Evaluation of Potential Furin Protease Inhibitory Properties of Pioglitazone, Rosiglitazone, and Pirfenidone: An In Silico Docking and Molecular Dynamics Simulation Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Structures
2.2. Preparation of Protein and Ligand
2.3. Molecular Docking Analysis
2.4. Molecular Dynamics Simulation
3. Results
3.1. Molecular Docking
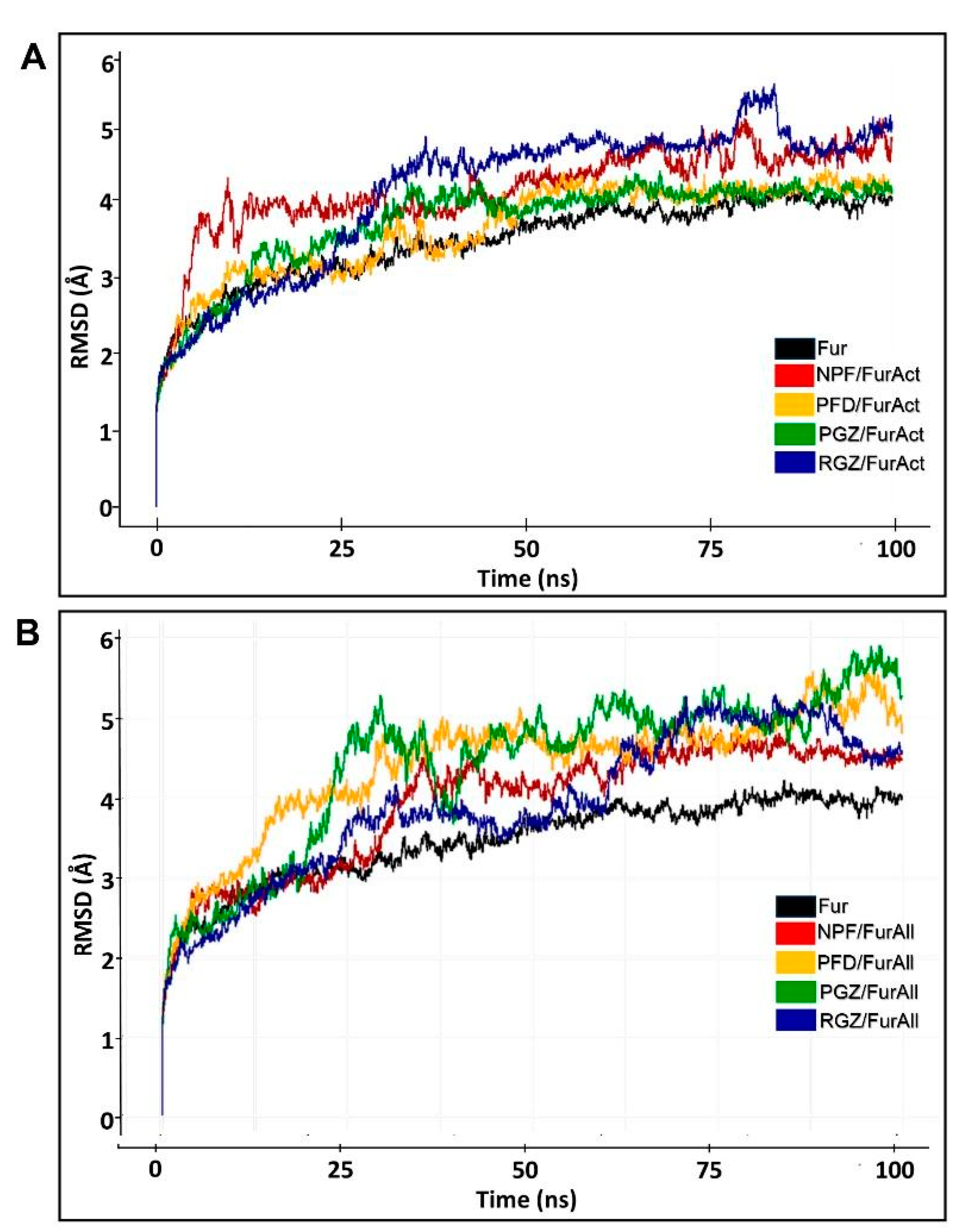
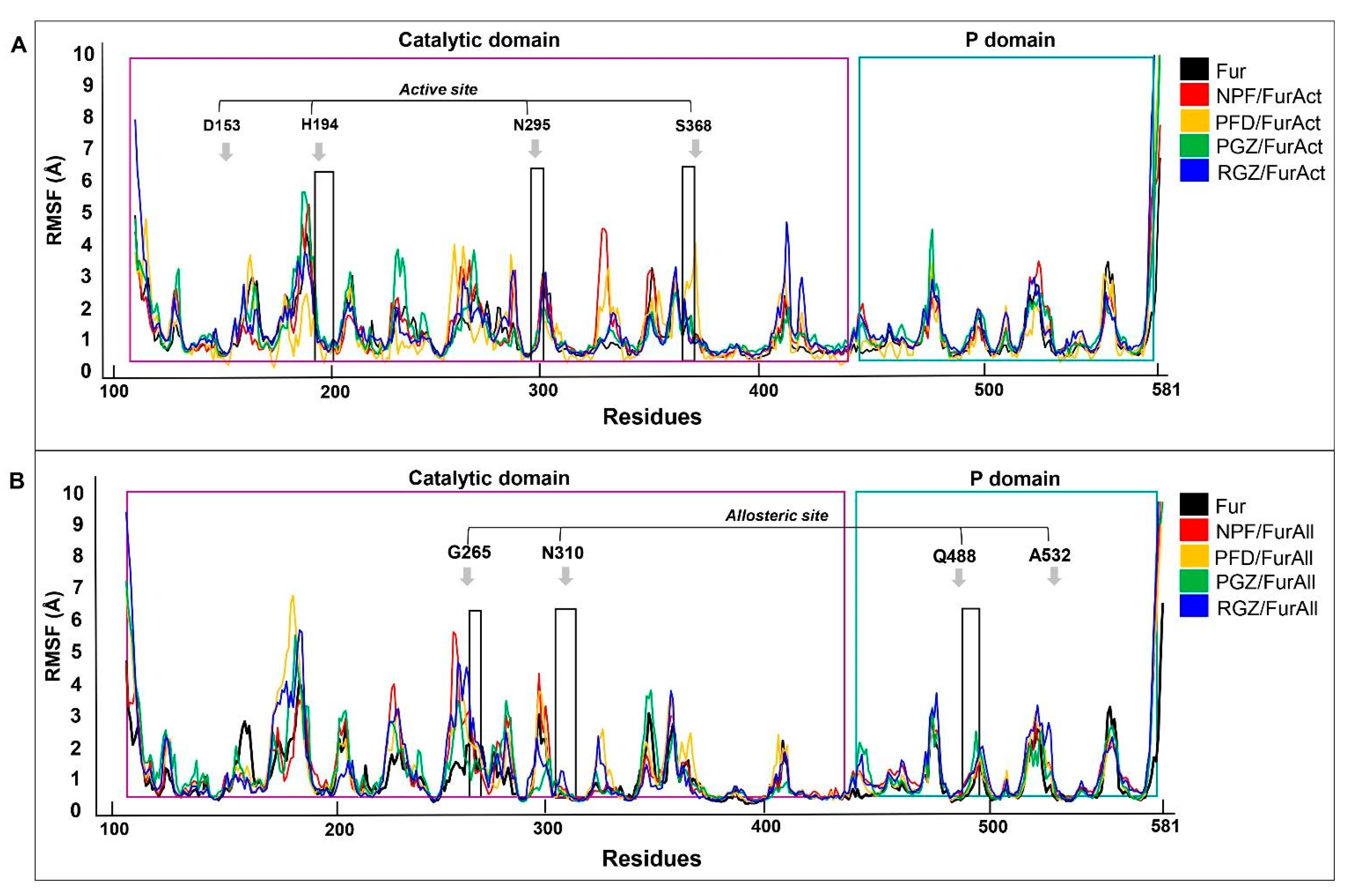
3.2. Molecular Dynamic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Praznik, A.; Fink, T.; Franko, N.; Lonzarić, J.; Benčina, M.; Jerala, N.; Plaper, T.; Roškar, S.; Jerala, R. Regulation of protein secretion through chemical regulation of endoplasmic reticulum retention signal cleavage. Nat. Commun. 2022, 13, 1323. [Google Scholar] [CrossRef] [PubMed]
- Solovyeva, N.I.; Gureeva, T.A.; Timoshenko, O.S.; Moskvitina, T.A.; Kugaevskaya, E.V. Furin as proprotein convertase and its role in normal and pathological biological processes. Biochem. Mosc. Suppl. B Biomed. Chem. 2017, 11, 87–100. [Google Scholar] [CrossRef]
- Tian, S.; Huang, Q.; Fang, Y.; Wu, J. FurinDB: A Database of 20-residue furin cleavage site motifs, substrates and their associated drugs. Int. J. Mol. Sci. 2011, 12, 1060–1065. [Google Scholar] [CrossRef]
- Steiner, D.F. The proprotein convertases. Curr. Opin. Chem. Biol. 1998, 2, 31–39. [Google Scholar] [CrossRef]
- Osman, E.E.A.; Rehemtulla, A.; Neamati, N. Why All the Fury over Furin? J. Med. Chem. 2022, 65, 2747–2784. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Sadr, M.S.; Chrétien, M.; Mbikay, M. The multifaceted proprotein convertases: Their unique, redundant, complementary, and opposite functions. J. Biol. Chem. 2013, 288, 21473–21481. [Google Scholar] [CrossRef]
- Cameron, A.; Appel, J.; Houghten, R.A.; Lindberg, I. Polyarginines are potent furin inhibitors. J. Biol. Chem. 2000, 275, 36741–36749. [Google Scholar] [CrossRef]
- Braun, E.; Sauter, D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl. Immunol. 2019, 8, e1073. [Google Scholar] [CrossRef] [PubMed]
- Bassi, D.E.; Mahloogi, H.; De Cicco, R.L.; Klein-Szanto, A. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am. J. Pathol. 2003, 162, 439–447. [Google Scholar] [CrossRef]
- He, Z.; Khatib, A.M.; Creemers, J.W.M. Loss of the proprotein convertase Furin in T cells represses mammary tumorigenesis in oncogene-driven triple negative breast cancer. Cancer Lett. 2020, 484, 40–49. [Google Scholar] [CrossRef]
- Farhat, D.; Léon, S.; Ghayad, S.E.; Gadot, N.; Icard, P.; Le Romancer, M.; Hussein, N.; Lincet, H. Lipoic acid decreases breast cancer cell proliferation by inhibiting IGF-1R via furin downregulation. Br. J. Cancer. 2020, 122, 885–894. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Thorrez, L.; Siegfried, G.; Meulemans, S.; Evrard, S.; Tejpar, S.; Khatib, A.-M.; Creemers, J.W.M. The proprotein convertase furin is a pro-oncogenic driver in KRAS and BRAF driven colorectal cancer. Oncogene 2020, 39, 3571–3587. [Google Scholar] [CrossRef] [PubMed]
- Page, R.E.; Klein-Szanto, A.J.P.; Litwin, S.; Nicolas, E.; Al-Jumaily, R.; Alexander, P.; Godwin, A.K.; Ross, E.A.; Schilder, R.J.; Bassi, D.E. Increased expression of the pro-protein convertase furin predicts decreased survival in ovarian cancer. Cell Oncol. 2007, 29, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, M.; Wei, H.; Zhou, H.; He, J.; Lu, Y.; Wang, D.; Chen, B.; Zeng, J.; Peng, W.; et al. Furin promotes epithelial-mesenchymal transition in pancreatic cancer cells via Hippo-YAP pathway. Int. J. Oncol. 2017, 50, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Sancho, E.; Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer. 2022, 22, 25–44. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Coppola, J.M.; Hamilton, C.A.; Bhojani, M.S.; Larsen, M.J.; Ross, B.D.; Rehemtulla, A. Identification of inhibitors using a cell-based assay for monitoring Golgi-resident protease activity. Anal. Biochem. 2007, 364, 19–29. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zheng, X.L.; Zhao, G.J. Proprotein convertase furin/PCSK3 and atherosclerosis: New insights and potential therapeutic targets. Atherosclerosis 2017, 262, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Kioon, M.-D.A.; Lalou, C.; Larghero, J.; Launay, J.-M.; Khatib, A.-M.; Cohen-Solal, M. Protective role of systemic furin in immune response-induced arthritis. Arthritis Rheum. 2012, 64, 2878–2886. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, Y.; Du, J.; Chen, S.; Wang, N.; Ying, H.; Shen, B. Increased FURIN expression in rheumatoid arthritis patients and its anti-inflammatory effect. J. Clin. Lab. Anal. 2020, 34, e23530. [Google Scholar] [CrossRef]
- Wu, T.; Ding, H.; Han, J.; Arriens, C.; Wei, C.; Han, W.; Pedroza, C.; Jiang, S.; Anolik, J.; Petri, M.; et al. Antibody-Array-Based Proteomic Screening of Serum Markers in Systemic Lupus Erythematosus: A Discovery Study. J. Proteome Res. 2016, 15, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Ranta, N.; Valli, A.; Grönholm, A.; Silvennoinen, O.; Isomäki, P.; Pesu, M.; Pertovaara, M. Proprotein convertase enzyme FURIN is upregulated in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S112), 47–50. Available online: https://europepmc.org/article/med/29465367 (accessed on 30 July 2024).
- Gordon, V.M.; Benz, R.; Fujii, K.; Leppla, S.H.; Tweten, R.K. Clostridium septicum alpha-toxin is proteolytically activated by furin. Infect. Immun. 1997, 65, 4130–4134. [Google Scholar] [CrossRef]
- Shiryaev, S.A.; Remacle, A.G.; Ratnikov, B.I.; Nelson, N.A.; Savinov, A.Y.; Wei, G.; Bottini, M.; Rega, M.F.; Parent, A.; Desjardins, R.; et al. Targeting host cell furin proprotein convertases as a therapeutic strategy against bacterial toxins and viral pathogens. J. Biol. Chem. 2007, 282, 20847–20853. [Google Scholar] [CrossRef]
- Couture, F.; Kwiatkowska, A.; Dory, Y.L.; Day, R. Therapeutic uses of furin and its inhibitors: A patent review. Expert Opin. Ther. Pat. 2015, 25, 379–396. [Google Scholar] [CrossRef]
- Logeat, F.; Bessia, C.; Brou, C.; LeBail, O.; Jarriault, S.; Seidah, N.G.; Israël, A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl. Acad. Sci. USA 1998, 95, 8108–8112. [Google Scholar] [CrossRef]
- Izaguirre, G. The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases. Viruses 2019, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. Degrees of maturity: The complex structure and biology of flaviviruses. Curr. Opin. Virol. 2012, 2, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Dejnirattisai, W.; Carrique, L.; Martin, I.S.; Karia, D.; Ilca, S.L.; Ho, S.F.; Kotecha, A.; Keown, J.R.; Mongkolsapaya, J.; et al. Flavivirus maturation leads to the formation of an occupied lipid pocket in the surface glycoproteins. Nat. Commun. 2021, 12, 1238. [Google Scholar] [CrossRef]
- Pang, Y.J.; Tan, X.J.; Li, D.M.; Zheng, Z.H.; Lei, R.X.; Peng, X.M. Therapeutic potential of furin inhibitors for the chronic infection of hepatitis B virus. Liver International. 2013, 33, 1230–1238. [Google Scholar] [CrossRef]
- Messageot, F.; Salhi, S.; Eon, P.; Rossignol, J.M. Proteolytic processing of the Hepatitis B virus e antigen precursor: Cleavage at two furin consensus sequences. J. Biol. Chem. 2003, 278, 891–895. [Google Scholar] [CrossRef]
- Tse, L.V.; Hamilton, A.M.; Friling, T.; Whittaker, G.R. A Novel Activation Mechanism of Avian Influenza Virus H9N2 by Furin. J. Virol. 2014, 88, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, S. Furin cleavage sites naturally occur in coronaviruses. Stem Cell Res. 2021, 50, 102115. [Google Scholar] [CrossRef]
- Johnson, B.A.; Xie, X.; Kalveram, B.; Lokugamage, K.G.; Muruato, A.; Zou, J.; Zhang, X.; Juelich, T.; Smith, J.K.; Zhang, L.; et al. Furin Cleavage Site Is Key to SARS-CoV-2 Pathogenesis. bioRxiv. 2020, preprint. [Google Scholar] [CrossRef]
- Johnson, B.A.; Xie, X.; Bailey, A.L.; Kalveram, B.; Lokugamage, K.G.; Muruato, A.; Zou, J.; Zhang, X.; Juelich, T.; Smith, J.K.; et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature 2021, 591, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.A.; Zhan, S.H. The Emergence of the Spike Furin Cleavage Site in SARS-CoV-2. Mol. Biol. Evol. 2022, 39, msab327. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Chao, T.-L.; Li, C.-L.; Chiu, M.-F.; Kao, H.-C.; Wang, S.-H.; Pang, Y.-H.; Lin, C.-H.; Tsai, Y.-M.; Lee, W.-H.; et al. Furin Inhibitors Block SARS-CoV-2 Spike Protein Cleavage to Suppress Virus Production and Cytopathic Effects. Cell Rep. 2020, 33, 108254. [Google Scholar] [CrossRef] [PubMed]
- Margaritopoulos, G.A.; Kokosi, M.A.; Wells, A.U. Diagnosing complications and co-morbidities of fibrotic interstitial lung disease. Expert Rev. Respir. Med. 2019, 13, 645–658. [Google Scholar] [CrossRef]
- Seifirad, S. Pirfenidone: A novel hypothetical treatment for COVID-19. Med. Hypotheses 2020, 144, 110005. [Google Scholar] [CrossRef]
- Burghardt, I.; Tritschler, F.; Opitz, C.A.; Frank, B.; Weller, M.; Wick, W. Pirfenidone inhibits TGF-β expression in malignant glioma cells. Biochem. Biophys. Res. Commun. 2007, 354, 542–547. [Google Scholar] [CrossRef]
- Villoutreix, B.O.; Creemers, J.; Léger, Y.; Siegfried, G.; Decroly, E.; Evrard, S.; Khatib, A.M. Targeting furin activity through in silico and in vitro drug repurposing strategy for SARS-CoV-2 spike glycoprotein cleavage repression. Res. Sq. 2020, preprint. [Google Scholar] [CrossRef]
- Miller, B.W.; Willett, K.C.; Desilets, A.R. Rosiglitazone and Pioglitazone for the Treatment of Alzheimer’s Disease. Ann. Pharmacother. 2011, 45, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Orasanu, G.; Ziouzenkova, O.; Devchand, P.R.; Nehra, V.; Hamdy, O.; Horton, E.S.; Plutzky, J. The Peroxisome Proliferator-Activated Receptor-γ Agonist Pioglitazone Represses Inflammation in a Peroxisome Proliferator-Activated Receptor-α-Dependent Manner In vitro and In vivo in Mice. J. Am. Coll. Cardiol. 2008, 52, 869–881. [Google Scholar] [CrossRef]
- Tilekar, K.; Shelke, O.; Upadhyay, N.; Lavecchia, A.; Ramaa, C.S. Current status and future prospects of molecular hybrids with thiazolidinedione (TZD) scaffold in anticancer drug discovery. J. Mol. Struct. 2022, 1250, 131767. [Google Scholar] [CrossRef]
- Adeshara, K.A.; Agrawal, S.B.; Gaikwad, S.M.; Tupe, R.S. Pioglitazone inhibits advanced glycation induced protein modifications and down-regulates expression of RAGE and NF-κB in renal cells. Int. J. Biol. Macromol. 2018, 119, 1154–1163. [Google Scholar] [CrossRef]
- Nhu, Q.M.; Hsieh, L.; Dohil, L.; Dohil, R.; Newbury, R.O.; Kurten, R.; Moawad, F.J.; Aceves, S.S. Antifibrotic Effects of the Thiazolidinediones in Eosinophilic Esophagitis Pathologic Remodeling: A Preclinical Evaluation. Clin. Transl. Gastroenterol. 2020, 11, e00164. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, D.; Zhang, Y.; Chen, Y.; Zhang, S.; Li, Q.; Zeng, P.; Li, X.; Zhang, W.; Duan, Y.; et al. Rosiglitazone ameliorates bile duct ligation-induced liver fibrosis by down-regulating NF-κB-TNF-α signaling pathway in a PPARγ-dependent manner. Biochem. Biophys. Res. Commun. 2019, 519, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Németh, Á.; Mózes, M.M.; Calvier, L.; Hansmann, G.; Kökény, G. The PPARγagonist pioglitazone prevents TGF-β induced renal fibrosis by repressing EGR-1 and STAT3. BMC Nephrol. 2019, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Setz, C.; Große, M.; Auth, J.; Fröba, M.; Rauch, P.; Bausch, A.; Wright, M.; Schubert, U. Synergistic Antiviral Activity of Pamapimod and Pioglitazone against SARS-CoV-2 and Its Variants of Concern. Int. J. Mol. Sci. 2022, 23, 6830. [Google Scholar] [CrossRef]
- Dahms, S.O.; Schnapp, G.; Winter, M.; Büttner, F.H.; Schlepütz, M.; Gnamm, C.; Pautsch, A.; Brandstetter, H. Dichlorophenylpyridine-Based Molecules Inhibit Furin through an Induced-Fit Mechanism. ACS Chem. Biol. 2022, 17, 816–821. [Google Scholar] [CrossRef]
- Douglas, L.E.; Reihill, J.A.; Ho, M.W.; Axten, J.M.; Campobasso, N.; Schneck, J.L.; Rendina, A.R.; Wilcoxen, K.M.; Martin, S.L. A highly selective, cell-permeable furin inhibitor BOS-318 rescues key features of cystic fibrosis airway disease. Cell Chem. Biol. 2022, 29, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Dahms, S.O.; Jiao, G.S.; Than, M.E. Structural Studies Revealed Active Site Distortions of Human Furin by a Small Molecule Inhibitor. ACS Chem. Biol. 2017, 12, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Dahms, S.O.; Haider, T.; Klebe, G.; Steinmetzer, T.; Brandstetter, H. OFF-State-Specific inhibition of the proprotein convertase furin. ACS Chem. Biol. 2021, 16, 1692–1700. [Google Scholar] [CrossRef]
- Irwin, J.J.; Shoichet, B.K. ZINC—A free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 2005, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity-a rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Jaqueline, A.; Zúñiga, P.; Rojo Domínguez, A. SIMULACIÓN DEL RECONOCIMIENTO ENTRE PROTEÍNAS Y MOLÉCULAS ORGÁNICAS O DOCKING. APLICACIÓN AL DISEÑO DE FÁRMACOS. Mensaje Bioquímico 2002, 26, 129–145. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Plewczynski, D.; Łaźniewski, M.; Augustyniak, R.; Ginalski, K. Can we trust docking results? Evaluation of seven commonly used programs on PDBbind database. J. Comput. Chem. 2011, 32, 742–755. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Linares, I.; Pérez-Sánchez, H.; Cecilia, J.M.; García, J.M. High-Throughput parallel blind Virtual Screening using BINDSURF. BMC Bioinform. 2012, 13 (Suppl. S14), S13. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: http://www.pymol.org/pymol (accessed on 30 July 2024).
- McRee, D.E. 3—Computational techniques. In Practical Protein Cristalographyc, 2nd ed.; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Kalé, L.; Skeel, R.; Bhandarkar, M.; Brunner, R.; Gursoy, A.; Krawetz, N.; Phillips, J.; Shinozaki, A.; Varadarajan, K.; Schulten, K. NAMD2: Greater Scalability for Parallel Molecular Dynamics. J. Comput. Phys. 1999, 151, 283–312. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Andrew, D.; Klaus, S. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Glykos, N.M. Software news and updates carma: A molecular dynamics analysis program. J. Comput. Chem. 2006, 27, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Reva, B.A.; Finkelstein, A.V.; Sanner, M.F.; Olson, A.J. Residue-residue mean-force potentials for protein structure recognition. Protein Eng. 1997, 10, 865–876. [Google Scholar] [CrossRef]
- Abdalla, M.; Eltayb, W.A.; El-Arabey, A.A.; Singh, K.; Jiang, X. Molecular dynamic study of SARS-CoV-2 with various S protein mutations and their effect on thermodynamic properties. Comput. Biol. Med. 2022, 141, 105025. [Google Scholar] [CrossRef]
- Anwar, M.A.; Choi, S. Structure-activity relationship in tlr4 mutations: Atomistic molecular dynamics simulations and residue interaction network analysis. Sci. Rep. 2017, 7, 43807. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Couture, F.; Kwiatkowska, A. The Path to Therapeutic Furin Inhibitors: From Yeast Pheromones to SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 3435. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Ren, L.; Wu, J.; Guo, L.; Han, Z.; Yang, J.; Xie, W.; Wang, Y.; Xu, F.; Su, X.; et al. Permethrin as a Potential Furin Inhibitor through a Novel Non-Competitive Allosteric Inhibition. Molecules 2023, 28, 1883. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Pourkarim, M.R.; Thijssen, M.; Sureda, A.; Khayatkashani, M.; Cismaru, C.A.; Neagoe, I.B.; Habtemariam, S.; Razmjouei, S.; Kashani, H.R.K. A perspective on the applications of furin inhibitors for the treatment of SARS-CoV-2. Pharmacol. Rep. 2022, 74, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Fukui, Y.; Kondoh, Y.; Honda, K.; Shimizu, T.; Hara, T.; Hayashi, T.; Saitoh, Y.; Murakami, Y.; Inoue, J.-I.; et al. Pharmacological inhibition of Mint3 attenuates tumour growth, metastasis, and endotoxic shock. Commun. Biol. 2021, 4, 1165. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Lick, A.N.; Blanco, E.H.; Posada-Salgado, J.A.; Martinez-Mayorga, K.; Johnson, A.T.; Jiao, G.-S.; Lindberg, I. Identification of potent and compartment-selective small molecule furin inhibitors using cell-based assays. Biochem. Pharmacol. 2015, 96, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Mohanta, B.C.; Chowdhury, D.R.; Banik, R.; Dinda, B.; Basak, A. Proprotein Convertase Inhibitory Activities of Flavonoids Isolated from Oroxylum Indicum. Curr. Med. Chem. 2010, 17, 2049–2058. [Google Scholar] [CrossRef]
- Tanaka, N.; Sakamoto, T. Mint3 as a Potential Target for Cooling Down HIF-1α-Mediated Inflammation and Cancer Aggressiveness. Biomedicines 2023, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Peiter, G.C.; de Bem Torquato de Souza, C.; de Oliveira, L.M.; Pagliarin, L.G.; Anjos, V.N.F.D.; da Silva, F.A.F.; de Melo, F.F.; Teixeira, K.N. COVID-19 liver and gastroenterology findings: An in silico analysis of SARS-CoV-2 interactions with liver molecules. World J. Hepatol. 2022, 14, 1131–1141. [Google Scholar] [CrossRef]
- Henrich, S.; Cameron, A.; Bourenkov, G.P.; Kiefersauer, R.; Huber, R.; Lindberg, I.; Bode, W.; Than, M.E. The crystal structure of the proprotein processing proteinase furin explains its stringent specificity. Nat. Struct. Biol. 2003, 10, 520–526. [Google Scholar] [CrossRef]
- Pearce, K.H.; Overton, L.K.; Gampe, R.T.; Barrett, G.B.; Taylor, J.D.; McKee, D.D.; Campobasso, N.; Nolte, R.T.; Reid, R.A. BacMam production and crystal structure of nonglycosylated apo human furin at 1.89 Å resolution. Acta Crystallogr. F Struct. Biol. Commun. 2019, 75, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Friedman, S.L.; Aloman, C. Hepatic fibrosis. Curr. Opin. Gastroenterol. 2009, 25, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Dahms, S.O.; Arciniega, M.; Steinmetzer, T.; Huber, R.; Than, M.E. Structure of the unliganded form of the proprotein convertase furin suggests activation by a substrate-induced mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, 11196–11201. [Google Scholar] [CrossRef] [PubMed]
- Ferreira De Freitas, R.; Schapira, M. A systematic analysis of atomic protein-ligand interactions in the PDB. Medchemcomm 2017, 8, 1970–1981. [Google Scholar] [CrossRef] [PubMed]
- Klebe, G. Protein–Ligand Interactions as the Basis for Drug Action. In Multifaceted Roles of Crystallography in Modern Drug Discovery; Springer: Dordrecht, The Netherlands, 2013; pp. 83–92. [Google Scholar]
- Jampilek, J. Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Long, N.; Le Gresley, A.; Wren, S.P. Thiazolidinediones: An In–Depth Study of Their Synthesis and Application to Medicinal Chemistry in the Treatment of Diabetes Mellitus. ChemMedChem 2021, 16, 1717–1736. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, Z.; Dokoohaki, M.H.; Negahdaripour, M.; Dehdashti, M.; Zolghadr, H.; Moghadami, M.; Masoompour, S.M.; Zolghadr, A.R. The interactions of folate with the enzyme furin: A computational study. RSC Adv. 2021, 11, 23815. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nguyen, T.H.; Pham, T.N.H.; Huy, N.T.; Van Bay, M.; Pham, M.Q.; Nam, P.C.; Vu, V.V.; Ngo, S.T. Autodock Vina Adopts More Accurate Binding Poses but Autodock4 Forms Better Binding Affinity. J. Chem. Inf. Model. 2020, 60, 204–211. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, S. An in-silico evaluation of dietary components for structural inhibition of SARS-Cov-2 main protease. J. Biomol. Struct. Dyn. 2022, 40, 136–142. [Google Scholar] [CrossRef]





| ID | Name and Abbreviation | Structure |
|---|---|---|
| PubChem CID 3124834 | Naphthofluorescein NPF | 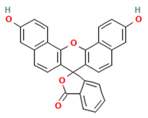 |
| ZINC1958 | Pirfenidone PFD |  |
| ZINC968326 | Pioglitazone PGZ |  |
| ZINC968328 | Rosiglitazone RGZ | 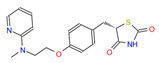 |
| Ligands | Site Interaction | Free Energy of Binding (kcal/mol) AutoDock4 | Free Energy of Binding (kcal/mol) Vina | H-Bond Classical and Non-Classical | Distance H-Bond (Å) | Electrostatic | Hydrophobic |
|---|---|---|---|---|---|---|---|
| Naphthofluorescein (NPF) | Active | NA (NA) | −9.9 (0.00) | Asp154—H Asp191—H | 2.7 3.1 | Asp258 | Val231 Leu227 |
| Pirfenidone (PFD) | Active | −6.28 (0.00) | −6.9 (1.709) | Gly255—O | 3.4 | Glu236 | Gly255 Trp254 Trp291 Leu227 Tyr308 Val231 Pro256 |
| Pioglitazone (PGZ) | Active | −8.04 (0.00) | −8.6 (0.00) | Asp154—H Asp191—H Asn192—O | 2.6 2.5 2.6 | NA | Leu227 Val231 Leu152 Met226 Trp254 Leu240 Tyr308 Trp291 |
| Rosiglitazone (RGZ) | Active | −7.47 (0.00) | −8.1 (0.00) | Asp154—H | 2.2 | NA | Leu227 Val231 Met226 Trp291 Ala252 Tyr308 |
| Ligands | Site Interaction | Free Energy of Binding (kcal/mol) Blind Docking Server | H-Bond Classical and Non-Classical | Distance H-Bond (Å) | Electrostatic | Hydrophobic |
|---|---|---|---|---|---|---|
| Naphthofluorescein (NPF) | Allosteric | −10.9 | Gln488—H Asn310—O Gly265—O | 2.3 2.4 2.3 | NA | Pro266 Trp531 Ser311 Ala532 Glu271 |
| Pirfenidone (PFD) | Allosteric | −7.1 | Asn310—O | 2.6 | Glu271 | Ala532 Trp531 Tyr313 Ile312 |
| Pioglitazone (PGZ) | Allosteric | −8.7 | Ser311—H Gln488—O | 2.2 2.7 | NA | Trp531 Val263 Ile312 |
| Rosiglitazone (RGZ) | Allosteric | −7.9 | Asn310—H Ser311—O Val263—N Glu271—O | 1.9 2.2 3.3 3.4 | NA | Trp531 Val263 Asp264 Gly307 |
| Inhibition Constant (Ki) | ||
|---|---|---|
| Ligands | Localized Docking (FurAct) | Blind Docking (FurAll) |
| NFP | NA | 201.04 nM |
| PFD | 24.87 μM | NA |
| PGZ | 1.28 μM | 970.36 nM |
| RGZ | 3.33 μM | 1.49 μM |
| Complexes | RMSD (Å) of Active Site (100 ns) | RMSD (Å) of Allosteric Site (100 ns) |
|---|---|---|
| Ligand-free Fur | 3.98 ± 0.07 | 3.98 ± 0.07 |
| NPF/Fur | 4.67 ± 0.13 | 4.52 ± 0.05 |
| PFD/Fur | 4.21 ± 0.08 | 5.22 ± 0.17 |
| PGZ/Fur | 4.12 ± 0.06 | 4.66 ± 0.17 |
| RGZ/Fur | 4.77 ± 0.14 | 5.57 ± 0.17 |
| Complexes | Rg (Å) of Active Site (100 ns) | Rg (Å) of Allosteric Site (100 ns) |
|---|---|---|
| Ligand-free Fur | 22.45 ± 0.06 | 22.4 ± 0.06 |
| NPF/Fur | 22.62 ± 0.09 | 22.56 ± 0.06 |
| PFD/Fur | 22.57 ± 0.05 | 22.90 ± 0.06 |
| PGZ/Fur | 22.65 ± 0.06 | 22.93 ± 0.06 |
| RGZ/Fur | 22.77 ± 0.10 | 23.22 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carranza-Aranda, A.S.; Diaz-Palomera, C.D.; Lepe-Reynoso, E.; Santerre, A.; Muñoz-Valle, J.F.; Viera-Segura, O. Evaluation of Potential Furin Protease Inhibitory Properties of Pioglitazone, Rosiglitazone, and Pirfenidone: An In Silico Docking and Molecular Dynamics Simulation Approach. Curr. Issues Mol. Biol. 2024, 46, 8665-8684. https://doi.org/10.3390/cimb46080511
Carranza-Aranda AS, Diaz-Palomera CD, Lepe-Reynoso E, Santerre A, Muñoz-Valle JF, Viera-Segura O. Evaluation of Potential Furin Protease Inhibitory Properties of Pioglitazone, Rosiglitazone, and Pirfenidone: An In Silico Docking and Molecular Dynamics Simulation Approach. Current Issues in Molecular Biology. 2024; 46(8):8665-8684. https://doi.org/10.3390/cimb46080511
Chicago/Turabian StyleCarranza-Aranda, Ahtziri Socorro, Carlos Daniel Diaz-Palomera, Eduardo Lepe-Reynoso, Anne Santerre, José Francisco Muñoz-Valle, and Oliver Viera-Segura. 2024. "Evaluation of Potential Furin Protease Inhibitory Properties of Pioglitazone, Rosiglitazone, and Pirfenidone: An In Silico Docking and Molecular Dynamics Simulation Approach" Current Issues in Molecular Biology 46, no. 8: 8665-8684. https://doi.org/10.3390/cimb46080511







