Extracellular Vesicle-Derived Non-Coding RNAs: Key Mediators in Remodelling Heart Failure
Abstract
:1. Introduction
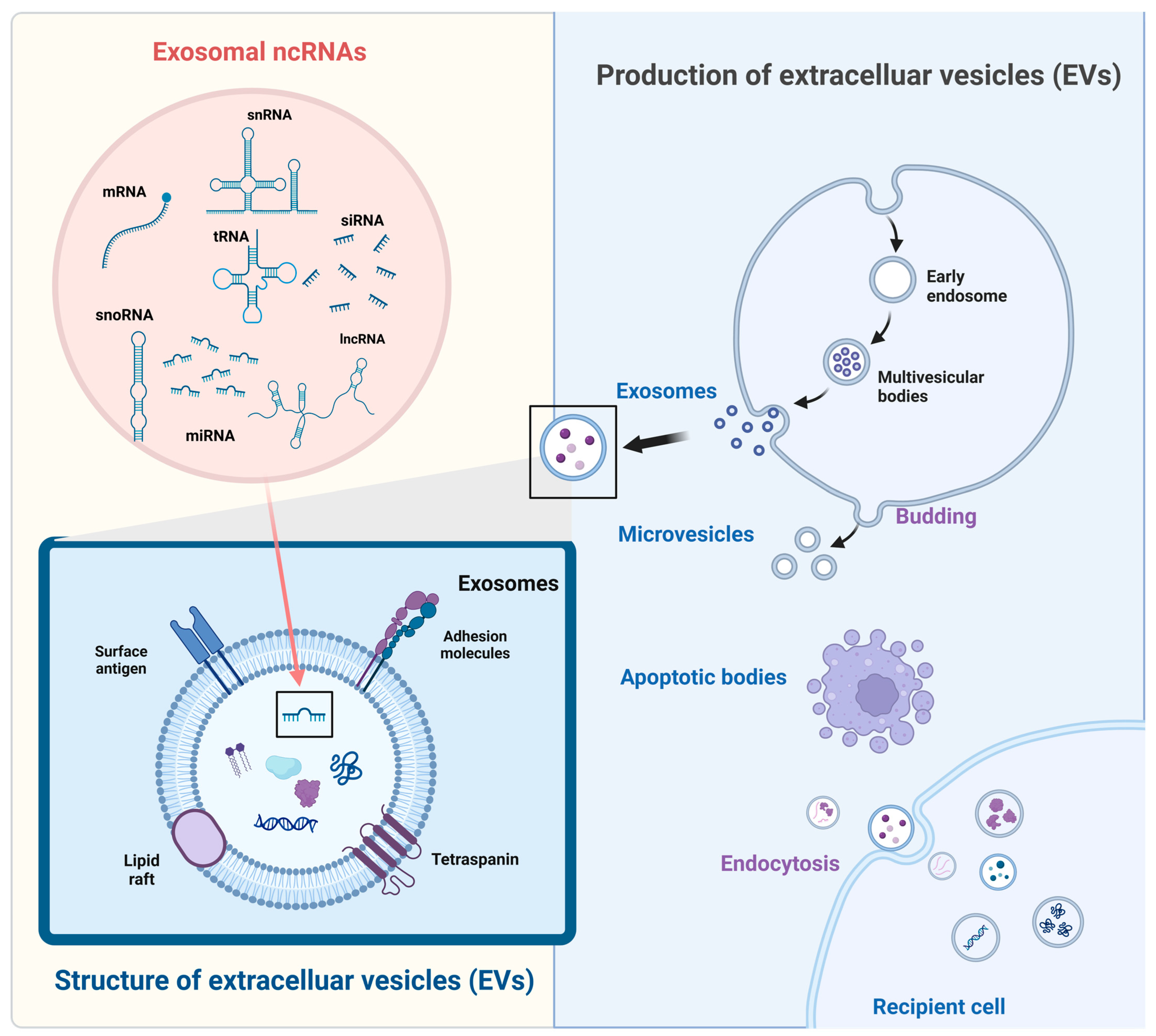
2. Size Classification of EVs, Generation Process, and Sorting Mechanism of EV-ncRNAs
2.1. Size Classification
2.2. Biogenesis of the EVs
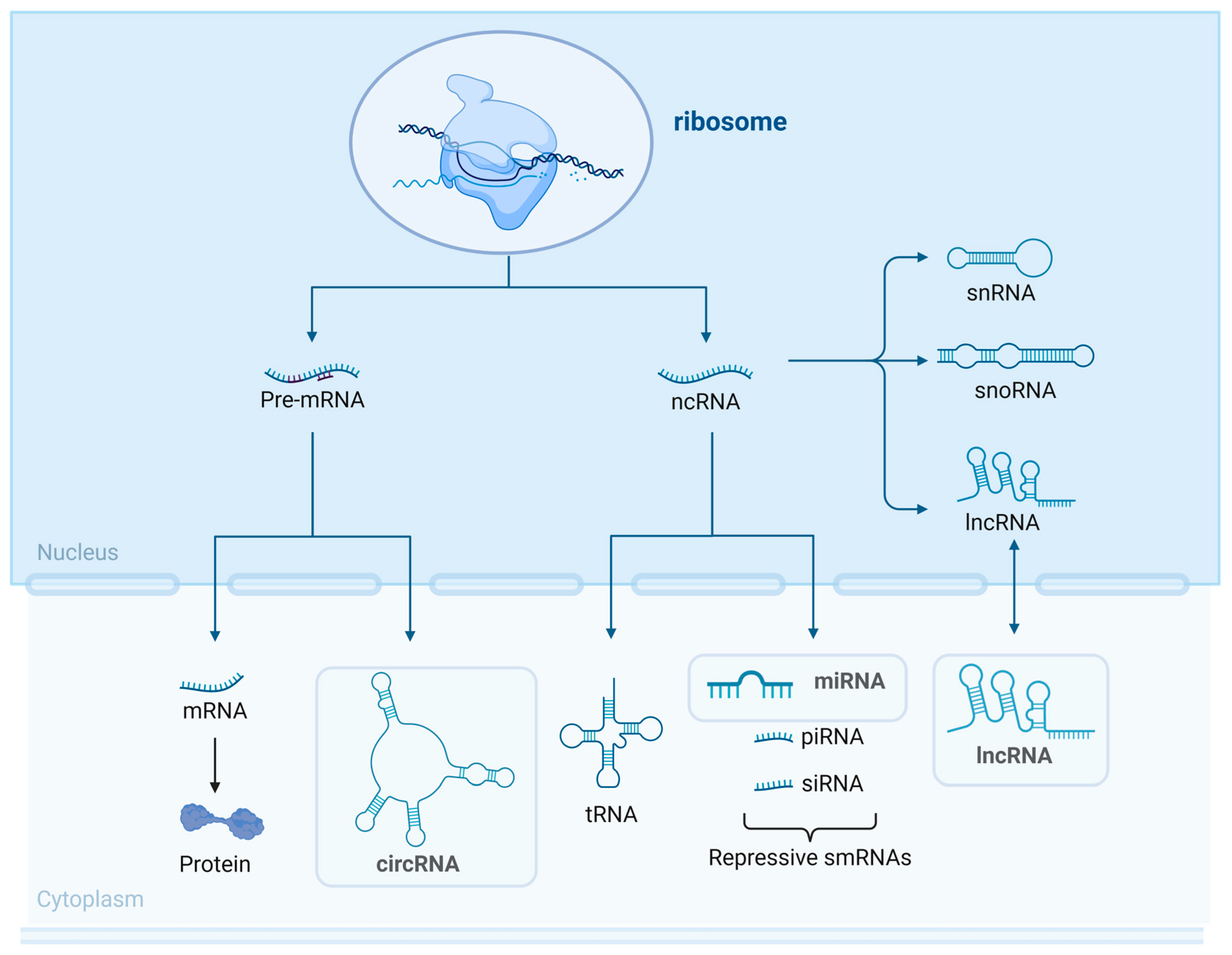
2.3. ncRNA Sorting
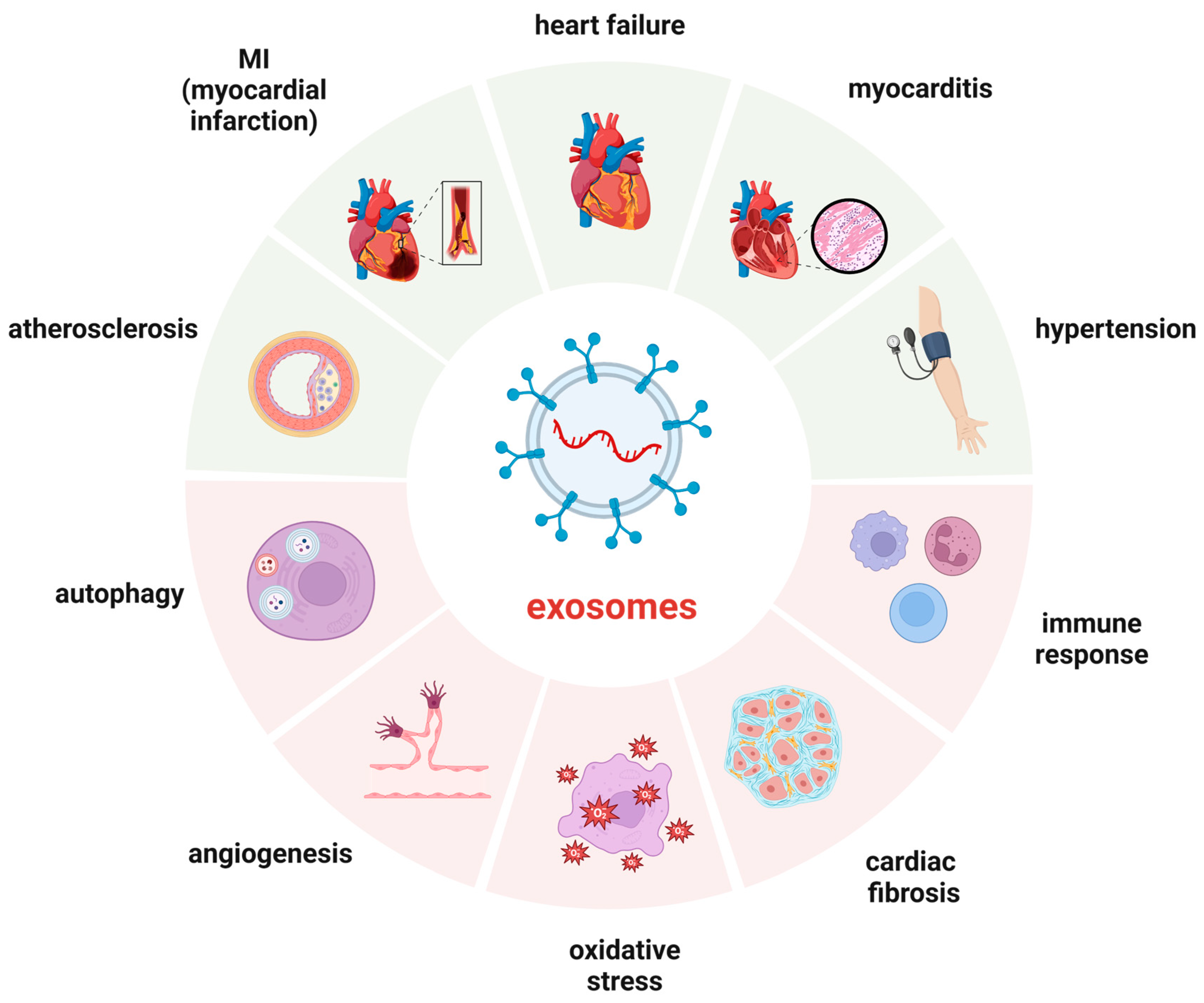
3. Participation Mechanisms
3.1. Oxidative Stress
3.2. Angiogenesis
3.3. Inflammation Response
3.4. Autophagy and Apoptosis
3.5. Immunomodulation
3.6. Fibrosis
4. Diagnosis and Prognosis
4.1. Diagnosis of Heart Failure
4.2. Prognosis of Heart Failure
| Exosomal Cargo | Functional Mechanism | Cell Source | Functions | Ref. |
|---|---|---|---|---|
| miR-21a-5p | miR-21a-5p/ITGAV/Col1α | FPC | Reduces PO-induced cardiac fibrosis and improves cardiac function | [122] |
| miR-126 | TGF-β/Smad3 | EPC | Improves cardiac fibrosis | [123] |
| miR-9-5p | VPO1/ERK | iPSC | Protects against Dox-induced cardiomyopathy | [124] |
| miR-125a-5p | Klf13, Tgfbr1, Daam1 | MSC | Alleviates myocardial ischemia/reperfusion (I/R) injury | [125] |
| miRNA-205 | metalloproteinase-3 | ADSC | Reduces myocardial fibrosis, inhibits myocardial apoptosis, promote angiogenesis | [126] |
| miR-22-3p | FURIN | CM | Increases the risk of HF damage | [127] |
| miR-129-5p | TRAF3, NF-κB | MSC | Protects the heart from HF | [59] |
| miR-195-3p | PTEN/AKT | CF | Promotes cardiac fibrosis and dysfunction after MI | [128] |
| miR-98-5p | TLR4, PI3K/Akt | BMSC | Protects against MI/RI | [129] |
| miR-217 | PTEN | CM | Therapeutic target for CHF | [130] |
| circ_LAS1L | circ_LAS1L/miR-125b/SFRP5 | CF | Regulates myocardial fibrosis after MI | [131] |
| circ_BMP2K | miR-455-3p, SUMO1 | CF | Regulates myocardial fibrosis | [132] |
| circ_NSD1 | miR-429-3p, Wnt/β-catenin | CF | Treatment of cardiac fibrosis | [133] |
| circ_0023461 | miR-370-3p/PDE4D | AC16 | Silencing protects cardiomyocytes from hypoxia-induced dysfunction | [134] |
| circ_0097435 | Sponging multiple miRNAs | DOX-treated CM | Influences HF | [135] |
5. Engineered EVs for HF Therapy
5.1. Self-Derived ncRNA Therapy
5.2. Combined and Targeted Drug Delivery
5.3. Current Limitations and Challenges of Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davidson, S.M.; Padró, T.; Bollini, S.; Vilahur, G.; Duncker, D.J.; Evans, P.C.; Guzik, T.; Hoefer, I.E.; Waltenberger, J.; Wojta, J.; et al. Progress in cardiac research: From rebooting cardiac regeneration to a complete cell atlas of the heart. Cardiovasc. Res. 2021, 117, 2161–2174. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Baman, J.R.; Ahmad, F.S. Heart Failure. JAMA 2020, 324, 1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Chai, K.; Long, Z.; Yang, Z.; Du, M.; Wang, S.; Zhan, S.; Liu, Y.; Wan, Y.; et al. Mortality in patients admitted to hospital with heart failure in China: A nationwide Cardiovascular Association Database-Heart Failure Centre Registry cohort study. Lancet Glob. Health 2024, 12, e611–e622. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart. Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure: Biological and Translational Perspectives. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Nomura, S.; Satoh, M.; Fujita, T.; Higo, T.; Sumida, T.; Ko, T.; Yamaguchi, T.; Tobita, T.; Naito, A.T.; Ito, M.; et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat. Commun. 2018, 9, 4435. [Google Scholar] [CrossRef]
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef]
- Kotani, A.; Ito, M.; Kudo, K. Non-coding RNAs and lipids mediate the function of extracellular vesicles in cancer cross-talk. Semin. Cancer Biol. 2021, 74, 121–133. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, X.; Huang, S. Extracellular vesicle long non-coding RNAs and circular RNAs: Biology, functions and applications in cancer. Cancer Lett. 2020, 489, 111–120. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart. Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovasc. Res. 2023, 119, 45–63. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Karnati, H.K.; Garcia, J.H.; Tweedie, D.; Becker, R.E.; Kapogiannis, D.; Greig, N.H. Neuronal Enriched Extracellular Vesicle Proteins as Biomarkers for Traumatic Brain Injury. J. Neurotrauma 2019, 36, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.W.; Sheehan, C.S.; Boomgarden, A.C.; D’Souza-Schorey, C. Recruitment of DNA to tumor-derived microvesicles. Cell Rep. 2022, 38, 110443. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.W.; Zhang, Y.; Sheehan, C.; D’Souza-Schorey, C. An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat. Cell Biol. 2019, 21, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef]
- Nakamura, Y.; Dryanovski, D.I.; Kimura, Y.; Jackson, S.N.; Woods, A.S.; Yasui, Y.; Tsai, S.Y.; Patel, S.; Covey, D.P.; Su, T.P.; et al. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. eLife 2019, 8, e47209. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Marie, P.P.; Fan, S.J.; Mason, J.; Wells, A.; Mendes, C.C.; Wainwright, S.M.; Scott, S.; Fischer, R.; Harris, A.L.; Wilson, C.; et al. Accessory ESCRT-III proteins are conserved and selective regulators of Rab11a-exosome formation. J. Extracell. Vesicles 2023, 12, e12311. [Google Scholar] [CrossRef] [PubMed]
- Bissig, C.; Gruenberg, J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2014, 24, 19–25. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Verweij, F.J.; Revenu, C.; Arras, G.; Dingli, F.; Loew, D.; Pegtel, D.M.; Follain, G.; Allio, G.; Goetz, J.G.; Zimmermann, P.; et al. Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Dev. Cell 2019, 48, 573–589.e4. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219, e201904113. [Google Scholar] [CrossRef]
- Dores, M.R.; Paing, M.M.; Lin, H.; Montagne, W.A.; Marchese, A.; Trejo, J. AP-3 regulates PAR1 ubiquitin-independent MVB/lysosomal sorting via an ALIX-mediated pathway. Mol. Biol. Cell 2012, 23, 3612–3623. [Google Scholar] [CrossRef]
- Liu, C.; Liu, D.; Wang, S.; Gan, L.; Yang, X.; Ma, C. Identification of the SNARE complex that mediates the fusion of multivesicular bodies with the plasma membrane in exosome secretion. J. Extracell. Vesicles 2023, 12, e12356. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, Y. The emerging roles of exosome-derived noncoding RNAs in the tumor immune microenvironment and their future applications. Biomed. Pharmacother. 2022, 156, 113863. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2020, 8, 616161. [Google Scholar] [CrossRef] [PubMed]
- Hinger, S.A.; Cha, D.J.; Franklin, J.L.; Higginbotham, J.N.; Dou, Y.; Ping, J.; Shu, L.; Prasad, N.; Levy, S.; Zhang, B.; et al. Diverse Long RNAs Are Differentially Sorted into Extracellular Vesicles Secreted by Colorectal Cancer Cells. Cell Rep. 2018, 25, 715–725.e4. [Google Scholar] [CrossRef]
- O’Grady, T.; Njock, M.S.; Lion, M.; Bruyr, J.; Mariavelle, E.; Galvan, B.; Boeckx, A.; Struman, I.; Dequiedt, F. Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC Biol. 2022, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef]
- Xu, X.; Jin, K.; Bais, A.S.; Zhu, W.; Yagi, H.; Feinstein, T.N.; Nguyen, P.K.; Criscione, J.D.; Liu, X.; Beutner, G.; et al. Uncompensated mitochondrial oxidative stress underlies heart failure in an iPSC-derived model of congenital heart disease. Cell Stem. Cell 2022, 29, 840–855.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, G.; Dasgupta, S.; Niewold, E.L.; Li, C.; Li, Q.; Luo, X.; Tan, L.; Ferdous, A.; Lorenzi, P.L.; et al. ATF4 Protects the Heart from Failure by Antagonizing Oxidative Stress. Circ. Res. 2022, 131, 91–105. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, R.; Chen, Y.; Wang, M.; Du, J. Crosstalk between Oxidative Stress and Exosomes. Oxid. Med. Cell. Longev. 2022, 2022, 3553617. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Li, H.; Li, Y.; Cheng, D.; Tang, Y.; Sang, H. Serum extracellular vesicles containing MIAT induces atrial fibrosis, inflammation and oxidative stress to promote atrial remodeling and atrial fibrillation via blockade of miR-485-5p-mediated CXCL10 inhibition. Clin. Transl. Med. 2021, 11, e482. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Rudebush, T.L.; Yu, L.; Zucker, I.H. Extracellular Vesicles Regulate Sympatho-Excitation by Nrf2 in Heart Failure. Circ. Res. 2022, 131, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Hu, G.; Gao, L.; Hackfort, B.T.; Zucker, I.H. Extracellular vesicular MicroRNA-27a* contributes to cardiac hypertrophy in chronic heart failure. J. Mol. Cell Cardiol. 2020, 143, 120–131. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Heart Failure: Insights from Gene Transfer Studies. Biomedicines 2021, 9, 1645. [Google Scholar] [CrossRef]
- Lee, D.Y.; Song, M.Y.; Kim, E.H. Role of Oxidative Stress and Nrf2/KEAP1 Signaling in Colorectal Cancer: Mechanisms and Therapeutic Perspectives with Phytochemicals. Antioxidants 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zucker, I.H. Regulation of Nrf2 signaling pathway in heart failure: Role of extracellular vesicles and non-coding RNAs. Free Radic. Biol. Med. 2021, 167, 218–231. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H928–H939. [Google Scholar] [CrossRef]
- Mugoni, V.; Ciani, Y.; Nardella, C.; Demichelis, F. Circulating RNAs in prostate cancer patients. Cancer Lett. 2022, 524, 57–69. [Google Scholar] [CrossRef]
- Yan, F.; Cui, W.; Chen, Z. Mesenchymal Stem Cell-Derived Exosome-Loaded microRNA-129-5p Inhibits TRAF3 Expression to Alleviate Apoptosis and Oxidative Stress in Heart Failure. Cardiovasc. Toxicol. 2022, 22, 631–645. [Google Scholar] [CrossRef]
- Liu, N.; Kataoka, M.; Wang, Y.; Pu, L.; Dong, X.; Fu, X.; Zhang, F.; Gao, F.; Liang, T.; Pei, J.; et al. LncRNA LncHrt preserves cardiac metabolic homeostasis and heart function by modulating the LKB1-AMPK signaling pathway. Basic Res. Cardiol. 2021, 116, 48. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Y.; Zhang, W.; Deng, S.Q.; Ge, Z.R. lncRNA-NRF is a Potential Biomarker of Heart Failure After Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2020, 13, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kadomatsu, T.; Miyata, K.; Warren, J.S.; Tian, Z.; Zhu, S.; Horiguchi, H.; Makaju, A.; Bakhtina, A.; Morinaga, J.; et al. The lncRNA Caren antagonizes heart failure by inactivating DNA damage response and activating mitochondrial biogenesis. Nat. Commun. 2021, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Huang, S.; Wei, G.; Sun, Y.; Li, C.; Si, X.; Chen, Y.; Tang, Z.; Li, X.; Chen, Y.; et al. CircRNA Samd4 induces cardiac repair after myocardial infarction by blocking mitochondria-derived ROS output. Mol. Ther. 2022, 30, 3477–3498. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.; Nazari-Jahantigh, M.; Wei, Y.; Bidzhekov, K.; Gremse, F.; Grommes, J.; Megens, R.T.; Heyll, K.; Noels, H.; Hristov, M.; et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat. Med. 2014, 20, 368–376. [Google Scholar] [CrossRef]
- Lin, X.; Zhan, J.K.; Wang, Y.J.; Tan, P.; Chen, Y.Y.; Deng, H.Q.; Liu, Y.S. Function, Role, and Clinical Application of MicroRNAs in Vascular Aging. Biomed. Res. Int. 2016, 2016, 6021394. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Shen, C.; Liu, W.; Yuan, J.; Li, C.; Deng, W.; Wang, Z.; Zhang, W.; Ge, J.; et al. Exosomal CircHIPK3 Released from Hypoxia-Induced Cardiomyocytes Regulates Cardiac Angiogenesis after Myocardial Infarction. Oxid. Med. Cell Longev. 2020, 2020, 8418407. [Google Scholar] [CrossRef]
- Liu, S.; Chen, J.; Shi, J.; Zhou, W.; Wang, L.; Fang, W.; Zhong, Y.; Chen, X.; Chen, Y.; Sabri, A.; et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res. Cardiol. 2020, 115, 22. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Q.; Hosen, M.R.; Zietzer, A.; Flender, A.; Levermann, P.; Schmitz, T.; Frühwald, D.; Goody, P.; Nickenig, G.; et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ. Res. 2019, 124, 575–587. [Google Scholar] [CrossRef]
- Ohayon, L.; Zhang, X.; Dutta, P. The role of extracellular vesicles in regulating local and systemic inflammation in cardiovascular disease. Pharmacol. Res. 2021, 170, 105692. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef]
- Duan, Q.; Yang, L.; Gong, W.; Chaugai, S.; Wang, F.; Chen, C.; Wang, P.; Zou, M.H.; Wang, D.W. MicroRNA-214 Is Upregulated in Heart Failure Patients and Suppresses XBP1-Mediated Endothelial Cells Angiogenesis. J. Cell. Physiol. 2015, 230, 1964–1973. [Google Scholar] [CrossRef]
- Caller, T.; Rotem, I.; Shaihov-Teper, O.; Lendengolts, D.; Schary, Y.; Shai, R.; Glick-Saar, E.; Dominissini, D.; Motiei, M.; Katzir, I.; et al. Small Extracellular Vesicles from Infarcted and Failing Heart Accelerate Tumor Growth. Circulation 2024, 149, 1729–1748. [Google Scholar] [CrossRef]
- Ginckels, P.; Holvoet, P. Oxidative Stress and Inflammation in Cardiovascular Diseases and Cancer: Role of Non-coding RNAs. Yale J. Biol. Med. 2022, 95, 129–152. [Google Scholar]
- Wang, D.; Wang, X.; Song, Y.; Si, M.; Sun, Y.; Liu, X.; Cui, S.; Qu, X.; Yu, X. Exosomal miR-146a-5p and miR-155-5p promote CXCL12/CXCR7-induced metastasis of colorectal cancer by crosstalk with cancer-associated fibroblasts. Cell Death Dis. 2022, 13, 380. [Google Scholar] [CrossRef]
- Wen, Q.; Wang, Y.; Pan, Q.; Tian, R.; Zhang, D.; Qin, G.; Zhou, J.; Chen, L. MicroRNA-155-5p promotes neuroinflammation and central sensitization via inhibiting SIRT1 in a nitroglycerin-induced chronic migraine mouse model. J. Neuroinflamm. 2021, 18, 287. [Google Scholar] [CrossRef]
- Sciarretta, S.; Maejima, Y.; Zablocki, D.; Sadoshima, J. The Role of Autophagy in the Heart. Annu. Rev. Physiol. 2018, 80, 1–26. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, W.; Liu, Y.; Zhou, J.; Cui, K.; Chen, Y. Autophagy protects mitochondrial health in heart failure. Heart Fail. Rev. 2024, 29, 113–123. [Google Scholar] [CrossRef]
- Gonzalez, C.; Cimini, M.; Cheng, Z.; Benedict, C.; Wang, C.; Trungcao, M.; Mallaredy, V.; Rajan, S.; Garikipati, V.N.S.; Kishore, R. Role of circular RNA cdr1as in modulation of macrophage phenotype. Life Sci. 2022, 309, 121003. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Chen, X.; Cheng, X.; Liao, Y.; Yu, X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J. Mol. Med. 2016, 94, 711–724. [Google Scholar] [CrossRef]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ. Res. 2021, 128, e1–e23. [Google Scholar] [CrossRef]
- Ge, X.; Meng, Q.; Wei, L.; Liu, J.; Li, M.; Liang, X.; Lin, F.; Zhang, Y.; Li, Y.; Liu, Z.; et al. Myocardial ischemia-reperfusion induced cardiac extracellular vesicles harbour proinflammatory features and aggravate heart injury. J. Extracell. Vesicles 2021, 10, e12072. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, C.; Wang, W.; Li, M.; Ma, C.; Gao, B. SIRT1 is a regulator of autophagy: Implications for the progression and treatment of myocardial ischemia-reperfusion. Pharmacol. Res. 2024, 199, 106957. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Zhu, J.; You, A.; Huang, X.; Yi, X.; Xue, M. Mangiferin prevents myocardial infarction-induced apoptosis and heart failure in mice by activating the Sirt1/FoxO3a pathway. J. Cell Mol. Med. 2021, 25, 2944–2955. [Google Scholar] [CrossRef]
- Chen, C.; Shen, H.; Huang, Q.; Li, Q. The Circular RNA CDR1as Regulates the Proliferation and Apoptosis of Human Cardiomyocytes Through the miR-135a/HMOX1 and miR-135b/HMOX1 Axes. Genet. Test Mol. Biomark. 2020, 24, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Guo, J.; Jia, R. Roles of Regulatory T Cell-Derived Extracellular Vesicles in Human Diseases. Int. J. Mol. Sci. 2022, 23, 11206. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Rogers, R.; de Couto, G.; Valle, J.; Li, L.; Ibrahim, A.; Sanchez, L.; Zhang, R.; Lin, Y.N.; Liu, W.; et al. Regulatory T cell activation, proliferation, and reprogramming induced by extracellular vesicles. J. Heart Lung Transpl. 2021, 40, 1387–1395. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, K.; Chen, F.; Qian, J.; Wang, D.; Wu, Y.; Zhou, C.; Yu, Y.; Chen, K.; Hwa, J.; et al. Bone marrow-derived naïve B lymphocytes improve heart function after myocardial infarction: A novel cardioprotective mechanism for empagliflozin. Basic Res. Cardiol. 2022, 117, 47. [Google Scholar] [CrossRef]
- Suchanek, O.; Ferdinand, J.R.; Tuong, Z.K.; Wijeyesinghe, S.; Chandra, A.; Clauder, A.K.; Almeida, L.N.; Clare, S.; Harcourt, K.; Ward, C.J.; et al. Tissue-resident B cells orchestrate macrophage polarisation and function. Nat. Commun. 2023, 14, 7081. [Google Scholar] [CrossRef]
- Dosil, S.G.; Lopez-Cobo, S.; Rodriguez-Galan, A.; Fernandez-Delgado, I.; Ramirez-Huesca, M.; Milan-Rois, P.; Castellanos, M.; Somoza, A.; Gómez, M.J.; Reyburn, H.T.; et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. Elife 2022, 11, e76319. [Google Scholar] [CrossRef]
- Martini, E.; Kunderfranco, P.; Peano, C.; Carullo, P.; Cremonesi, M.; Schorn, T.; Carriero, R.; Termanini, A.; Colombo, F.S.; Jachetti, E.; et al. Single-Cell Sequencing of Mouse Heart Immune Infiltrate in Pressure Overload-Driven Heart Failure Reveals Extent of Immune Activation. Circulation 2019, 140, 2089–2107. [Google Scholar] [CrossRef]
- Curran, S.A.; Shyer, J.A.; St Angelo, E.T.; Talbot, L.R.; Sharma, S.; Chung, D.J.; Heller, G.; Hsu, K.C.; Betts, B.C.; Young, J.W. Human Dendritic Cells Mitigate NK-Cell Dysfunction Mediated by Nonselective JAK1/2 Blockade. Cancer Immunol. Res. 2017, 5, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, P.; Clements, T.; Venø, M.; Abrahams, V.M.; Holder, B. Distinct non-coding RNA cargo of extracellular vesicles from M1 and M2 human primary macrophages. J. Extracell. Vesicles 2022, 11, e12293. [Google Scholar] [CrossRef]
- Liu, X.L.; Pan, Q.; Cao, H.X.; Xin, F.Z.; Zhao, Z.H.; Yang, R.X.; Zeng, J.; Zhou, H.; Fan, J.G. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 72, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, X.; Spanos, M.; Wang, H.; Yang, Z.; Li, G.; Xiao, J.; Zhou, L. Exosomal Non-Coding RNA Mediates Macrophage Polarization: Roles in Cardiovascular Diseases. Biology 2023, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Brigstock, D.R. Extracellular Vesicles in Organ Fibrosis: Mechanisms, Therapies, and Diagnostics. Cells 2021, 10, 1596. [Google Scholar] [CrossRef]
- Shaihov-Teper, O.; Ram, E.; Ballan, N.; Brzezinski, R.Y.; Naftali-Shani, N.; Masoud, R.; Ziv, T.; Lewis, N.; Schary, Y.; Levin-Kotler, L.P.; et al. Extracellular Vesicles from Epicardial Fat Facilitate Atrial Fibrillation. Circulation 2021, 143, 2475–2493. [Google Scholar] [CrossRef]
- Tang, C.; Hou, Y.X.; Shi, P.X.; Zhu, C.H.; Lu, X.; Wang, X.L.; Que, L.L.; Zhu, G.Q.; Liu, L.; Chen, Q.; et al. Cardiomyocyte-specific Peli1 contributes to the pressure overload-induced cardiac fibrosis through miR-494-3p-dependent exosomal communication. FASEB J. 2023, 37, e22699. [Google Scholar] [CrossRef]
- Kmiotek-Wasylewska, K.; Bobis-Wozowicz, S.; Karnas, E.; Orpel, M.; Woźnicka, O.; Madeja, Z.; Dawn, B.; Zuba-Surma, E.K. Anti-inflammatory, Anti-fibrotic and Pro-cardiomyogenic Effects of Genetically Engineered Extracellular Vesicles Enriched in miR-1 and miR-199a on Human Cardiac Fibroblasts. Stem Cell Rev. Rep. 2023, 19, 2756–2773. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, R.; Qiu, Z.; Shen, C.; Wang, Z.; Liu, W.; Zhang, W.; Ge, J.; Shi, B. CircUbe3a from M2 macrophage-derived small extracellular vesicles mediates myocardial fibrosis after acute myocardial infarction. Theranostics 2021, 11, 6315–6333. [Google Scholar] [CrossRef]
- Huang, X.H.; Li, J.L.; Li, X.Y.; Wang, S.X.; Jiao, Z.H.; Li, S.Q.; Liu, J.; Ding, J. miR-208a in Cardiac Hypertrophy and Remodeling. Front. Cardiovasc. Med. 2021, 8, 773314. [Google Scholar] [CrossRef]
- Liu, L.; Luo, F.; Lei, K. Exosomes Containing LINC00636 Inhibit MAPK1 through the miR-450a-2-3p Overexpression in Human Pericardial Fluid and Improve Cardiac Fibrosis in Patients with Atrial Fibrillation. Mediat. Inflamm. 2021, 2021, 9960241. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Liu, I.F.; Kuo, H.F.; Li, C.Y.; Lian, W.S.; Chang, C.Y.; Chen, Y.H.; Liu, W.L.; Lu, C.Y.; Liu, Y.R.; et al. miR-29a-3p/THBS2 Axis Regulates PAH-Induced Cardiac Fibrosis. Int. J. Mol. Sci. 2021, 22, 10574. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic peptides: Role in the diagnosis and management of heart failure: A scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur. J. Heart Fail. 2023, 25, 616–631. [Google Scholar] [CrossRef]
- Magnussen, C.; Blankenberg, S. Biomarkers for heart failure: Small molecules with high clinical relevance. J. Intern Med. 2018, 283, 530–543. [Google Scholar] [CrossRef]
- Caporali, A.; Anwar, M.; Devaux, Y.; Katare, R.; Martelli, F.; Srivastava, P.K.; Pedrazzini, T.; Emanueli, C. Non-coding RNAs as therapeutic targets and biomarkers in ischaemic heart disease. Nat. Rev. Cardiol. 2024, 21, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, Y.; Hu, L.; Xue, S.; Wang, Y.; Zhang, L.; Zhang, Y.; Qi, H.; Yu, H.; Aung, L.H.H.; et al. Combined detection of miR-21-5p, miR-30a-3p, miR-30a-5p, miR-155-5p, miR-216a and miR-217 for screening of early heart failure diseases. Biosci. Rep. 2020, 40, BSR20191653. [Google Scholar] [CrossRef]
- Sabia, C.; Picascia, A.; Grimaldi, V.; Amarelli, C.; Maiello, C.; Napoli, C. The epigenetic promise to improve prognosis of heart failure and heart transplantation. Transpl. Rev. 2017, 31, 249–256. [Google Scholar] [CrossRef]
- Galluzzo, A.; Gallo, S.; Pardini, B.; Birolo, G.; Fariselli, P.; Boretto, P.; Vitacolonna, A.; Peraldo-Neia, C.; Spilinga, M.; Volpe, A.; et al. Identification of novel circulating microRNAs in advanced heart failure by next-generation sequencing. ESC Heart Fail. 2021, 8, 2907–2919. [Google Scholar] [CrossRef]
- Tian, C.; Ziegler, J.N.; Zucker, I.H. Extracellular Vesicle MicroRNAs in Heart Failure: Pathophysiological Mediators and Therapeutic Targets. Cells 2023, 12, 2145. [Google Scholar] [CrossRef]
- Watson, C.J.; Gupta, S.K.; O’Connell, E.; Thum, S.; Glezeva, N.; Fendrich, J.; Gallagher, J.; Ledwidge, M.; Grote-Levi, L.; McDonald, K.; et al. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur. J. Heart Fail. 2015, 17, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Parvan, R.; Hosseinpour, M.; Moradi, Y.; Devaux, Y.; Cataliotti, A.; da Silva, G.J.J. Diagnostic performance of microRNAs in the detection of heart failure with reduced or preserved ejection fraction: A systematic review and meta-analysis. Eur. J. Heart Fail. 2022, 24, 2212–2225. [Google Scholar] [CrossRef]
- Li, G.; Song, Y.; Li, Y.D.; Jie, L.J.; Wu, W.Y.; Li, J.Z.; Zhang, Q.; Wang, Y. Circulating miRNA-302 family members as potential biomarkers for the diagnosis of acute heart failure. Biomark. Med. 2018, 12, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Zhou, Z.; Yang, Z. Serum Exosomal MiR-92b-5p as a Potential Biomarker for Acute Heart Failure Caused by Dilated Cardiomyopathy. Cell Physiol. Biochem. 2018, 46, 1939–1950. [Google Scholar] [CrossRef]
- Goren, Y.; Kushnir, M.; Zafrir, B.; Tabak, S.; Lewis, B.S.; Amir, O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012, 14, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ma, F.; Zhang, Y.; Wang, C.; Qiu, D.; Zhou, K.; Hua, Y.; Li, Y. miRNAs as biomarkers for diagnosis of heart failure: A systematic review and meta-analysis. Medicine 2017, 96, e6825. [Google Scholar] [CrossRef]
- Das, S.; Lyon, C.J.; Hu, T. A Panorama of Extracellular Vesicle Applications: From Biomarker Detection to Therapeutics. ACS Nano 2024, 18, 9784–9797. [Google Scholar] [CrossRef]
- Sardu, C.; Santulli, G.; Savarese, G.; Trotta, M.C.; Sacra, C.; Santamaria, M.; Volpicelli, M.; Ruocco, A.; Mauro, C.; Signoriello, G.; et al. Endothelial Dysfunction Drives CRTd Outcome at 1-Year Follow-Up: A Novel Role as Biomarker for miR-130a-5p. Int. J. Mol. Sci. 2023, 24, 1510. [Google Scholar] [CrossRef]
- D’Souza, A.; Pearman, C.M.; Wang, Y.; Nakao, S.; Logantha, S.; Cox, C.; Bennett, H.; Zhang, Y.; Johnsen, A.B.; Linscheid, N.; et al. Targeting miR-423-5p Reverses Exercise Training-Induced HCN4 Channel Remodeling and Sinus Bradycardia. Circ. Res. 2017, 121, 1058–1068. [Google Scholar] [CrossRef]
- Seronde, M.F.; Vausort, M.; Gayat, E.; Goretti, E.; Ng, L.L.; Squire, I.B.; Vodovar, N.; Sadoune, M.; Samuel, J.L.; Thum, T.; et al. Circulating microRNAs and Outcome in Patients with Acute Heart Failure. PLoS ONE 2015, 10, e0142237. [Google Scholar] [CrossRef]
- van Boven, N.; Kardys, I.; van Vark, L.C.; Akkerhuis, K.M.; de Ronde, M.W.J.; Khan, M.A.F.; Merkus, D.; Liu, Z.; Voors, A.A.; Asselbergs, F.W.; et al. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur. J. Heart Fail. 2018, 20, 89–96. [Google Scholar] [CrossRef]
- Ranjan, P.; Dutta, R.K.; Colin, K.; Li, J.; Zhang, Q.; Lal, H.; Qin, G.; Verma, S.K. Bone marrow-fibroblast progenitor cell-derived small extracellular vesicles promote cardiac fibrosis via miR-21-5p and integrin subunit αV signalling. J. Extracell. Biol. 2024, 3, e152. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Wang, Z.; Hu, S. Exercise improves cardiac fibrosis by stimulating the release of endothelial progenitor cell-derived exosomes and upregulating miR-126 expression. Front. Cardiovasc. Med. 2024, 11, 1323329. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liang, X.; Liu, B.; Huang, X.; Shen, Y.; Lin, F.; Chen, J.; Gao, X.; He, H.; Li, W.; et al. Exosomal miR-9-5p derived from iPSC-MSCs ameliorates doxorubicin-induced cardiomyopathy by inhibiting cardiomyocyte senescence. J. Nanobiotechnol. 2024, 22, 195. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Qiu, F.; Cao, H.; Li, H.; Dai, G.; Ma, T.; Gong, Y.; Luo, W.; Zhu, D.; Qiu, Z.; et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics 2023, 13, 685–703. [Google Scholar] [CrossRef]
- Wang, T.; Li, T.; Niu, X.; Hu, L.; Cheng, J.; Guo, D.; Ren, H.; Zhao, R.; Ji, Z.; Liu, P.; et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol. Direct. 2023, 18, 6. [Google Scholar] [CrossRef]
- Lu, W.; Liu, X.; Zhao, L.; Yan, S.; Song, Q.; Zou, C.; Li, X. MiR-22-3p in exosomes increases the risk of heart failure after down-regulation of FURIN. Chem. Biol. Drug Des. 2023, 101, 550–567. [Google Scholar] [CrossRef]
- Carvalho, A.; Ji, Z.; Zhang, R.; Zuo, W.; Qu, Y.; Chen, X.; Tao, Z.; Ji, J.; Yao, Y.; Ma, G. Inhibition of miR-195-3p protects against cardiac dysfunction and fibrosis after myocardial infarction. Int. J. Cardiol. 2023, 387, 131128. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Q.; Liu, X.; Zhang, T.; Wang, S.; Zhou, L.; Zou, L.; Fan, F.; Chi, H.; Sun, J.; et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int. Immunopharmacol. 2021, 101, 107592. [Google Scholar] [CrossRef]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther. Nucleic Acids 2018, 12, 254–266. [Google Scholar] [CrossRef]
- Sun, L.Y.; Zhao, J.C.; Ge, X.M.; Zhang, H.; Wang, C.M.; Bie, Z.D. Circ_LAS1L regulates cardiac fibroblast activation, growth, and migration through miR-125b/SFRP5 pathway. Cell Biochem. Funct. 2020, 38, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bian, Y.F.; Bai, R.; Song, X.S.; Liang, B.; Xiao, C.S. Circ_BMP2K enhances the regulatory effects of miR-455-3p on its target gene SUMO1 and thereby inhibits the activation of cardiac fibroblasts. Biochem. Cell Biol. 2020, 98, 583–590. [Google Scholar] [CrossRef]
- Ji, D.N.; Jin, S.D.; Jiang, Y.; Xu, F.Y.; Fan, S.W.; Zhao, Y.L.; Liu, X.Q.; Sun, H.; Cheng, W.Z.; Zhang, X.Y.; et al. CircNSD1 promotes cardiac fibrosis through targeting the miR-429-3p/SULF1/Wnt/β-catenin signaling pathway. Acta Pharmacol. Sin. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Li, B.; Jiang, L.; Liu, Z.; Wu, F.; Zhang, Y.; Liu, J.; Duan, W. circ_0023461 Silencing Protects Cardiomyocytes from Hypoxia-Induced Dysfunction through Targeting miR-370-3p/PDE4D Signaling. Oxid. Med. Cell Longev. 2021, 2021, 8379962. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, L.; Hu, L.; Yu, H.; Xu, F.; Yang, B.; Zhang, R.; Zhang, Y.; An, Y. Circular RNA-Expression Profiling Reveals a Potential Role of Hsa_circ_0097435 in Heart Failure via Sponging Multiple MicroRNAs. Front. Genet. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, T.; Dahlman, J.; Eniola-Adefeso, L.; Ghiran, I.C.; Kurre, P.; Lam, W.A.; Lang, J.K.; Marbán, E.; Martín, P.; et al. Current challenges and future directions for engineering extracellular vesicles for heart, lung, blood and sleep diseases. J. Extracell. Vesicles 2023, 12, e12305. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Lee, H.W.; Kalimuthu, S.; Hong, C.M.; Jeong, S.Y.; Lee, S.W.; Lee, J.; Ahn, B.C. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control Release 2017, 264, 112–126. [Google Scholar] [CrossRef]
- Xiao, X.; Li, W.; Xu, Z.; Sun, Z.; Ye, H.; Wu, Y.; Zhang, Y.; Xie, L.; Jiang, D.; Jia, R.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells reduce lipopolysaccharide-induced spinal cord injury neuronal apoptosis by mediating miR-29b-3p/PTEN. Connect. Tissue Res. 2022, 63, 634–649. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, H.; Liu, C.; Shao, L.; Zhang, H.; Lu, K.; Wang, J.; Wang, Y.; Yu, Q.; Zhang, Y.; et al. Microneedle Patch Loaded with Exosomes Containing MicroRNA-29b Prevents Cardiac Fibrosis after Myocardial Infarction. Adv. Healthc. Mater. 2023, 12, e2202959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, Y.; Wu, H.; Mao, Y.; Qi, Y. LncRNA HOX transcript antisense RNA mitigates cardiac function injury in chronic heart failure via regulating microRNA-30a-5p to target KDM3A. J. Cell Mol. Med. 2022, 26, 1473–1485. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.; Fan, X.; Ning, X.; Yu, B.; Ou, C.; Chen, M. Targeted delivery of extracellular vesicles in heart injury. Theranostics 2021, 11, 2263–2277. [Google Scholar] [CrossRef]
- Pei, Y.; Xie, S.; Li, J.; Jia, B. Bone marrow-mesenchymal stem cell-derived exosomal microRNA-141 targets PTEN and activates β-catenin to alleviate myocardial injury in septic mice. Immunopharmacol. Immunotoxicol. 2021, 43, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.B.; Ahmed, S.; Sridharan, D.; Naseer, Z.; Pracha, N.; Wang, H.; Boudoulas, K.D.; Zhu, W.; Sayed, N.; Khan, M. De novo Drug Delivery Modalities for Treating Damaged Hearts: Current Challenges and Emerging Solutions. Front. Cardiovasc. Med. 2021, 8, 742315. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Schumacher, S.M.; Gao, E.; Chuprun, J.K.; Ibetti, J.; Roy, R.; Khan, M.; Kishore, R.; Koch, W.J. Characterization of βARKct engineered cellular extracellular vesicles and model specific cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1276–H1289. [Google Scholar] [CrossRef]
- de Freitas, R.C.C.; Hirata, R.D.C.; Hirata, M.H.; Aikawa, E. Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases. Biomolecules 2021, 11, 388. [Google Scholar] [CrossRef]
- Sharma, I.; Bhardwaj, S.; Karwasra, R.; Kaushik, D.; Sharma, S. The Emergence of Nanotechnology in the Prognosis and Treatment of Myocardial Infarctions. Recent. Pat. Nanotechnol. 2023. ahead of print. [Google Scholar] [CrossRef]
- Qasim, M.; Arunkumar, P.; Powell, H.M.; Khan, M. Current research trends and challenges in tissue engineering for mending broken hearts. Life Sci. 2019, 229, 233–250. [Google Scholar] [CrossRef]
- Kancheva, M.; Aronson, L.; Pattilachan, T.; Sautto, F.; Daines, B.; Thommes, D.; Shar, A.; Razavi, M. Bubble-Based Drug Delivery Systems: Next-Generation Diagnosis to Therapy. J. Funct. Biomater. 2023, 14, 373. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Huang, H. Extracellular Vesicle-Derived Non-Coding RNAs: Key Mediators in Remodelling Heart Failure. Curr. Issues Mol. Biol. 2024, 46, 9430-9448. https://doi.org/10.3390/cimb46090559
Zhao J, Huang H. Extracellular Vesicle-Derived Non-Coding RNAs: Key Mediators in Remodelling Heart Failure. Current Issues in Molecular Biology. 2024; 46(9):9430-9448. https://doi.org/10.3390/cimb46090559
Chicago/Turabian StyleZhao, Jiayi, and Huang Huang. 2024. "Extracellular Vesicle-Derived Non-Coding RNAs: Key Mediators in Remodelling Heart Failure" Current Issues in Molecular Biology 46, no. 9: 9430-9448. https://doi.org/10.3390/cimb46090559




