The Effect of Autologous Dendritic Cell Immunotherapy on Kidney Function and Endothelial Dysfunction of Patients with Diabetic Kidney Disease (DKD): An Open Label Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Subjects
2.3. Study Procedure
2.4. Statistics
3. Results
3.1. Subject Characteristics
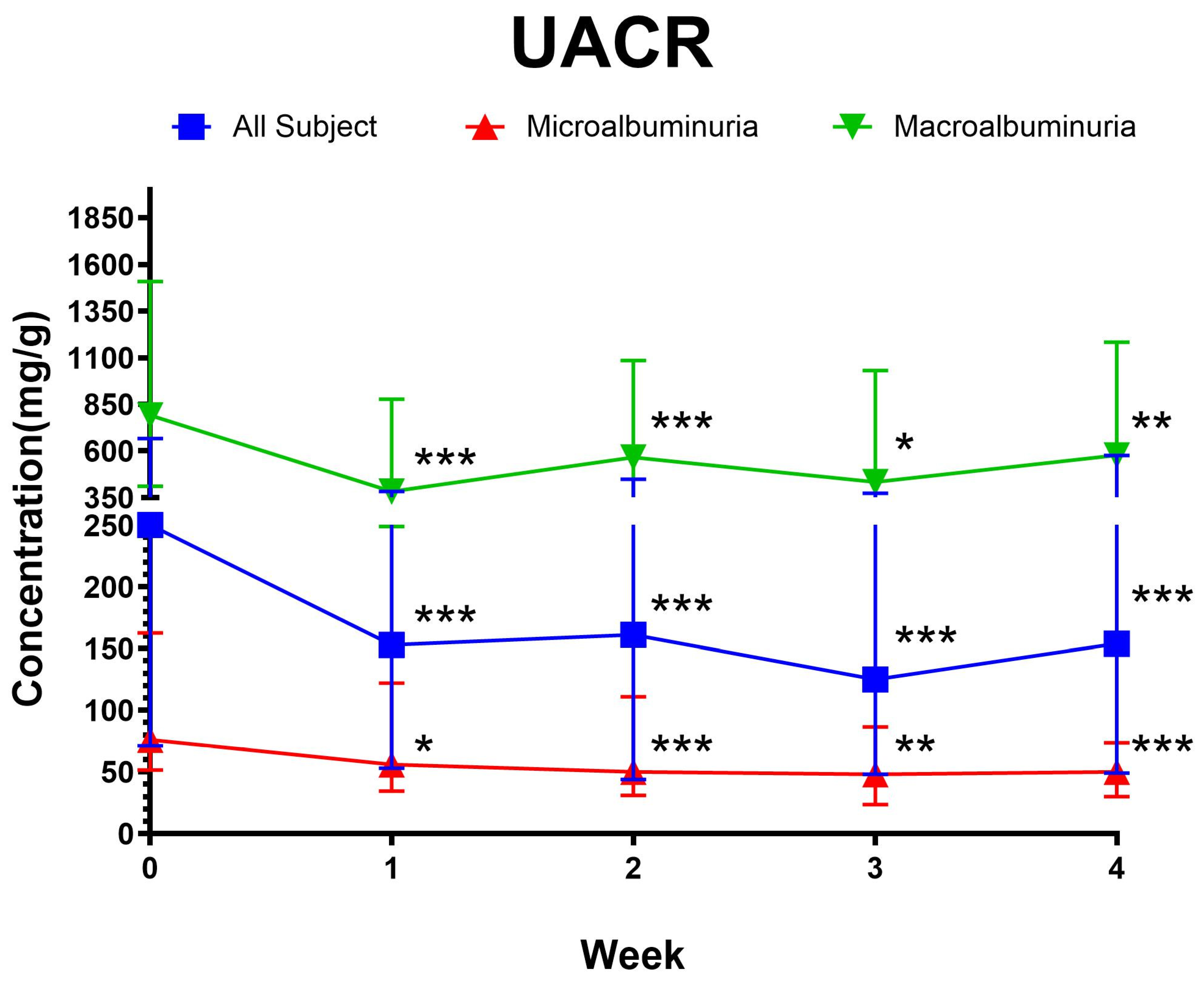
3.2. Clinical Outcome
3.3. Predictors of UACR Change
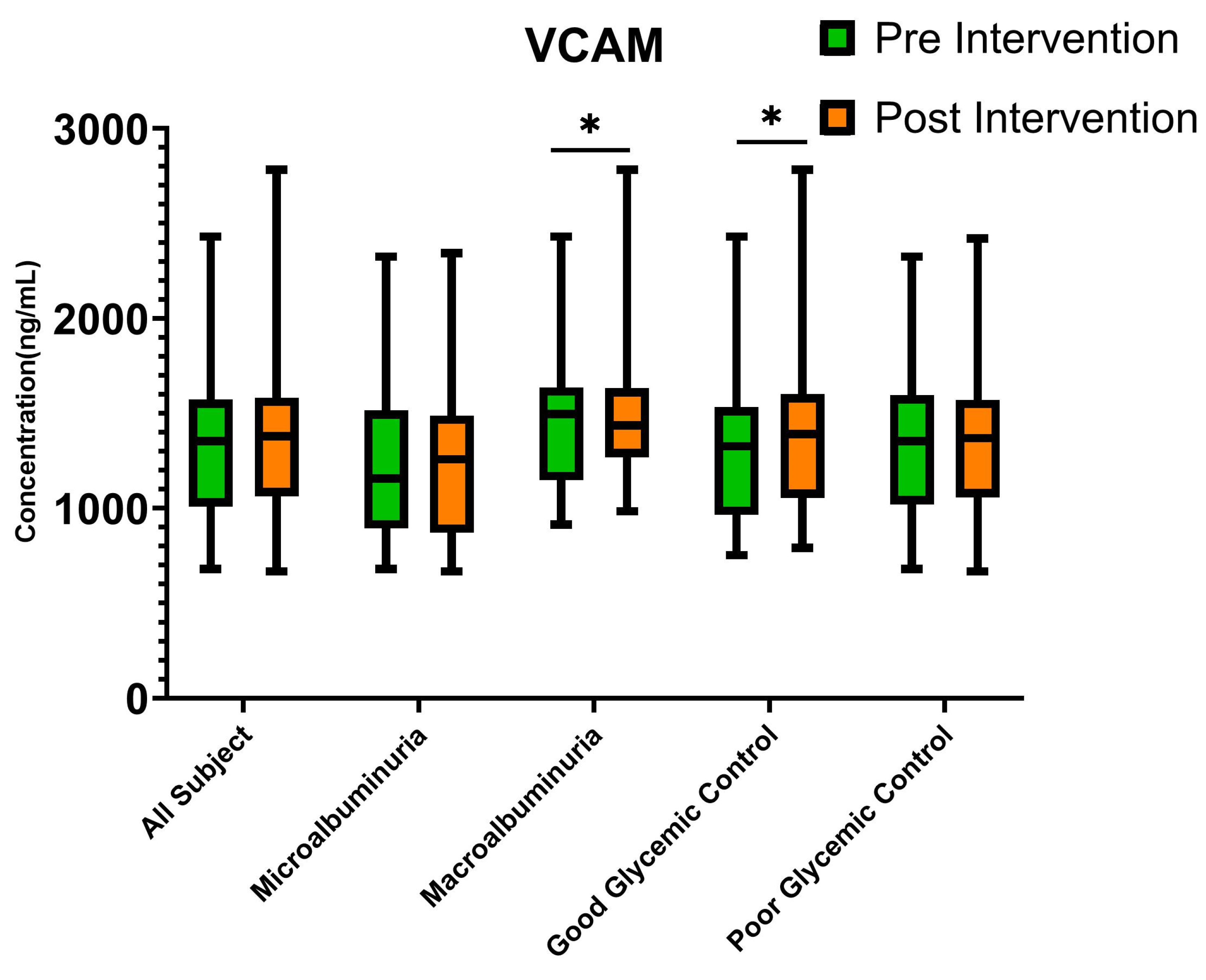
3.4. Endothelial Dysfunction Biomarkers
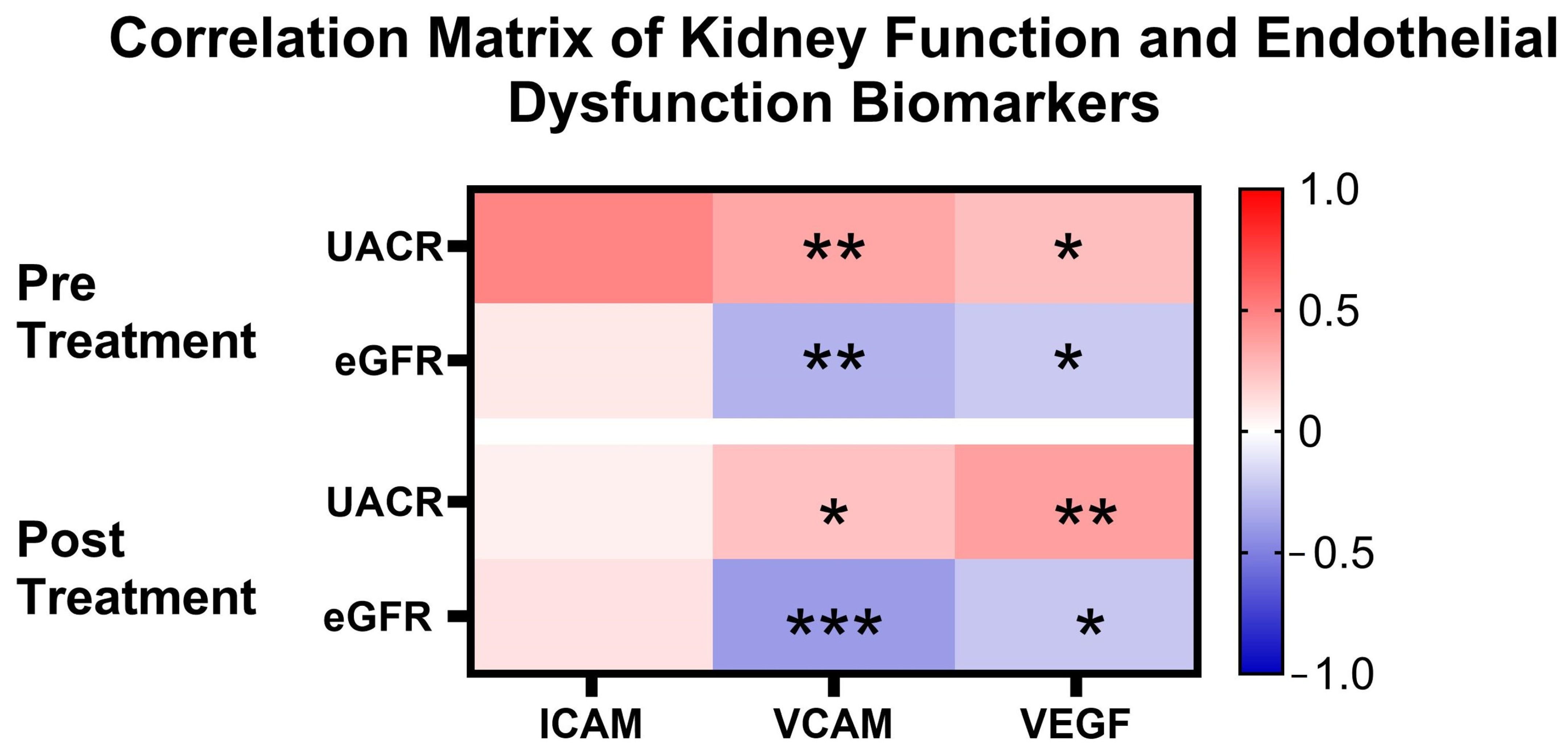
3.5. Correlation of Clinical Outcome Parameters with Endothelial Dysfunction Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, R.; Koya, D.; Haneda, M. Progression of diabetic nephropathy. Am. J. Kidney Dis. 2003, 41, S19–S21. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Bhatti, R. Cellular and molecular mechanisms involved in metabolic disorders. In Drug Delivery Systems for Metabolic Disorders; Academic Press: Cambridge, MA, USA, 2022; pp. 21–29. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Zhu, J.; Liu, Y.; Jin, X.; Chen, G.; Ye, L. Current application status and structure–activity relationship of selective and non-selective JAK inhibitors in diseases. Int. Immunopharmacol. 2023, 122, 110660. [Google Scholar] [CrossRef] [PubMed]
- Lourido, F.; Quenti, D.; Salgado-Canales, D.; Tobar, N. Domeless receptor loss in fat body tissue reverts insulin resistance induced by a high-sugar diet in Drosophila melanogaster. Sci. Rep. 2021, 11, 3263. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Liu, W.; Wang, S.; Jiang, R.; Hu, K.; Sheng, L.; Xu, G.; Kou, X.; Song, Y. NF-κB-Inducing Kinase Provokes Insulin Resistance in Skeletal Muscle of Obese Mice. Inflammation 2023, 46, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.J.; Lee, E.K.; Park, T.J.; Kim, W. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res. Rev. 2015, 24, 66–76. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Litovkina, Z.І.; Susla, O.B. Cardiovascular Features of Chronic Inflammation and Endothelial Dysfunction in Patients with Diabetic Nephropathy on Programmed Hemodialysis. J. Educ. Health Sport 2020, 10, 144–157. [Google Scholar] [CrossRef]
- Yun, J.-S.; Ko, S.-H.; Kim, J.-H.; Moon, K.-W.; Park, Y.-M.; Yoo, K.-D.; Ahn, Y.-B. Diabetic Retinopathy and Endothelial Dysfunction in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2013, 37, 262. [Google Scholar] [CrossRef]
- Lassén, E.; Daehn, I.S. Molecular Mechanisms in Early Diabetic Kidney Disease: Glomerular Endothelial Cell Dysfunction. Int. J. Mol. Sci. 2020, 21, 9456. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Adeva-Contreras, L.; Fernández-Fernández, C.; Carneiro-Freire, N.; Domínguez-Montero, A. Histological Manifestations of Diabetic Kidney Disease and its Relationship with Insulin Resistance. Curr. Diabetes Rev. 2023, 19, e280322202705. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, J.; Ahlberg, G.; Andreasen, L.; Banasik, K.; Brunak, S.; Schwinn, M.; Larsen, I.H.; Petersen, O.; Sørensen, E.; Ullum, H.; et al. Association of Variants Near the Bradykinin Receptor B2 Gene with Angioedema in Patients Taking ACE Inhibitors. J. Am. Coll. Cardiol. 2021, 78, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, H.A.; Palmer, B.F. Management of Hyperkalemia in Renin-Angiotensin-Aldosterone System Inhibitor: Strategies to Maintain Chronic Kidney Disease Patients with Type II Diabetes on Therapy. Cardiorenal. Med. 2024, 14, 191–201. [Google Scholar] [CrossRef]
- Sidorenkov, G.; Navis, G. Safety of ACE inhibitor therapies in patients with chronic kidney disease. Expert Opin. Drug Saf. 2014, 13, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Balan, S.; Saxena, M.; Bhardwaj, N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019, 348, 1–68. [Google Scholar] [PubMed]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef]
- Cechim, G.; Chies, J.A.B. In vitro generation of human monocyte-derived dendritic cells methodological aspects in a comprehensive review. An. Acad. Bras. Ciências 2019, 91, e20190310. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0001-37652019000700614&tlng=en (accessed on 4 August 2024). [CrossRef]
- Jonny, J.; Sitepu, E.C.; Lister, I.N.E.; Chiuman, L.; Putranto, T.A. The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines 2024, 12, 972. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, A.; Takahashi, H.; Ishikawa, H.; Yoshida, S.; Kazuta, K.; Utsuno, A.; Ueyama, E. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: Results of the Long-Term ASP1941 Safety Evaluation in Patients with Type 2 Dia. Diabetes Obes. Metab. 2015, 17, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Jonny, J.; Putranto, T.A.; Yana, M.L.; Sitepu, E.C.; Irfon, R.; Ramadhani, B.P.; Sofro, M.A.U.; Nency, Y.M.; Lestari, E.S.; Triwardhani, R.; et al. Safety and efficacy of dendritic cell vaccine for COVID-19 prevention after 1-Year follow-up: Phase I and II clinical trial final result. Front. Immunol. 2023, 14, 1122389. [Google Scholar] [CrossRef]
- Nistor, G.I.; Dillman, R.O.; Robles, R.M.; Langford, J.L.; Poole, A.J.; Sofro, M.A.U.; Nency, Y.M.; Jonny, J.; Yana, M.L.; Karyana, M.; et al. A personal COVID-19 dendritic cell vaccine made at point-of-care: Feasibility, safety, and antigen-specific cellular immune responses. Hum. Vaccines Immunother. 2022, 18, 2100189. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kataru, R.P.; Park, H.J.; Baik, J.E.; Li, C.; Shin, J.; Mehrara, B.J. Regulation of Lymphatic Function in Obesity. Front. Physiol. 2020, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Curovic, V.R.; Jongs, N.; Kroonen, M.Y.; Zobel, E.H.; Hansen, T.W.; Sen, T.; Laverman, G.D.; Kooy, A.; Persson, F.; Rossing, P.; et al. Optimization of Albuminuria-Lowering Treatment in Diabetes by Crossover Rotation to Four Different Drug Classes: A Randomized Crossover Trial. Diabetes Care 2023, 46, 593–601. [Google Scholar] [CrossRef]
- Mathu Selvarajah, M.; Mendis, S. Randomized Controlled Trial of Treatment of Chronic Kidney Disease of Uncertain Aetiolgy with Enalapril. J. Clin. Toxicol. 2016, 6, 281. [Google Scholar] [CrossRef]
- Yarlagadda, C.; Abutineh, M.; Reddy, A.J.; Landau, A.B.; Travis, L.M.; Perrone, C.G.; Idriss, A.; Patel, R. An Investigation on the Efficacy of Glucagon-Like Peptide 1 Receptor Agonists Drugs in Reducing Urine Albumin-to-Creatinine Ratio in Patients with Type 2 Diabetes: A Potential Treatment for Diabetic Nephropathy. Cureus 2023, 15, e36438. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, X.; Xia, S.; Xiao, X.; Chen, J.; Li, L. Relationship between dapagliflozin and urinary albumin-to-creatinine ratio in patients with diabetes mellitus and cardiovascular disease: An observational study. Cardiol. Plus. 2023, 8, 263–268. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S83–S96. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xue, Y.; Chen, Q.; Han, X.; Cai, M.; Tian, J.; Jin, S.; Lu, H. Identifying Distinct Risk Thresholds of Glycated Hemoglobin and Systolic Blood Pressure for Rapid Albuminuria Progression in Type 2 Diabetes from NHANES (1999–2018). Front. Med. 2022, 9, 928825. [Google Scholar] [CrossRef]
- Tsuda, A.; Mori, K.; Nakatani, S.; Machiba, Y.; Uedono, H.; Kurajoh, M.; Yamada, S.; Morioka, T.; Inaba, M.; Ishimura, E.; et al. Dissociation of Glycated Albumin and HbA1c Is Associated with a line of Glomerular Filtration Rate as Evaluated by Inulin Clearance. Diabetes Care 2021, 44, e188–e189. [Google Scholar] [CrossRef]
- Güler, S.; Cakir, B.; Demirbas, B.; Yönem, A.; Odabasi, E.; Önde, U.; Aykut; Gürsoy, G. Plasma Soluble Intercellular Adhesion Molecule 1 Levels Are Increased in Type 2 Diabetic Patients with Nephropathy. Horm. Res. Paediatr. 2002, 58, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.; George, T.P.; Mujammami, M.; Isnani, A.; Alfadda, A.A. The association of cell adhesion molecules and selectins (VCAM-1, ICAM-1, E-selectin, L-selectin, and P-selectin) with microvascular complications in patients with type 2 diabetes: A follow-up study. Front. Endocrinol. 2023, 14, 1072288. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Advani, A. VEGF and the diabetic kidney: More than too much of a good thing. J. Diabetes Complicat. 2017, 31, 273–279. [Google Scholar] [CrossRef]
- Pertseva, N.; Borysova, I.; Chub, D. TGF-β1 and VCAM-1 Serum Concentrations as Diagnostic Biomarkers of Diabetic Kidney Disease Progression. Rom. J. Diabetes Nutr. Metab. Dis. 2019, 26, 169–175. [Google Scholar] [CrossRef]
- Melchinger, I.; Guo, K.; Guo, J.; Xu, L. Inflammation-mediated Upregulation of VCAM-1 but not KIM-1 during Acute Kidney Injury to Chronic Kidney Disease Transition. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, K.B.; Kim, D.L.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Kang, Y.S.; Han, S.Y.; Han, K.H.; et al. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in Type 2 diabetes mellitus. Diabet. Med. 2004, 21, 545–551. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. Rev. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Beirão, I.; Alves, R.; Belo, L.; Santos-Silva, A. New Potential Biomarkers for Chronic Kidney Disease Management—A Review of the Literature. Int. J. Mol. Sci. 2020, 22, 43. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Díaz-Vesga, M.C.; Sanhueza-Olivares, F.; Riquelme, J.A.; Müller, M.; Garrido, L.; Gabrielli, L.; Chiong, M.; Corbalan, R.; Castro, P.F.; et al. Targeting VCAM-1: A therapeutic opportunity for vascular damage. Expert Opin. Ther. Targets 2023, 27, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Sun, L.; Ma, B.; Jin, R.; Ban, Y.; Li, R.; Wang, J.; Lian, H.; Yue, H. VCAM-1 Promotes Angiogenesis of Bone, row Mesenchymal Stem Cells Derived from Patients with Trauma-Induced Osteonecrosis of the Femoral Head by Regulating the Apelin/CCN2 Pathway. Stem. Cells Int. 2023, 2023, 6684617. [Google Scholar] [CrossRef]

| Parameter | Pre Intervention | Post Intervention | p-Value |
|---|---|---|---|
| Creatinine (mg/dL) | 1.04 (0.82–1.62) | 1.12 (0.78–1.66) | 0.467 |
| eGFR (mL/min/1.73 m2) | 60.35 (38.78–86.31) | 56.26 (37.59–90.07) | 0.478 |
| Parameter | Pre Intervention | Post Intervention | p-Value |
|---|---|---|---|
| ICAM (ng/mL) | 314.50 ± 93.12 | 315.12 ± 78.98 | 0.905 |
| VCAM (ng/mL) | 1354.25 (1015.90–1567.03) | 1380.65 (1067.92–1572.03) | 0.827 |
| VEGF (pg/mL) | 504.44 (294.54–790.72) | 494.57 (319.39–775.10) | 0.664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yana, M.L.; Sitepu, E.C.; Jonny; Chiuman, L.; Lister, I.N.E.; Putranto, T.A. The Effect of Autologous Dendritic Cell Immunotherapy on Kidney Function and Endothelial Dysfunction of Patients with Diabetic Kidney Disease (DKD): An Open Label Clinical Trial. Curr. Issues Mol. Biol. 2025, 47, 31. https://doi.org/10.3390/cimb47010031
Yana ML, Sitepu EC, Jonny, Chiuman L, Lister INE, Putranto TA. The Effect of Autologous Dendritic Cell Immunotherapy on Kidney Function and Endothelial Dysfunction of Patients with Diabetic Kidney Disease (DKD): An Open Label Clinical Trial. Current Issues in Molecular Biology. 2025; 47(1):31. https://doi.org/10.3390/cimb47010031
Chicago/Turabian StyleYana, Martina Lily, Enda Cindylosa Sitepu, Jonny, Linda Chiuman, I Nyoman Ehrich Lister, and Terawan Agus Putranto. 2025. "The Effect of Autologous Dendritic Cell Immunotherapy on Kidney Function and Endothelial Dysfunction of Patients with Diabetic Kidney Disease (DKD): An Open Label Clinical Trial" Current Issues in Molecular Biology 47, no. 1: 31. https://doi.org/10.3390/cimb47010031
APA StyleYana, M. L., Sitepu, E. C., Jonny, Chiuman, L., Lister, I. N. E., & Putranto, T. A. (2025). The Effect of Autologous Dendritic Cell Immunotherapy on Kidney Function and Endothelial Dysfunction of Patients with Diabetic Kidney Disease (DKD): An Open Label Clinical Trial. Current Issues in Molecular Biology, 47(1), 31. https://doi.org/10.3390/cimb47010031






