Patch-Based Far-Infrared Radiation (FIR) Therapy Does Not Impact Cell Tracking or Motility of Human Melanoma Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Patch-Based FIR Therapy
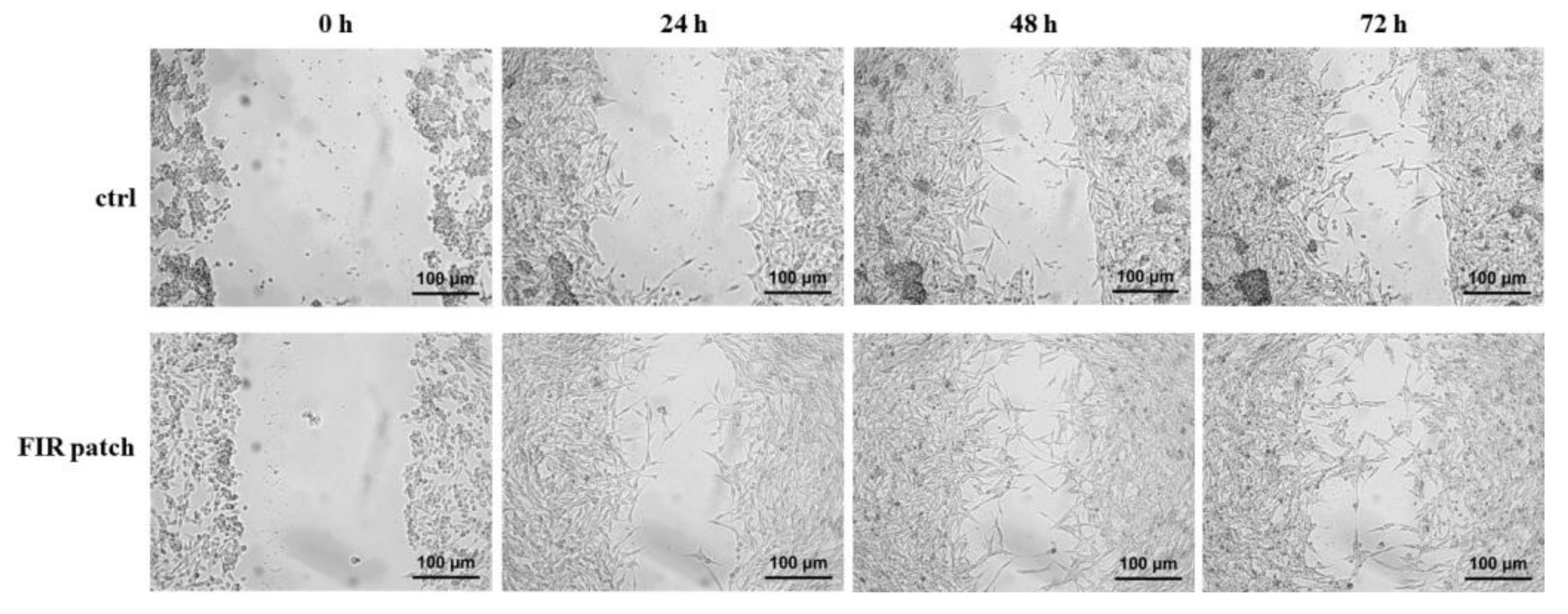
2.2. Scratch Assay
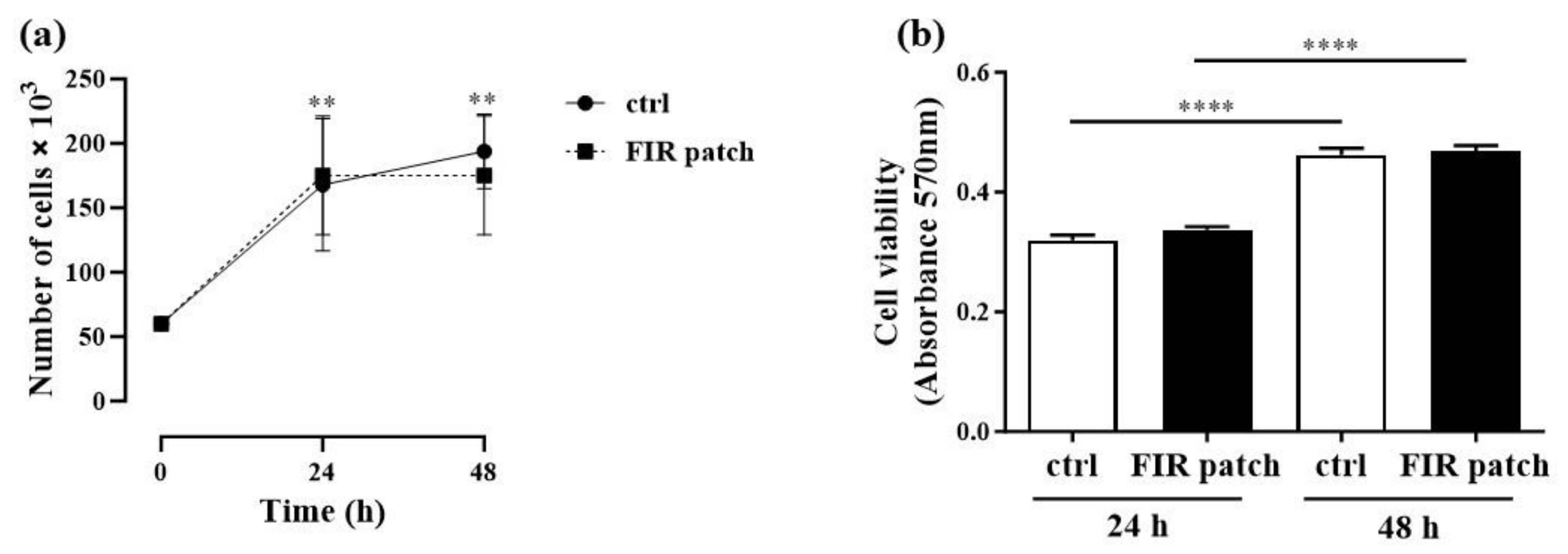
2.3. Cell Proliferation Assay
2.4. Cell Viability Assay
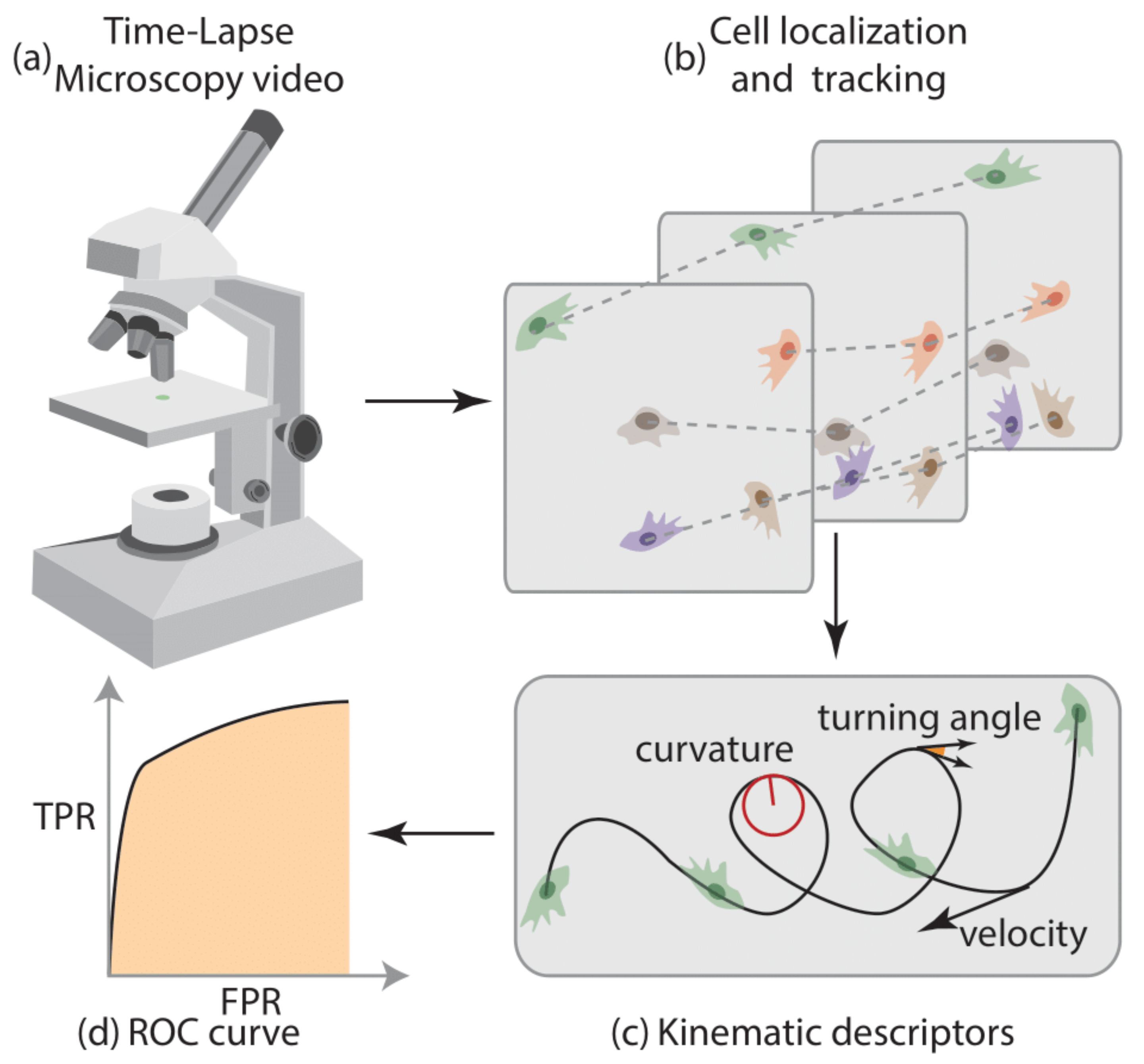
2.5. Platform for the Analysis of Cell Motility
2.6. Time-Lapse Microscopy Details
2.7. Cell Localization and Tracking
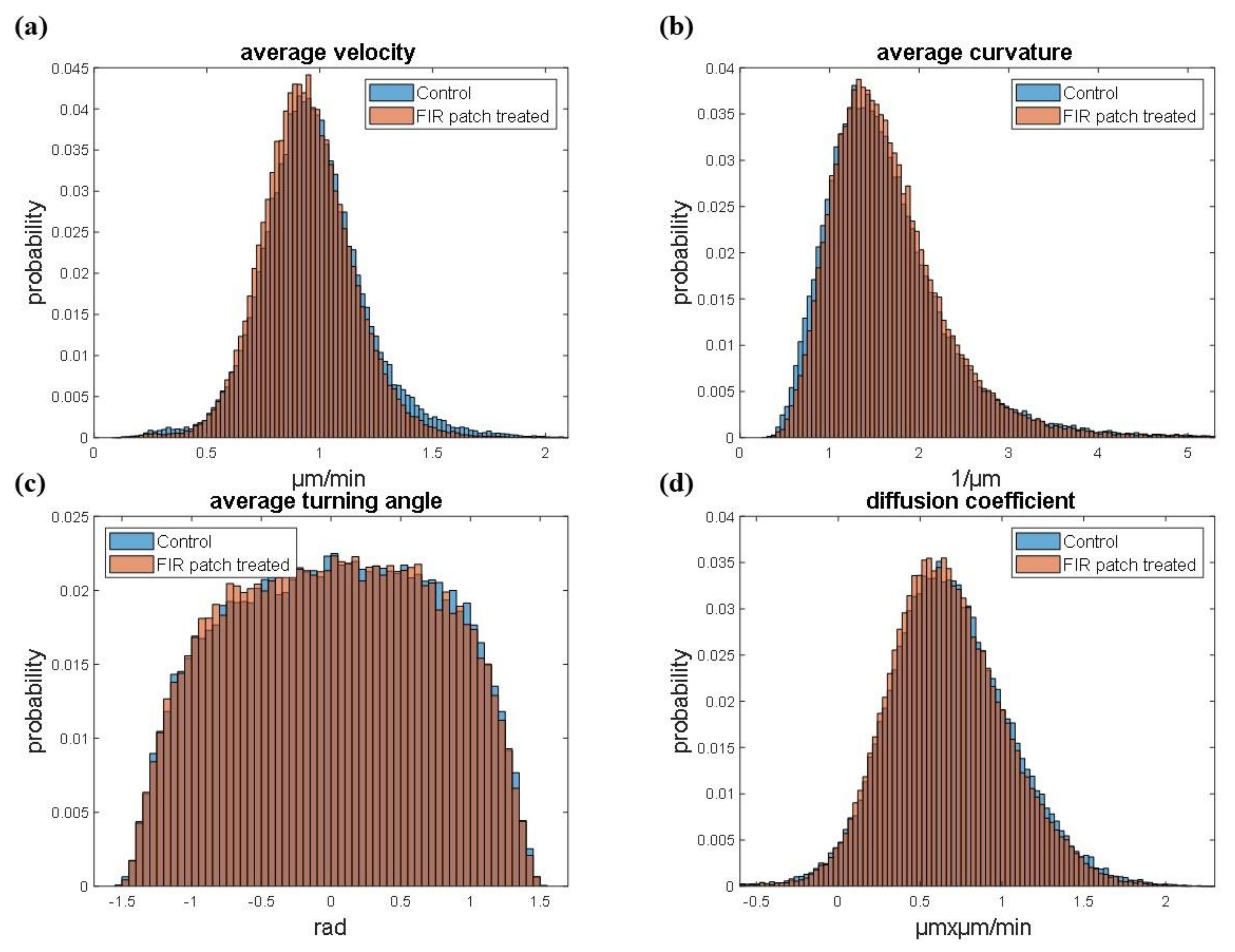
2.8. Cell Motility Analysis
- a.
- Tangential speed norm
- b.
- Curvature
- c.
- Turning angle
- d.
- Diffusion coefficient
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Effects of Patch-Based FIR Therapy on Cell Proliferation and Cell Survival
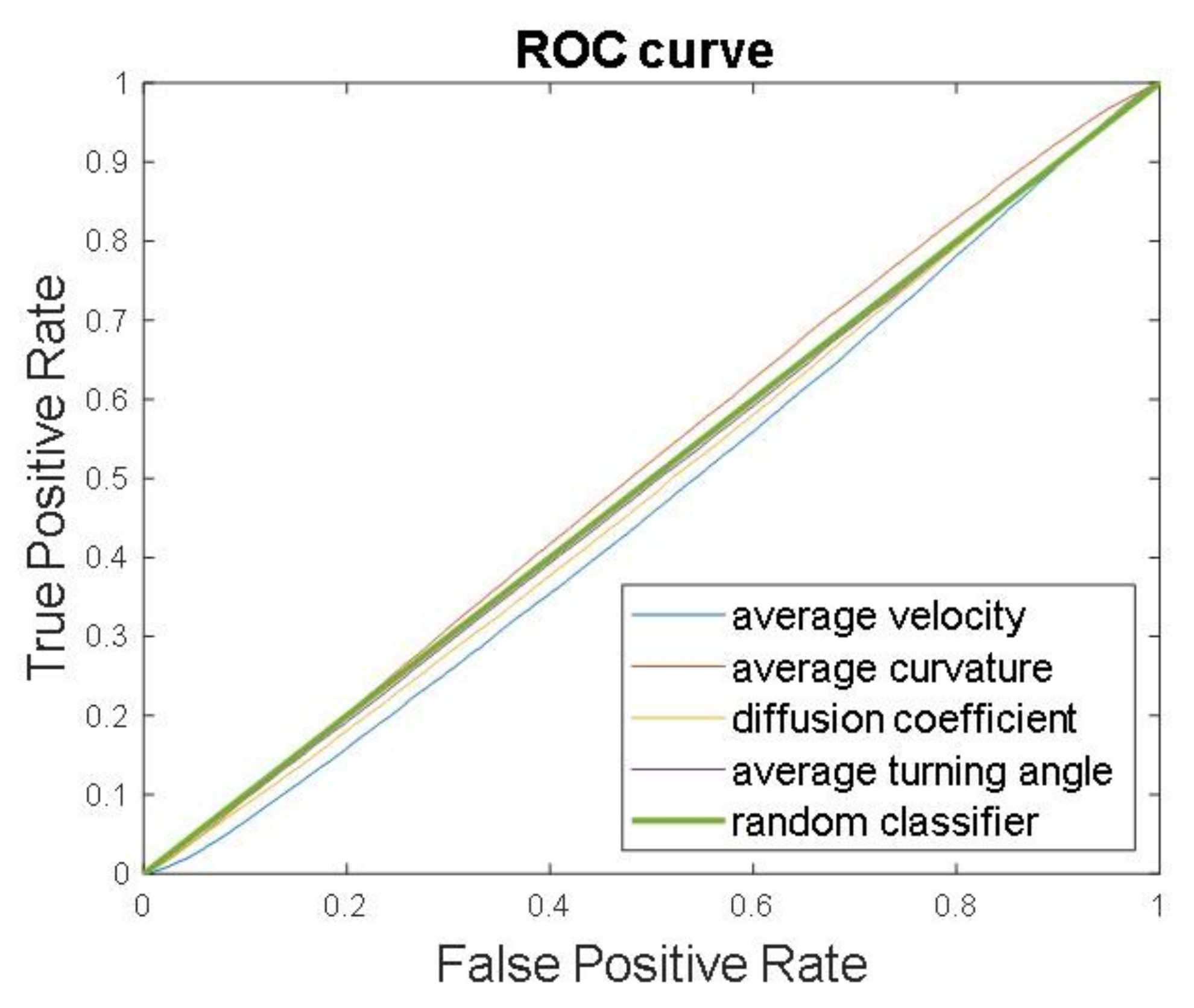
3.2. Cell Tracking and Motility Assessment
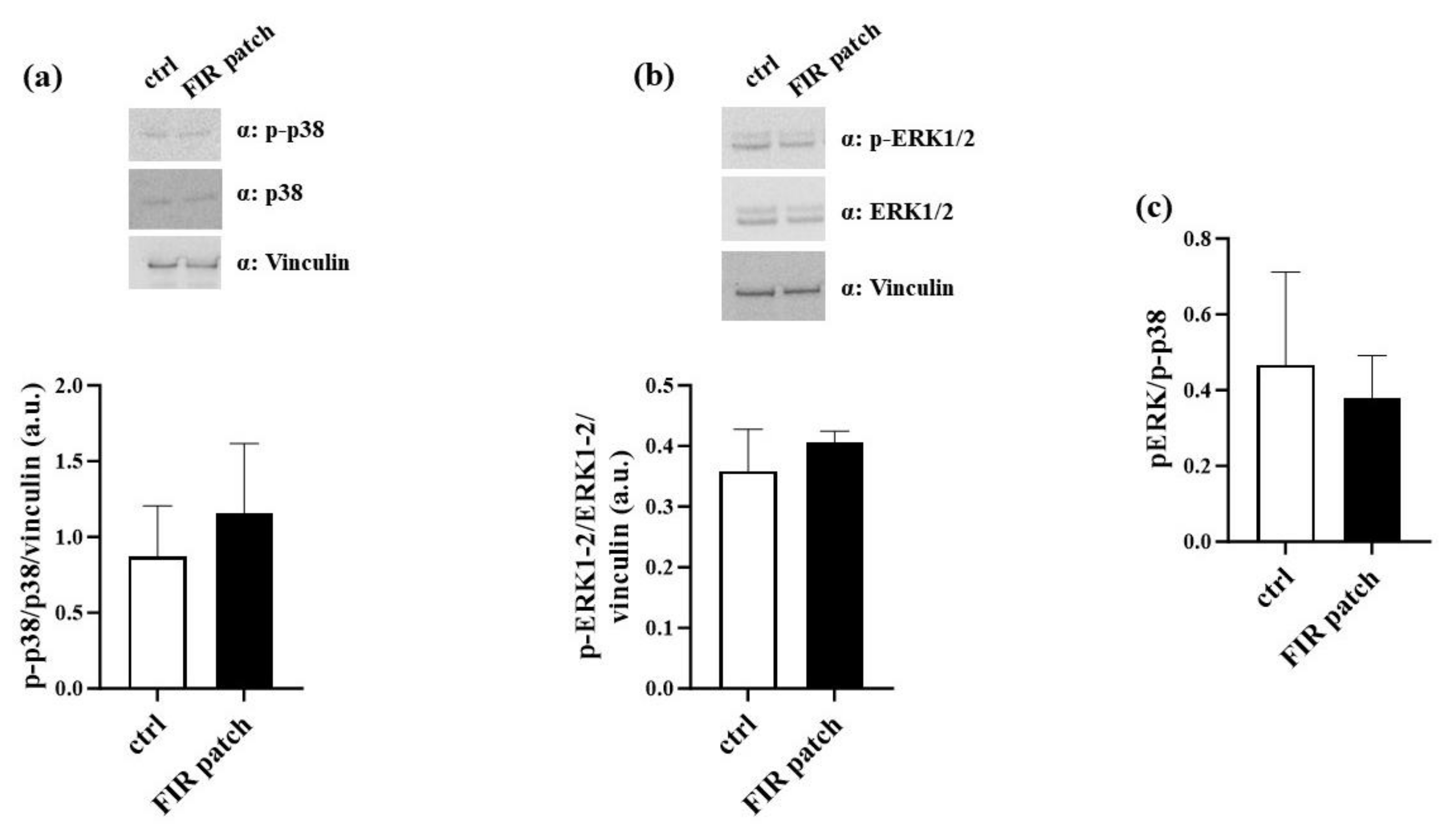
3.3. Patch-Based FIR Therapy Does Not Promote the Activation of ERK1/2 MAPK Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photonics Lasers Med. 2012, 4, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y. Infrared Spectroscopy-Mid-infrared, Near-infrared, and Far-infrared/Terahertz Spectroscopy. Anal. Sci. 2021, 37, 1193–1212. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Ikeda, Y.; Miyata, M.; Shinsato, T.; Kubozono, T.; Kuwahata, S.; Hamada, N.; Miyauchi, T.; Yamaguchi, T.; Torii, H.; et al. Effect of Waon therapy on oxidative stress in chronic heart failure. Circ. J. 2011, 75, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Beever, R. The effects of repeated thermal therapy on quality of life in patients with type II diabetes mellitus. J. Altern. Complement. Med. 2010, 16, 677–681. [Google Scholar] [CrossRef]
- Oosterveld, F.G.; Rasker, J.J.; Floors, M.; Landkroon, R.; van Rennes, B.; Zwijnenberg, J.; van de Laar, M.A.; Koel, G.J. Infrared sauna in patients with rheumatoid arthritis and ankylosing spondylitis. A pilot study showing good tolerance, short-term improvement of pain and stiffness, and a trend towards long-term beneficial effects. Clin. Rheumatol. 2009, 28, 29–34. [Google Scholar] [CrossRef]
- Hu, K.H.; Li, W.T. Clinical effects of far-infrared therapy in patients with allergic rhinitis. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2007, 2007, 1479–1482. [Google Scholar] [CrossRef]
- Hausswirth, C.; Louis, J.; Bieuzen, F.; Pournot, H.; Fournier, J.; Filliard, J.R.; Brisswalter, J. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS ONE 2011, 6, e27749. [Google Scholar] [CrossRef]
- Herr, G.E.G.; da Silva, F.G.; Cidral-Filho, F.J.; Petronilho, F.; Danielski, L.G.; de Souza Goldim, M.P.; Salgado, A.S.I.; Bobinski, F.; Martins, D.F.; Winkelmann, E.R. Effects of the use of bioceramic wraps in patients with lower limb venous ulcers: A randomized double-blind placebo-controlled trial. J. Integr. Med. 2020, 18, 26–34. [Google Scholar] [CrossRef]
- van Kraaij, S.J.W.; Hamblin, M.R.; Pickering, G.; Giannokopoulos, B.; Kechemir, H.; Heinz, M.; Igracki-Turudic, I.; Yavuz, Y.; Rissmann, R.; Gal, P. A Phase 1 randomized, open-label clinical trial to evaluate the effect of a far-infrared emitting patch on local skin perfusion, microcirculation and oxygenation. Exp. Dermatol. 2024, 33, e14962. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, Y.C.; Chen, T.H.; Sue, Y.M.; Cheng, T.H.; Chen, J.R.; Chen, C.H. Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells. PLoS ONE 2012, 7, e30674. [Google Scholar] [CrossRef]
- Pastore, D.; Pacifici, F.; Ciao, G.; Bedin, V.; Pasquantonio, G.; Della-Morte, D. Far Infrared Technology (FIT) Therapy Patches, Protects from Inflammation, Oxidative Stress and Promotes Cellular Vitality. Curr. Pharm. Des. 2020, 26, 4323–4329. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Miyata, M.; Eto, H.; Shirasawa, T.; Hamada, N.; Ikeda, Y.; Biro, S.; Otsuji, Y.; Tei, C. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ. J. 2006, 70, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, H.; Matsui, Y.; Uhara, J.; Tsuchiya, H.; Teshima, S.; Nakanishi, H.; Kwon, A.H.; Azuma, Y.; Nagaoka, T.; Ogawa, T.; et al. Promotive effects of far-infrared ray on full-thickness skin wound healing in rats. Exp. Biol. Med. 2003, 228, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Lee, H.J.; Lee, J.Y.; Park, J.H.; Jo, I. Far-infrared irradiation inhibits breast cancer cell proliferation independently of DNA damage through increased nuclear Ca2+/calmodulin binding modulated-activation of checkpoint kinase 2. J. Photochem. Photobiol. B 2021, 219, 112188. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, J.; Yamashita, K.; Ishikawa, T.; Hosokawa, H.; Sumida, K.; Nagayama, M.; Kitamura, S. The effects inhibiting the proliferation of cancer cells by far-infrared radiation (FIR) are controlled by the basal expression level of heat shock protein (HSP) 70A. Med. Oncol. 2008, 25, 229–237. [Google Scholar] [CrossRef]
- Leung, T.-K.; Chan, C.-F.; Lai, P.-S.; Yang, C.-H.; Hsu, C.-Y.; Lin, Y.-S. Inhibitory Effects of Far-Infrared Irradiation Generated by Ceramic Material on Murine Melanoma Cell Growth. Int. J. Photoenergy 2012, 2012, 646845. [Google Scholar] [CrossRef]
- Naffa, R.; Vogel, L.; Hegedus, L.; Paszty, K.; Toth, S.; Kelemen, K.; Singh, N.; Remenyi, A.; Kallay, E.; Cserepes, M.; et al. P38 MAPK Promotes Migration and Metastatic Activity of BRAF Mutant Melanoma Cells by Inducing Degradation of PMCA4b. Cells 2020, 9, 1209. [Google Scholar] [CrossRef]
- Ruffini, F.; D’Atri, S.; Lacal, P.M. Neuropilin-1 expression promotes invasiveness of melanoma cells through vascular endothelial growth factor receptor-2-dependent and -independent mechanisms. Int. J. Oncol. 2013, 43, 297–306. [Google Scholar] [CrossRef]
- Pacifici, F.; Salimei, C.; Pastore, D.; Malatesta, G.; Ricordi, C.; Donadel, G.; Bellia, A.; Rovella, V.; Tafani, M.; Garaci, E.; et al. The Protective Effect of a Unique Mix of Polyphenols and Micronutrients against Neurodegeneration Induced by an In Vitro Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 3110. [Google Scholar] [CrossRef]
- Comes, M.C.; Mencattini, A.; Di Giuseppe, D.; Filippi, J.; D’Orazio, M.; Casti, P.; Corsi, F.; Ghibelli, L.; Di Natale, C.; Martinelli, E. A Camera Sensors-Based System to Study Drug Effects On In Vitro Motility: The Case of PC-3 Prostate Cancer Cells. Sens. 2020, 20, 1531. [Google Scholar] [CrossRef]
- Mencattini, A.; Rizzuto, V.; Antonelli, G.; Di Giuseppe, D.; D’Orazio, M.; Filippi, J.; Comes, M.C.; Casti, P.; Vives Corrons, J.L.; Garcia-Bravo, M.; et al. Machine learning microfluidic based platform: Integration of Lab-on-Chip devices and data analysis algorithms for red blood cell plasticity evaluation in Pyruvate Kinase Disease monitoring. Sens. Actuators A Phys. 2023, 351, 114187. [Google Scholar] [CrossRef]
- Mencattini, A.; Spalloni, A.; Casti, P.; Comes, M.C.; Di Giuseppe, D.; Antonelli, G.; D’Orazio, M.; Filippi, J.; Corsi, F.; Isambert, H.; et al. NeuriTES. Monitoring neurite changes through transfer entropy and semantic segmentation in bright-field time-lapse microscopy. Patterns 2021, 2, 100261. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, M.; Corsi, F.; Mencattini, A.; Di Giuseppe, D.; Colomba Comes, M.; Casti, P.; Filippi, J.; Di Natale, C.; Ghibelli, L.; Martinelli, E. Deciphering Cancer Cell Behavior from Motility and Shape Features: Peer Prediction and Dynamic Selection to Support Cancer Diagnosis and Therapy. Front. Oncol. 2020, 10, 580698. [Google Scholar] [CrossRef] [PubMed]
- Comes, M.C.; Filippi, J.; Mencattini, A.; Corsi, F.; Casti, P.; De Ninno, A.; Di Giuseppe, D.; D’Orazio, M.; Ghibelli, L.; Mattei, F.; et al. Accelerating the experimental responses on cell behaviors: A long-term prediction of cell trajectories using Social Generative Adversarial Network. Sci. Rep. 2020, 10, 15635. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Wintz, P. (Eds.) Digital Image Processing, 2nd ed.; Addison-Wesley Longman Publishing Co.: Boston, MA, USA, 2001. [Google Scholar]
- Munkres, J. Algorithms for the Assignment and Transportation Problems. J. Soc. Ind. Appl. Math. 1957, 5, 32–38. [Google Scholar] [CrossRef]
- D’Orazio, M.; Murdocca, M.; Mencattini, A.; Casti, P.; Filippi, J.; Antonelli, G.; Di Giuseppe, D.; Comes, M.C.; Di Natale, C.; Sangiuolo, F.; et al. Machine learning phenomics (MLP) combining deep learning with time-lapse-microscopy for monitoring colorectal adenocarcinoma cells gene expression and drug-response. Sci. Rep. 2022, 12, 8545. [Google Scholar] [CrossRef]
- Comes, M.C.; Casti, P.; Mencattini, A.; Di Giuseppe, D.; Mermet-Meillon, F.; De Ninno, A.; Parrini, M.C.; Businaro, L.; Di Natale, C.; Martinelli, E. The influence of spatial and temporal resolutions on the analysis of cell-cell interaction: A systematic study for time-lapse microscopy applications. Sci. Rep. 2019, 9, 6789. [Google Scholar] [CrossRef]
- Sbalzarini, I.F.; Koumoutsakos, P. Feature point tracking and trajectory analysis for video imaging in cell biology. J. Struct. Biol. 2005, 151, 182–195. [Google Scholar] [CrossRef]
- Estrada, Y.; Dong, J.; Ossowski, L. Positive crosstalk between ERK and p38 in melanoma stimulates migration and in vivo proliferation. Pigment. Cell Melanoma Res. 2009, 22, 66–76. [Google Scholar] [CrossRef]
- Ricci, M.; Micheloni, G.M.; Perusi, F.; Corbo, V.R.; Vecchini, E.; Magnan, B. Use of a non-medicated plaster in shoulder tendinopathies. Acta Biomed. 2016, 87 (Suppl. 1), 90–94. [Google Scholar]
- Palmieri, B.; Vadalà, M.; Laurino, C. The FIT therapy for the treatment of musculoskeletal and neurological disorders related symptoms: A retrospective observational study. Asian J. Med. Sci. 2019, 10, 6–12. [Google Scholar] [CrossRef]
- Ricci, M.; Mulone, A.; Elena, N.; Vecchini, E.; Valentini, R.; Gelmini, M. Use of a non-medicated plaster in chronic lumbar back pain: A randomized controlled trial. Acta Biomed. 2022, 93, e2022260. [Google Scholar] [CrossRef]
- Imamura, M.; Biro, S.; Kihara, T.; Yoshifuku, S.; Takasaki, K.; Otsuji, Y.; Minagoe, S.; Toyama, Y.; Tei, C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J. Am. Coll. Cardiol. 2001, 38, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Liu, X.M.; Peyton, K.; Wang, H.; Yang, W.C.; Lin, S.J.; Durante, W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Lin, C.S.; Tsai, T.N.; Cheng, S.M.; Lin, W.S.; Cheng, C.C.; Wu, C.H.; Hsu, C.H. Stimulatory Influences of Far Infrared Therapy on the Transcriptome and Genetic Networks of Endothelial Progenitor Cells Receiving High Glucose Treatment. Acta Cardiol. Sin. 2015, 31, 414–428. [Google Scholar] [CrossRef]
- Yamashita, K.; Dalkhsuren, S.-O.; Ishikawa, T.; Sumida, K.; Ishibashi, J.; Hosokawa, H.; Ueno, A.; Nasu, F.; Kitamura, S. Far Infrared Ray Radiation Inhibits the Proliferation of A549, HSC3 and Sa3 Cancer Cells through Enhancing the Expression of ATF3 Gene. J. Electromagn. Anal. Appl. 2010, 2. [Google Scholar] [CrossRef]
- Watmough, D.J.; Oliver, R. The emission of infrared radiation from human skin--implications for clinical thermography. Br. J. Radiol. 1969, 42, 411–415. [Google Scholar] [CrossRef]
- Sharma, G.D.; He, J.; Bazan, H.E. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: Evidence of cross-talk activation between MAP kinase cascades. J. Biol. Chem. 2003, 278, 21989–21997. [Google Scholar] [CrossRef]
- Colone, M.; Calcabrini, A.; Toccacieli, L.; Bozzuto, G.; Stringaro, A.; Gentile, M.; Cianfriglia, M.; Ciervo, A.; Caraglia, M.; Budillon, A.; et al. The multidrug transporter P-glycoprotein: A mediator of melanoma invasion? J. Invest. Dermatol. 2008, 128, 957–971. [Google Scholar] [CrossRef]
- Budina-Kolomets, A.; Webster, M.R.; Leu, J.I.; Jennis, M.; Krepler, C.; Guerrini, A.; Kossenkov, A.V.; Xu, W.; Karakousis, G.; Schuchter, L.; et al. HSP70 Inhibition Limits FAK-Dependent Invasion and Enhances the Response to Melanoma Treatment with BRAF Inhibitors. Cancer Res. 2016, 76, 2720–2730. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacifici, F.; Chiereghin, F.; D’Orazio, M.; Malatesta, G.; Infante, M.; Fazio, F.; Bertinato, C.; Donadel, G.; Martinelli, E.; De Lorenzo, A.; et al. Patch-Based Far-Infrared Radiation (FIR) Therapy Does Not Impact Cell Tracking or Motility of Human Melanoma Cells In Vitro. Curr. Issues Mol. Biol. 2024, 46, 10026-10037. https://doi.org/10.3390/cimb46090599
Pacifici F, Chiereghin F, D’Orazio M, Malatesta G, Infante M, Fazio F, Bertinato C, Donadel G, Martinelli E, De Lorenzo A, et al. Patch-Based Far-Infrared Radiation (FIR) Therapy Does Not Impact Cell Tracking or Motility of Human Melanoma Cells In Vitro. Current Issues in Molecular Biology. 2024; 46(9):10026-10037. https://doi.org/10.3390/cimb46090599
Chicago/Turabian StylePacifici, Francesca, Francesca Chiereghin, Michele D’Orazio, Gina Malatesta, Marco Infante, Federica Fazio, Chiara Bertinato, Giulia Donadel, Eugenio Martinelli, Antonino De Lorenzo, and et al. 2024. "Patch-Based Far-Infrared Radiation (FIR) Therapy Does Not Impact Cell Tracking or Motility of Human Melanoma Cells In Vitro" Current Issues in Molecular Biology 46, no. 9: 10026-10037. https://doi.org/10.3390/cimb46090599










