Construction of a Synergy Combination Model for Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) Extracts: Enhanced Anticancer Activity against A549 and NCI-H292 Human Lung Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction of Curcumin and Piperine
2.3. Component Analysis
2.4. Cell Culture and Viability
2.5. Response Surface Methodology
2.5.1. Central Composite Design
2.5.2. Model Construction
2.5.3. Desirability Function
2.6. Synergistic Effect
2.6.1. Combination Index
2.6.2. Curcumin Consumption Activity
2.7. Statistical Analysis
3. Results and Discussion
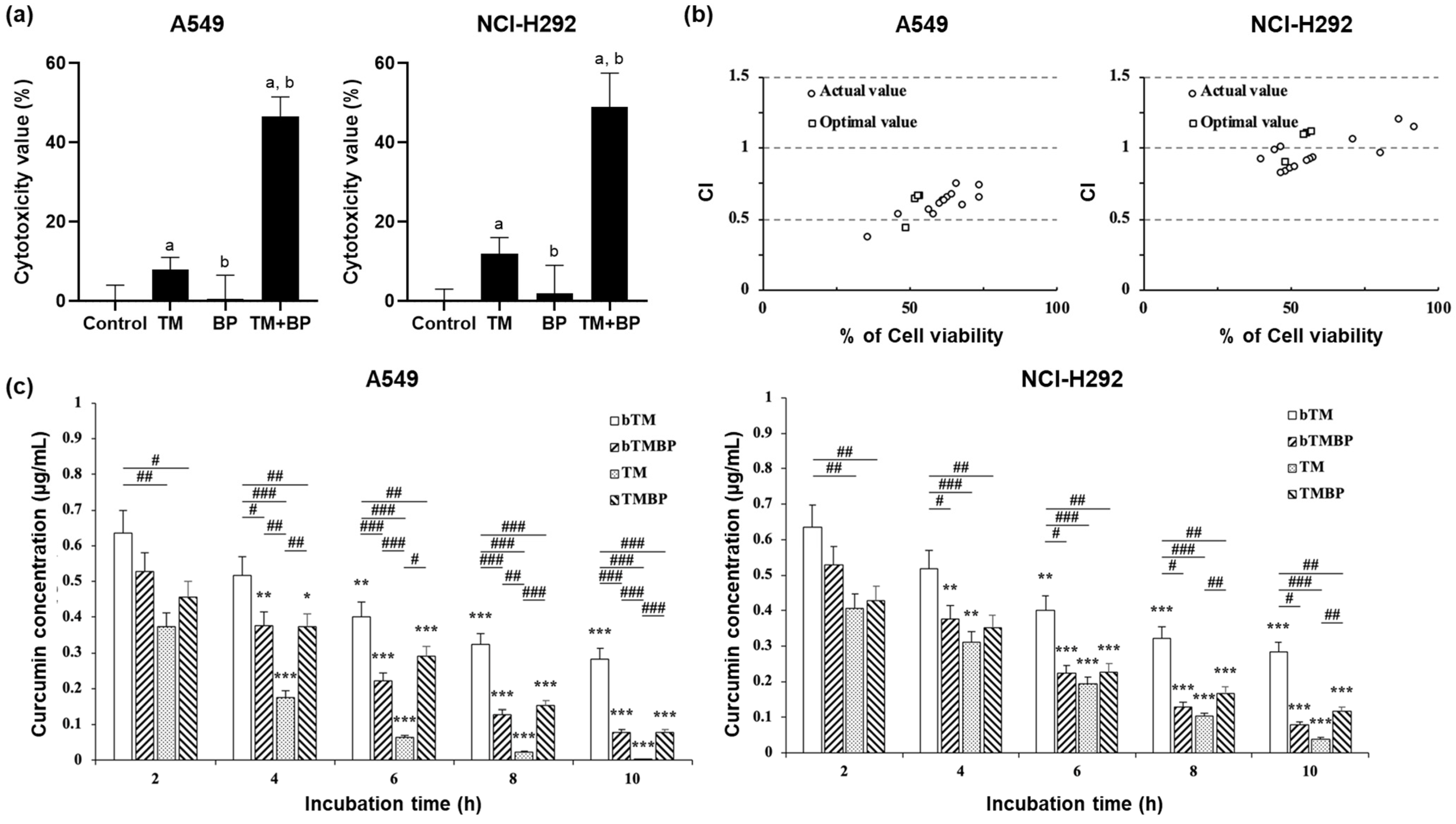
3.1. Cytotoxicity
3.2. Response Surface Analysis
3.3. Synergistic Effect and Consumption Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tesfaw, L.M.; Dessie, Z.G.; Mekonnen Fenta, H. Lung cancer mortality and associated predictors: Systematic review using 32 scientific research findings. Front. Oncol. 2023, 13, 1308897. [Google Scholar] [CrossRef] [PubMed]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non–Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Shah, A.J.; Ghayur, M.N.; Majeed, K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005, 76, 3089–3105. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Turmeric. In Drugs and Lactation Database (LactMed®); National Institute of Child Health and Human Development: Bethesda, MD, USA, 2006.
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Ahmad, N.; Fazal, H.; Abbasi, B.H.; Farooq, S.; Ali, M.; Khan, M.A. Biological role of Piper nigrum L. (Black pepper): A review. Asian Pac. J. Trop. Biomed. 2012, 2, S1945–S1953. [Google Scholar] [CrossRef]
- Patial, V.; S, M.; Sharma, S.; Pratap, K.; Singh, D.; Padwad, Y.S. Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats. Environ. Toxicol. Pharmacol. 2015, 40, 445–452. [Google Scholar] [CrossRef]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage. Food Chem. Toxicol. 2011, 49, 3002–3006. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Sankha, B. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; Palanikumar, K., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Eckert, G.P.; Rimbach, G.; Frank, J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal. Bioanal Chem. 2010, 397, 1917–1925. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Guo, H.; King, L.E.; Edgerton, E.S.; Weber, R.J. A semi-automated system for quantifying the oxidative potential of ambient particles in aqueous extracts using the dithiothreitol (DTT) assay: Results from the Southeastern Center for Air Pollution and Epidemiology (SCAPE). Atmos. Meas. Tech. 2015, 8, 471–482. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Abbruzzese, J.; Abdelrahim, M.; Hedrick, E. Specificity Protein Transcription Factors and Cancer: Opportunities for Drug Development. Cancer Prev. Res. 2018, 11, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Ngai, C.S. Curcumin Sensitizes Cancers Towards TRAIL-induced Apoptosis via Extrinsic and Intrinsic Apoptotic Pathways. Curr. Drug Targets 2020, 21, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Saleem, A.; Iqbal, Y.; Tehseen Hussain, M.; Tahir, S.; Shabbir, H. Analysis of folate and curcumin-conjugated cadmium sulfide cystein quantum dots for targeted cancer therapy. Pak. J. Pharm. Sci. 2023, 36, 659–663. [Google Scholar] [PubMed]
- Dwivedi, V.; Shrivastava, R.; Hussain, S.; Ganguly, C.; Bharadwaj, M. Cytotoxic potential of Indian spices (extracts) against esophageal squamous carcinoma cells. Asian Pac. J. Cancer Prev. 2011, 12, 2069–2073. [Google Scholar] [PubMed]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Mozafari, L.; Bueso, M.C.; Kessler, M.; Artés-Hernández, F. Response Surface Methodology to Optimize the Extraction of Carotenoids from Horticultural By-Products—A Systematic Review. Foods 2023, 12, 4456. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef] [PubMed]
- Banji, D.; Banji, O.J.; Dasaroju, S.; Annamalai, A.R. Piperine and curcumin exhibit synergism in attenuating D-galactose induced senescence in rats. Eur. J. Pharmacol. 2013, 703, 91–99. [Google Scholar] [CrossRef]
- Khajuria, A.; Thusu, N.; Zutshi, U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: Influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine 2002, 9, 224–231. [Google Scholar] [CrossRef]
- Chearwae, W.; Anuchapreeda, S.; Nandigama, K.; Ambudkar, S.V.; Limtrakul, P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem. Pharmacol. 2004, 68, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Kumar, M.; Jain, M.; Dhawan, D.K. Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum. Exp. Toxicol. 2012, 31, 473–482. [Google Scholar] [CrossRef] [PubMed]

| Rank | Desirability Value | Concentration (μg/mL) | Predicted Value (%) | Experimental Value (%) | |||
|---|---|---|---|---|---|---|---|
| Turmeric | Black Pepper | A549 | NCI-H292 | A549 | NCI-H292 | ||
| 1 | 1 | 48.5 | 241.7 | 50 | 50 | 47.6 ± 5.9 | 48.6 ± 6.2 |
| 2 | 0.816 | 29.7 | 400 | 53.3 | 49.9 | 53.1 ± 6.1 | 52.3 ± 4.5 |
| 3 | 0.816 | 29.6 | 400 | 53.3 | 50 | 51.6 ± 5.6 | 52.2 ± 4.4 |
| 4 | 0.815 | 29.7 | 400 | 53.2 | 49.8 | 52.5 ± 3.8 | 51.8 ± 3.9 |
| 5 | 0.814 | 29.8 | 400 | 53.1 | 49.6 | 52.1 ± 5.3 | 51.5 ± 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.-K.; Park, C.-G.; Lim, H.-B. Construction of a Synergy Combination Model for Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) Extracts: Enhanced Anticancer Activity against A549 and NCI-H292 Human Lung Cancer Cells. Curr. Issues Mol. Biol. 2024, 46, 5551-5560. https://doi.org/10.3390/cimb46060332
Cho H-K, Park C-G, Lim H-B. Construction of a Synergy Combination Model for Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) Extracts: Enhanced Anticancer Activity against A549 and NCI-H292 Human Lung Cancer Cells. Current Issues in Molecular Biology. 2024; 46(6):5551-5560. https://doi.org/10.3390/cimb46060332
Chicago/Turabian StyleCho, Hyun-Ki, Chang-Gyun Park, and Heung-Bin Lim. 2024. "Construction of a Synergy Combination Model for Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) Extracts: Enhanced Anticancer Activity against A549 and NCI-H292 Human Lung Cancer Cells" Current Issues in Molecular Biology 46, no. 6: 5551-5560. https://doi.org/10.3390/cimb46060332
APA StyleCho, H.-K., Park, C.-G., & Lim, H.-B. (2024). Construction of a Synergy Combination Model for Turmeric (Curcuma longa L.) and Black Pepper (Piper nigrum L.) Extracts: Enhanced Anticancer Activity against A549 and NCI-H292 Human Lung Cancer Cells. Current Issues in Molecular Biology, 46(6), 5551-5560. https://doi.org/10.3390/cimb46060332






