate-solubilizing Microorganisms in Agricultural Soil. Microbes Environ. 2022, 37, ME21041. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guzmán, P.; Santoyo, G. Action mechanisms, biodiversity, and omics approaches in biocontrol and plant growth-promoting Pseudomonas: An updated review. Biocontrol. Sci. Technol. 2022, 32, 527–550. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.; Yadav, A.N.; Suman, A.; Ahluwalia, A.S.; Saxena, A.K. Minerals solubilizing and mobilizing microbiomes: A sustainable approach for managing minerals’ deficiency in agricultural soil. J. Appl. Microbiol. 2022, 133, 1245–1272. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Fahad, S.; Saleem, M.H.; Ali, B.; Mussart, M.; Ullah, R.; Jr, A.; Arif, M.; Ahmad, M.; Shah, W.A.; et al. Comparative efficacy of phosphorous supplements with phosphate solubilizing bacteria for optimizing wheat yield in calcareous soils. Sci. Rep. 2022, 12, 11997. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, R.; Cao, Y.; Turner, B.L.; Ma, L.Q. Enhancing Phytate Availability in Soils and Phytate-P Acquisition by Plants: A Review. Environ. Sci. Technol. 2022, 56, 9196–9219. [Google Scholar] [CrossRef]
- de Freitas Duarte, N.; Paiva, C.A.O.; Pagano, M.C.; Correa, E.J.A. Phosphate solubilization by microorganisms. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 257–282. [Google Scholar]
- Ramamoorthy, P.; Karthikeyan, M.; Nirubana, V. Role of Phosphate Solubilizing Microorganisms in Agriculture. Agric. Mirror Future India 2020, 1, 104. [Google Scholar]
- Mandal, P.; Tiru, Z. Soil application of plant growth promoting fungi for sustainable agriculture in the new decade. In Plant Stress: Challenges and Management in the New Decade; Springer Int. Publishing: Cham, Switzerland, 2022; pp. 321–330. [Google Scholar]
- Zhao, S.; Zheng, B.-W.; Wang, Y.-C.; He, F.; Wang, L.-J.; Lin, X.; Luo, X.-M.; Feng, J.-X. Environmentally-friendly biorecovery of manganese from electrolytic manganese residue using a novel Penicillium oxalicum strain Z6-5-1: Kinetics and mechanism. J. Hazard. Mater. 2023, 446, 130662. [Google Scholar] [CrossRef]
- Aberathna, A.A.A.U.; Satharasinghe, D.A.; Jayasooriya, A.P.; Jinadasa, R.N.; Manopriya, S.; Jayaweera, B.P.A.; Fernando, C.A.N.; Weerathilake, W.A.D.V.; Prathapasinghe, G.A.; Liyanage, J.A.; et al. Increasing the Bioavailability of Phosphate by Using Microorganisms. Int. J. Agron. 2022, 2022, 4305501. [Google Scholar] [CrossRef]
- Aberathna, A.A.U.; Satharasinghe, D.A.; Jayasooriya, A.P.; Jinadasa, H.R.N.; Manopriya, S.; Jayaweera, B.A.; Fernando, C.A.N.; Weerathilake, W.A.D.V.; Prathapasinghe, G.A.; Liyanage, J.A.; et al. Managing Soil and Plant Nutrients: Role of Microbial Phosphate Solubilisation. In Phosphorus in Soils and Plants; IntechOpen: London, UK, 2023. [Google Scholar]
- Yadav, A.N. Phosphate-Solubilizing Microorganisms for Agricultural Sustainability. J. Appl. Biol. Biotechnol. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Ghoulam, C.; Barakat, A.; Zeroual, Y.; Bargaz, A. Phosphate bacterial solubilization: A key rhizosphere driving force enabling higher P use efficiency and crop productivity. J. Adv. Res. 2022, 38, 13–28. [Google Scholar] [CrossRef]
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate Solubilizing Rhizobacteria Could Have a Stronger Influence on Wheat Root Traits and Aboveground Physiology Than Rhizosphere P Solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Bargaz, A.; Elhaissoufi, W.; Khourchi, S.; Benmrid, B.; Borden, K.A.; Rchiad, Z. Benefits of phosphate solubilizing bacteria on belowground crop performance for improved crop acquisition of phosphorus. Microbiol. Res. 2021, 252, 126842. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2021, 3, 100094. [Google Scholar] [CrossRef]
- Crichton, R.R.; Wilmet, S.; Legssyer, R.; Ward, R.J. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 2002, 91, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology; The Benyamin. Cummings: Redwood City, CA, USA, 1991. [Google Scholar]
- Tognetti, V.B.; Zurbriggen, M.D.; Morandi, E.N.; Fillat, M.F.; Valle, E.M.; Hajirezaei, M.-R.; Carrillo, N. Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc. Natl. Acad. Sci. USA 2007, 104, 11495–11500. [Google Scholar] [CrossRef] [PubMed]
- Bienfait, H.F.; Van der Mark, F. Phytoferritin and its role in iron metabolism. In Metals and Micronutrients. Uptake and Utilization by Plants; Academic Press: Cambridge, MA, USA, 1983; pp. 111–123. [Google Scholar]
- Kobayashi, T.; Nakayama, Y.; Itai, R.N.; Nakanishi, H.; Yoshihara, T.; Mori, S.; Nishizawa, N.K. Identification of novel cis-acting elements, IDE1 and IDE2, of the barley IDS2 gene promoter conferring iron-deficiency-inducible, root-specific expression in heterogeneous tobacco plants. Plant J. 2003, 36, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Miyagi, A.; Tazoe, Y.; Suganami, M.; Kawai-Yamada, M.; Ueda, A.; Suzuki, Y.; Noguchi, K.; Hirotsu, N.; Makino, A. Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defence systems by phytic acid accumulation in leaves. Plant Cell Environ. 2020, 43, 2033–2053. [Google Scholar] [CrossRef] [PubMed]
- Gikaara, D.M.; Johnston, M.E.; Edwards, D.G. Phosphorus management of Australian native plants. In V International Symposium on New Floricultural Crops; International Society for Horticultural Science: Leuven, Belgium, 2003; Volume 683, pp. 133–140. [Google Scholar]
- Verma, L.; Pandey, N. The effect of Fe toxicity on seed germination and early seedling growth of green gram (Vigna radiata L. Wilczek). Int. J. Sci. Res. 2017, 6, 1427–1430. [Google Scholar]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; LimaBrito, J. Seed priming with Fe and zinc in bread wheat: Effects in germination, mitosis and grain yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Ifie, J.E.; Ifie-Etumah, S.O.; Ikhajiagbe, B. Physiological and biochemical responses of selected cowpea (Vigna unguiculata (L.) Walp.) accessions to Fe toxicity. Acta Agric. Slov. 2020, 115, 25–38. [Google Scholar] [CrossRef]
- El Rasafi, T.; Nouri, M.; Bouda, S.; Haddioui, A. The Effect of Cd, Zn and Fe on Seed Germination and Early Seedling Growth of Wheat and Bean. Ekologia 2016, 35, 213–223. [Google Scholar] [CrossRef]
- Nozoe, T.; Agbisit, R.; Fukuta, Y.; Rodriguez, R.; Yanagihara, S. Characteristics of Fe tolerant rice lines developed at IRRI under field conditions. Jpn. Agric. Res. Q. 2008, 42, 187–192. [Google Scholar] [CrossRef]
- Rodrigues Filho, J.; Corte, V.B.; Perin, I.T.; dos Santos, C.R.; da Silva, R.W. Effects of Fe toxicity on germination and initial growth of Carica papaya L. Sci. Plena 2020, 16, 101201. [Google Scholar] [CrossRef]
- Peña-Olmos, J.E.; Casierra-Posada, F.; Olmos-Cubides, M.A. The effect of high Fe doses (Fe2+) on the growth of broccoli plants (Brassica oleracea var. italica). Agron. Colomb. 2014, 32, 22–28. [Google Scholar] [CrossRef]
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2005, 168, 558–573. [Google Scholar] [CrossRef]
- dos Santos, M.S.; Sanglard, L.M.; Barbosa, M.L.; Namorato, F.A.; de Melo, D.C.; Franco, W.C.; Pérez-Molina, J.P.; Martins, S.C.V.; DaMatta, F.M. Silicon nutrition mitigates the negative impacts of Fe toxicity on rice photosynthesis and grain yield. Ecotoxicol. Environ. Saf. 2020, 189, 110008. [Google Scholar] [CrossRef] [PubMed]
- Tadaiesky, L.B.A.; da Silva, B.R.S.; Batista, B.L.; Lobato, A.K.D.S. Brassinosteroids trigger tolerance to iron toxicity in rice. Physiol. Plant. 2020, 171, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Onyango, D.A.; Entila, F.; Dida, M.M.; Ismail, A.M.; Drame, K.N. Mechanistic understanding of Fe toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responses. Funct. Plant Biol. 2019, 46, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Sahrawat, K.L. Reducing Fe toxicity in lowland rice with tolerant genotypes and plant nutrition. Plant Stress 2010, 4, 70–75. [Google Scholar]
- Mengel, K.; Kirkby, E. Principles of Plant Nutrition; International Potash Institute: Bern, Switzerland, 1987; pp. 687–695. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Amsterdam, The Netherlands, 2011; pp. 7–44. [Google Scholar]
- Audebert, A. Iron toxicity in rice: Environmental conditions and symptoms. In Iron Toxicity in Rice-Based Systems in West Africa; Audebert, A., Narteh, L.T., Kiepe, P., Millar, D., Beks, B., Eds.; WARDA [Africa Rice Center]: Cotonou, Benin, 2006; pp. 18–33. [Google Scholar]
- Olaleye, A.; Ogunkunle, A.; Singh, B.; Akinbola, G.; Tabi, F.; Fayinminu, O.; Iji, M.E. Ratios of nutrients in lowland rice grown on two Fe toxic soils in Nigeria. J. Plant Nutr. 2009, 32, 1336–1352. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Phosphorus toxicity in tomato plants: When and how does it occur? Commun. Soil Sci. Plant Anal. 1998, 29, 1779–1784. [Google Scholar] [CrossRef]
- Dixit, R.K.; Thomas, M.B.; Farooqui, U.M.; Patra, A.K.; Spurway, M.I. Phosphorus response and toxicity in Acacia spp. Indian For. 1999, 125, 770–774. [Google Scholar]
- Sahrawat, K.L. Iron Toxicity in Wetland Rice and the Role of Other Nutrients. J. Plant Nutr. 2005, 27, 1471–1504. [Google Scholar] [CrossRef]
- Majerus, V.; Bertin, P.; Lutts, S. Effects of iron toxicity on osmotic potential, osmolytes and polyamines concentrations in the African rice (Oryza glaberrima Steud.). Plant Sci. 2007, 173, 96–105. [Google Scholar] [CrossRef]
- Olaleye, A.; Ogunkunle, A. Management of two potentially Fe toxic benchmark wetlands using integrated approach for rice production in Nigeria. J. Agron. Crop Sci. 2008, 194, 237–243. [Google Scholar] [CrossRef]
- Mehraban, P.; Zadeh, A.A.; Sadeghipour, H.R. Iron toxicity in rice (Oryza sativa L.), under different potassium nutrition. Asian J. Plant Sci. 2008, 7, 251–259. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.G.; Beardsell, D.V. Interactions of calcium, nitrogen and potassium with phosphorus on the symptoms of toxicity in Grevillea cv. ‘Poorinda Firebird’. Plant Soil 1981, 61, 437–445. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 110, pp. 251–331. [Google Scholar]
- Zheng, L.; Huang, F.; Narsai, R.; Wu, J.; Giraud, E.; He, F.; Cheng, L.; Wang, F.; Wu, P.; Whelan, J.; et al. Physiological and Transcriptome Analysis of Iron and Phosphorus Interaction in Rice Seedlings. Plant Physiol. 2009, 151, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Sanagala, R.; Sinilal, B.; Yadav, S.; Sarkar, A.K.; Dantu, P.K.; Jain, A. Iron Availability Affects Phosphate Deficiency-Mediated Responses, and Evidence of Cross-Talk with Auxin and Zinc in Arabidopsis. Plant Cell Physiol. 2015, 56, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The Effect of Iron on the Primary Root Elongation of Arabidopsis during Phosphate Deficiency. Plant Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef]
- Jain, A.; Sinilal, B.; Dhandapani, G.; Meagher, R.B.; Sahi, S.V. Effects of Deficiency and Excess of Zinc on Morphophysiological Traits and Spatiotemporal Regulation of Zinc-Responsive Genes Reveal Incidence of Cross Talk between Micro- and Macronutrients. Environ. Sci. Technol. 2013, 47, 5327–5335. [Google Scholar] [CrossRef]
- Ferrol, N.; Tamayo, E.; Vargas, P. The heavy metal paradox in arbuscular mycorrhizas: From mechanisms to biotechnological applications. J. Exp. Bot. 2016, 67, 6253–6265. [Google Scholar] [CrossRef] [PubMed]
- Hoseinzade, H.; Ardakani, M.R.; Shahdi, A.; Rahmani, H.A.; Noormohammadi, G.; Miransari, M. Rice (Oryza sativa L.) nutrient management using mycorrhizal fungi and endophytic Herbaspirillum seropedicae. J. Integr. Agric. 2016, 15, 1385–1394. [Google Scholar] [CrossRef]
- Nafady, N.A.; Elgharably, A. Mycorrhizal symbiosis and phosphorus fertilization effects on Zea mays growth and heavy metals uptake. Int. J. Phytoremediation 2018, 20, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Hirsch, J.; Marin, E.; Floriani, M.; Chiarenza, S.; Richaud, P.; Nussaume, L.; Thibaud, M. Phosphate deficiency promotes modification of iron distribution in Arabidopsis plants. Biochimie 2006, 88, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Delhaize, E.; Ma, J.F.; Ryan, P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012, 17, 341–348. [Google Scholar] [CrossRef] [PubMed]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Cumbus, I.P.; Hornsey, D.J.; Robinson, L.W. The influence of phosphorus, zinc and manganese on absorption and translocation of iron in watercress. Plant Soil 1977, 48, 651–660. [Google Scholar] [CrossRef]
- Mathan, K.K.; Amberger, A. Influence of iron on the uptake of phosphorus by maize. Plant Soil 1977, 46, 413–422. [Google Scholar] [CrossRef]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed]
- Thibaud, M.-C.; Arrighi, J.-F.; Bayle, V.; Chiarenza, S.; Creff, A.; Bustos, R.; Paz-Ares, J.; Poirier, Y.; Nussaume, L. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J. 2010, 64, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Bournier, M.; Tissot, N.; Mari, S.; Boucherez, J.; Lacombe, E.; Briat, J.-F.; Gaymard, F. Arabidopsis Ferritin 1 (AtFer1) Gene Regulation by the Phosphate Starvation Response 1 (AtPHR1) Transcription Factor Reveals a Direct Molecular Link between Iron and Phosphate Homeostasis. J. Biol. Chem. 2013, 288, 22670–22680. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.; Castrillo, G.; Linhares, F.; Puga, M.I.; Rubio, V.; Pérez-Pérez, J.; Solano, R.; Leyva, A.; Paz-Ares, J. A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis. PLoS Genet. 2010, 6, e1001102. [Google Scholar] [CrossRef] [PubMed]
- Godon, C.; Mercier, C.; Wang, X.; David, P.; Richaud, P.; Nussaume, L.; Liu, D.; Desnos, T. Under phosphate starvation conditions, Fe and Al trigger accumulation of the transcription factor STOP1 in the nucleus of Arabidopsis root cells. Plant J. 2019, 99, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Hanikenne, M.; Esteves, S.M.; Fanara, S.; Rouached, H. Coordinated homeostasis of essential mineral nutrients: A focus on iron. J. Exp. Bot. 2021, 72, 2136–2153. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Gu, T.-Y.; Qi, Z.-A.; Yan, J.; Fang, Z.-J.; Lu, Y.-T.; Li, H.; Gong, J.-M. Two NPF transporters mediate iron long-distance transport and homeostasis in Arabidopsis. Plant Commun. 2021, 2, 100244. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Y.; Liang, G. FIT and bHLH Ib transcription factors modulate iron and copper crosstalk in Arabidopsis. Plant Cell Environ. 2021, 44, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Eyster, H.C. Auxin action. Science 1943, 97, 358–359. [Google Scholar] [CrossRef]
- Phinney, B.O. Growth Response of single-gene dwarf mutants in maize to gibberellic acid. Proc. Natl. Acad. Sci. USA 1956, 42, 185–189. [Google Scholar] [CrossRef]
- Braun, P.; Wild, A. The Influence of Brassinosteroid on Growth and Parameters of Photosynthesis of Wheat and Mustard Plants. J. Plant Physiol. 1984, 116, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Nakashita, H.; Yasuda, M.; Nitta, T.; Asami, T.; Fujioka, S.; Arai, Y.; Sekimata, K.; Takatsuto, S.; Yamaguchi, I.; Yoshida, S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003, 33, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; McRoberts, N. Plants and biotrophs: A pivotal role for cytokinins? Trends Plant Sci. 2006, 11, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. Linking development to defense: Auxin in plant–pathogen interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, I.A.; Eggermont, K.; Terras, F.R.; Thomma, B.P.; De Samblanx, G.W.; Buchala, A.; Métraux, J.P.; Manners, J.M.; Broekaert, W.F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 1996, 8, 2309–2323. [Google Scholar] [PubMed]
- Gaffney, T.; Friedrich, L.; Vernooij, B.; Negrotto, D.; Nye, G.; Uknes, S.; Ward, E.; Kessmann, H.; Ryals, J. Requirement of Salicylic Acid for the Induction of Systemic Acquired Resistance. Science 1993, 261, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Flors, V.; Mauch-Mani, B. The multifaceted role of ABA in disease resistance. Trends Plant Sci. 2009, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, E.C. Fe stress induced transfer cell formation: Regulated by auxin. Plant Physiol. 1981, 67, 563. [Google Scholar]
- Schmidt, W.; Tittel, J.; Schikora, A. Role of Hormones in the Induction of Iron Deficiency Responses in Arabidopsis Roots. Plant Physiol. 2000, 122, 1109–1118. [Google Scholar] [CrossRef]
- Landsberg, E.C. Hormonal regulation of iron-stress response in sunflower roots: A morphological and cytological investigation. Protoplasma 1996, 194, 69–80. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996, 19, 529–538. [Google Scholar] [CrossRef]
- Chen, W.W.; Yang, J.L.; Qin, C.; Jin, C.W.; Mo, J.H.; Ye, T.; Zheng, S.J. Nitric Oxide Acts Downstream of Auxin to Trigger Root Ferric-Chelate Reductase Activity in Response to Iron Deficiency in Arabidopsis. Plant Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef]
- Romera, F.J.; Alcantara, E.; DE LA Guardia, M.D. Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by strategy I plants. Ann. Bot. 1999, 83, 51–55. [Google Scholar] [CrossRef]
- Lucena, C.; Waters, B.M.; Romera, F.J.; García, M.J.; Morales, M.; Alcántara, E.; Pérez-Vicente, R. Ethylene could influence ferric reductase, iron transporter, and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. J. Exp. Bot. 2006, 57, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Lucena, C.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene and nitric oxide involvement in the up-regulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J. Exp. Bot. 2010, 61, 3885–3899. [Google Scholar] [CrossRef] [PubMed]
- Maurer, F.; Müller, S.; Bauer, P. Suppression of Fe deficiency gene expression by jasmonate. Plant Physiol. Biochem. 2011, 49, 530–536. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; García-Pineda, E.; Valencia-Cantero, E. Bacterial compound N, N-dimethylhexadecylamine modulates expression of iron deficiency and defense response genes in Medicago truncatula independently of the jasmonic acid pathway. Plants 2020, 9, 624. [Google Scholar] [CrossRef]
- Kang, H.G.; Foley, R.C.; Oñate-Sánchez, L.; Lin, C.; Singh, K.B. Target genes for OBP3, a Dof transcription factor, include novel basic helix-loop-helix domain proteins inducible by salicylic acid. Plant J. 2003, 35, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Dong, Y.; Xu, L.; Liu, S.; Bai, X. Effects of Exogenous Salicylic Acid on Alleviating Chlorosis Induced by Iron Deficiency in Peanut Seedlings (Arachis hypogaea L.). J. Plant Growth Regul. 2014, 33, 715–729. [Google Scholar] [CrossRef]
- García, M.J.; Suárez, V.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe-acquisition genes in Strategy I plants. Plant Physiol. Biochem. 2011, 49, 537–544. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, B.; Song, W.F.; Zheng, S.J.; Shen, R.F. Putrescine Alleviates Iron Deficiency via NO-Dependent Reutilization of Root Cell-Wall Fe in Arabidopsis. Plant Physiol. 2016, 170, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Mari, S. New routes for plant iron mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhu, C.; Liu, Y.; Karthikeyan, A.S.; Bressan, R.A.; Raghothama, K.G.; Liu, D. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytol. 2011, 189, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.K.; Smith, A.P. Ethylene’s role in phosphate starvation signaling: More than just a root growth regulator. Plant Cell Physiol. 2012, 53, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.K.; Jain, A.; Poling, M.D.; Lewis, A.J.; Raghothama, K.G.; Smith, A.P. Arabidopsis Pht1;5 Mobilizes Phosphate between Source and Sink Organs and Influences the Interaction between Phosphate Homeostasis and Ethylene Signaling. Plant Physiol. 2011, 156, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Gao, Y.; Tian, Q.-Y.; Shi, F.-L.; Li, L.-H.; Zhang, W.-H. Stimulation of root acid phosphatase by phosphorus deficiency is regulated by ethylene in Medicago falcata. Environ. Exp. Bot. 2011, 71, 114–120. [Google Scholar] [CrossRef]
- García, M.J.; Romera, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R. Ethylene and the Regulation of Physiological and Morphological Responses to Nutrient Deficiencies. Plant Physiol. 2015, 169, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Horgan, J.M.; Wareing, P.F. Cytokinins and the growth responses of seedlings of Betula pendula Roth. and Acer pseudoplatanus L. to nitrogen and phosphorus deficiency. J. Exp. Bot. 1980, 31, 525–532. [Google Scholar] [CrossRef]
- Martín, A.C.; Del Pozo, J.C.; Iglesias, J.; Rubio, V.; Solano, R.; De La Peña, A.; Leyva, A.; Paz-Ares, J. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. Plant J. 2000, 24, 559–567. [Google Scholar] [CrossRef]
- Séguéla, M.; Briat, J.; Vert, G.; Curie, C. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J. 2008, 55, 289–300. [Google Scholar] [CrossRef]
| Phosphate-Solubilizing Microorganisms | Predominantly Produced Acids | Ecological Niche | References |
|---|---|---|---|
| Escherichia freundii | Lactic acid | Soil | [174,175] |
| Penicillium sp., Aspergillus niger | Citric acid, succinic acid, oxalic acid, glycolic acid, gluconic acid, lactic acid | Soil | [176,177] |
| Bacillus subtilus, Bacillus megaterium, Pseudomonas sp. | Lactic acid, malic acid | Soil rizoshpere | [177] |
| Arthrobacter sp., Bacillus sp. Bacillus firmus B-7650 | Citric acid, lactic acid | Cowpea and wheat rhizospheres | [178] |
| Chaetomium nigricolor, Penicillium sp., Aspergillus sp. | Oxalic acid, succinic acid, citric acid, 2-ketogluconic acid | Lateritic soil | [179,180] |
| A. foetidus, A. japonicus | Citric acid, tartaric acid, succinic acid, oxalic acid, gluconic acid | Indian rock phosphate | [175] |
| P. radicum | Gluconic acid | Wheat rhizosphere | [181] |
| Enterobacter agglomerans | Citric acid, oxalic acid | Wheat rhizosphere | [182] |
| Enterobacter aerogenes, E. asburiae, E. taylorae, Penibacillus macerans, Vibrio proteolyticus, Kluyvera cryocrescens, Xanthobacter agilis, Pseudomonas aeromonassens, Bacillus amyloliquefaciens, B. atrophaeus, B. licheniformis | Acetic acid, itaconic acid, isobutyric acid, isovaleric acid, lactic acid | Mangrove | [177,183] |
| Penicillium rugulosum | Gluconic acid, citric acid | Venezuelan phosphate rocks | [184] |
| Enterobacter intermedius | 2-ketogluconic acid | Grass rhizosphere | [185] |
| Penicillium canescens, Aspergillus flavus, A. niger | Citric acid, gluconic acid, oxalic acid, succinic acid | Wheat grains | [176,186] |
| Pseudomonas fluorescens | Tartaric acid, citric acid, malic acid, gluconic acid | Oil palm rhizosphere | [182,187] |
| Aspergillus niger | Gluconic acid, oxalic acid | Tropical and subtropical soils | [188] |
| P. trivialis | Lactic acid, formic acid | Rhizosphere (Hippophae rhamnoides) (Trans-Himalayas, cold Howl and Spiti deserts) | [177] |
| Actinomadura oligospora; B. pumilus var.2; B. subtilis var.2; Citrobacter sp. | Propionic acid, gluconic acid, isovaleric acid, caproic acid, heptonic acid, isocaproic acid, formic acid, valeric acid, succinic acid, oxalic acid, oxaloacetic acid, malonic acid | Giant cardon cactus (P. pringlei) | [189,190] |
| B. pumilus CHOO8A; B. fusiformis | Succinic acid, citric acid, gluconic acid, oxalic acid, 2-ketogluconic acid, lactic acid, malic acid, formic acid | Opuntia cholla | [177] |
| Bacillus sp. SENDO 6 | Gluconic acid, isovaleric acid, propionic acid, lactic acid, formic acid, succinic acid | P. pringlei | [177] |
| Bacillus megaterium M1PCa, Enterobacter sakazakii M2PFe, Pseudomonas putida M5TSA | Acetic acid, formic acid, gluconic acid, lactic acid, oxalic acid, propionic acid, succinic acid | Mammillaria fraileana cactus | [173,191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, M.A.; Mishra, A.K.; Shah, S.N.; Bhat, M.A.; Jan, S.; Rahman, S.; Baek, K.-H.; Jan, A.T. Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants. Curr. Issues Mol. Biol. 2024, 46, 5194-5222. https://doi.org/10.3390/cimb46060312
Bhat MA, Mishra AK, Shah SN, Bhat MA, Jan S, Rahman S, Baek K-H, Jan AT. Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants. Current Issues in Molecular Biology. 2024; 46(6):5194-5222. https://doi.org/10.3390/cimb46060312
Chicago/Turabian StyleBhat, Mujtaba Aamir, Awdhesh Kumar Mishra, Sheezma Nazir Shah, Mudasir Ahmad Bhat, Saima Jan, Safikur Rahman, Kwang-Hyun Baek, and Arif Tasleem Jan. 2024. "Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants" Current Issues in Molecular Biology 46, no. 6: 5194-5222. https://doi.org/10.3390/cimb46060312
APA StyleBhat, M. A., Mishra, A. K., Shah, S. N., Bhat, M. A., Jan, S., Rahman, S., Baek, K.-H., & Jan, A. T. (2024). Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants. Current Issues in Molecular Biology, 46(6), 5194-5222. https://doi.org/10.3390/cimb46060312








