The Diterpene Isopimaric Acid Modulates the Phytohormone Pathway to Promote Oryza sativa L. Rice Seedling Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Culture Conditions
2.2. Extraction of Isopimaric Acid
2.3. Preparation of Reagent Solutions
2.4. Growth of Rice with Different Concentrations of Isopimaric and Gibberellic Acids
2.5. Analysis of Seedling Growth
2.6. Extraction and Quantitative Analysis of Phytohormones
2.7. Phytohormone Standard Curve
2.8. Statistical Analyses
3. Results
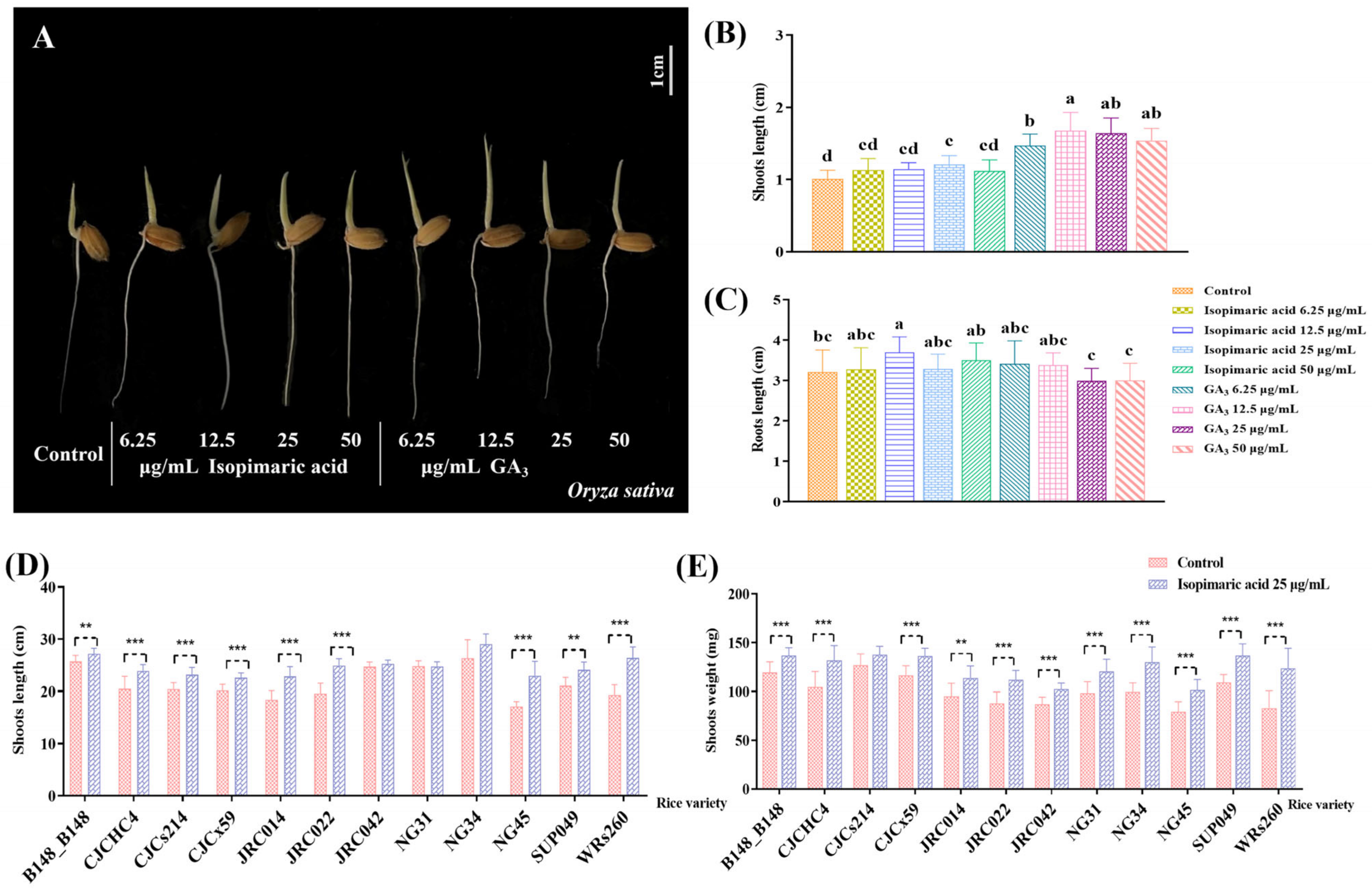
3.1. Isopimaric Acid Promotes Rice Seedling Growth
3.2. Isopimaric Acid Activates the Endogenous Phytohormone Pathway Affecting Rice Growth
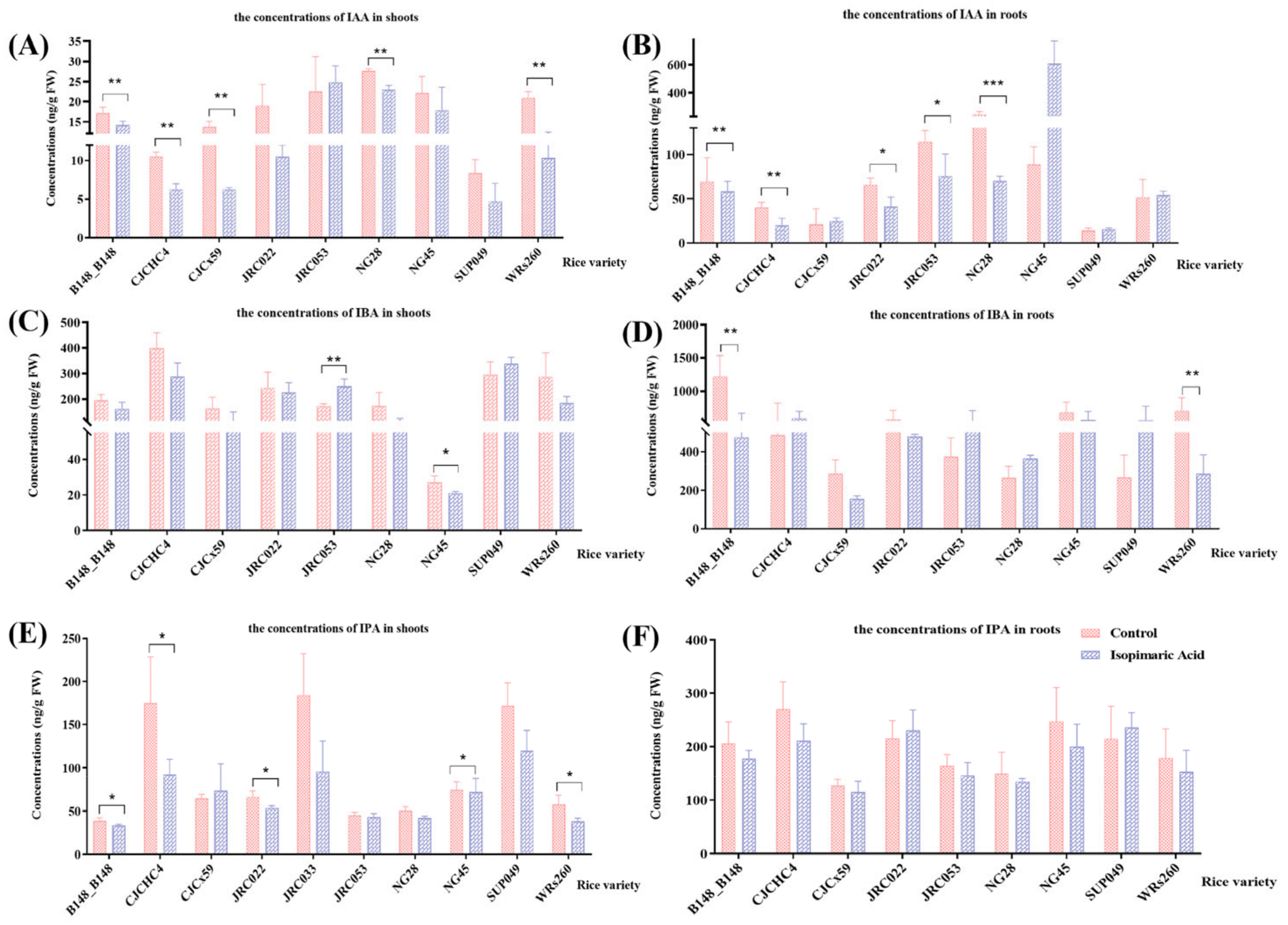
3.2.1. Quantification of IAA-Related Phytohormones in Seedlings Showing Growth Promotion
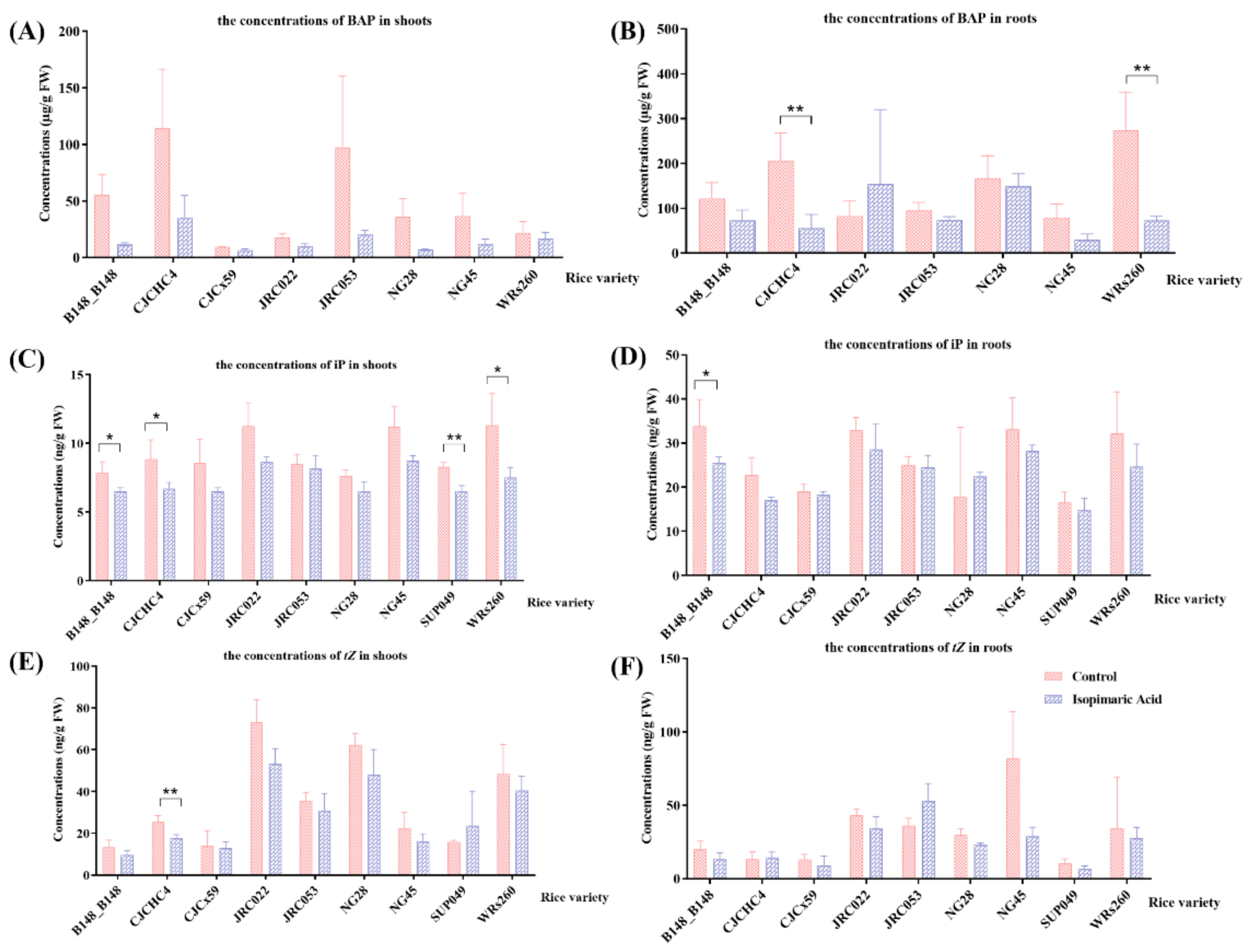
3.2.2. Quantification of Cytokinin Concentrations in Rice Seedlings Following Isopimaric Acid Treatment
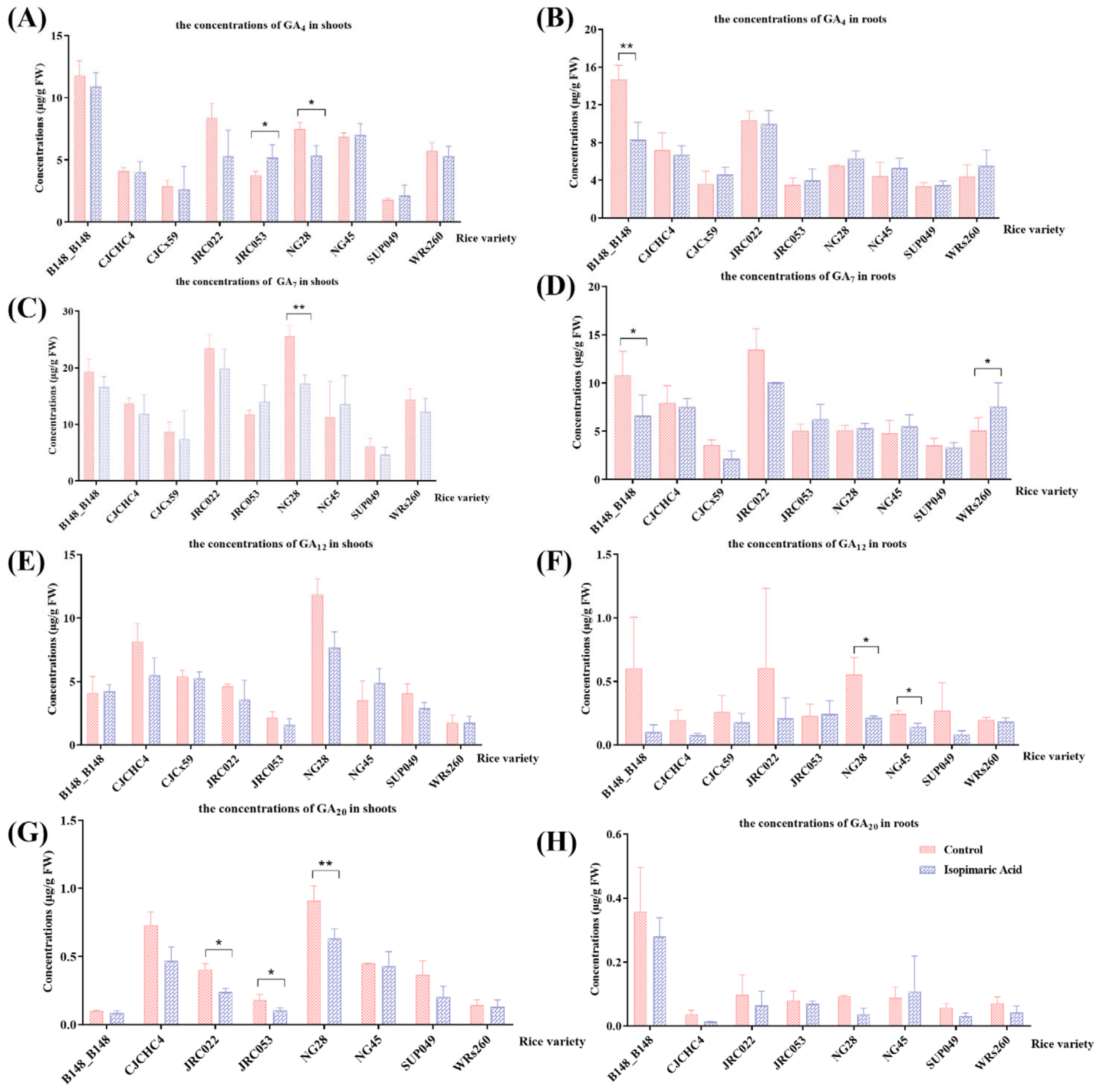
3.2.3. Quantitative Analysis of Gibberellin-Related (GA-Related) Phytohormones in Rice Seedlings Following Treatment with Isopimaric Acid
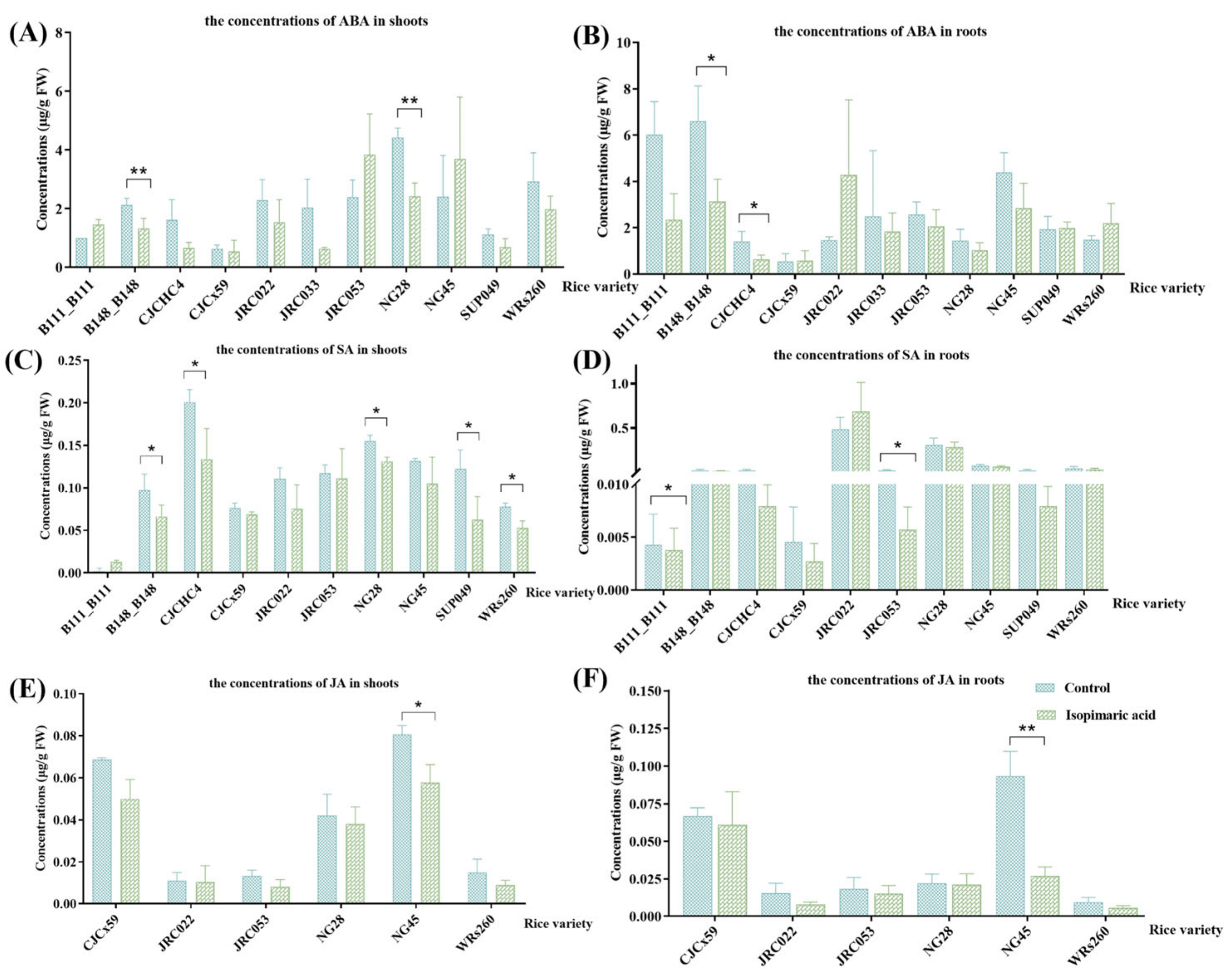
3.2.4. Quantification of Defense-Related Phytohormone Concentrations in Rice Seedlings Following Treatment with Isopimaric Acid
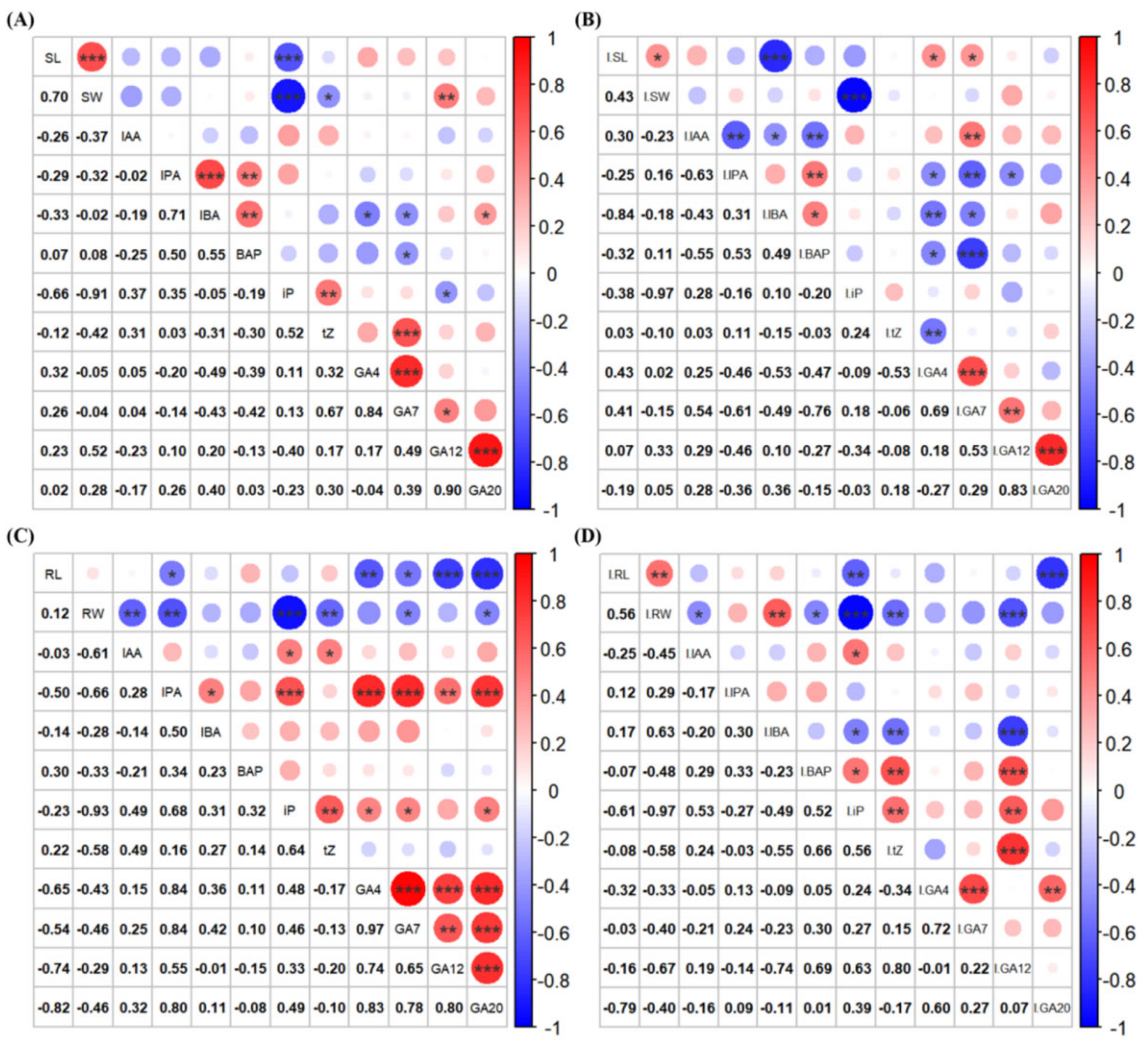
3.3. Correlation Analysis of Phytohormone Concentrations with Growth Characteristics in Rice Seedlings Following Treatment with Isopimaric Acid
3.3.1. Correlation Analysis of Growth-Related Phytohormone Concentrations with Growth Characteristics in Rice Seedlings
3.3.2. Correlation of Concentrations of Defense-Related Phytohormones with Growth Characteristics in Rice Seedlings
4. Discussion
4.1. The Diterpene Isopimaric Acid Could Be Developed as a Promising Plant-Derived Growth Regulator
4.2. Isopimaric Acid May Act through Phytohormone Pathways to Promote Rice Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mafu, S.; Ding, Y.; Murphy, K.M.; Yaacoobi, O.; Addison, J.B.; Wang, Q.; Shen, Z.; Briggs, S.P.; Bohlmann, J.; Castro-Falcon, G.; et al. Discovery, Biosynthesis and Stress-Related Accumulation of Dolabradiene-Derived Defenses in Maize. Plant Physiol. 2018, 176, 2677–2690. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Dowd, T.; Khalil, A.; Char, S.N.; Yang, B.; Endelman, B.J.; Shih, P.M.; Topp, C.; Schmelz, E.A.; Zerbe, P. A dolabralexin-deficient mutant provides insight into specialized diterpenoid metabolism in maize. Plant Physiol. 2023, 192, 1338–1358. [Google Scholar] [CrossRef]
- Bano, C.; Amist, N.; Sunaina; Singh, N.B. UV-B radiation escalate allelopathic effect of benzoic acid on Solanum lycopersicum L. Sci. Hortic. 2017, 220, 199–205. [Google Scholar] [CrossRef]
- He, C.N.; Gao, W.W.; Yang, J.X.; Bi, W.; Zhang, X.S.; Zhao, Y.J. Identification of autotoxic compounds from fibrous roots of Panax quinquefolium L. Plant Soil 2008, 318, 63–72. [Google Scholar] [CrossRef]
- Xie, Z.L.; Zhao, S.; Li, Y.; Deng, Y.H.; Shi, Y.B.; Chen, X.Y.; Li, Y.; Li, H.W.; Chen, C.T.; Wang, X.W.; et al. Phenolic acid-induced phase separation and translation inhibition mediate plant interspecific competition. Nat. Plants 2023, 9, 1481–1499. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.C.; Oliver, J.; Casson, S.; Gray, J.E. Putting the brakes on: Abscisic acid as a central environmental regulator of stomatal development. New Phytol. 2014, 202, 376–391. [Google Scholar] [CrossRef]
- Huang, A.C.; Jiang, T.; Liu, Y.X.; Bai, Y.C.; Reed, J.; Qu, B.; Goossens, A.; Nützmann, H.W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Li, C.H.; Li, D.S.; Jing, S.X.; Hua, J.; Luo, S.H.; Liu, Y.; Li, S.H. Peltate glandular trichomes of colquhounia vestita harbor diterpenoid acids that contribute to plant adaptation to UV radiation and cold stresses. Phytochemistry 2020, 172, 112285. [Google Scholar] [CrossRef] [PubMed]
- Köhler, C.; Fu, J.Y.; Pei, W.Z.; He, L.Q.; Ma, B.; Tang, C.; Zhu, L.; Wang, L.P.; Zhong, Y.Y.; Chen, G.; et al. ZmEREB92 plays a negative role in seed germination by regulating ethylene signaling and starch mobilization in maize. PLoS Genet. 2023, 19, e1011052. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Yu, Z.P.; Duan, X.B.; Luo, L.; Dai, S.j.; Ding, Z.j.; Xia, G.m. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid signaling in plant development and adaptation to stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Vukašinović, N.; Wang, Y.; Vanhoutte, I.; Fendrych, M.; Guo, B.; Kvasnica, M.; Jiroutová, P.; Oklestkova, J.; Strnad, M.; Russinova, E. Local brassinosteroid biosynthesis enables optimal root growth. Nat. Plants 2020, 7, 619–632. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Rao, S.S.R. Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 2002, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Burr, B. International rice genome sequencing project: The effort to completely sequence the rice genome. Curr. Opin. Plant Biol. 2000, 3, 138–142. [Google Scholar] [CrossRef]
- Li, W.J.; Yan, J.J.; Zhang, Y.; Zhang, F.; Guan, Z.Y.; Yao, Y.L.; Chang, Y.; Tu, H.F.; Li, X.K.; Wang, H.J.; et al. Serine protease NAL1 exerts pleiotropic functions through degradation of TOPLESS-related corepressor in rice. Nat. Plants 2023, 9, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Wang, H.R.; Yin, W.C.; Meng, W.J.; Xiao, Y.H.; Liu, D.P.; Zhang, X.X.; Dong, N.N.; Liu, J.H.; Yang, Y.Z.; et al. Rice DWARF and LOW-TILLERING and the homeodomain protein OSH15 interact to regulate internode elongation via orchestrating brassinosteroid signaling and metabolism. Plant Cell 2022, 34, 3754–3772. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Bai, L.; Cao, P.; Li, S.S.; Huang, S.X.; Wang, J.D.; Li, L.; Zhang, J.; Zhao, J.; Song, J.; et al. Novel plant growth regulator guvermectin from plant growth-promoting rhizobacteria boosts biomass and grain yield in rice. J. Agric. Food Chem. 2022, 70, 16229–16240. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.J.; Tan, G.F.; Zhou, W.Q.; Wang, G.L. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2019, 257, 853–861. [Google Scholar] [CrossRef]
- Li, W.H.; Chang, S.T.; Chang, S.C.; Chang, H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef]
- Patrick, d.J. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Kitaoka, N.; Zhang, J.; Oyagbenro, R.K.; Brown, B.; Wu, Y.; Yang, B.; Li, Z.; Peters, R.J. Interdependent evolution of biosynthetic gene clusters for momilactone production in rice. Plant Cell 2021, 33, 290–305. [Google Scholar] [CrossRef]
- Chen, R.B.; Bu, Y.J.; Ren, J.Z.; Pelot, K.A.; Hu, X.Y.; Diao, Y.; Chen, W.S.; Zerbe, P.; Zhang, L. Discovery and modulation of diterpenoid metabolism improves glandular trichome formation, artemisinin production and stress resilience in Artemisia annua. New Phytol. 2021, 230, 2387–2403. [Google Scholar] [CrossRef]
- Ding, L.; Jing, H.W.; Wang, T.; Li, J.; Liu, G.A. Regulation of root growth in Lactuca sativa L. seedlings by the ent-kaurane diterpenoid epinodosin. J. Plant Growth Regul. 2010, 29, 419–427. [Google Scholar] [CrossRef]
- Ali, J.; Mukarram, M.; Ojo, J.; Dawam, N.; Riyazuddin, R.; Ghramh, H.A.; Khan, K.A.; Chen, R.; Kurjak, D.; Bayram, A. Harnessing phytohormones: Advancing plant growth and defence strategies for sustainable agriculture. Physiol. Plant. 2024, 176. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Kang, Z.L.; Hua, J.; Feng, Y.L.; Luo, S.H. Root exudate sesquiterpenoids from the invasive weed Ambrosia trifida regulate rhizospheric proteobacteria. Sci. Total Environ. 2022, 834, 155263. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, T.; Shenton, M.R.; Okada, K. Evolution of labdane-related diterpene synthases in cereals. Plant Cell Physiol. 2020, 61, 1850–1859. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Zhao, J.; Huang, Q.Q.; Peng, L.L.; Huang, Z.B.; Li, W.W.; Sun, S.; He, Y.Q.; Wang, Z.F. OsNAC3 regulates seed germination involving abscisic acid pathway and cell elongation in rice. New Phytol. 2023, 241, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lu, Y.J.; Wang, J.; Bi, L.W.; Zhao, Z.D. Synthesis and bioactivity evaluation of acylthiourea derivatives based on isopimaric acid. Chin. J. Org. Chem. 2017, 37, 731–738. [Google Scholar] [CrossRef]
- Zhou, H.W.; Hua, J.; Zhang, J.M.; Luo, S.H. Negative interactions balance growth and defense in plants confronted with herbivores or pathogens. J. Agric. Food Chem. 2022, 70, 12723–12732. [Google Scholar] [CrossRef]
- Mudbhari, S.; Lofgren, L.; Appidi, M.R.; Vilgalys, R.; Hettich, R.L.; Abraham, P.E. Decoding the chemical language of Suillus fungi: Genome mining and untargeted metabolomics uncover terpene chemical diversity. mSystems 2024, 9, e01225-23. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Hua, J.; Peng, L.; Bai, L.; Luo, S. The Diterpene Isopimaric Acid Modulates the Phytohormone Pathway to Promote Oryza sativa L. Rice Seedling Growth. Curr. Issues Mol. Biol. 2024, 46, 9772-9784. https://doi.org/10.3390/cimb46090580
Huang J, Hua J, Peng L, Bai L, Luo S. The Diterpene Isopimaric Acid Modulates the Phytohormone Pathway to Promote Oryza sativa L. Rice Seedling Growth. Current Issues in Molecular Biology. 2024; 46(9):9772-9784. https://doi.org/10.3390/cimb46090580
Chicago/Turabian StyleHuang, Jiaqi, Juan Hua, Luying Peng, Liping Bai, and Shihong Luo. 2024. "The Diterpene Isopimaric Acid Modulates the Phytohormone Pathway to Promote Oryza sativa L. Rice Seedling Growth" Current Issues in Molecular Biology 46, no. 9: 9772-9784. https://doi.org/10.3390/cimb46090580
APA StyleHuang, J., Hua, J., Peng, L., Bai, L., & Luo, S. (2024). The Diterpene Isopimaric Acid Modulates the Phytohormone Pathway to Promote Oryza sativa L. Rice Seedling Growth. Current Issues in Molecular Biology, 46(9), 9772-9784. https://doi.org/10.3390/cimb46090580





