Emerging Therapeutic Innovations for Vitiligo Treatment
Abstract
:1. Introduction
2. Literature Search Strategy
3. JAK Inhibitors
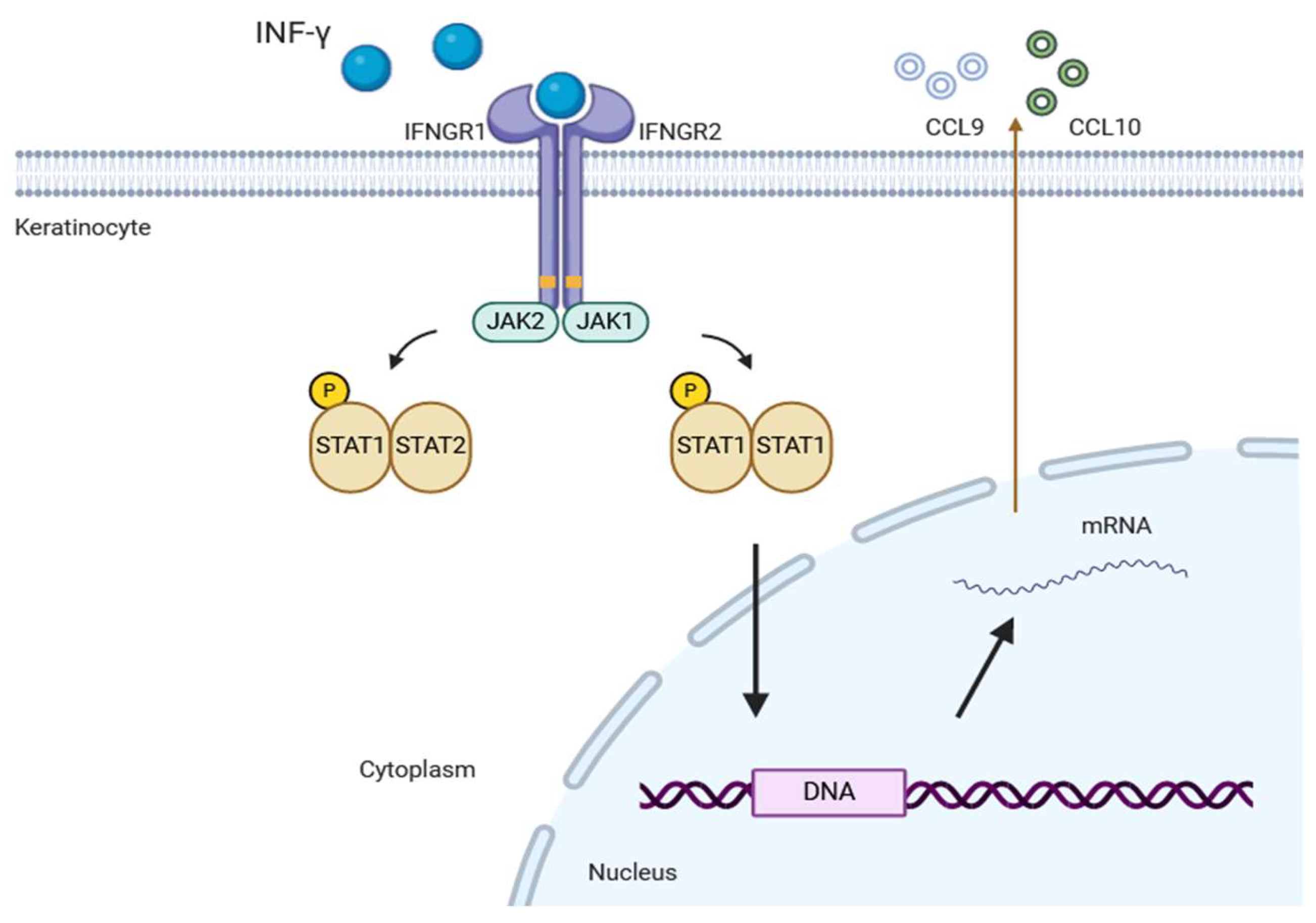
3.1. JAK-STAT Signaling Pathway
3.2. Ruxolitinib
3.3. Tofacitinib
3.4. Baricitinib
3.5. Upadacitinib
3.6. Ritlecitinib
3.7. Other JAK Inhibitors
4. STAT Inhibitors
5. Cytokines
6. Immune Checkpoints
7. T-Cell Metabolism
8. α-Melanocyte-Stimulating Hormone
9. Phosphodiesterase-4
10. 5-Fluorouracil
11. Trichloroacetic Acid
12. Pseudocatalase
13. Prostaglandins
14. Considerations for the Future
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| cAMP | Cyclic adenosine monophosphate |
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen-4 |
| DCs | Dendritic cells |
| HSP | Heat shock protein |
| IFN | Interferon |
| IL | Interleukin |
| JAK | Janus kinase |
| mTOR | Mammalian target of rapamycin |
| PDE-4 | Phosphodiesterase-4 |
| PGE2 | Prostaglandin E2 |
| PGF2α | Prostaglandin F2 alpha |
| STAT | Signal transducer and activator of transcription |
| TCA | Trichloroacetic acid |
| TNF-α | Tumor necrosis factor α |
| Tregs | Regulatory functions of regulatory T cells |
| TRMs | Tissue-resident memory T cells |
| TYK2 | Tyrosine kinase 2 |
| TYR | Tyrosinase |
| U.S. FDA | United States Food and Drug Administration |
| VASI | Vitiligo Area Scoring Index |
| α-MSH | α-melanocyte-stimulating hormone |
References
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Whitton, M.E.; Pinart, M.; Batchelor, J.; Leonardi-Bee, J.; Gonzalez, U.; Jiyad, Z.; Eleftheriadou, V.; Ezzedine, K. Interventions for vitiligo. Cochrane Database Syst. Rev. 2015, 2015, CD003263. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Ott, G.; Kunsebeck, H.W.; Jecht, E.; Shimshoni, R.; Lazaroff, I.; Schallmayer, S.; Calliess, I.T.; Malewski, P.; Lamprecht, F.; Gotz, A. Stigmatization experience, coping and sense of coherence in vitiligo patients. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ongenae, K.; Van Geel, N.; De Schepper, S.; Naeyaert, J.M. Effect of vitiligo on self-reported health-related quality of life. Br. J. Dermatol. 2005, 152, 1165–1172. [Google Scholar] [CrossRef]
- Bibeau, K.; Ezzedine, K.; Harris, J.E.; van Geel, N.; Grimes, P.; Parsad, D.; Tulpule, M.; Gardner, J.; Valle, Y.; Tlhong Matewa, G.; et al. Mental Health and Psychosocial Quality-of-Life Burden Among Patients with Vitiligo: Findings from the Global VALIANT Study. JAMA Dermatol. 2023, 159, 1124–1128. [Google Scholar] [CrossRef]
- Sehgal, V.N.; Srivastava, G. Vitiligo: Compendium of clinico-epidemiological features. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 149–156. [Google Scholar] [CrossRef]
- Kundu, R.V.; Mhlaba, J.M.; Rangel, S.M.; Le Poole, I.C. The convergence theory for vitiligo: A reappraisal. Exp. Dermatol. 2019, 28, 647–655. [Google Scholar] [CrossRef]
- Faraj, S.; Kemp, E.H.; Gawkrodger, D.J. Patho-immunological mechanisms of vitiligo: The role of the innate and adaptive immunities and environmental stress factors. Clin. Exp. Immunol. 2022, 207, 27–43. [Google Scholar] [CrossRef]
- Chaudhary, A.; Patel, M.; Singh, S. Current Debates on Etiopathogenesis and Treatment Strategies for Vitiligo. Curr. Drug Targets 2022, 23, 1219–1238. [Google Scholar] [CrossRef]
- Dogra, S.; Sharma, A.; Mehta, H.; Sarkar, R. Emerging role of topical Janus kinase inhibitors in dermatological disorders: A review. Clin. Exp. Dermatol. 2023, 48, 1102–1112. [Google Scholar] [CrossRef]
- Sheikh, A.; Rafique, W.; Owais, R.; Malik, F.; Ali, E. FDA approves Ruxolitinib (Opzelura) for Vitiligo Therapy: A breakthrough in the field of dermatology. Ann. Med. Surg. 2022, 81, 104499. [Google Scholar] [CrossRef] [PubMed]
- Rosmarin, D.; Pandya, A.G.; Lebwohl, M.; Grimes, P.; Hamzavi, I.; Gottlieb, A.B.; Butler, K.; Kuo, F.; Sun, K.; Ji, T.; et al. Ruxolitinib cream for treatment of vitiligo: A randomised, controlled, phase 2 trial. Lancet 2020, 396, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; Ferdinand, J.R.; O’Shea, J.J. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 2015, 194, 21–27. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’Shea, J.J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef]
- Abdel Motaleb, A.A.; Tawfik, Y.M.; El-Mokhtar, M.A.; Elkady, S.; El-Gazzar, A.F.; ElSayed, S.K.; Awad, S.M. Cutaneous JAK Expression in Vitiligo. J. Cutan. Med. Surg. 2021, 25, 157–162. [Google Scholar] [CrossRef]
- Rashighi, M.; Harris, J.E. Interfering with the IFN-gamma/CXCL10 pathway to develop new targeted treatments for vitiligo. Ann. Transl. Med. 2015, 3, 343. [Google Scholar] [CrossRef]
- Qi, F.; Liu, F.; Gao, L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 2021, 12, 790125. [Google Scholar] [CrossRef]
- Mobasher, P.; Guerra, R.; Li, S.J.; Frangos, J.; Ganesan, A.K.; Huang, V. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br. J. Dermatol. 2020, 182, 1047–1049. [Google Scholar] [CrossRef]
- Mesa, R.A. Ruxolitinib, a selective JAK1 and JAK2 inhibitor for the treatment of myeloproliferative neoplasms and psoriasis. IDrugs 2010, 13, 394–403. [Google Scholar]
- Harris, J.E.; Rashighi, M.; Nguyen, N.; Jabbari, A.; Ulerio, G.; Clynes, R.; Christiano, A.M.; Mackay-Wiggan, J. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J. Am. Acad. Dermatol. 2016, 74, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Heine, A.; Held, S.A.; Daecke, S.N.; Wallner, S.; Yajnanarayana, S.P.; Kurts, C.; Wolf, D.; Brossart, P. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood 2013, 122, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, B.; Joshipura, D.; Saraiya, A.; Abdat, R.; Ashkar, H.; Turkowski, Y.; Sheth, V.; Huang, V.; Au, S.C.; Kachuk, C.; et al. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib. J. Am. Acad. Dermatol. 2017, 76, 1054–1060.e1. [Google Scholar] [CrossRef]
- Joshipura, D.; Alomran, A.; Zancanaro, P.; Rosmarin, D. Treatment of vitiligo with the topical Janus kinase inhibitor ruxolitinib: A 32-week open-label extension study with optional narrow-band ultraviolet B. J. Am. Acad. Dermatol. 2018, 78, 1205–1207.e1. [Google Scholar] [CrossRef]
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and future status of JAK inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef]
- Mease, P.; Hall, S.; FitzGerald, O.; van der Heijde, D.; Merola, J.F.; Avila-Zapata, F.; Cieslak, D.; Graham, D.; Wang, C.; Menon, S.; et al. Tofacitinib or Adalimumab versus Placebo for Psoriatic Arthritis. N. Engl. J. Med. 2017, 377, 1537–1550. [Google Scholar] [CrossRef]
- Zhou, S.; Qi, F.; Gong, Y.; Zhang, J.; Zhu, B. Biological Therapies for Atopic Dermatitis: A Systematic Review. Dermatology 2021, 237, 542–552. [Google Scholar] [CrossRef]
- Craiglow, B.G.; King, B.A. Tofacitinib Citrate for the Treatment of Vitiligo: A Pathogenesis-Directed Therapy. JAMA Dermatol. 2015, 151, 1110–1112. [Google Scholar] [CrossRef]
- Kim, S.R.; Heaton, H.; Liu, L.Y.; King, B.A. Rapid Repigmentation of Vitiligo Using Tofacitinib Plus Low-Dose, Narrowband UV-B Phototherapy. JAMA Dermatol. 2018, 154, 370–371. [Google Scholar] [CrossRef]
- Mumford, B.P.; Gibson, A.; Chong, A.H. Repigmentation of vitiligo with oral baricitinib. Australas. J. Dermatol. 2020, 61, 374–376. [Google Scholar] [CrossRef]
- Dong, J.; Huang, X.; Ma, L.P.; Qi, F.; Wang, S.N.; Zhang, Z.Q.; Wei, S.N.; Gao, L.; Liu, F. Baricitinib is Effective in Treating Progressing Vitiligo in vivo and in vitro. Dose Response 2022, 20, 15593258221105370. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, X.; Zhao, K.; Meng, F.; Li, L.; Mu, Z.; Han, X. Efficacy and Safety of Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis. Dermatology 2022, 238, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Seneschal, J.; Guyon, M.; Merhi, R.; Mazereeuw-Hautier, J.; Andreu, N.; Cazenave, S.; Ezzedine, K.; Passeron, T.; Boniface, K. Combination of Baricitinib and Phototherapy in Adults With Active Vitiligo: A Randomized Clinical Trial. JAMA Dermatol. 2025. Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Mu, Y.; Shi, X.; Chen, L. Concurrent vitiligo and atopic dermatitis successfully treated with upadacitinib: A case report. J. Dermatol. Treat. 2023, 34, 2200873. [Google Scholar] [CrossRef]
- Su, X.; Luo, R.; Ruan, S.; Zhong, Q.; Zhuang, Z.; Xiao, Z.; Zhang, P.; Cheng, B.; Gong, T.; Ji, C. Efficacy and tolerability of oral upadacitinib monotherapy in patients with recalcitrant vitiligo. J. Am. Acad. Dermatol. 2023, 89, 1257–1259. [Google Scholar] [CrossRef]
- Ezzedine, K.; Peeva, E.; Yamaguchi, Y.; Cox, L.A.; Banerjee, A.; Han, G.; Hamzavi, I.; Ganesan, A.K.; Picardo, M.; Thaci, D.; et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: A randomized phase 2b clinical trial. J. Am. Acad. Dermatol. 2023, 88, 395–403. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef]
- Dainichi, T.; Iwata, M.; Kaku, Y. Alopecia areata: What’s new in the diagnosis and treatment with JAK inhibitors? J. Dermatol. 2024, 51, 196–209. [Google Scholar] [CrossRef]
- Cheuk, S.; Schlums, H.; Gallais Serezal, I.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef]
- Kirby, J.S.; Okun, M.M.; Alavi, A.; Bechara, F.G.; Zouboulis, C.C.; Brown, K.; Santos, L.L.; Wang, A.; Bibeau, K.B.; Kimball, A.B.; et al. Efficacy and safety of the oral Janus kinase 1 inhibitor povorcitinib (INCB054707) in patients with hidradenitis suppurativa in a phase 2, randomized, double-blind, dose-ranging, placebo-controlled study. J. Am. Acad. Dermatol. 2024, 90, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Hussain, K.; Namiq, K.S.; Firoz, A.; Bouchama, M.; Raza, M.; Haris, M.; Khan, S. Vitiligo: The Association with Metabolic Syndrome and the Role of Simvastatin as an Immunomodulator. Cureus 2021, 13, e14029. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rashighi, M.; Essien, K.I.; Richmond, J.M.; Randall, L.; Pazoki-Toroudi, H.; Hunter, C.A.; Harris, J.E. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J. Investig. Dermatol. 2015, 135, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Gagne, C.; Bergeron, J.; Jobin, J.; Poirier, P. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004, 3, 7. [Google Scholar] [CrossRef]
- Shaker, E.S.E.; Allam, S.H.; Mabrouk, M.M.; Elgharbawy, N.M.; Salaam, S.F.A. Simvastatin and non-segmental vitiligo: A new potential treatment option? Dermatol. Ther. 2022, 35, e15969. [Google Scholar] [CrossRef]
- Zhang, S.; Zdravkovic, T.P.; Wang, T.; Liu, Y.; Jin, H. Efficacy and safety of oral simvastatin in the treatment of patients with vitiligo. J. Investig. Med. 2021, 69, 393–396. [Google Scholar] [CrossRef]
- Vanderweil, S.G.; Amano, S.; Ko, W.C.; Richmond, J.M.; Kelley, M.; Senna, M.M.; Pearson, A.; Chowdary, S.; Hartigan, C.; Barton, B.; et al. A double-blind, placebo-controlled, phase-II clinical trial to evaluate oral simvastatin as a treatment for vitiligo. J. Am. Acad. Dermatol. 2017, 76, 150–151.e3. [Google Scholar] [CrossRef]
- Chang, Y.; Li, S.; Guo, W.; Yang, Y.; Zhang, W.; Zhang, Q.; He, Y.; Yi, X.; Cui, T.; An, Y.; et al. Simvastatin Protects Human Melanocytes from H2O2-Induced Oxidative Stress by Activating Nrf2. J. Investig. Dermatol. 2017, 137, 1286–1296. [Google Scholar] [CrossRef]
- Nguyen, S.; Chuah, S.Y.; Fontas, E.; Khemis, A.; Jhingan, A.; Thng, S.T.G.; Passeron, T. Atorvastatin in Combination with Narrowband UV-B in Adult Patients with Active Vitiligo: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 725–726. [Google Scholar] [CrossRef]
- Niezgoda, A.; Winnicki, A.; Kosmalski, T.; Kowaliszyn, B.; Krysinski, J.; Czajkowski, R. The Evaluation of Vitiligous lesions Repigmentation after the Administration of Atorvastatin calcium salt and Simvastatin-acid sodium salt in patients with active vitiligo (EVRAAS), a pilot study: Study protocol for a randomized controlled trial. Trials 2019, 20, 78. [Google Scholar] [CrossRef]
- Jacquemin, C.; Rambert, J.; Guillet, S.; Thiolat, D.; Boukhedouni, N.; Doutre, M.S.; Darrigade, A.S.; Ezzedine, K.; Blanco, P.; Taieb, A.; et al. Heat shock protein 70 potentiates interferon alpha production by plasmacytoid dendritic cells: Relevance for cutaneous lupus and vitiligo pathogenesis. Br. J. Dermatol. 2017, 177, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Mosenson, J.A.; Zloza, A.; Klarquist, J.; Barfuss, A.J.; Guevara-Patino, J.A.; Poole, I.C. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012, 25, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kahlenberg, J.M. Emerging biologic therapies for systemic lupus erythematosus. Curr. Opin. Rheumatol. 2024, 36, 169–175. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.; Yang, B.; Yan, G.; Dong, X.; Ding, Y.; Fan, W.; Li, L.; Yang, D.; Fang, H.; et al. A randomized, double-blind, placebo-controlled phase II study to evaluate the efficacy and safety of ivarmacitinib (SHR0302) in adult patients with moderate-to-severe alopecia areata. J. Am. Acad. Dermatol. 2023, 89, 911–919. [Google Scholar] [CrossRef]
- Cavalie, M.; Ezzedine, K.; Fontas, E.; Montaudie, H.; Castela, E.; Bahadoran, P.; Taieb, A.; Lacour, J.P.; Passeron, T. Maintenance therapy of adult vitiligo with 0.1% tacrolimus ointment: A randomized, double blind, placebo-controlled study. J. Investig. Dermatol. 2015, 135, 970–974. [Google Scholar] [CrossRef]
- Dwivedi, M.; Kemp, E.H.; Laddha, N.C.; Mansuri, M.S.; Weetman, A.P.; Begum, R. Regulatory T cells in vitiligo: Implications for pathogenesis and therapeutics. Autoimmun. Rev. 2015, 14, 49–56. [Google Scholar] [CrossRef]
- Tahvildari, M.; Dana, R. Low-Dose IL-2 Therapy in Transplantation, Autoimmunity, and Inflammatory Diseases. J. Immunol. 2019, 203, 2749–2755. [Google Scholar] [CrossRef]
- Lykhopiy, V.; Malviya, V.; Humblet-Baron, S.; Schlenner, S.M. IL-2 immunotherapy for targeting regulatory T cells in autoimmunity. Genes Immun. 2023, 24, 248–262. [Google Scholar] [CrossRef]
- Tokura, Y.; Phadungsaksawasdi, P.; Kurihara, K.; Fujiyama, T.; Honda, T. Pathophysiology of Skin Resident Memory T Cells. Front. Immunol. 2020, 11, 618897. [Google Scholar] [CrossRef]
- Atwa, M.A.; Ali, S.M.M.; Youssef, N.; Mahmoud Marie, R.E. Elevated serum level of interleukin-15 in vitiligo patients and its correlation with disease severity but not activity. J. Cosmet. Dermatol. 2021, 20, 2640–2644. [Google Scholar] [CrossRef]
- Cellier, C.; Bouma, G.; van Gils, T.; Khater, S.; Malamut, G.; Crespo, L.; Collin, P.; Green, P.H.R.; Crowe, S.E.; Tsuji, W.; et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: A phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol. 2019, 4, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; van Geel, N. Targeting CTLA-4, PD-L1 and IDO to modulate immune responses in vitiligo. Exp. Dermatol. 2017, 26, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, L.; Singh, J.A. Abatacept for rheumatoid arthritis. Cochrane Database Syst Rev. 2009, 2009, CD007277. [Google Scholar] [CrossRef]
- Dwivedi, M.; Laddha, N.C.; Arora, P.; Marfatia, Y.S.; Begum, R. Decreased regulatory T-cells and CD4+/CD8+ ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res. 2013, 26, 586–591. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Raffa, S.; Delle Macchie, M.; Pacifico, A.; Iacovelli, P.; Torrisi, M.R.; Picardo, M. A protective role for autophagy in vitiligo. Cell Death Dis. 2021, 12, 318. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Nie, H.Q.; Wang, P.; Zhang, X.Y.; Ding, C.; Liu, J.; Xu, A.E. Relationship between autophagy of melanocytes in patients with vitiligo and clinical types. Zhonghua Yi Xue Za Zhi 2016, 96, 2064–2069. [Google Scholar] [CrossRef]
- Lai, Z.W.; Kelly, R.; Winans, T.; Marchena, I.; Shadakshari, A.; Yu, J.; Dawood, M.; Garcia, R.; Tily, H.; Francis, L.; et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: A single-arm, open-label, phase 1/2 trial. Lancet 2018, 391, 1186–1196. [Google Scholar] [CrossRef]
- Chatterjee, S.; Eby, J.M.; Al-Khami, A.A.; Soloshchenko, M.; Kang, H.K.; Kaur, N.; Naga, O.S.; Murali, A.; Nishimura, M.I.; Caroline Le Poole, I.; et al. A quantitative increase in regulatory T cells controls development of vitiligo. J. Investig. Dermatol. 2014, 134, 1285–1294. [Google Scholar] [CrossRef]
- Ursini, F.; Russo, E.; Pellino, G.; D’Angelo, S.; Chiaravalloti, A.; De Sarro, G.; Manfredini, R.; De Giorgio, R. Metformin and Autoimmunity: A “New Deal” of an Old Drug. Front. Immunol. 2018, 9, 1236. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Choi, S.C.; Xu, Z.; Perry, D.J.; Seay, H.; Croker, B.P.; Sobel, E.S.; Brusko, T.M.; Morel, L. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 2015, 7, 274ra18. [Google Scholar] [CrossRef] [PubMed]
- Langendonk, J.G.; Balwani, M.; Anderson, K.E.; Bonkovsky, H.L.; Anstey, A.V.; Bissell, D.M.; Bloomer, J.; Edwards, C.; Neumann, N.J.; Parker, C.; et al. Afamelanotide for Erythropoietic Protoporphyria. N. Engl. J. Med. 2015, 373, 48–59. [Google Scholar] [CrossRef]
- Minder, E.I.; Barman-Aksoezen, J.; Schneider-Yin, X. Pharmacokinetics and Pharmacodynamics of Afamelanotide and its Clinical Use in Treating Dermatologic Disorders. Clin. Pharmacokinet. 2017, 56, 815–823. [Google Scholar] [CrossRef]
- Fabrikant, J.; Touloei, K.; Brown, S.M. A review and update on melanocyte stimulating hormone therapy: Afamelanotide. J. Drugs Dermatol. 2013, 12, 775–779. [Google Scholar]
- Grimes, P.E.; Hamzavi, I.; Lebwohl, M.; Ortonne, J.P.; Lim, H.W. The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatol. 2013, 149, 68–73. [Google Scholar] [CrossRef]
- Lim, H.W.; Grimes, P.E.; Agbai, O.; Hamzavi, I.; Henderson, M.; Haddican, M.; Linkner, R.V.; Lebwohl, M. Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: A randomized multicenter trial. JAMA Dermatol. 2015, 151, 42–50. [Google Scholar] [CrossRef]
- Toh, J.J.H.; Chuah, S.Y.; Jhingan, A.; Chong, W.S.; Thng, S.T.G. Afamelanotide implants and narrow-band ultraviolet B phototherapy for the treatment of nonsegmental vitiligo in Asians. J. Am. Acad. Dermatol. 2020, 82, 1517–1519. [Google Scholar] [CrossRef]
- Lim, H.W.; Grimes, P.E.; Lebwohl, M. Indications and limitations of afamelanotide for treating vitiligo-reply. JAMA Dermatol. 2015, 151, 350. [Google Scholar] [CrossRef]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Huff, S.B.; Gottwald, L.D. Repigmentation of Tenacious Vitiligo on Apremilast. Case Rep. Dermatol. Med. 2017, 2017, 2386234. [Google Scholar] [CrossRef]
- Majid, I.; Imran, S.; Batool, S. Apremilast is effective in controlling the progression of adult vitiligo: A case series. Dermatol. Ther. 2019, 32, e12923. [Google Scholar] [CrossRef] [PubMed]
- Khemis, A.; Fontas, E.; Moulin, S.; Montaudie, H.; Lacour, J.P.; Passeron, T. Apremilast in Combination with Narrowband UVB in the Treatment of Vitiligo: A 52-Week Monocentric Prospective Randomized Placebo-Controlled Study. J. Investig. Dermatol. 2020, 140, 1533–1537.e2. [Google Scholar] [CrossRef]
- Kim, H.J.; Singer, G.K.; Del Duca, E.; Abittan, B.J.; Chima, M.A.; Kimmel, G.; Bares, J.; Gagliotti, M.; Genece, J.; Chu, J.; et al. Combination of apremilast and narrowband ultraviolet B light in the treatment of generalized vitiligo in skin phototypes IV to VI: A randomized split-body pilot study. J. Am. Acad. Dermatol. 2021, 85, 1657–1660. [Google Scholar] [CrossRef]
- Sharma, S.; Bhardwaj, A.; Dwivedi, P.; Yadav, S.S.; Shamim, M.A.; Singh, S.; Sharma, P.P.; Ambwani, S.; SIngh, K. Apremilast Add-On Benefits Over Conventional Drugs (ABCD) in Unstable Non-segmental Vitiligo: A 12-Week Single-Center Randomized Controlled Trial. Cureus 2023, 15, e37180. [Google Scholar] [CrossRef]
- Tam, I.; Kahn, J.S.; Rosmarin, D. Repigmentation in a patient with vitiligo on crisaborole 2% ointment. JAAD Case Rep. 2021, 11, 99–101. [Google Scholar] [CrossRef]
- Sun, X.; Sheng, A.; Xu, A.E. Successful treatment of vitiligo with crisaborole ointment: A report of two cases. Br. J. Dermatol. 2023, 188, 436–437. [Google Scholar] [CrossRef]
- Marasca, C.; Fabbrocini, G.; D’Andrea, M.; Luciano, M.A.; De Maio, G.; Ruggiero, A. Low dose oral corticosteroids, microneedling, and topical 5-fluorouracil: A novel treatment for recalcitrant pediatric vitiligo. Pediatr. Dermatol. 2021, 38, 322–323. [Google Scholar] [CrossRef]
- Kumar, A.; Bharti, R.; Agarwal, S. Microneedling with Dermaroller 192 needles along with 5-fluorouracil solution in the treatment of stable vitiligo. J. Am. Acad. Dermatol. 2019, 81, e67–e69. [Google Scholar] [CrossRef] [PubMed]
- Shashikiran, A.R.; Gandhi, S.; Murugesh, S.B.; Kusagur, M. Efficacy of topical 5% fluorouracil needling in vitiligo. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Attwa, E.M.; Khashaba, S.A.; Ezzat, N.A. Evaluation of the additional effect of topical 5-fluorouracil to needling in the treatment of localized vitiligo. J. Cosmet. Dermatol. 2020, 19, 1473–1478. [Google Scholar] [CrossRef]
- Abdou, A.G.; Farag, A.G.A.; Rashwan, M.; Shehata, W.A. The clinical and pathological effectiveness of microneedling and topical 5-fluorouracil in vitiligo treatment: An association with matrix metalloproteinase 2 immunohistochemical expression. J. Cosmet. Dermatol. 2022, 21, 2153–2161. [Google Scholar] [CrossRef]
- Zahra, F.T.; Adil, M.; Amin, S.S.; Mohtashim, M.; Bansal, R.; Khan, H.Q. Efficacy of Topical 5% 5-Fluorouracil with Needling versus 5% 5-Fluorouracil Alone in Stable Vitiligo: A Randomized Controlled Study. J. Cutan. Aesthet. Surg. 2020, 13, 197–203. [Google Scholar] [CrossRef]
- Mina, M.; Elgarhy, L.; Al-Saeid, H.; Ibrahim, Z. Comparison between the efficacy of microneedling combined with 5-fluorouracil vs microneedling with tacrolimus in the treatment of vitiligo. J. Cosmet. Dermatol. 2018, 17, 744–751. [Google Scholar] [CrossRef]
- Pazyar, N.; Hatami, M.; Yaghoobi, R.; Parvar, S.Y.; Radmanesh, M.; Hadibarhaghtalab, M. The efficacy of adding topical 5-fluorouracil to micro-needling in the treatment of vitiligo: A randomized controlled trial. J. Cosmet. Dermatol. 2023, 22, 1513–1520. [Google Scholar] [CrossRef]
- Saad, M.A.; Tawfik, K.M.; Abdelaleem, H.L. Efficacy and safety of micro-needling combined with topical 5-fluorouracil and excimer light vs. excimer light alone in treatment of non-segmental vitiligo: A comparative study. J. Cosmet. Dermatol. 2023, 22, 810–821. [Google Scholar] [CrossRef]
- Sethi, S.; Mahajan, B.B.; Gupta, R.R.; Ohri, A. Comparative evaluation of the therapeutic efficacy of dermabrasion, dermabrasion combined with topical 5% 5-fluorouracil cream, and dermabrasion combined with topical placentrex gel in localized stable vitiligo. Int. J. Dermatol. 2007, 46, 875–879. [Google Scholar] [CrossRef]
- Garg, T.; Chander, R.; Jain, A. Combination of microdermabrasion and 5-fluorouracil to induce repigmentation in vitiligo: An observational study. Dermatol. Surg. 2011, 37, 1763–1766. [Google Scholar] [CrossRef]
- Zohdy, H.A.; Hussein, M.S. Intradermal injection of Fluorouracil versus triamcinolone in localized vitiligo treatment. J. Cosmet. Dermatol. 2019, 18, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Samad, Z.; Shaaban, D. Treatment of localized non-segmental vitiligo with intradermal 5-flurouracil injection combined with narrow-band ultraviolet B: A preliminary study. J. Dermatol. Treat. 2012, 23, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.; Fawzy, M.M.; Alakad, R. Trichloroacetic Acid in Different Concentrations: A Promising Treatment Modality for Vitiligo. Dermatol. Surg. 2021, 47, e53–e57. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Kanazawa, N.; Li, H.J.; Yonei, N.; Yamamoto, Y.; Furukawa, F. Influence of trichloroacetic acid peeling on the skin stress response system. J. Dermatol. 2011, 38, 740–747. [Google Scholar] [CrossRef]
- Khater, M.; Nasr, M.; Salah, S.; Khattab, F.M. Clinical evaluation of the efficacy of trichloroacetic acid 70% after microneedling vs intradermal injection of 5-fluorouracil in the treatment of nonsegmental vitiligo; A prospective comparative study. Dermatol. Ther. 2020, 33, e13532. [Google Scholar] [CrossRef]
- Nofal, A.; Eldeeb, F.; Shalaby, M.; Al-Balat, W. Microneedling combined with pimecrolimus, 5-fluorouracil, and trichloroacetic acid in the treatment of vitiligo: A comparative study. Dermatol. Ther. 2022, 35, e15294. [Google Scholar] [CrossRef]
- Bialczyk, A.; Welniak, A.; Kaminska, B.; Czajkowski, R. Oxidative Stress and Potential Antioxidant Therapies in Vitiligo: A Narrative Review. Mol. Diagn. Ther. 2023, 27, 723–739. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Dermatol. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Kruger, C.; Wurfel, B.A.; Panske, A.; Wood, J.M. From basic research to the bedside: Efficacy of topical treatment with pseudocatalase PC-KUS in 71 children with vitiligo. Int. J. Dermatol. 2008, 47, 743–753. [Google Scholar] [CrossRef]
- Patel, D.C.; Evans, A.V.; Hawk, J.L. Topical pseudocatalase mousse and narrowband UVB phototherapy is not effective for vitiligo: An open, single-centre study. Clin. Exp. Dermatol. 2002, 27, 641–644. [Google Scholar] [CrossRef]
- Bakis-Petsoglou, S.; Le Guay, J.L.; Wittal, R. A randomized, double-blinded, placebo-controlled trial of pseudocatalase cream and narrowband ultraviolet B in the treatment of vitiligo. Br. J. Dermatol. 2009, 161, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Alshiyab, D.M.; Al-Qarqaz, F.A.; Muhaidat, J.M.; Alkhader, Y.S.; Al-Sheyab, R.F.; Jafaar, S.I. Comparison of the efficacy of Tacrolimus 0.1% ointment and Tacrolimus 0.1% plus topical pseudocatalase/superoxide dismutase gel in children with limited vitiligo: A randomized controlled trial. J. Dermatol. Treat. 2022, 33, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Galloway, G.D.; Eke, T.; Broadway, D.C. Periocular cutaneous pigmentary changes associated with bimatoprost use. Arch. Ophthalmol. 2005, 123, 1609–1610. [Google Scholar] [CrossRef]
- Prota, G.; Vincensi, M.R.; Napolitano, A.; Selen, G.; Stjernschantz, J. Latanoprost stimulates eumelanogenesis in iridial melanocytes of cynomolgus monkeys. Pigment Cell Res. 2000, 13, 147–150. [Google Scholar] [CrossRef]
- Nagui, N.A.; El-Tartoushy, S.A.; Rashed, L.A.; Elmasry, M.F. Assessment of prostaglandin F2-alpha (PGF2alpha) in lesional and nonlesional skin of vitiligo patients. Int. J. Dermatol. 2022, 61, 1390–1396. [Google Scholar] [CrossRef]
- Nowroozpoor Dailami, K.; Hosseini, A.; Rahmatpour Rokni, G.; Saeedi, M.; Morteza-Semnani, K.; Sadeghi, Z.; Ghasemzadeh Diva, S.M.; Goldust, M.; Lotti, T.; Vojvodic, A.; et al. Efficacy of topical latanoprost in the treatment of eyelid vitiligo: A randomized, double-blind clinical trial study. Dermatol. Ther. 2020, 33, e13175. [Google Scholar] [CrossRef]
- Neinaa, Y.M.E.; Lotfy, S.S.; Ghaly, N.R.; Doghaim, N.N. A comparative study of combined microneedling and narrowband ultraviolet B phototherapy versus their combination with topical latanoprost in the treatment of vitiligo. Dermatol. Ther. 2021, 34, e14813. [Google Scholar] [CrossRef]
- Silpa-Archa, N.; Likittanasombat, S.; Apinuntham, C.; Pruksaeakanan, C.; Charoenpipatsin, N.; Chaiyabutr, C.; Wongpraparut, C. The efficacy of bimatoprost ophthalmic solution combined with NB-UVB phototherapy in non-segmental and segmental vitiligo: A single-blind randomized controlled study. Sci. Rep. 2023, 13, 6438. [Google Scholar] [CrossRef]
- Kanokrungsee, S.; Pruettivorawongse, D.; Rajatanavin, N. Clinical outcomes of topical bimatoprost for nonsegmental facial vitiligo: A preliminary study. J. Cosmet. Dermatol. 2021, 20, 812–818. [Google Scholar] [CrossRef]
- Neinaa, Y.M.E.; Mahmoud, M.A.E.; El Maghraby, G.M.; Ibrahim, Z.A.E. Efficacy of prostaglandin E2 versus prostaglandin F2 alpha assisted with narrowband-UVB in stable vitiligo. Arch. Dermatol. Res. 2023, 315, 2647–2653. [Google Scholar] [CrossRef]
- Azzolino, V.; Zapata, L., Jr.; Garg, M.; Gjoni, M.; Riding, R.L.; Strassner, J.P.; Richmond, J.M.; Harris, J.E. Jak Inhibitors Reverse Vitiligo in Mice but Do Not Deplete Skin Resident Memory T Cells. J. Investig. Dermatol. 2021, 141, 182–184.e1. [Google Scholar] [CrossRef]
- Zubair, R.; Hamzavi, I.H. Phototherapy for Vitiligo. Dermatol. Clin. 2020, 38, 55–62. [Google Scholar] [CrossRef]

| Drug | NCT/Country | Study Design | Outcome | Side Effects |
|---|---|---|---|---|
| Ruxolitinib JAK1/2 inhibitor | NCT04896385 The USA and Canada Trial phase: 2 | Subjects: 60. Treatment period: 24 weeks. Randomized, double-blind. Group1: 1.5% ruxolitinib cream twice a day, Group2: vehicle. | Completed; no results published. | Not available. |
| NCT02809976 The USA Trial phase: 2 | Subjects: 11. Treatment period: 20 weeks. Single group, open-label. Group 1: ruxolitinib 1.5% phosphate cream twice daily. | Completed; four patients presented significant facial improvement, 23% of patients decreased VASI. | Only mild side effects. | |
| NCT03099304 The United Kingdom Trial phase: 2 | Subjects: 157. Treatment period: 24 weeks. Randomized, double-blind. Group 1: ruxolitinib cream 1.5% twice daily, Group 2: ruxolitinib cream 1.5% once daily, Group 3: ruxolitinib cream 0.5% once daily, Group 4: ruxolitinib 0.15% once daily, Group 5: vehicle. | Completed; more patients in cream 1.5% twice daily, 1.5% once daily, 0.5% once-daily groups achieved F-VASI50 than the control groups. Extended treatment to 104 weeks: 54.9% (39/71) achieved F-VASI75, 50.0% (53/106) achieved T-VASI50. | All treatment-related adverse events were mild or moderate in severity. | |
| NCT05247489 The USA Trial phase: 2 | Subjects: 55. Treatment period: 48 weeks. Randomized, open-label. Group 1: 1.5% ruxolitinib cream + narrow-band ultraviolet B phototherapy (NB-UVB), Group 2: 1.5% ruxolitinib cream monotherapy. | Completed; no results published. | Not available. | |
| NCT05750823 The USA Trial phase: 2 | Subjects: 49. Treatment period: 48 weeks. Single group, open-label. Group 1: non-segmental vitiligo, apply ruxolitinib 1.5% cream twice a day to all depigmented areas. | Active, not recruiting; no results published. | Not available. | |
| Tofacitinib JAK1/3 inhibitor | NCT: not available ([19]) The USA Trial phase: 2 | Subjects: 16. Treatment period: mean time of 153 days (63–367). Single group, open-label. Group 1: Topical use of 2% tofacitinib twice daily. | Thirteen experienced repigmentation with four patients experiencing > 90% repigmentation, five patients experiencing 25–75% repigmentation, and four patients experiencing 5–15% repigmentation. | Only mild side effects. |
| NCT05293119 The USA Trial phase: 1 | Subjects: 80. Treatment period: 12 weeks. Randomized, open-label. Group 1: will receive 5mg Tofacitinib (oral), Group 2: will receive topical 0.1%Mometasone furoate. | Not yet recruiting | Not available. | |
| Baricitinib JAK1/2 inhibitor | NCT04822584 France Trial phase: 2 | Subjects: 49. Treatment period: 48 weeks. Randomized, open-label. Group 1: baricitinib 4 mg/day + narrowband UVB TL01 arm, Group 2: placebo. | Completed. The mean change in total VASI at week 36 was −44.8% (95% CI, −58.4% to −31.3%) for the baricitinib group and −9.2% (95% CI, −27.7% to 24.7%) for the placebo group. | One participant (5%) experienced back pain and one (5%) experienced a pulmonary embolism in the baricitinib group. |
| Upadacitinib JAK 1 inhibitor | NCT06118411 The USA Trial phase: 3 | Subjects: 614. In Studies 1 and 2: Period A, participants take daily upadacitinib or placebo tablets for 48 weeks. Period B, 15 mg upadacitinib tablets daily for 112 weeks. In Study 3, participants get upadacitinib alone or with NB-UBV phototherapy for ≥24 weeks, then upadacitinib only. | Active, not recruiting. | Not available. |
| NCT04927975 The USA Trial phase: 2 | Subjects: 185. Treatment period: 24 weeks. Participants were randomized to 6-, 11-, or 22-mg/day upadacitinib or placebo. | Completed. Treatment with a 22 mg dose of upadacitinib led to a greater proportion of patients reporting less conspicuous vitiligo lesions compared to placebo (11.6% vs. 0%; p < 0.05). | Not available. | |
| Ritlecitinib JAK 3 and TEC inhibitor | NCT05583526 The USA Trial phase: 3 | Subjects: 581. Treatment period: 52 weeks. Randomized, double-blind. Group 1: ritilecitinib 50 mg QD arm, Group 2: placebo arm. Out of approximately 200 participants, | Active, not recruiting. | Not available. |
| NCT03715829 The USA Trial phase: 2 | 187 patients subsequently received ritlecitinib at 200/50 mg daily in a 24-week extension period. Phase 2b, randomized, double-blind, placebo-controlled, parallel-group, multicenter, and dose-ranging study of 364 patients with face and body vitiligo treated for a 24-week dose-ranging period and 24-week extension period. | Completed. Significant differences from placebo in percent change from baseline in Facial-Vitiligo Area Scoring Index were observed for the 50 mg ritlecitinib groups with (−21.2 vs. 2.1; p < 0.001) or without (−18.5 vs. 2.1; p < 0.001) a loading dose and 30 mg ritlecitinib group (−14.6 vs. 2.1; p = 0.01). Accelerated improvement was observed after treatment with 200/50 mg ritlecitinib in the extension period (n = 187). | Only mild side effects. | |
| Povorcitinib JAK1 inhibitor | NCT04818346 The USA Trial phase: 2 | Subjects: 171. Treatment period: 24-week placebo-controlled double-blind treatment period, followed by a 28-week double-blind extension period; 171 patients were randomized to receive 15, 45, or 75 mg povorcitinib or placebo for 24 weeks, followed by extension 28 weeks. | Completed; no results published. | Not available. |
| NCT06113471 The USA Trial phase: 3 | Subjects: 444. Treatment period: 52 weeks. Randomized, double-blind. Povorcitinib or placebo (dose information not available). | Recruiting. | Not available. | |
| NCT06113445 The USA Trial phase: 3 | Subjects: 444. Treatment period: 52 weeks. Randomized, double-blind. Povorcitinib or placebo (dose information not available). | Recruiting. | Not available. | |
| Cerdulatinib SYK and JAK inhibitor (without JAK2) | NCT04103060 The USA Trial phase: 2a | Subjects: 33. Treatment period: 6 weeks. Randomized, double-blind. Group 1: Cerdulatinib 0.37% gel applied topically twice daily. Group 2: Vehicle gel applied topically twice daily. | Completed; no results published. | Not available. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Dong, P.; Zhang, G.; Hu, J.; Yang, S. Emerging Therapeutic Innovations for Vitiligo Treatment. Curr. Issues Mol. Biol. 2025, 47, 191. https://doi.org/10.3390/cimb47030191
Li W, Dong P, Zhang G, Hu J, Yang S. Emerging Therapeutic Innovations for Vitiligo Treatment. Current Issues in Molecular Biology. 2025; 47(3):191. https://doi.org/10.3390/cimb47030191
Chicago/Turabian StyleLi, Weiran, Penghao Dong, Guiyuan Zhang, Junjie Hu, and Sen Yang. 2025. "Emerging Therapeutic Innovations for Vitiligo Treatment" Current Issues in Molecular Biology 47, no. 3: 191. https://doi.org/10.3390/cimb47030191
APA StyleLi, W., Dong, P., Zhang, G., Hu, J., & Yang, S. (2025). Emerging Therapeutic Innovations for Vitiligo Treatment. Current Issues in Molecular Biology, 47(3), 191. https://doi.org/10.3390/cimb47030191




