Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential
Abstract
1. Introduction
2. Biosynthesis and Bioavailability of Pinosylvin
2.1. Synthesis of Pinosylvin in Plants
2.2. Pharmacokinetics of Pinosylvin
2.3. Methods of In Vitro Pinosylvin Synthesis
| Pinosylvin Producer | Method | Result | Year | References |
|---|---|---|---|---|
| Cells of P. resinosa | Stimulation of plant cells in response to desiccation | Production of pinosylvin and pinosylvin monomethyl ether | 1961 | [97] |
| Cells of P. resinosa | Stimulation of plant cells in response to desiccation | Production of pinosylvin and pinosylvin monomethyl ether | 1969 | [96] |
| Pinus sylvestris L. cells | Treatment of cells with an elicitor preparation from the pine needle pathogen L. seditiosum | Production of pinosylvin and pinosylvin-3-O-methyl ether | 1994 | [100] |
| Metabolically engineered E. coli | Construction of a pathway for stilbene biosynthesis inside E. coli cells | Production of stilbene polyketides | 2006 | [102] |
| Metabolically engineered E. coli | Construction and subsequent modification of a pathway for stilbene biosynthesis inside E. coli cells | Production of stilbene methyl ethers | 2007 | [103] |
| Metabolically engineered E. coli | Construction of a pathway for stilbene biosynthesis inside E. coli cells | Production of pinosylvin | 2015 | [104] |
| Metabolically engineered E. coli | Establishment of a variety of biosynthetic pathways in E. coli cells using enzymes from different sources | Production of various phenylpropanoid derivatives | 2015 | [105] |
| Metabolically engineered E. coli | Development of three different bioengineering strategies | Production of pinosylvin | 2016 | [106] |
| Metabolically engineered C. glutamicium | Construction of a pathway for stilbene biosynthesis inside C. glutamicium cells | Production of pinosylvin and other associated compounds | 2016 | [110] |
| Metabolically engineered E. coli | Construction and subsequent modification of a pathway for stilbene biosynthesis inside E. coli cells | Production of pinosylvin | 2018 | [107] |
| Metabolically engineered E. coli | Reduction of specific gene expression in order to increase pinosylvin production in already-modified E. coli | Production of pinosylvin (increased compared to the originally modified strain) | 2018 | [108] |
| Callus cells of P. strobus L. | Aging of callus cells in a specially modified culture medium | Production of pinosylvin stilbenes | 2022 | [99] |
| Metabolically engineered P. taiwanensis | Construction of a pathway for polyketide biosynthesis inside P. taiwanensis cells | Production of polyketides | 2023 | [109] |
3. Pinosylvin as an Antibacterial Agent
3.1. Antibacterial Properties Against Achromobacter xylosoxidans
3.2. Antibacterial Properties Against Arcobacter butzleri
3.3. Antibacterial Properties Against Bacillus spp.
3.3.1. Bacillus cereus
3.3.2. Bacillus coagulans
3.3.3. Bacillus subtilis
3.4. Antibacterial Properties Against Burkholderia multivorans
3.5. Antibacterial Properties Against Campylobacter spp.
3.6. Antibacterial Properties Against Escherichia coli
3.7. Antibacterial Properties Against Listeria monocytogenes
3.8. Antibacterial Activity Against Proteus vulgaris
3.9. Antibacterial Activity Against Pseudomonas spp.
3.9.1. Pseudomonas aeruginosa
3.9.2. Pseudomonas fluorescens
3.10. Antibacterial Activity Against Salmonella spp.
3.11. Antibacterial Activity Against Staphylococcus spp.
3.11.1. Staphylococcus aureus
3.11.2. Staphylococcus epidermidis
4. Pinosylvin as an Antifungal Agent
4.1. Antifungal Activity Against Aspergillus fumigatus
4.2. Antifungal Activity Against Candida albicans
4.3. Antifungal Activity Against Cladosporium herbarum
4.4. Antifungal Activity Against Plasmopara viticola
4.5. Antifungal Activity Against Penicillium brevirocompactum
4.6. Antifungal Activity Against Saccharomyces cerevisiae
5. Pinosylvin as an Antiparasitic and Antiviral Agent
6. Antioxidant Properties of Pinosylvin
| Compound | Source | Test Type | Mechanism | Concentration | Administration | Year | Reference |
|---|---|---|---|---|---|---|---|
| Pinosylvin | n/a (pure compound) | In vitro—pulse radiolysis experiments | Free radical scavenging in pH values between 2 and 12 | 0.1 mM aqueous solution | n/a | 2002 | [271] |
| Pinosylvin | n/a (laboratory synthesis) | In vivo—rat model | Inhibition of neutrophil infiltration | n/a | oral daily dose of 30 mg/kg b.w. for 28 d | 2010 | [265] |
| Pinosylvin | n/a (pure compound) | In vitro—LPS-stimulated RAW 264.7 cells | (probable) TRIF-mediated signalling, iNOS and mRNA expression inhibition | 39.9 μΜ (IC50) | Pretreatment of cells with pinosylvin before LPS stimulation | 2011 | [266] |
| Pinosylvin | n/a (laboratory synthesis) | In vitro—human neutrophils | (probable) inhibition of protein kinase C | 14.16 ± 1.46 μΜ/L (EC50) | Incubation of cells with pinosylvin | 2012 | [267] |
| In vivo—rat model | Reduction in neutrophilia and oxidants production | n/a | Oral daily dose of 30 mg/kg for 21 d | ||||
| Pinosylvin | n/a (laboratory synthesis) | In vitro—bovine aortic endothelial cells | Mediation of NO production | Various (depending on different experimental protocols) | Incubation of cells with pinosylvin | 2012 | [268] |
| Pinosylvin | n/a (pure compound) | In vivo—rat model | Reduction in pro-oxidative processes | n/a | Oral daily dose of 30 mg/kg b.w. per os for 28 d | 2012 | [269] |
| Pinosylvin | n/a (pure compound) | In vitro—human retinal pigment epithelial (ARPE-19) cells | Promotion of HO-1 expression | Various (depending on different experimental protocols) | Incubation of cells with pinosylvin | 2014 | [282] |
| Pinosylvin | n/a (laboratory synthesis) | In vivo—rat model | Promotion of hepatic and pulmonary NF-κB activation, increase in lung lipo-oxygenase and promotion of plasma antioxidant status | n/a | Oral daily dose of 50 mg/kg b.w. twice a week for 28 d | 2015 | [270] |
| Pinosylvin monomethyl ether, pinosylvin, pinosylvin dimethyl ether | P. merkusii | In vitro—free radical scavenging experiments | Uptake of reactive oxygen species | 11.4–25.8 mg/L (EC50 for extract) | n/a | 2015 | [69] |
| Pinosylvin | n/a (pure compound) | In vitro—ORAC-FL, ABTS and FRAP assays | Free radical scavenging | Various (depending on the assay) | n/a | 2017 | [272] |
| Pinosylvin | n/a (laboratory synthesis) | In vitro—mouse model | Activation of the Nrf2-ARE pathway | n/a | Intragastric daily administration of 100 mg/kg b.w for 2 w | 2020 | [291] |
| Pinosylvin, pinosylvin monomethyl ether | P. caribaea | In vitro—antioxidant assays using DPPH and ABTS methods | Free radical scavenging (electron donation/cation scavenging) | 17.25 ± 0.78 μg/mL (IC50 for extract) | n/a | 2023 | [63] |
7. Anti-Inflammatory and Anti-Allergic Properties of Pinosylvin
| Compound | Plant | Tested on | Mechanism | Concentration | Administration | Year | Reference |
|---|---|---|---|---|---|---|---|
| Pinosylvin (and other derivatives) | n/a (laboratory synthesis) | In vitro—LPS-stimulated murine RAW 264.7 cells | Inhibition of COX-2-induced PGE production | 10.6 μΜ (IC50) | Pretreatment of cells with pinosylvin before LPS stimulation | 2004 | [297] |
| Pinosylvin, dihydropinosylvin | n/a (laboratory synthesis) and S. tuberosa (dihydrop.) | In vitro—activated human neutrophils | Inhibition of leukotriene biosynthesis | 50 μΜ (IC50) | Incubation of cells with test compounds | 2005 | [81] |
| Pinosylvin | n/a (pure compound) | In vitro—human THP-1 monocytes | Inhibition of LPS-induced NF-κB activation | Various | Incubation of cells with pinosylvin | 2006 | [306] |
| Pinosylvin | n/a (pure compound) | In vitro—LPS-stimulated murine RAW 264.7 cells | (probable) TRIF-mediated signalling, iNOS and mRNA expression inhibition | 39.9 μΜ (IC50) | Pretreatment of cells with pinosylvin before LPS stimulation | 2011 | [266] |
| Pinosylvin, monomethylpinosylvin | P. sylvestris | In vitro—murine J774 macrophages | Decreased iNOS expression and NO production, decreased NF-κB transcription | 13–15 μΜ, 8–12 μΜ (ΕC50) | Addition in fresh culture medium post-cell growth (for 72 h) | 2015 | [76] |
| In vivo—male C57BL/6 mice | Reduction of paw oedema | 100 mg/kg | Administered via intraperitoneal injection once | ||||
| Pinosylvin | H. dulcis Thunb | In vitro—RBL-2H3 basophilic leukaemia cell line | Inhibition of released and/or expressions of inflammatory mediators | 5–20 μg/mL | Treatment of cells with pinosylvin for 1 h | 2015 | [54] |
| Pinosylvin | n/a (pure compound) | In vitro—HEK293 (human embryonic kidney) cells | Inhibition of TRPA1 activation | 0.1–100 μΜ (IC50 = 16.7–26.5 μΜ) | Pre-incubation of cells with pinosylvin | 2016 | [315] |
| In vivo—male C57BL/6N mice | Reduction of IL-6 in inflamed tissue | 10 mg/kg | Intraperitoneal injection (pinosylvin dissolved in 250 μL of phosphate buffered saline solution) | ||||
| Pinosylvin monomethylether | C. cajan | In vitro—LPS-stimulated murine RAW 264.7 cells | Activation of PPARγ and inhibition of IL-6 activation | Various IC50 values | Incubation of cells with solution containing the target compound | 2016 | [53] |
| Pinosylvin | n/a (pure compound) | In vitro—mouse 3T3-L1 preadipocyte fibroblasts | Downregulation of PPARγ and C/EBPa | 116.8 ± 7.5 μΜ (ΕC50) | Incubation of cells with pinosylvin | 2017 | [331] |
| Pinosylvin, monomethylpinosylvin | n/a (laboratory synthesis) | In vitro—murine J774 macrophages | Inhibition of PI3K/Akt activation and of IL-6, NO, and MCP-1 expression | Various 1 | Incubation of cells with pinosylvin | 2018 | [312] |
| In vivo—male C57BL/6 mice | Reduction in carrageenan-induced paw oedema via inhibition of IL-6 and MCP-1 | 30 mg/kg | Intraperitoneal injection 1 h prior to inflammation induction | ||||
| Pinosylvin | n/a (pure compound) | In vitro—human THP-1 monocytes and human U937 cells | Promotion of leucocyte apoptosis via upregulation of ALOX15 expression | Various 1 | Treatment of cells with pinosylvin | 2018 | [320] |
| (Z)-pinosylvin mono methyl ether, (Z)-pinosylvin-3-O-b-D-glucoside | A. flexuosa | In vitro—U937 human monocytes | Inhibition of histamine release | Various (less than the IC50 of ciprofloxacin) 1 | Incubation of cells with target compounds | 2020 | [56] |
| Pinosylvin, monomethyl pinosylvin | n/a (pure compound) | In vitro—murine J774 macrophages | Downregulation of classical M1 macrophage activation and upregulation of alternative M2 activation | 10, 30, 60 μΜ | Addition of target compounds in fresh culture medium after differentiation of monocytes to macrophages | 2021 | [319] |
| Pinosylvin, pinosylvin monomethylether | P. abies, P. sylvestris | In vivo—Drosophila melanogaster | 1 (TrpA1)-dependent antagonism of NF-kB-mediated intestinal immune responses | 100 μΜ or 500 μΜ | 24 h feeding of larvae of indicated concentrations mixed with fly food | 2023 | [57] |
| Pinosylvin | P. nigra laricio var. calabrica | In vitro—LPS-stimulated murine RAW 264.7 cells | Inhibition of TNFα and IL-6 expression, via inhibition of the JAK/STAT pathway | 40 μΜ (IC50 = 10.6 μΜ) | Pretreatment of cells with target compounds | 2023 | [264] |
8. Anti-Cancer Properties of Pinosylvin
9. Neuroprotective Properties of Pinosylvin
10. Traditional Medical Applications of Pinosylvin-Producing Plants
10.1. Traditional Uses in Europe
10.2. Traditional Uses in Africa
10.3. Traditional Uses in Asia
10.4. Traditional Uses in North America
11. Discussion
11.1. Health-Related Properties of Pinosylvin and Future Research Perspectives
11.2. Non-Medical Uses of Pinosylvin and Its Derivatives
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaitin, K.I.; DiMasi, J.A. Pharmaceutical innovation in the 21st century: New drug approvals in the first decade, 2000–2009. Clin. Pharmacol. Ther. 2011, 89, 183–188. [Google Scholar] [CrossRef]
- De la Torre, B.G.; Albericio, F. The pharmaceutical industry in 2023: An analysis of FDA drug approvals from the perspective of molecules. Molecules 2024, 29, 585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Innovative Drug Discovery Research by Pharmaceutical Companies in India and China; ACS Publications: Washington, DC, USA, 2024. [Google Scholar]
- Butt, J.; Barthel, J.; Hosokawa, M.; Moore, R. NSAIDs: A clinical approach to the problems of gastrointestinal side-effects. Aliment. Pharmacol. Ther. 1988, 2, 121–129. [Google Scholar] [CrossRef]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Mackin, P. Cardiac side effects of psychiatric drugs. Hum. Psychopharmacol. Clin. Exp. 2008, 23, S3–S14. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Richardson, W.L.; Hammert, W.C. Adverse effects of common oral antibiotics. J. Hand Surg. 2014, 39, 989–991. [Google Scholar] [CrossRef]
- Rousan, T.A.; Mathew, S.T.; Thadani, U. The risk of cardiovascular side effects with anti-anginal drugs. Expert Opin. Drug Saf. 2016, 15, 1609–1623. [Google Scholar] [CrossRef]
- Bețiu, A.M.; Noveanu, L.; Hâncu, I.M.; Lascu, A.; Petrescu, L.; Maack, C.; Elmér, E.; Muntean, D.M. Mitochondrial effects of common cardiovascular medications: The good, the bad and the mixed. Int. J. Mol. Sci. 2022, 23, 13653. [Google Scholar] [CrossRef]
- McManus, M.C. Mechanisms of bacterial resistance to antimicrobial agents. Am. J. Health-Syst. Pharm. 1997, 54, 1420–1433. [Google Scholar] [CrossRef]
- Tenover, F.C. Development and spread of bacterial resistance to antimicrobial agents: An overview. Clin. Infect. Dis. 2001, 33, S108–S115. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of bacterial resistance to antimicrobial agents. In Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 51–82. [Google Scholar]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial resistance to antimicrobial agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef]
- Gilca, M.; Gaman, L.; Panait, E.; Stoian, I.; Atanasiu, V. Chelidonium majus—An integrative review: Traditional knowledge versus modern findings. Forsch. Komplementmed. 2010, 17, 241–248. [Google Scholar] [CrossRef]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, B.; Verma, P.; Bhalla, M.; Gilca, M. Phyto-pharmacotherapeutics of Cyperus rotundus Linn. (Motha): An overview. IJNPR 2012, 3, 467–476. [Google Scholar]
- Petran, M.; Dragos, D.; Gilca, M. Historical ethnobotanical review of medicinal plants used to treat children diseases in Romania (1860s–1970s). J. Ethnobiol. Ethnomed. 2020, 16, 15. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. AYU (Int. Q. J. Res. Ayurveda) 2012, 33, 10–19. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Konishi, T. Natural Products and Drug Discovery: An Integrated Approach; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Seidel, V. Plant-Derived Chemicals: A Source of Inspiration for New Drugs. Plants 2020, 9, 1562. [Google Scholar] [CrossRef]
- Tesso, H.; König, W.A.; Kubeczka, K.H.; Bartnik, M.; Glowniak, K. Secondary metabolites of Peucedanum tauricum fruits. Phytochemistry 2005, 66, 707–713. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska, M.; Zgrajka, W.; Bartnik, M.; Kocki, T.; Turski, W.A. Distribution, synthesis, and absorption of kynurenic acid in plants. Planta Med. 2011, 77, 858–864. [Google Scholar] [CrossRef]
- Koch, W.; Kukula-Koch, W.; Głowniak, K. Catechin Composition and Antioxidant Activity of Black Teas in Relation to Brewing Time. J. AOAC Int. 2017, 100, 1694–1699. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Grabarska, A.; Łuszczki, J.; Czernicka, L.; Nowosadzka, E.; Gumbarewicz, E.; Jarząb, A.; Audo, G.; Upadhyay, S.; Głowniak, K.; et al. Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposed to individual curcuminoids separated by centrifugal partition chromatography. Phytother. Res. 2018, 32, 933–942. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W.; et al. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2020, 11, 599959. [Google Scholar] [CrossRef]
- Füchtbauer, S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Antibacterial properties of capsaicin and its derivatives and their potential to fight antibiotic resistance—A literature survey. Eur. J. Microbiol. Immunol. 2021, 11, 10–17. [Google Scholar] [CrossRef]
- Periferakis, A.-T.; Periferakis, A.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; Savulescu-Fiedler, I.; Scheau, C.; Caruntu, C. Antimicrobial Properties of Capsaicin: Available Data and Future Research Perspectives. Nutrients 2023, 15, 4097. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Dumitrache, M.D.; Jieanu, A.S.; Scheau, C.; Badarau, I.A.; Popescu, G.D.A.; Caruntu, A.; Costache, D.O.; Costache, R.S.; Constantin, C.; Neagu, M.; et al. Comparative effects of capsaicin in chronic obstructive pulmonary disease and asthma (Review). Exp. Ther. Med. 2021, 22, 917. [Google Scholar] [CrossRef]
- Kumar, R.; Vijayalakshmi, S.; Nadanasabapathi, S. Health benefits of quercetin. Def. Life Sci. J. 2017, 2, 142–151. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Noorafshan, A.; Ashkani-Esfahani, S. A review of therapeutic effects of curcumin. Curr. Pharm. Des. 2013, 19, 2032–2046. [Google Scholar]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Scheau, A.-E.; Savulescu-Fiedler, I.; Caruntu, A.; Badarau, I.A.; Caruntu, C.; Scheau, C. Kaempferol: A Review of Current Evidence of Its Antiviral Potential. Int. J. Mol. Sci. 2023, 24, 16299. [Google Scholar] [CrossRef]
- Castelli, G.; Bruno, F.; Vitale, F.; Roberti, M.; Colomba, C.; Giacomini, E.; Guidotti, L.; Cascio, A.; Tolomeo, M. In vitro antileishmanial activity of trans-stilbene and terphenyl compounds. Exp Parasitol 2016, 166, 1–9. [Google Scholar] [CrossRef]
- Bakrim, S.; Machate, H.; Benali, T.; Sahib, N.; Jaouadi, I.; Omari, N.E.; Aboulaghras, S.; Bangar, S.P.; Lorenzo, J.M.; Zengin, G.; et al. Natural Sources and Pharmacological Properties of Pinosylvin. Plants 2022, 11, 1541. [Google Scholar] [CrossRef]
- Rivière, C.; Pawlus, A.D.; Mérillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Chu, L.L.; Tran, C.T.B.; Pham, D.T.K.; Nguyen, H.T.A.; Nguyen, M.H.; Pham, N.M.; Nguyen, A.T.V.; Phan, D.T.; Do, H.M.; Nguyen, Q.H. Metabolic Engineering of Corynebacterium glutamicum for the Production of Flavonoids and Stilbenoids. Molecules 2024, 29, 2252. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, V.S.; Horn, B.W.; Potter, T.L.; Deyrup, S.T.; Gloer, J.B. Production of Stilbenoids and Phenolic Acids by the Peanut Plant at Early Stages of Growth. J. Agric. Food Chem. 2006, 54, 3505–3511. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of Environmental Factors on Stilbene Biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Erdtman, V.H. Tallvedkärnans extraktivämnen och deras inverkan på uppslutningen enligt sulfitmetoden. Sven. Papperstidning 1939, 42, 344–349. [Google Scholar]
- Shrestha, A.; Pandey, R.P.; Sohng, J.K. Biosynthesis of resveratrol and piceatannol in engineered microbial strains: Achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2959–2972. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef]
- Schöppner, A.; Kindl, H. Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut. J. Biol. Chem. 1984, 259, 6806–6811. [Google Scholar] [CrossRef]
- Mendonça, E.L.S.S.; Xavier, J.A.; Fragoso, M.B.T.; Silva, M.O.; Escodro, P.B.; Oliveira, A.C.M.; Tucci, P.; Saso, L.; Goulart, M.O.F. E-Stilbenes: General Chemical and Biological Aspects, Potential Pharmacological Activity Based on the Nrf2 Pathway. Pharmaceuticals 2024, 17, 232. [Google Scholar] [CrossRef]
- Schuster, R.; Holzer, W.; Doerfler, H.; Weckwerth, W.; Viernstein, H.; Okonogi, S.; Mueller, M. Cajanus cajan-a source of PPARγ activators leading to anti-inflammatory and cytotoxic effects. Food Funct. 2016, 7, 3798–3806. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, M.; Randy, A.; Nho, C.W. Inhibitory effect of the branches of Hovenia dulcis Thunb. and its constituent pinosylvin on the activities of IgE-mediated mast cells and passive cutaneous anaphylaxis in mice. Food Funct. 2015, 6, 1361–1370. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Wang, L.; Chen, S. Pharmacokinetics and Tissue Distribution Study of Pinosylvin in Rats by Ultra-High-Performance Liquid Chromatography Coupled with Linear Trap Quadrupole Orbitrap Mass Spectrometry. Evid. Based Complement. Altern. Med. 2018, 2018, 4181084. [Google Scholar] [CrossRef]
- Labib, R.M.; Malak, L.G.; Youssef, F.S.; Ross, S.A. A new stilbene from Agonis flexuosa leaves and verification of its histamine release inhibitory activity using in silico and in vitro studies. S. Afr. J. Bot. 2020, 135, 384–390. [Google Scholar] [CrossRef]
- Aalto, A.L.; Saadabadi, A.; Lindholm, F.; Kietz, C.; Himmelroos, E.; Marimuthu, P.; Salo-Ahen, O.M.H.; Eklund, P.; Meinander, A. Stilbenoid compounds inhibit NF-κB-mediated inflammatory responses in the Drosophila intestine. Front. Immunol. 2023, 14, 1253805. [Google Scholar] [CrossRef]
- Celimene, C.C.; Micales, J.A.; Ferge, L.; Young, R.A. Efficacy of pinosylvins against white-rot and brown-rot fungi. Holzforschung 1999. [Google Scholar] [CrossRef]
- Rowe, J.W.; Bower, C.L.; Wagner, E.R. Extractives of jack pine bark: Occurrence of cis- and trans-pinosylvin dimethyl ether and ferulic acid esters. Phytochemistry 1969, 8, 235–241. [Google Scholar] [CrossRef]
- Lindberg, L.E.; Willför, S.M.; Holmbom, B.R. Antibacterial effects of knotwood extractives on paper mill bacteria. J. Ind. Microbiol. Biotechnol. 2004, 31, 137–147. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Yildirim, H.; Holmbom, B. Investigations on the wood extractives of pine species from Turkey. Part III. Nonvolatile, nonpolar components in Pinus brutia (Henry). Acta Acad. Abo B 1977, 37, 1–9. [Google Scholar]
- Sinyeue, C.; Maerker, L.; Guentas, L.; Medevielle, V.; Bregier, F.; Chaleix, V.; Sol, V.; Lebouvier, N. Polyphenol Content, Antioxidant, and Antibiotic Activities of Pinus Caribaea Morelet Forestry Coproducts. Nat. Prod. Commun. 2023, 18, 1934578X231211958. [Google Scholar] [CrossRef]
- Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Reunanen, M.H.; Eklund, P.C.; Sjöholm, R.E.; Eckerman, C.S.; Pohjamo, S.P.; Holmbom, B.R. Antioxidant activity of knotwood extractives and phenolic compounds of selected tree species. J. Agric. Food Chem. 2003, 51, 7600–7606. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.T.; Hubbes, M.; Strunz, G.M. Identification of some compounds associated with resistance of Pinus densiflora to Fomes annosus. Eur. J. For. Pathol. 1983, 13, 151–160. [Google Scholar] [CrossRef]
- Kodan, A.; Kuroda, H.; Sakai, F. A stilbene synthase from Japanese red pine (Pinus densiflora): Implications for phytoalexin accumulation and down-regulation of flavonoid biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 3335–3339. [Google Scholar] [CrossRef]
- Benouadah, N.; Pranovich, A.; Aliouche, D.; Hemming, J.; Smeds, A.; Willför, S. Analysis of extractives from Pinus halepensis and Eucalyptus camaldulensis as predominant trees in Algeria. Holzforschung 2018, 72, 97–104. [Google Scholar] [CrossRef]
- El Omari, N.; Ezzahrae Guaouguaou, F.; El Menyiy, N.; Benali, T.; Aanniz, T.; Chamkhi, I.; Balahbib, A.; Taha, D.; Shariati, M.A.; Zengin, G.; et al. Phytochemical and biological activities of Pinus halepensis mill., and their ethnomedicinal use. J. Ethnopharmacol. 2021, 268, 113661. [Google Scholar] [CrossRef]
- Wijayanto, A.; Dumarçay, S.; Gérardin-Charbonnier, C.; Sari, R.K.; Syafii, W.; Gérardin, P. Phenolic and lipophilic extractives in Pinus merkusii Jungh. et de Vries knots and stemwood. Ind. Crops Prod. 2015, 69, 466–471. [Google Scholar] [CrossRef]
- Ioannidis, K.; Melliou, E.; Alizoti, P.; Magiatis, P. Identification of black pine (Pinus nigra Arn.) heartwood as a rich source of bioactive stilbenes by qNMR. J. Sci. Food Agric. 2017, 97, 1708–1716. [Google Scholar] [CrossRef]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogenous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Conde, E.; Fang, W.; Hemming, J.; Willför, S.; Domínguez, H.; Parajó, J.C. Recovery of bioactive compounds from Pinus pinaster wood by consecutive extraction stages. Wood Sci. Technol. 2014, 48, 311–323. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Palos Pinto, A.; Dufour, M.C.; Corio-Costet, M.F.; Mérillon, J.M. Pinus pinaster Knot: A Source of Polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef]
- Gabaston, J.; Leborgne, C.; Waffo-Téguo, P.; Pedrot, E.; Richard, T.; Mérillon, J.-M.; Valls Fonayet, J. Separation and isolation of major polyphenols from maritime pine (Pinus pinaster) knots by two-step centrifugal partition chromatography monitored by LC-MS and NMR spectroscopy. J. Sep. Sci. 2020, 43, 1080–1088. [Google Scholar] [CrossRef]
- Kaushik, P.; Kaushik, D.; Khokra, S.L. Ethnobotany and phytopharmacology of Pinus roxburghii Sargent: A plant review. J. Integr. Med. 2013, 11, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Laavola, M.; Nieminen, R.; Leppänen, T.; Eckerman, C.; Holmbom, B.; Moilanen, E. Pinosylvin and monomethylpinosylvin, constituents of an extract from the knot of Pinus sylvestris, reduce inflammatory gene expression and inflammatory responses in vivo. J. Agric. Food Chem. 2015, 63, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, R.W.; McGraw, G.W.; Barras, S.J. Polyphenols in Ceratocystis minor infected Pinus taeda: Fungal metabolites, phloem and xylem phenols. J. Agric. Food Chem. 1977, 25, 717–722. [Google Scholar] [CrossRef]
- Kostecki, K.; Engelmeier, D.; Pacher, T.; Hofer, O.; Vajrodaya, S.; Greger, H. Dihydrophenanthrenes and other antifungal stilbenoids from Stemona cf. pierrei. Phytochemistry 2004, 65, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Pacher, T.; Seger, C.; Engelmeier, D.; Vajrodaya, S.; Hofer, O.; Greger, H. Antifungal Stilbenoids from Stemona collinsae. J. Nat. Prod. 2002, 65, 820–827. [Google Scholar] [CrossRef]
- Brem, B.; Seger, C.; Pacher, T.; Hofer, O.; Vajrodaya, S.; Greger, H. Feeding deterrence and contact toxicity of Stemona alkaloids-a source of potent natural insecticides. J. Agric. Food Chem. 2002, 50, 6383–6388. [Google Scholar] [CrossRef]
- Adams, M.; Pacher, T.; Greger, H.; Bauer, R. Inhibition of leukotriene biosynthesis by stilbenoids from Stemona species. J. Nat. Prod. 2005, 68, 83–85. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and Metabolism of Piceatannol in Rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Roupe, K.A.; Remsberg, C.M.; Yáñez, J.A.; Davies, N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L.R. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, S.; Wang, M.; Tian, Y.; Zhang, Z.; Song, R. Intestinal metabolism of Polygonum cuspidatum in vitro and in vivo. Biomed. Chromatogr. 2018, 32, e4190. [Google Scholar] [CrossRef]
- Miksits, M.; Maier-Salamon, A.; Aust, S.; Thalhammer, T.; Reznicek, G.; Kunert, O.; Haslinger, E.; Szekeres, T.; Jaeger, W. Sulfation of resveratrol in human liver: Evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica 2005, 35, 1101–1119. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Poljanšek, I.; Oven, P.; Vek, V.; Raitanen, J.-E.; Hemming, J.; Willför, S. Isolation of pure pinosylvins from industrial knotwood residue with non-chlorinated solvents. Holzforschung 2019, 73, 475–484. [Google Scholar] [CrossRef]
- Kim, H.; Rencoret, J.; Elder, T.J.; Del Río, J.C.; Ralph, J. Biomimetic oxidative copolymerization of hydroxystilbenes and monolignols. Sci. Adv. 2023, 9, eade5519. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, Y.; Xu, X.; Shi, W.; Chen, S. [Synthesis of artificial diethylstilbestrol antigen for preparation of polyclonal antibodies]. Zhejiang Da Xue Xue Bao Yi Xue Ban 2013, 42, 25–31. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Lin, M.B.; Hou, Q.; Yao, C.S.; Shi, J.G. Biomimetic synthesis of active isorhapontigenin dimers. J. Asian Nat. Prod. Res. 2014, 16, 511–521. [Google Scholar] [CrossRef]
- Jorgensen, E.; Balsillie, D. Formation of heartwood phenols in callus tissue cultures of red pine (Pinus resinosa). Can. J. Bot. 1969, 47, 1015–1016. [Google Scholar] [CrossRef]
- Jorgensen, E. The formation of pinosylvin and its monomethyl ether in the sapwood of Pinus resinosa Ait. Can. J. Bot. 1961, 39, 1765–1772. [Google Scholar] [CrossRef]
- von Rudloff, E.; Jorgensen, E. The biosynthesis of pinosylvin in the sapwood of Pinus resinosa AIT. Phytochemistry 1963, 2, 297–304. [Google Scholar] [CrossRef]
- Koo, H.B.; Hwang, H.S.; Han, J.Y.; Cheong, E.J.; Kwon, Y.S.; Choi, Y.E. Enhanced production of pinosylvin stilbene with aging of Pinus strobus callus and nematicidal activity of callus extracts against pinewood nematodes. Sci. Rep. 2022, 12, 770. [Google Scholar] [CrossRef]
- Lange, B.M.; Trost, M.; Heller, W.; Langebartels, C.; Sandermann, H. Elicitor-induced formation of free and cell-wall-bound stilbenes in cell-suspension cultures of Scots pine (Pinus sylvestris L.). Planta 1994, 194, 143–148. [Google Scholar] [CrossRef]
- Mahdizade Ari, M.; Dadgar, L.; Elahi, Z.; Ghanavati, R.; Taheri, B. Genetically Engineered Microorganisms and Their Impact on Human Health. Int. J. Clin. Pract. 2024, 2024, 6638269. [Google Scholar] [CrossRef]
- Watts, K.T.; Lee, P.C.; Schmidt-Dannert, C. Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol. 2006, 6, 22. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Funa, N.; Horinouchi, S. Precursor-directed biosynthesis of stilbene methyl ethers in Escherichia coli. Biotechnol. J. 2007, 2, 1286–1293. [Google Scholar] [CrossRef]
- van Summeren-Wesenhagen, P.V.; Marienhagen, J. Metabolic engineering of Escherichia coli for the synthesis of the plant polyphenol pinosylvin. Appl. Environ. Microbiol. 2015, 81, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, S.; Xiao, A.; Rasmussen, M.; Skidmore, C.; Zhan, J. Metabolic engineering of Escherichia coli for the biosynthesis of various phenylpropanoid derivatives. Metab. Eng. 2015, 29, 153–159. [Google Scholar] [CrossRef]
- Liang, J.L.; Guo, L.Q.; Lin, J.F.; He, Z.Q.; Cai, F.J.; Chen, J.F. A novel process for obtaining pinosylvin using combinatorial bioengineering in Escherichia coli. World J. Microbiol. Biotechnol. 2016, 32, 102. [Google Scholar] [CrossRef]
- Xu, J.Y.; Xu, Y.; Chu, X.; Tan, M.; Ye, B.C. Protein Acylation Affects the Artificial Biosynthetic Pathway for Pinosylvin Production in Engineered E. coli. ACS Chem. Biol. 2018, 13, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Salas-Navarrete, C.; Hernández-Chávez, G.; Flores, N.; Martínez, L.M.; Martinez, A.; Bolívar, F.; Barona-Gomez, F.; Gosset, G. Increasing pinosylvin production in Escherichia coli by reducing the expression level of the gene fabI-encoded enoyl-acyl carrier protein reductase. Electron. J. Biotechnol. 2018, 33, 11–16. [Google Scholar] [CrossRef]
- Schwanemann, T.; Otto, M.; Wynands, B.; Marienhagen, J.; Wierckx, N. A Pseudomonas taiwanensis malonyl-CoA platform strain for polyketide synthesis. Metab. Eng. 2023, 77, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kallscheuer, N.; Vogt, M.; Kappelmann, J.; Krumbach, K.; Noack, S.; Bott, M.; Marienhagen, J. Identification of the phd gene cluster responsible for phenylpropanoid utilization in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2016, 100, 1871–1881. [Google Scholar] [CrossRef]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Makuwa, S.C.; Serepa-Dlamini, M.H. The antibacterial activity of crude extracts of secondary metabolites from bacterial endophytes associated with Dicoma anomala. Int. J. Microbiol. 2021, 2021, 8812043. [Google Scholar] [CrossRef]
- Aminah, N.S.; Laili, E.; Rafi, M.; Rochman, A.; Insanu, M.; Tun, K. Secondary metabolite compounds from Sida genus and their bioactivity. Heliyon 2021, 7, e06682. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid.-Based Complement. Altern. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Sharma, A.; George, A.J.; Anvitha, D.; Kumar, P.; Dwivedi, V.P.; Chandra, N.S. Antibacterial activity of medicinal plants against ESKAPE: An update. Heliyon 2021, 7, e06310. [Google Scholar] [CrossRef] [PubMed]
- Dorobăţ, O.-M.; Moisoiu, A.; Tălăpan, D. Bacteria isolated from pleural fluid and their resistance to antimicrobials. Pneumologia 2006, 55, 47–51. [Google Scholar]
- Dorobăţ, O.M.; Moisoiu, A.; Tălăpan, D. Incidence and resistance patterns of pathogens from lower respiratory tract infections (LRTI). Pneumologia 2007, 56, 7–15. [Google Scholar]
- Dorobăţ, O.M.; Bădicuţ, I.; Tălăpan, D.; Tenea, C.; Rafila, A. Antibiotic resistance of Gram-positive cocci isolated in 2008. Bacteriol. Virusol. Parazitol. Epidemiol. (Buchar. Rom. 1990) 2010, 55, 83–92. [Google Scholar]
- Popescu, G.A.; Șerban, R.; Iosif, I.; Codiță, I.; Dorobăț, O.; Tălăpan, D.; Buzea, M.; Szekely, E.; Dorneanu, O.; Bota, K. Antimicrobial resistance of germs isolated from invasive infections—Romania 2012. BMC Infect. Dis. 2013, 13, O16. [Google Scholar] [CrossRef]
- Rafila, A.; Talapan, D.; Dorobăţ, O.M.; Popescu, G.A.; Piţigoi, D.; Florea, D.; Buicu, F.C. Emergence of Carbapenemase-producing Enterobacteriaceae, a Public Health Threat: A Romanian Infectious Disease Hospital Based Study/Emergenţa Enterobacteriaceaelor producătoare de carbapenemaze, o ameninţare pentru sănătatea publică: Un studiu realizat într-un spital romanesc de boli infectioase. Rev. Romana Med. Lab. 2015, 23, 295–301. [Google Scholar]
- Kohler, P.P.; Melano, R.G.; Patel, S.N.; Shafinaz, S.; Faheem, A.; Coleman, B.L.; Green, K.; Armstrong, I.; Almohri, H.; Borgia, S. Emergence of carbapenemase-producing Enterobacteriaceae, south-central Ontario, Canada. Emerg. Infect. Dis. 2018, 24, 1674. [Google Scholar] [CrossRef]
- Tălăpan, D.; Rafila, A. Five-Year Survey of Asymptomatic Colonization with Multidrug-Resistant Organisms in a Romanian Tertiary Care Hospital. Infect. Drug Resist. 2022, 15, 2959–2967. [Google Scholar] [CrossRef]
- Cireșă, A.; Tălăpan, D.; Vasile, C.-C.; Popescu, C.; Popescu, G.-A. Evolution of Antimicrobial Resistance in Klebsiella pneumoniae over 3 Years (2019–2021) in a Tertiary Hospital in Bucharest, Romania. Antibiotics 2024, 13, 431. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Iliopoulos, I.; Kokkaliari, M.; Moforis, L.; Economou-Amilli, A. Microbial Mat Stratification in Travertine Depositions of Greek Hot Springs and Biomineralization Processes. Minerals 2022, 12, 1408. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Iliopoulos, I.; Kokkaliari, M.; Moforis, L.; Economou-Amilli, A. Speleothems and Biomineralization Processes in Hot Spring Environment: The Case of Aedipsos (Edipsos), Euboea (Evia) Island, Greece. J. Mar. Sci. Eng. 2022, 10, 1909. [Google Scholar] [CrossRef]
- Kanellopoulos, C.; Lamprinou, V.; Politi, A.; Voudouris, P.; Economou-Amilli, A. Pioneer species of Cyanobacteria in hot springs and their role to travertine formation: The case of Aedipsos hot springs, Euboea (Evia), Greece. Depos. Rec. 2022, 8, 1079–1092. [Google Scholar] [CrossRef]
- Ackerman, S.; Gonzales, R. The context of antibiotic overuse. Ann. Intern. Med. 2012, 157, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Shallcross, L.J.; Davies, D.S.C. Antibiotic overuse: A key driver of antimicrobial resistance. Br. J. Gen. Pract. 2014, 64, 604–605. [Google Scholar] [CrossRef]
- Denyer Willis, L.; Chandler, C. Quick fix for care, productivity, hygiene and inequality: Reframing the entrenched problem of antibiotic overuse. BMJ Glob. Health 2019, 4, e001590. [Google Scholar] [CrossRef]
- Kitsos, N.; Cassimos, D.; Xinias, I.; Agakidis, C.; Mavroudi, A. Management of antibiotic allergy in children: A practical approach. Allergol. Immunopathol. 2022, 50, 30–38. [Google Scholar] [CrossRef]
- Green, E.A.; Fogarty, K.; Ishmael, F.T. Penicillin Allergy: Mechanisms, Diagnosis, and Management. Prim. Care 2023, 50, 221–235. [Google Scholar] [CrossRef]
- Wrynn, A.F. An overview of penicillin allergies for nurses. Nursing 2023, 53, 27–31. [Google Scholar] [CrossRef]
- Westphal, J.F.; Vetter, D.; Brogard, J.M. Hepatic side-effects of antibiotics. J. Antimicrob. Chemother. 1994, 33, 387–401. [Google Scholar] [CrossRef]
- Thiim, M.; Friedman, L.S. Hepatotoxicity of antibiotics and antifungals. Clin. Liver Dis. 2003, 7, 381–399. [Google Scholar] [CrossRef]
- Polson, J.E. Hepatotoxicity due to antibiotics. Clin. Liver Dis. 2007, 11, 549–561. [Google Scholar] [CrossRef]
- Andrade, R.J.; Tulkens, P.M. Hepatic safety of antibiotics used in primary care. J. Antimicrob. Chemother. 2011, 66, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Kamran, S.H.; Hamid Akash, M.S. Chapter 16—Toxicity of antibiotics. In Antibiotics and Antimicrobial ResistanceGenes in the Environment; Hashmi, M.Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 234–252. [Google Scholar]
- Sousa, V.; Luís, Â.; Oleastro, M.; Domingues, F.; Ferreira, S. Polyphenols as resistance modulators in Arcobacter butzleri. Folia Microbiol. 2019, 64, 547–554. [Google Scholar] [CrossRef]
- Välimaa, A.L.; Honkalampi-Hämäläinen, U.; Pietarinen, S.; Willför, S.; Holmbom, B.; von Wright, A. Antimicrobial and cytotoxic knotwood extracts and related pure compounds and their effects on food-associated microorganisms. Int. J. Food Microbiol. 2007, 115, 235–243. [Google Scholar] [CrossRef]
- Hou, W.; Sun, P.-H.; Geng, Z.-Z.; Xu, H.-G.; Lin, J.; Chen, W.-M. Design, Synthesis and Antibacterial Assay of Pinosylvin Acid Derivatives. J. Pharm. Biomed. Sci. 2016, 6, 369–373. [Google Scholar]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Silva, F.; Nerín, C.; Domingues, F.C. Stilbene phytoallexins inclusion complexes: A natural-based strategy to control foodborne pathogen Campylobacter. Food Control 2015, 54, 66–73. [Google Scholar] [CrossRef]
- Silva, F.; Domingues, F.C.; Nerín, C. Control microbial growth on fresh chicken meat using pinosylvin inclusion complexes based packaging absorbent pads. LWT 2018, 89, 148–154. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, H.J.; Min, H.Y.; Park, E.J.; Lee, K.M.; Ahn, Y.H.; Cho, Y.J.; Pyee, J.H. Antibacterial and antifungal activity of pinosylvin, a constituent of pine. Fitoterapia 2005, 76, 258–260. [Google Scholar] [CrossRef] [PubMed]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.E.; Eklund, P.; Willför, S.; Alakomi, H.L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, W.J.C.; Araya-Cloutier, C.; Bijlsma, J.; de Swart, A.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.-P. Antibacterial prenylated stilbenoids from peanut (Arachis hypogaea). Phytochem. Lett. 2018, 28, 13–18. [Google Scholar] [CrossRef]
- Marion-Sanchez, K.; Olive, C.; Platon, M.G.; Cesarine, M.; Derancourt, C.; Pailla, K. Achromobacter xylosoxidans in hospital environments: Still waters run deep! Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 470–472. [Google Scholar] [CrossRef]
- Bonis, B.M.; Hunter, R.C. JMM Profile: Achromobacter xylosoxidans: The cloak-and-dagger opportunist. J. Med. Microbiol. 2022, 71, 001505. [Google Scholar] [CrossRef]
- Alkan, S. Ecthyma gangrenosum caused by Achromobacter xylosoxidans bacteremia. Rev. Soc. Bras. Med. Trop. 2023, 56, e00712023. [Google Scholar] [CrossRef]
- Jiménez-Guerra, G.; Casanovas MorenoTorres, I.; Moldovan, T.D.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Arcobacter butzleri and intestinal colonization. Rev. Esp. Quim. 2020, 33, 73–75. [Google Scholar] [CrossRef]
- Ruiz de Alegría Puig, C.; Fernández Martínez, M.; Pablo Marcos, D.; Agüero Balbín, J.; Calvo Montes, J. Outbreak of Arcobacter butzleri? An emerging enteropathogen. Enferm. Infecc. Microbiol. Clin. 2023, 41, 169–172. [Google Scholar] [CrossRef]
- Chieffi, D.; Fanelli, F.; Fusco, V. Arcobacter butzleri: Up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2071–2109. [Google Scholar] [CrossRef]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Brown, K.L. Control of bacterial spores. Br. Med. Bull. 2000, 56, 158–171. [Google Scholar] [CrossRef]
- Peláez Bejarano, A.; García de Lomas, J.M.; Franco-Huertas, M.; Martínez-Marcos, F.J.; Jiménez-Hidalgo, A. Successful treatment of postsurgical meningitis caused by Bacillus cereus: A case report and literature review. J. Chemother. 2023, 35, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Drobniewski, F.A. Bacillus cereus and related species. Clin. Microbiol. Rev. 1993, 6, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.S.; Korel, F.; King, J.H. Bacillus cereus: A review of “fried rice syndrome” causative agents. Microb. Pathog. 2023, 185, 106418. [Google Scholar] [CrossRef]
- Mu, Y.; Cong, Y. Bacillus coagulans and its applications in medicine. Benef. Microbes 2019, 10, 679–688. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maity, C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: A prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT Compliant]. Medicine 2021, 100, e23641. [Google Scholar] [CrossRef]
- Kovács, Á.T. Bacillus subtilis. Trends Microbiol. 2019, 27, 724–725. [Google Scholar] [CrossRef]
- Lv, P.; Song, Y.; Liu, C.; Yu, L.; Shang, Y.; Tang, H.; Sun, S.; Wang, F. Application of Bacillus subtilis as a live vaccine vector: A review. J. Vet. Med. Sci. 2020, 82, 1693–1699. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the antibacterial effects of a variety of essential oils on respiratory tract pathogens, using a modified dilution assay method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- Flanagan, J.N.; Kavanaugh, L.; Steck, T.R. Burkholderia multivorans Exhibits Antibiotic Collateral Sensitivity. Microb. Drug Resist. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Dogra, S.; Angrup, A.; Kanaujia, R.; Vig, S.; Kaur, R.; Paul, R.A.; Biswal, M.; Samujh, R.; Ray, P. Burkholderia multivorans Sepsis Outbreak in a Neonatal Surgical Unit of a Tertiary Care Hospital. Indian. J. Pediatr. 2021, 88, 725. [Google Scholar] [CrossRef]
- Courtney, J.M.; Dunbar, K.E.; McDowell, A.; Moore, J.E.; Warke, T.J.; Stevenson, M.; Elborn, J.S. Clinical outcome of Burkholderia cepacia complex infection in cystic fibrosis adults. J. Cyst. Fibros. 2004, 3, 93–98. [Google Scholar] [CrossRef]
- Peralta, D.P.; Chang, A.Y.; Ariza-Hutchinson, A.; Ho, C.A. Burkholderia multivorans: A rare yet emerging cause of bacterial meningitis. IDCases 2018, 11, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeyer, N.; Sharafutdinov, I.; Harrer, A.; Soltan Esmaeili, D.; Linz, B.; Backert, S. Campylobacter Virulence Factors and Molecular Host-Pathogen Interactions. Curr. Top. Microbiol. Immunol. 2021, 431, 169–202. [Google Scholar] [CrossRef] [PubMed]
- Elmi, A.; Nasher, F.; Dorrell, N.; Wren, B.; Gundogdu, O. Revisiting Campylobacter jejuni Virulence and Fitness Factors: Role in Sensing, Adapting, and Competing. Front. Cell Infect. Microbiol. 2020, 10, 607704. [Google Scholar] [CrossRef]
- Gao, F.; Tu, L.; Chen, M.; Chen, H.; Zhang, X.; Zhuang, Y.; Luo, J.; Chen, M. Erythromycin resistance of clinical Campylobacter jejuni and Campylobacter coli in Shanghai, China. Front. Microbiol. 2023, 14, 1145581. [Google Scholar] [CrossRef]
- Barker, C.R.; Painset, A.; Swift, C.; Jenkins, C.; Godbole, G.; Maiden, M.C.J.; Dallman, T.J. Microevolution of Campylobacter jejuni during long-term infection in an immunocompromised host. Sci. Rep. 2020, 10, 10109. [Google Scholar] [CrossRef]
- Mariette, F.; Amrane, S.; Couteau, C.; Lagier, J.C.; Eldin, C. Campylobacter jejuni infection associated with miscarriage, a case report and literature review. J. Reprod. Immunol. 2020, 141, 103153. [Google Scholar] [CrossRef]
- Shang, P.; Zhu, M.; Wang, Y.; Zheng, X.; Wu, X.; Zhu, J.; Feng, J.; Zhang, H.L. Axonal variants of Guillain-Barré syndrome: An update. J. Neurol. 2021, 268, 2402–2419. [Google Scholar] [CrossRef]
- Adrianza, A.; Pourfarrokh, N.; Choi, H.; Hwang, M.; Lukey, J.; Jinadatha, C.; Navarathna, D.H. Campylobacter coli bacteremia associated with diarrhea. IDCases 2023, 31, e01734. [Google Scholar] [CrossRef]
- Gomes, S.A.; Trigo, C.; Pinto, F.F. Campylobacter coli Myocarditis: A case report. Cardiol. Young 2022, 32, 1172–1174. [Google Scholar] [CrossRef]
- Martinson, J.N.V.; Walk, S.T. Escherichia coli Residency in the Gut of Healthy Human Adults. EcoSal Plus 2020, 9, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Cerceo, E.; Deitelzweig, S.B.; Sherman, B.M.; Amin, A.N. Multidrug-Resistant Gram-Negative Bacterial Infections in the Hospital Setting: Overview, Implications for Clinical Practice, and Emerging Treatment Options. Microb. Drug Resist. 2016, 22, 412–431. [Google Scholar] [CrossRef]
- Farber, J.M.; Peterkin, P.I. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef]
- Disson, O.; Moura, A.; Lecuit, M. Making Sense of the Biodiversity and Virulence of Listeria monocytogenes. Trends Microbiol. 2021, 29, 811–822. [Google Scholar] [CrossRef]
- Finn, L.; Onyeaka, H.; O’Neill, S. Listeria monocytogenes Biofilms in Food-Associated Environments: A Persistent Enigma. Foods 2023, 12, 3339. [Google Scholar] [CrossRef]
- Rogalla, D.; Bomar, P.A. Listeria Monocytogenes. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef]
- Matthews, S.J.; Lancaster, J.W. Urinary Tract Infections in the Elderly Population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L. Urinary tract infections in the elderly: A review of disease characteristics and current treatment options. Drugs Context 2020. [Google Scholar] [CrossRef]
- Charkhian, H.; Bodaqlouie, A.; Soleimannezhadbari, E.; Lotfollahi, L.; Shaykh-Baygloo, N.; Hosseinzadeh, R.; Yousefi, N.; Khodayar, M. Comparing the Bacteriostatic Effects of Different Metal Nanoparticles Against Proteus vulgaris. Curr. Microbiol. 2020, 77, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Buhl, M.; Peter, S.; Willmann, M. Prevalence and risk factors associated with colonization and infection of extensively drug-resistant Pseudomonas aeruginosa: A systematic review. Expert. Rev. Anti Infect. Ther. 2015, 13, 1159–1170. [Google Scholar] [CrossRef]
- Paz-Zarza, V.M.; Mangwani-Mordani, S.; Martínez-Maldonado, A.; Álvarez-Hernández, D.; Solano-Gálvez, S.G.; Vázquez-López, R. [Pseudomonas aeruginosa: Pathogenicity and antimicrobial resistance in urinary tract infection]. Rev. Chil. Infectol. 2019, 36, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, J.; Tümmler, B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research 2017, 6, 1261. [Google Scholar] [CrossRef]
- Murphy, T.F. Pseudomonas aeruginosa in adults with chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 2009, 15, 138–142. [Google Scholar] [CrossRef]
- Gonçalves-de-Albuquerque, C.F.; Silva, A.R.; Burth, P.; Rocco, P.R.; Castro-Faria, M.V.; Castro-Faria-Neto, H.C. Possible mechanisms of Pseudomonas aeruginosa-associated lung disease. Int. J. Med. Microbiol. 2016, 306, 20–28. [Google Scholar] [CrossRef]
- Silverio, M.P.; Kraychete, G.B.; Rosado, A.S.; Bonelli, R.R. Pseudomonas fluorescens Complex and Its Intrinsic, Adaptive, and Acquired Antimicrobial Resistance Mechanisms in Pristine and Human-Impacted Sites. Antibiotics 2022, 11, 985. [Google Scholar] [CrossRef]
- Ishii, H.; Kushima, H.; Koide, Y.; Kinoshita, Y. Pseudomonas fluorescens pneumonia. Int. J. Infect. Dis. 2024, 140, 92–94. [Google Scholar] [CrossRef]
- Mitra, S.; Rath, S.; Das, S.; Basu, S. Ocular infection by a psychrophile: Pseudomonas fluorescens. Indian. J. Med. Microbiol. 2019, 37, 289–291. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Holistic Strategies to Control Salmonella Infantis: An Emerging Challenge in the European Broiler Sector. Microorganisms 2023, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.M.; Barrón-Montenegro, R.; Conejeros, J.; Rivera, D.; Undurraga, E.A.; Moreno-Switt, A.I. A review of the global emergence of multidrug-resistant Salmonella enterica subsp. enterica Serovar Infantis. Int. J. Food Microbiol. 2023, 403, 110297. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, F.; Long, J.; Duan, G.; Yang, H. A review on circular RNAs and bacterial infections. Int. J. Biol. Macromol. 2023, 244, 125391. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R.C.; Liu, R.; Burgin, D.J.; Otto, M. Understanding mechanisms of virulence in MRSA: Implications for antivirulence treatment strategies. Expert. Rev. Anti Infect. Ther. 2023, 21, 911–928. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Leong, K.F. Staphylococcal-scalded skin syndrome: Evaluation, diagnosis, and management. World J. Pediatr. 2018, 14, 116–120. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, A.T.; Troumpata, L.; Dragosloveanu, S.; Timofticiuc, I.A.; Georgatos-Garcia, S.; Scheau, A.E.; Periferakis, K.; Caruntu, A.; Badarau, I.A.; et al. Use of Biomaterials in 3D Printing as a Solution to Microbial Infections in Arthroplasty and Osseous Reconstruction. Biomimetics 2024, 9, 154. [Google Scholar] [CrossRef]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef]
- Cristea, S.; Predescu, V.; Dragosloveanu, S.; Cuculici, S.; Marandici, N. Surgical Approaches for Total Knee Arthroplasty. In Arthroplasty—A Comprehensive Review; Bagaria, V., Ed.; IntechOpen: London, UK, 2016; pp. 25–47. [Google Scholar]
- Howden, B.P.; Davies, J.K.; Johnson, P.D.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef]
- Burke, T.L.; Rupp, M.E.; Fey, P.D. Staphylococcus epidermidis. Trends Microbiol. 2023, 31, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Armeftis, C.; Ioannou, A.; Lazarou, T.; Giannopoulos, A.; Dimitriadou, E.; Makrides, K.; Pana, Z.D. Staphylococcus epidermidis induced toxic shock syndrome (TSS) secondary to influenza infection. BMC Infect. Dis. 2023, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis-Skin friend or foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef]
- Zheng, Y.; Hunt, R.L.; Villaruz, A.E.; Fisher, E.L.; Liu, R.; Liu, Q.; Cheung, G.Y.C.; Li, M.; Otto, M. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe 2022, 30, 301–313.e309. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Alastruey-Izquierdo, A.; Gago, S.; Cuenca-Estrella, M.; León, C.; Miro, J.M.; Nuñez Boluda, A.; Ruiz Camps, I.; Sole, A.; Denning, D.W. Burden of serious fungal infections in Spain. Clin. Microbiol. Infect. 2015, 21, 183–189. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.A.; Cortes, J.A.; Denning, D.W. Burden of Fungal Infections in Colombia. J. Fungi 2018, 4, 41. [Google Scholar] [CrossRef]
- Tufa, T.B.; Denning, D.W. The Burden of Fungal Infections in Ethiopia. J. Fungi 2019, 5, 109. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R.; Kontoyiannis, D.P. Resistance to Antifungal Drugs. Infect. Dis. Clin. N. Am. 2021, 35, 279–311. [Google Scholar] [CrossRef]
- Matthaiou, E.I.; Sass, G.; Stevens, D.A.; Hsu, J.L. Iron: An essential nutrient for Aspergillus fumigatus and a fulcrum for pathogenesis. Curr. Opin. Infect. Dis. 2018, 31, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, P.A. Allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 2002, 110, 685–692. [Google Scholar] [CrossRef]
- Amona, F.M.; Oladele, R.O.; Resendiz-Sharpe, A.; Denning, D.W.; Kosmidis, C.; Lagrou, K.; Zhong, H.; Han, L. Triazole resistance in Aspergillus fumigatus isolates in Africa: A systematic review. Med. Mycol. 2022, 60, myac059. [Google Scholar] [CrossRef]
- Hurley, R.; De Louvois, J. Candida vaginitis. Postgrad. Med. J. 1979, 55, 645–647. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef]
- Pedro Abreu, J.; Esteves, J.; Boncoraglio, M.T.; Pereira, F.M.; Costa, C.; Oliveira, C. Cladosporium herbarum Hot-Tub Lung Hypersensitivity Pneumonitis in a Greenhouse Worker. Eur. J. Case Rep. Intern. Med. 2020, 7, 001565. [Google Scholar] [CrossRef]
- Planté-Bordeneuve, T.; Gilbert, O.; Latinne, D.; Bruffaerts, N.; Ghaye, B.; Froidure, A. Familial hypersensitivity pneumonitis triggered by Cladosporium herbarum exposure during carpooling. ERJ Open Res. 2020, 6, 00233-2020. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, M.; Simon-Nobbe, B. The allergens of Cladosporium herbarum and Alternaria alternata. Chem. Immunol. 2002, 81, 48–72. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2023, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Koledenkova, K.; Esmaeel, Q.; Jacquard, C.; Nowak, J.; Clément, C.; Ait Barka, E. Plasmopara viticola the Causal Agent of Downy Mildew of Grapevine: From Its Taxonomy to Disease Management. Front. Microbiol. 2022, 13, 889472. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, Y.; Yang, J.; Jiao, Y.; Li, W.; Yang, F.; Yin, X.; Shang, B.; Liu, R.; Zhang, Y.; et al. Plasmopara viticola RxLR effector PvAvh77 triggers cell death and governs immunity responses in grapevine. J. Exp. Bot. 2023, 74, 2047–2066. [Google Scholar] [CrossRef]
- Gouveia, C.; Santos, R.B.; Paiva-Silva, C.; Buchholz, G.; Malhó, R.; Figueiredo, A. The pathogenicity of Plasmopara viticola: A review of evolutionary dynamics, infection strategies and effector molecules. BMC Plant Biol. 2024, 24, 327. [Google Scholar] [CrossRef]
- Louw, J.P.; Korsten, L. Pathogenic Penicillium spp. on Apple and Pear. Plant Dis. 2014, 98, 590–598. [Google Scholar] [CrossRef]
- Rand, T.G.; Giles, S.; Flemming, J.; Miller, J.D.; Puniani, E. Inflammatory and cytotoxic responses in mouse lungs exposed to purified toxins from building isolated Penicillium brevicompactum Dierckx and P. chrysogenum Thom. Toxicol. Sci. 2005, 87, 213–222. [Google Scholar] [CrossRef]
- Vincent, M.; Percier, P.; De Prins, S.; Huygen, K.; Potemberg, G.; Muraille, E.; Romano, M.; Michel, O.; Denis, O. Investigation of inflammatory and allergic responses to common mold species: Results from in vitro experiments, from a mouse model of asthma, and from a group of asthmatic patients. Indoor Air 2017, 27, 933–945. [Google Scholar] [CrossRef]
- Eldarov, M.A.; Kishkovskaia, S.A.; Tanaschuk, T.N.; Mardanov, A.V. Genomics and Biochemistry of Saccharomyces cerevisiae Wine Yeast Strains. Biochemistry 2016, 81, 1650–1668. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Santos, A.; Van Wyk, N.; Pretorius, I.S. Saccharomyces cerevisiae. Trends Genet. 2019, 35, 956–957. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Inácio, M.L.; Mota, M. The Potential of Esteya spp. for the Biocontrol of the Pinewood Nematode, Bursaphelenchus xylophilus. Microorganisms 2022, 10, 168. [Google Scholar] [CrossRef]
- Koenning, S.R.; Overstreet, C.; Noling, J.W.; Donald, P.A.; Becker, J.O.; Fortnum, B.A. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. J Nematol 1999, 31, 587–618. [Google Scholar]
- Yang, H.; Rao, Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Daia, C.; Scheau, C.; Neagu, G.; Andone, I.; Spanu, A.; Popescu, C.; Stoica, S.I.; Verenca, M.C.; Onose, G. Nerve conduction study and electromyography findings in patients recovering from COVID-19-Case report. Int. J. Infect. Dis. 2021, 103, 420–422. [Google Scholar] [CrossRef]
- Fertig, T.E.; Chitoiu, L.; Terinte-Balcan, G.; Peteu, V.-E.; Marta, D.; Gherghiceanu, M. The atomic portrait of SARS-CoV-2 as captured by cryo-electron microscopy. J. Cell. Mol. Med. 2022, 26, 25–34. [Google Scholar] [CrossRef]
- Lupașcu, R.E.; Ilie, M.I.; Velescu, B.Ș.; Udeanu, D.I.; Sultana, C.; Ruță, S.; Arsene, A.L. COVID-19-Current Therapeutical Approaches and Future Perspectives. Processes 2022, 10, 1053. [Google Scholar] [CrossRef]
- Zamfir, M.A.; Moraru, L.; Dobrea, C.; Scheau, A.E.; Iacob, S.; Moldovan, C.; Scheau, C.; Caruntu, C.; Caruntu, A. Hematologic Malignancies Diagnosed in the Context of the mRNA COVID-19 Vaccination Campaign: A Report of Two Cases. Medicina 2022, 58, 874. [Google Scholar] [CrossRef] [PubMed]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef]
- Santos-López, G.; Cortés-Hernández, P.; Vallejo-Ruiz, V.; Reyes-Leyva, J. SARS-CoV-2: Basic concepts, origin and treatment advances. Gac. Med. Mex. 2021, 157, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Talevi, D.; Socci, V.; Carai, M.; Carnaghi, G.; Faleri, S.; Trebbi, E.; Di Bernardo, A.; Capelli, F.; Pacitti, F. Mental health outcomes of the COVID-19 pandemic. Riv. Di Psichiatr. 2020, 55, 137–144. [Google Scholar]
- Pedrosa, A.L.; Bitencourt, L.; Fróes, A.C.F.; Cazumbá, M.L.B.; Campos, R.G.B.; de Brito, S.B.C.S.; Simões e Silva, A.C. Emotional, behavioral, and psychological impact of the COVID-19 pandemic. Front. Psychol. 2020, 11, 566212. [Google Scholar] [CrossRef]
- Cullen, W.; Gulati, G.; Kelly, B.D. Mental health in the COVID-19 pandemic. QJM Int. J. Med. 2020, 113, 311–312. [Google Scholar] [CrossRef]
- Mihailescu, A.; Graur, A.; Ioniță, I.; Styliadis, V.; Serban, T.; Bubulac, L.; Diaconescu, L.; Popa-Velea, O. Associations between personality characteristics and perceived quality of life in medical students during the COVID-19 pandemic. J. Psychosom. Res. 2022, 157, 110883. [Google Scholar] [CrossRef]
- Papandreou, A.; Mavrogalou, A.; Periferakis, A.-T.; Periferakis, A.; Badarau, I.A.; Popa-Velea, O.; Scheau, C. The Effects of COVID-19 on the Emotional and Social Stability, Motivation and Attitudes of Gifted and Non-Gifted Children in Greece. Children 2023, 10, 706. [Google Scholar] [CrossRef]
- Mihăilescu, A.I.; Popa-Velea, O.; Ciobanu, A.M.; Diaconescu, L.V.; Graur, A.; Ioniţă, I.; Carsote, M. Psychological Factors Associated with General Quality of Life in the Context of COVID-19 Pandemic: A Cross-Sectional Study on a Multicultural Sample of Romanian Medical Students. Healthcare 2024, 12, 1243. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Andrews, H.S.; Herman, J.D.; Gandhi, R.T. Treatments for COVID-19. Annu. Rev. Med. 2024, 75, 145–157. [Google Scholar] [CrossRef]
- Naseem, A.; Rasool, F.; Ahmed, A.; Carter, W.G. The Potential of Stilbene Compounds to Inhibit M(pro) Protease as a Natural Treatment Strategy for Coronavirus Disease-2019. Curr. Issues Mol. Biol. 2022, 45, 12–32. [Google Scholar] [CrossRef]
- Li, Y.Q.; Li, Z.L.; Zhao, W.J.; Wen, R.X.; Meng, Q.W.; Zeng, Y. Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication. Eur. J. Med. Chem. 2006, 41, 1084–1089. [Google Scholar] [CrossRef]
- Chen, M.-K.; Liu, Y.-T.; Lin, J.-T.; Lin, C.-C.; Chuang, Y.-C.; Lo, Y.-S.; Hsi, Y.-T.; Hsieh, M.-J. Pinosylvin reduced migration and invasion of oral cancer carcinoma by regulating matrix metalloproteinase-2 expression and extracellular signal-regulated kinase pathway. Biomed. Pharmacother. 2019, 117, 109160. [Google Scholar] [CrossRef]
- Perri, M.R.; Pellegrino, M.; Marrelli, M.; Aquaro, S.; Cavaliere, F.; Grande, F.; Occhiuzzi, M.A.; Lupia, C.; Toma, C.C.; Conforti, F.; et al. Identification of Pinosylvin in Pinus nigra subsp. laricio: A Naturally Occurring Stilbenoid Suppressing LPS-Induced Expression of Pro-Inflammatory Cytokines and Mediators and Inhibiting the JAK/STAT Signaling Pathway. Pharmaceuticals 2023, 16, 718. [Google Scholar] [CrossRef]
- Macickova, T.; Drabikova, K.; Nosal, R.; Bauerova, K.; Mihalova, D.; Harmatha, J.; Pecivova, J. In vivo effect of pinosylvin and pterostilbene in the animal model of adjuvant arthritis. Neuro Endocrinol. Lett. 2010, 31 (Suppl. 2), 91–95. [Google Scholar]
- Park, E.J.; Min, H.Y.; Chung, H.J.; Ahn, Y.H.; Pyee, J.H.; Lee, S.K. Pinosylvin suppresses LPS-stimulated inducible nitric oxide synthase expression via the MyD88-independent, but TRIF-dependent downregulation of IRF-3 signaling pathway in mouse macrophage cells. Cell Physiol. Biochem. 2011, 27, 353–362. [Google Scholar] [CrossRef]
- Jančinová, V.; Perečko, T.; Nosáľ, R.; Harmatha, J.; Smidrkal, J.; Drábiková, K. The natural stilbenoid pinosylvin and activated neutrophils: Effects on oxidative burst, protein kinase C, apoptosis and efficiency in adjuvant arthritis. Acta Pharmacol. Sin. 2012, 33, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Lee, H.R.; Pyee, J.; Park, H. Pinosylvin induces cell survival, migration and anti-adhesiveness of endothelial cells via nitric oxide production. Phytother. Res. 2012, 27, 610–617. [Google Scholar] [CrossRef]
- Drafi, F.; Bauerova, K.; Kuncirova, V.; Ponist, S.; Mihalova, D.; Fedorova, T.; Harmatha, J.; Nosal, R. Pharmacological influence on processes of adjuvant arthritis: Effect of the combination of an antioxidant active substance with methotrexate. Interdiscip. Toxicol. 2012, 5, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Bauerova, K.; Acquaviva, A.; Ponist, S.; Gardi, C.; Vecchio, D.; Drafi, F.; Arezzini, B.; Bezakova, L.; Kuncirova, V.; Mihalova, D.; et al. Markers of inflammation and oxidative stress studied in adjuvant-induced arthritis in the rat on systemic and local level affected by pinosylvin and methotrexate and their combination. Autoimmunity 2015, 48, 46–56. [Google Scholar] [CrossRef]
- Stojanović, S.; Brede, O. Elementary reactions of the antioxidant action of trans-stilbene derivatives: Resveratrol, pinosylvin and 4-hydroxystilbene. Phys. Chem. Chem. Phys. 2002, 4, 757–764. [Google Scholar] [CrossRef]
- Rodríguez-Bonilla, P.; Gandía-Herrero, F.; Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Comparative Study of the Antioxidant Capacity of Four Stilbenes Using ORAC, ABTS+, and FRAP Techniques. Food Anal. Methods 2017, 10, 2994–3000. [Google Scholar] [CrossRef]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase-Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 22, 56. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Feng, Q.; Hedner, T. Endothelium-derived relaxing factor (EDRF) and nitric oxide (NO). II. Physiology, pharmacology and pathophysiological implications. Clin. Physiol. 1990, 10, 503–526. [Google Scholar] [CrossRef]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Patel, R.P.; McAndrew, J.; Sellak, H.; White, C.R.; Jo, H.; Freeman, B.A.; Darley-Usmar, V.M. Biological aspects of reactive nitrogen species. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1411, 385–400. [Google Scholar] [CrossRef]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-dependent TLR signaling, its functions in host defense and inflammation, and its potential as a therapeutic target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Slater, S.J.; Seiz, J.L.; Cook, A.C.; Stagliano, B.A.; Buzas, C.J. Inhibition of protein kinase C by resveratrol. Biochim. Biophys. Acta 2003, 1637, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, R.; Jaken, S. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 2000, 28, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Agnoletto, C.; Baldini, C.; Bononi, A.; Bonora, M.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A. Redox control of protein kinase C: Cell-and disease-specific aspects. Antioxid. Redox Signal. 2010, 13, 1051–1085. [Google Scholar] [CrossRef]
- Koskela, A.; Reinisalo, M.; Hyttinen, J.M.; Kaarniranta, K.; Karjalainen, R.O. Pinosylvin-mediated protection against oxidative stress in human retinal pigment epithelial cells. Mol. Vis. 2014, 20, 760–769. [Google Scholar]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme oxygenase-1: A metabolic nike. Antioxid. Redox Signal 2014, 20, 1709–1722. [Google Scholar] [CrossRef]
- Flück, C.E.; Rojas Velazquez, M.N.; Pandey, A.V. Chapter 12—P450 oxidoreductase deficiency. In Genetic Steroid Disorders, 2nd ed.; New, M.I., Ed.; Academic Press: San Diego, CA, USA, 2023; pp. 239–264. [Google Scholar] [CrossRef]
- Swan, R.; Kim, S.J.; Campbell, J.P.; Chan, R.V.P.; Sonmez, K.; Taylor, K.D.; Li, X.; Chen, Y.-D.I.; Rotter, J.I.; Simmons, C.; et al. The Genetics of Retinopathy of Prematurity: A Model for Neovascular Retinal Disease. Ophthalmol. Retin. 2018, 2, 949–962. [Google Scholar] [CrossRef]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxidative Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Dragosloveanu, C.D.M.; Potop, V.; Coviltir, V.; Dinu, V.; Păsărică, M.; Ducan, I.L.; Maier, C.; Dragosloveanu, Ş. Prematurity-Risk Factor or Coincidence in Congenital Glaucoma? Medicina 2022, 58, 334. [Google Scholar] [CrossRef]
- Srejovic, J.V.; Muric, M.D.; Jakovljevic, V.L.; Srejovic, I.M.; Sreckovic, S.B.; Petrovic, N.T.; Todorovic, D.Z.; Bolevich, S.B.; Sarenac Vulovic, T.S. Molecular and Cellular Mechanisms Involved in the Pathophysiology of Retinal Vascular Disease-Interplay Between Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2024, 25, 11850. [Google Scholar] [CrossRef]
- Dragosloveanu, C.D.M.; Celea, C.G.; Dragosloveanu, Ş. Comparison of 360° circumferential trabeculotomy and conventional trabeculotomy in primary pediatric glaucoma surgery: Complications, reinterventions and preoperative predictive risk factors. Int. Ophthalmol. 2020, 40, 3547–3554. [Google Scholar] [CrossRef]
- Su, E.; Kesavamoorthy, N.; Junge, J.A.; Zheng, M.; Craft, C.M.; Ameri, H. Comparison of Retinal Metabolic Activity and Structural Development between rd10 Mice and Normal Mice Using Multiphoton Fluorescence Lifetime Imaging Microscopy. Curr. Issues Mol. Biol. 2024, 46, 612–620. [Google Scholar] [CrossRef]
- Wang, C.N.; Sang, M.M.; Gong, S.N.; Yang, J.F.; Cheng, C.Y.; Sun, F. Two resveratrol analogs, pinosylvin and 4,4′-dihydroxystilbene, improve oligoasthenospermia in a mouse model by attenuating oxidative stress via the Nrf2-ARE pathway. Bioorg. Chem. 2020, 104, 104295. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Z.; Zhang, L.; Li, X.; Li, M.; Pan, Y.; Jiao, T.; Shi, X.; Liu, Q.; Wang, C.; et al. Efficacy and safety of nonpharmacological strategies for the treatment of oligoasthenospermia: A systematic review and Bayesian network meta-analysis. Eur. J. Med. Res. 2023, 28, 6. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; León, R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Bauerová, K.; Ponist, S.; Ondrejicková, O.; Komendová, D.; Mihalová, D. Association between tissue gamma-glutamyl-transferase and clinical markers of adjuvant arthritis in Lewis rats. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 2), 172–175. [Google Scholar]
- Drábiková, K.; Nosal, R.; Bauerova, K.; Mihalová, D.; Jancinová, V.; Petríková, M.; Košťálová, D. Chemiluminescence as a measure of oxidative stress in paws and spleen of rats with adjuvant arthritis. Chem. Listy 2007, 101, s180–s182. [Google Scholar]
- Jančinová, V.; Petríková, M.; Perečko, T.; Drábiková, K.; Nosáľ, R.; Bauerová, K.; Poništ, S.; Košťálová, D. Inhibition of neutrophil oxidative burst with arbutin. Effects in vitro and in adjuvant arthritis. Chem. Listy 2007, 101, 189–191. [Google Scholar]
- Park, E.J.; Min, H.Y.; Ahn, Y.H.; Bae, C.M.; Pyee, J.H.; Lee, S.K. Synthesis and inhibitory effects of pinosylvin derivatives on prostaglandin E2 production in lipopolysaccharide-induced mouse macrophage cells. Bioorg. Med. Chem. Lett. 2004, 14, 5895–5898. [Google Scholar] [CrossRef]
- Lee, S.K.; Min, H.Y.; Huh, S.K.; Kim, E.Y.; Lee, E.; Song, S.; Kim, S. Styrylheterocycles: A novel class of inhibitors on lipopolysaccharide-Induced nitric oxide production. Bioorg. Med. Chem. Lett. 2003, 13, 3689–3692. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- Hla, T.; Bishop-Bailey, D.; Liu, C.H.; Schaefers, H.J.; Trifan, O.C. Cyclooxygenase-1 and -2 isoenzymes. Int. J. Biochem. Cell Biol. 1999, 31, 551–557. [Google Scholar] [CrossRef]
- Adelizzi, R.A. COX-1 and COX-2 in health and disease. J. Am. Osteopath. Assoc. 1999, 99, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, N.V.; Dai, H.; Roos, K.L.; Evanson, N.K.; Tomsik, J.; Elton, T.S.; Simmons, D.L. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA 2002, 99, 13926–13931. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.E. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lim, J.; Lee, J.; Hur, S.; Kim, S.S.; Lim, S.; Hyun, C.G.; Kim, Y.S.; Park, D. Involvement of nuclear factor-kappaB in the inhibition of pro-inflammatory mediators by pinosylvin. Planta Med. 2006, 72, 801–806. [Google Scholar] [CrossRef]
- Yamamoto, K.; Arakawa, T.; Ueda, N.; Yamamoto, S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J. Biol. Chem. 1995, 270, 31315–31320. [Google Scholar] [CrossRef]
- Deng, J.; Miller, S.A.; Wang, H.Y.; Xia, W.; Wen, Y.; Zhou, B.P.; Li, Y.; Lin, S.Y.; Hung, M.C. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell 2002, 2, 323–334. [Google Scholar] [CrossRef]
- Haeggström, J.Z. Leukotriene biosynthetic enzymes as therapeutic targets. J. Clin. Investig. 2018, 128, 2680–2690. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and Down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT signaling in inflammation and stress-related diseases: Implications for therapeutic interventions. Mol. Biomed. 2023, 4, 40. [Google Scholar] [CrossRef]
- Moilanen, L.J.; Hämäläinen, M.; Lehtimäki, L.; Nieminen, R.M.; Muraki, K.; Moilanen, E. Pinosylvin Inhibits TRPA1-Induced Calcium Influx In Vitro and TRPA1-Mediated Acute Paw Inflammation In Vivo. Basic. Clin. Pharmacol. Toxicol. 2016, 118, 238–242. [Google Scholar] [CrossRef]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihöfner, C. TRPA1 and TRPM8 activation in humans: Effects of cinnamaldehyde and menthol. Neuroreport 2005, 16, 955–959. [Google Scholar] [CrossRef]
- Schütz, M.; Oertel, B.G.; Heimann, D.; Doehring, A.; Walter, C.; Dimova, V.; Geisslinger, G.; Lötsch, J. Consequences of a human TRPA1 genetic variant on the perception of nociceptive and olfactory stimuli. PLoS ONE 2014, 9, e95592. [Google Scholar] [CrossRef]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, K.; Leppänen, T.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, E. Pinosylvin Shifts Macrophage Polarization to Support Resolution of Inflammation. Molecules 2021, 26, 2772. [Google Scholar] [CrossRef]
- Kwon, O.; Seo, Y.; Park, H. Pinosylvin exacerbates LPS-induced apoptosis via ALOX 15 upregulation in leukocytes. BMB Rep. 2018, 51, 302–307. [Google Scholar] [CrossRef]
- Modi, S.; Yaluri, N.; Kokkola, T. Strigolactone GR24 and pinosylvin attenuate adipogenesis and inflammation of white adipocytes. Biochem. Biophys. Res. Commun. 2018, 499, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARγ and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun. 2020, 113, 102510. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Tao, L.L.; Zhai, Y.Z.; Ding, D.; Yin, W.H.; Liu, X.P.; Yu, G.Y. The role of C/EBP-α expression in human liver and liver fibrosis and its relationship with autophagy. Int. J. Clin. Exp. Pathol. 2015, 8, 13102–13107. [Google Scholar]
- Koleva, R.I.; Ficarro, S.B.; Radomska, H.S.; Carrasco-Alfonso, M.J.; Alberta, J.A.; Webber, J.T.; Luckey, C.J.; Marcucci, G.; Tenen, D.G.; Marto, J.A. C/EBPα and DEK coordinately regulate myeloid differentiation. Blood 2012, 119, 4878–4888. [Google Scholar] [CrossRef]
- Alvarado, M.G.; Thakore, P.; Earley, S. Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function. Cells 2021, 10, 1167. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; Yu, W.; Li, J.; Yao, J.; Zhang, J.; Wang, J.; Wang, C. Transient receptor potential ankyrin 1 (TRPA1) modulators: Recent update and future perspective. Eur. J. Med. Chem. 2023, 257, 115392. [Google Scholar] [CrossRef]
- White, M.V.; Kaliner, M.A. Mediators of allergic rhinitis. J. Allergy Clin. Immunol. 1992, 90, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Morita, H.; Sugita, K.; Akdis, C.A. Chapter 1—Introduction to Mechanisms of Allergic Diseases. In Middleton’s Allergy Essentials; O’Hehir, R.E., Holgate, S.T., Sheikh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Modi, S.; Yaluri, N.; Kokkola, T.; Laakso, M. Plant-derived compounds strigolactone GR24 and pinosylvin activate SIRT1 and enhance glucose uptake in rat skeletal muscle cells. Sci. Rep. 2017, 7, 17606. [Google Scholar] [CrossRef] [PubMed]
- Sirerol, J.A.; Rodríguez, M.L.; Mena, S.; Asensi, M.A.; Estrela, J.M.; Ortega, A.L. Role of Natural Stilbenes in the Prevention of Cancer. Oxid. Med. Cell Longev. 2016, 2016, 3128951. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Fantacuzzi, M.; Giampietro, L.; Maccallini, C.; Amoroso, R. Anticancer Activity of Stilbene-Based Derivatives. ChemMedChem 2017, 12, 558–570. [Google Scholar] [CrossRef]
- Yousuf, M.; Jinka, S.; Adhikari, S.S.; Banerjee, R. Methoxy-enriched cationic stilbenes as anticancer therapeutics. Bioorg. Chem. 2020, 98, 103719. [Google Scholar] [CrossRef]
- Borys, F.; Tobiasz, P.; Poterała, M.; Fabczak, H.; Krawczyk, H.; Joachimiak, E. Systematic Studies on Anti-Cancer Evaluation of Stilbene and Dibenzo[b,f]oxepine Derivatives. Molecules 2023, 28, 3558. [Google Scholar] [CrossRef]
- Scheau, C.; Mihai, L.G.; Badarau, I.A.; Caruntu, C. Emerging applications of some important natural compounds in the field of oncology. Farmacia 2020, 68, 984–991. [Google Scholar] [CrossRef]
- Duta-Bratu, C.-G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef]
- Hassan, A.H.E.; Wang, C.Y.; Oh, T.; Ham, G.; Lee, S.K.; Lee, Y.S. Discovery of a stilbenoid-flavanone hybrid as an antitumor Wnt/β-catenin signaling pathway inhibitor. Bioorganic Chem. 2024, 145, 107178. [Google Scholar] [CrossRef]
- Skinnider, L.; Stoessl, A. The effect of the phytoalexins, lubimin, (−)-maackiain, pinosylvin, and the related compounds dehydroloroglossol and hordatine M on human lymphoblastoid cell lines. Experientia 1986, 42, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Chung, H.J.; Park, H.J.; Kim, G.D.; Ahn, Y.H.; Lee, S.K. Suppression of Src/ERK and GSK-3/β-catenin signaling by pinosylvin inhibits the growth of human colorectal cancer cells. Food Chem. Toxicol. 2013, 55, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ketola, K.; Viitala, M.; Kohonen, P.; Fey, V.; Culig, Z.; Kallioniemi, O.; Iljin, K. High-throughput cell-based compound screen identifies pinosylvin methyl ether and tanshinone IIA as inhibitors of castration-resistant prostate cancer. J. Mol. Biochem. 2016, 5, 12–22. [Google Scholar]
- Pelaz, S.G.; Tabernero, A. Src: Coordinating metabolism in cancer. Oncogene 2022, 41, 4917–4928. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; Maestro, R.; et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: Opportunities for therapeutic intervention. Leukemia 2014, 28, 15–33. [Google Scholar] [CrossRef]
- Mellanen, P.; Petänen, T.; Lehtimäki, J.; Mäkelä, S.; Bylund, G.; Holmbom, B.; Mannila, E.; Oikari, A.; Santti, R. Wood-derived estrogens: Studies in vitro with breast cancer cell lines and in vivo in trout. Toxicol. Appl. Pharmacol. 1996, 136, 381–388. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Kong, Q.; Jiang, Y. Mechanisms for estrogen receptor expression in human cancer. Exp. Hematol. Oncol. 2018, 7, 24. [Google Scholar] [CrossRef]
- Tanwar, A.K.; Dhiman, N.; Kumar, A.; Jaitak, V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur. J. Med. Chem. 2021, 213, 113037. [Google Scholar] [CrossRef]
- Park, E.J.; Park, H.J.; Chung, H.J.; Shin, Y.; Min, H.Y.; Hong, J.Y.; Kang, Y.J.; Ahn, Y.H.; Pyee, J.H.; Lee, S.K. Antimetastatic activity of pinosylvin, a natural stilbenoid, is associated with the suppression of matrix metalloproteinases. J. Nutr. Biochem. 2012, 23, 946–952. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Hsieh, M.C.; Lin, C.C.; Lo, Y.S.; Ho, H.Y.; Hsieh, M.J.; Lin, J.T. Pinosylvin inhibits migration and invasion of nasopharyngeal carcinoma cancer cells via regulation of epithelial-mesenchymal transition and inhibition of MMP-2. Oncol. Rep. 2021, 46, 143. [Google Scholar] [CrossRef]
- Sun, J.; Nan, G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target (Review). Int. J. Mol. Med. 2017, 39, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Moten, A.; Lin, H.-K. Akt: A new activation mechanism. Cell Res. 2014, 24, 785–786. [Google Scholar] [CrossRef]
- Berr, A.L.; Wiese, K.; dos Santos, G.; Koch, C.M.; Anekalla, K.R.; Kidd, M.; Davis, J.M.; Cheng, Y.; Hu, Y.-S.; Ridge, K.M. Vimentin is required for tumor progression and metastasis in a mouse model of non–small cell lung cancer. Oncogene 2023, 42, 2074–2087. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef]
- Ram, A.K.; Vairappan, B. Role of zonula occludens in gastrointestinal and liver cancers. World J. Clin. Cases 2022, 10, 3647–3661. [Google Scholar] [CrossRef]
- Du, J.; Zhao, Y.; Hu, D.; Li, H.; Gao, L.; Xing, X.; Shi, K. Pinosylvin Inhibits Esophageal Squamous Cell Carcinoma Migration and Invasion by Regulating STX6/ITGA3/VASP Pathway. Rev. Bras. De Farmacogn. 2023, 33, 173–181. [Google Scholar] [CrossRef]
- Du, J.; Liu, X.; Wu, Y.; Zhu, J.; Tang, Y. Essential role of STX6 in esophageal squamous cell carcinoma growth and migration. Biochem. Biophys. Res. Commun. 2016, 472, 60–67. [Google Scholar] [CrossRef]
- Simard, F.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Isolation and identification of cytotoxic compounds from the wood of Pinus resinosa. Phytother. Res. 2008, 22, 919–922. [Google Scholar] [CrossRef]
- Song, J.; Seo, Y.; Park, H. Pinosylvin enhances leukemia cell death via down-regulation of AMPKα expression. Phytother. Res. 2018, 32, 2097–2104. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Park, E.-J.; Min, H.-Y.; Kim, M.-S.; Pyee, J.-H.; Ahn, Y.-H.; Lee, S.-K. Suppressive effects of pinosylvin on prostaglandin E2 and nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 cells. In Proceedings of the Annual Meeting of KSAP: International Symposium on Pharmaceutical and Biomedical Sciences on Obesity, Seoul, Republic of Korea, 1 November 2003; p. 102. [Google Scholar]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol Promotes Clearance of Alzheimer’s Disease Amyloid-β Peptides*. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Lu, K.T.; Ko, M.C.; Chen, B.Y.; Huang, J.C.; Hsieh, C.W.; Lee, M.C.; Chiou, R.Y.; Wung, B.S.; Peng, C.H.; Yang, Y.L. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J. Agric. Food Chem. 2008, 56, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.S.; Zhou, H.; Wilson, B.; Hong, J.S.; Gao, H.M. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef]
- Temsamani, H.; Krisa, S.; Mérillon, J.-M.; Richard, T. Promising neuroprotective effects of oligostilbenes. Nutr. Aging 2015, 3, 49–54. [Google Scholar] [CrossRef]
- Xu, H.; Deng, R.; Li, E.T.S.; Shen, J.; Wang, M. Pinosylvin provides neuroprotection against cerebral ischemia and reperfusion injury through enhancing PINK1/Parkin mediated mitophagy and Nrf2 pathway. J. Funct. Foods 2020, 71, 104019. [Google Scholar] [CrossRef]
- Lee, R.H.C.; Lee, M.H.H.; Wu, C.Y.C.; Couto, E.S.A.; Possoit, H.E.; Hsieh, T.H.; Minagar, A.; Lin, H.W. Cerebral ischemia and neuroregeneration. Neural Regen. Res. 2018, 13, 373–385. [Google Scholar] [CrossRef]
- Kunz, A.; Dirnagl, U.; Mergenthaler, P. Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 495–509. [Google Scholar] [CrossRef]
- Xing, C.; Arai, K.; Lo, E.H.; Hommel, M. Pathophysiologic cascades in ischemic stroke. Int. J. Stroke 2012, 7, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, W.; Traganos, F.; Jesionowska, H.; Darzynkiewicz, Z. Presence of DNA Strand Breaks and Increased Sensitivity of DNA in Situ to Denaturation in Abnormal Human Sperm Cells: Analogy to Apoptosis of Somatic Cells. Exp. Cell Res. 1993, 207, 202–205. [Google Scholar] [CrossRef]
- Moro, M.A.; Almeida, A.; Bolaños, J.P.; Lizasoain, I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic. Biol. Med. 2005, 39, 1291–1304. [Google Scholar] [CrossRef]
- Chan, P.H. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem. Res. 2004, 29, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Hanley, B.; Lamuela-Raventos, R.M. Isoflavones, lignans and stilbenes–Origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000, 80, 1044–1062. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Gil, L. Las Transformaciones Históricas del Paisaje: La Permanencia y la Extinción Local del Pino Piñonero. Los Montes y su Historia: Una Perspectiva Política, Económica y Social; Universidad de Huelva: Huelva, Spain, 1999; pp. 151–186. [Google Scholar]
- Whited, T.L.; Engels, J.I.; Hoffmann, R.C.; Ibsen, H.; Verstegen, W. Northern Europe: An Environmental History; Bloomsbury Publishing USA: New York, NY, USA, 2005. [Google Scholar]
- Tsoucalas, G.; Vladimiros, L. The medical use of Málaka wine and cola nuts in late 19th/early 20th century Greece. DELTOS 2024, 33, 85–92. [Google Scholar] [CrossRef]
- Rodríguez-Delgado, M.A.; González, G.; Pérez-Trujillo, J.P.; García-Montelongo, F.J. Trans-resveratrol in wines from the Canary Islands (Spain). Analysis by high performance liquid chromatography. Food Chem. 2002, 76, 371–375. [Google Scholar] [CrossRef]
- Suprun, A.R.; Dubrovina, A.S.; Tyunin, A.P.; Kiselev, K.V. Profile of Stilbenes and Other Phenolics in Fanagoria White and Red Russian Wines. Metabolites 2021, 11, 231. [Google Scholar] [CrossRef]
- Zhou, D.D.; Li, J.; Xiong, R.G.; Saimaiti, A.; Huang, S.Y.; Wu, S.X.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, N.; Haridas, M.; Abdulhameed, S. Stilbenes and Their Derivatives in Traditional Medicine. In Bioresources and Bioprocess in Biotechnology: Volume 2: Exploring Potential Biomolecules; Sugathan, S., Pradeep, N.S., Abdulhameed, S., Eds.; Springer: Singapore, 2017; pp. 407–418. [Google Scholar] [CrossRef]
- Lopes, R.M.; Agostini-Costa, T.d.S.; Gimenes, M.A.; Silveira, D. Chemical Composition and Biological Activities of Arachis Species. J. Agric. Food Chem. 2011, 59, 4321–4330. [Google Scholar] [CrossRef]
- Zu, X.Y.; Xiong, G.Q.; Geng, S.R.; Liao, T.; Li, X.; Zhang, Z.Y. Arachis hypogaea L. stem and leaf extract improves the sleep behavior of pentobarbital-treated rats. Biomed. Rep. 2014, 2, 388–391. [Google Scholar] [CrossRef]
- Al-Azawi, A.H.; Hassan, Z.H. Antibacterial Activity of Arachis hypogaea L. Seed Coat Extract Cultivated in Iraq. Pak. J. Biotechnol. Vol. 2017, 14, 601–605. [Google Scholar]
- Luo, Q.F.; Sun, L.; Si, J.Y.; Chen, D.H. Hypocholesterolemic effect of stilbenes containing extract-fraction from Cajanus cajan L. on diet-induced hypercholesterolemia in mice. Phytomedicine 2008, 15, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Fu, Y.-J.; Zu, Y.-G.; Chang, F.-R.; Chen, Y.-H.; Liu, X.-L.; Stelten, J.; Schiebel, H.-M. Cajanuslactone, a new coumarin with anti-bacterial activity from pigeon pea [Cajanus cajan (L.) Millsp.] leaves. Food Chem. 2010, 121, 1150–1155. [Google Scholar] [CrossRef]
- Nicholson, R.A.; David, L.S.; Pan, R.L.; Liu, X.M. Pinostrobin from Cajanus cajan (L.) Millsp. inhibits sodium channel-activated depolarization of mouse brain synaptoneurosomes. Fitoterapia 2010, 81, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Mishra, P.; Sachan, N.; Ghosh, A.K. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J. Adv. Pharm. Technol. Res. 2011, 2, 207–214. [Google Scholar] [CrossRef]
- Hyun, T.K.; Eom, S.H.; Yu, C.Y.; Roitsch, T. Hovenia dulcis–an Asian traditional herb. Planta Medica 2010, 76, 943–949. [Google Scholar] [CrossRef]
- Sferrazza, G.; Brusotti, G.; Zonfrillo, M.; Temporini, C.; Tengattini, S.; Bononi, M.; Tateo, F.; Calleri, E.; Pierimarchi, P. Hovenia dulcis Thumberg: Phytochemistry, Pharmacology, Toxicology and Regulatory Framework for Its Use in the European Union. Molecules 2021, 26, 903. [Google Scholar] [CrossRef]
- Ritch-Krc, E.M.; Thomas, S.; Turner, N.J.; Towers, G.H.N. Carrier herbal medicine: Traditional and contemporary plant use. J. Ethnopharmacol. 1996, 52, 85–94. [Google Scholar] [CrossRef]
- Ritch-Krc, E.M.; Turner, N.J.; Towers, G.H.N. Carrier herbal medicine: An evaluation of the antimicrobial and anticancer activity in some frequently used remedies. J. Ethnopharmacol. 1996, 52, 151–156. [Google Scholar] [CrossRef]
- Legault, J.; Girard-Lalancette, K.; Dufour, D.; Pichette, A. Antioxidant Potential of Bark Extracts from Boreal Forest Conifers. Antioxidants 2013, 2, 77–89. [Google Scholar] [CrossRef]
- Uprety, Y.; Lacasse, A.; Asselin, H. Traditional Uses of Medicinal Plants from the Canadian Boreal Forest for the Management of Chronic Pain Syndromes. Pain Pract. 2016, 16, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Ince, I.; Yesil-Celiktas, O.; Karabay-Yavasoglu, N.U.; Elgin, G. Effects of Pinus brutia bark extract and Pycnogenol® in a rat model of carrageenan induced inflammation. Phytomedicine 2009, 16, 1101–1104. [Google Scholar] [CrossRef]
- Satil, F.; Selvi, S.; Polat, R. Ethnic uses of pine resin production from Pinus brutia by native people on the Kazdag Mountain (Mt. Ida) in Western Turkey. J. Food Agric. Environ. 2011, 9, 1059–1063. [Google Scholar]
- Cretu, E.; Karonen, M.; Salminen, J.-P.; Mircea, C.; Trifan, A.; Charalambous, C.; Constantinou, A.I.; Miron, A. In Vitro Study on the Antioxidant Activity of a Polyphenol-Rich Extract from Pinus brutia Bark and Its Fractions. J. Med. Food 2013, 16, 984–991. [Google Scholar] [CrossRef]
- Secim-Karakaya, P.; Saglam-Metiner, P.; Yesil-Celiktas, O. Antimicrobial and wound healing properties of cotton fabrics functionalized with oil-in-water emulsions containing Pinus brutia bark extract and Pycnogenol® for biomedical applications. Cytotechnology 2021, 73, 423–431. [Google Scholar] [CrossRef]
- Süntar, I.; Demirel, M.A.; Taban, K.; Çeribaşı, A.O.; Gök, H.N.; Metkin, G. Assessment of the preventive activity of Pinus brutia Ten. (Pinaceae) against in vivo acute lung injury model. Phytochem. Lett. 2023, 58, 8–18. [Google Scholar] [CrossRef]
- Erol, K.F.; Kutlu, G.; Tornuk, F.; Guzel, M.; Donmez, I. Determination of antioxidant, anticancer, antidiabetic and antimicrobial activities of Turkish red pine (Pinus brutia Ten.) bark ultrasound-assisted extract as a functional food additive. Acta Aliment. 2023, 52, 102–112. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, J.E.; Choi, S.W. Quantitative Changes of Phenolic Compounds in Pine Twigs by Variety, Harvest Season, and Growing Region. Prev. Nutr. Food Sci. 2022, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Šarac, Z.; Matejić, J.S.; Stojanović-Radić, Z.Z.; Veselinović, J.B.; Džamić, A.M.; Bojović, S.; Marin, P.D. Biological activity of Pinus nigra terpenes—Evaluation of FtsZ inhibition by selected compounds as contribution to their antimicrobial activity. Comput. Biol. Med. 2014, 54, 72–78. [Google Scholar] [CrossRef]
- Milić, N.; Milanović, M.; Četojević-Simin, D.; Malenčić, Đ.; Prvulović, D.; Pavkov, N.; Radulović, Z.; Milošević, N.; Rašković, A.; Mandić, A. Phytochemical characterization and effects on cell proliferation of Pinus nigra Arn. bark. Arch. Pharm. 2021, 354, e2000416. [Google Scholar] [CrossRef]
- Papp, N.; Purger, D.; Czigle, S.; Czégényi, D.; Stranczinger, S.; Tóth, M.; Dénes, T.; Kocsis, M.; Takácsi-Nagy, A.; Filep, R. The Importance of Pine Species in the Ethnomedicine of Transylvania (Romania). Plants 2022, 11, 2331. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, İ.; Ozen, T.; Marah, S.; Mutlu, D.; Arslan, Ş.; Gül, F. Functional food components and activities of Pinus nigra and Pinus sylvestris barks as food supplements. Int. J. Chem. Technol. 2023, 7, 229–238. [Google Scholar] [CrossRef]
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of use on medicinal species in Valfurva (Sondrio, Italy). J. Ethnopharmacol. 2015, 163, 113–134. [Google Scholar] [CrossRef]
- Manzione, M.G.; Vitalini, S.; Sharopov, F.; Capó, X.; Iriti, M.; Martorell, M.; Pezzani, R. Pinus mugo Turra and its Therapeutic Potential: A Narrative Review. Nat. Prod. Commun. 2024, 19, 1934578X241265934. [Google Scholar] [CrossRef]
- Hussain, M.; Shah, G.M.; Khan, M.A. Traditional medicinal and economic uses of Gymnosperms of Kaghan valley, Pakistan. Ethnobot. Leafl. 2006, 2006, 7. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Siddiqui, M.; Mesaik, A.; Wahab, M.; Khan, N.; Khan, U.; Nazim, K.; Hussain, S. Phytosociology of Pinus roxburghii Sargent. (Chir pine) in Lesser Himalayan and Hindu Kush range of Pakistan. Pak. J. Bot. 2009, 41, 2357–2369. [Google Scholar]
- Abbasi, A.M.; Khan, M.A.; Ahmad, M.; Zafar, M.; Jahan, S.; Sultana, S. Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 2010, 128, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Matłok, N.; Balawejder, M. Extraction of phytochemicals from young shoots of Pinus sylvestris L. and analysis of their selected biological properties. LWT 2023, 188, 115404. [Google Scholar] [CrossRef]
- Stewart, C.D.; Jones, C.D.; Setzer, W.N. Essential oil compositions of Juniperus virginiana and Pinus virginiana, two important trees in Cherokee traditional medicine. Am. J. Essent. Oil Nat. Prod. 2014, 2, 17–24. [Google Scholar]
- Georghiou, K.; Delipetrou, P. Patterns and traits of the endemic plants of Greece. Bot. J. Linn. Soc. 2010, 162, 130–153. [Google Scholar] [CrossRef]
- Axiotis, E.; Halabalaki, M.; Skaltsounis, L.A. An Ethnobotanical Study of Medicinal Plants in the Greek Islands of North Aegean Region. Front. Pharmacol. 2018, 9, 409. [Google Scholar] [CrossRef]
- Sigerist, H.E. On Hippocrates. Bull. Inst. Hist. Med. 1934, 2, 190–214. [Google Scholar]
- Goldberg, H. Hippocrates: Father of Medicine; iUniverse: Lincoln, NE, USA, 2006. [Google Scholar]
- Scarborough, J. Theophrastus on herbals and herbal remedies. J. Hist. Biol. 1978, 11, 353–385. [Google Scholar] [CrossRef]
- Sharples, R.W.; Huby, P.M.; Fortenbaugh, W.W. Theophrastus of Eresus: Sources on Biology; Brill: Leiden, The Netherlands, 1995; Volume 64. [Google Scholar]
- Riddle, J.M. Dioscorides on Pharmacy and Medicine; University of Texas Press: Austin, TX, USA, 1985. [Google Scholar]
- Staub, P.O.; Casu, L.; Leonti, M. Back to the roots: A quantitative survey of herbal drugs in Dioscorides’ De Materia Medica (ex Matthioli, 1568). Phytomedicine 2016, 23, 1043–1052. [Google Scholar] [CrossRef]
- Wallner, C.; Moormann, E.; Lulof, P.; Drysch, M.; Lehnhardt, M.; Behr, B. Burn Care in the Greek and Roman Antiquity. Medicina 2020, 56, 657. [Google Scholar] [CrossRef]
- De Armas, E.; Sarracent, Y.; Marrero, E.; Fernández, O.; Branford-White, C. Efficacy of Rhizophora mangle aqueous bark extract (RMABE) in the treatment of aphthous ulcers: A pilot study. Curr. Med. Res. Opin. 2005, 21, 1711–1715. [Google Scholar] [CrossRef]
- Gonzales, M.V.M.; Tolentino, A.G. Extraction and isolation of the alkaloids from the Samanea saman (Acacia) bark: Its antiseptic potential. IInternational J. Sci. Technol. Res. 2014, 3, 119–124. [Google Scholar]
- Casas-Agustench, P.; Salas-Huetos, A.; Salas-Salvadó, J. Mediterranean nuts: Origins, ancient medicinal benefits and symbolism. Public. Health Nutr. 2011, 14, 2296–2301. [Google Scholar] [CrossRef]
- Ghalioungui, P. La Médecine des Pharaons: Magie et Science Médicale Dans l’Egypte Ancienne; Robert Laffont: Paris, France, 1983. [Google Scholar]
- Mattern, S.P. Galen and the Rhetoric of Healing; JHU Press: Baltimore, MD, USA, 2008. [Google Scholar]
- McNally, S. The maenad in early Greek art. Arethusa 1978, 11, 101–135. [Google Scholar]
- Hedreen, G. Silens, nymphs, and maenads. J. Hell. Stud. 1994, 114, 47–69. [Google Scholar]
- Brussell, D.E. Medicinal plants of Mt. Pelion, Greece. Econ. Bot. 2004, 58, S174–S202. [Google Scholar] [CrossRef]
- Seltman, C. Greek Sculpture and some festival coins. Hesperia J. Am. Sch. Class. Stud. Athens 1948, 17, 71–85. [Google Scholar] [CrossRef]
- Ghosh, S. Representation of Greek gods/goddesses in Graeco-Bactrian and Indo-Greek visual culture. In The Graeco-Bactrian and Indo-Greek World; Routledge: London, UK, 2020; pp. 570–579. [Google Scholar]
- Broneer, O. The Isthmian victory crown. Am. J. Archaeol. 1962, 66, 259–263. [Google Scholar] [CrossRef]
- Tillyard, E. Theseus, Sinis, and the Isthmian Games. J. Hell. Stud. 1913, 33, 296–312. [Google Scholar] [CrossRef]
- Ruiz Daniels, R.; Taylor, R.S.; González-Martínez, S.C.; Vendramin, G.G.; Fady, B.; Oddou-Muratorio, S.; Piotti, A.; Simioni, G.; Grivet, D.; Beaumont, M.A. Looking for local adaptation: Convergent microevolution in Aleppo pine (Pinus halepensis). Genes 2019, 10, 673. [Google Scholar] [CrossRef]
- Mauri, A.; Di Leo, M.; de Rigo, D.; Caudullo, G. Pinus halepensis and Pinus brutia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 122–123. [Google Scholar]
- Scherrer, A.M.; Motti, R.; Weckerle, C.S. Traditional plant use in the areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy). J. Ethnopharmacol. 2005, 97, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Passalacqua, N.G.; Guarrera, P.M.; De Fine, G. Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy). Fitoterapia 2007, 78, 52–68. [Google Scholar] [CrossRef]
- Motti, R.; Motti, P. An Ethnobotanical Survey of Useful Plants in the Agro Nocerino Sarnese (Campania, Southern Italy). Hum. Ecol. 2017, 45, 865–878. [Google Scholar] [CrossRef]
- Menale, B.; Muoio, R. Use of medicinal plants in the South-Eastern area of the Partenio Regional Park (Campania, Southern Italy). J. Ethnopharmacol. 2014, 153, 297–307. [Google Scholar] [CrossRef]
- Guri, A.; Kefalas, P.; Roussis, V. Antioxidant potential of six pine species. Phytother. Res. 2006, 20, 263–266. [Google Scholar] [CrossRef]
- Dhibi, M.; Mechri, B.; Brahmi, F.; Skhiri, F.; Alsaif, M.A.; Hammami, M. Fatty acid profiles, antioxidant compounds and antiradical properties of Pinus halepensis Mill. cones and seeds. J. Sci. Food Agric. 2012, 92, 1702–1708. [Google Scholar] [CrossRef]
- Dhibi, M.; Issaoui, M.; Brahmi, F.; Mechri, B.; Mnari, A.; Cheraif, I.; Skhiri, F.; Gazzah, N.; Hammami, M. Nutritional quality of fresh and heated Aleppo pine (Pinus halepensis Mill.) seed oil: Trans-fatty acid isomers profiles and antioxidant properties. J. Food Sci. Technol. 2014, 51, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Djerrad, Z.; Kadik, L.; Djouahri, A. Chemical variability and antioxidant activities among Pinus halepensis Mill. essential oils provenances, depending on geographic variation and environmental conditions. Ind. Crops Prod. 2015, 74, 440–449. [Google Scholar] [CrossRef]
- Djerrad, Z.; Djouahri, A.; Kadik, L. Variability of Pinus halepensis Mill. Essential Oils and Their Antioxidant Activities Depending on the Stage of Growth During Vegetative Cycle. Chem. Biodivers. 2017, 14, e1600340. [Google Scholar] [CrossRef] [PubMed]
- Salim, H.; Rimawi, W.H.; Shaheen, S.; Mjahed, A. Phytochemical Analysis and Antibacterial Activity of Extracts from Palestinian Aleppo Pine Seeds, Bark and Cones. Asian J. Chem. 2018, 31, 143–147. [Google Scholar] [CrossRef]
- Abbou, A.; Kadri, N.; Debbache, N.; Dairi, S.; Remini, H.; Dahmoune, F.; Berkani, F.; Adel, K.; Belbahi, A.; Madani, K. Effect of precipitation solvent on some biological activities of polysaccharides from Pinus halepensis Mill. seeds. Int. J. Biol. Macromol. 2019, 141, 663–670. [Google Scholar] [CrossRef]
- Meziti, H.; Bouriche, H.; Kada, S.; Demirtas, I.; Kizil, M.; Senator, A.; Garrido, G. Phytochemical analysis, and antioxidant, anti-hemolytic and genoprotective effects of Quercus ilex L. and Pinus halepensis Mill. methanolic extracts. J. Pharm. Pharmacogn. Res. 2019, 7, 260–272. [Google Scholar] [CrossRef]
- Bouyahya, A.; Belmehdi, O.; Abrini, J.; Dakka, N.; Bakri, Y. Chemical composition of Mentha suaveolens and Pinus halepensis essential oils and their antibacterial and antioxidant activities. Asian Pac. J. Trop. Med. 2019, 12, 117–122. [Google Scholar] [CrossRef]
- Duganath, N.; Kumar, S.R.; Kumanan, R.; Jayaveera, K.N. Evaluation of anti-denaturation property and anti-oxidant activity of traditionally used medicinal plants. Int. J. Pharma Biosci. 2010, 1, PS110. [Google Scholar]
- Leelaprakash, G.; Dass, S.M. Invitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int. J. Drug Dev. Res. 2011, 3, 189–196. [Google Scholar]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Evaluation of in vitro anti-inflammatory activity of coffee against the denaturation of protein. Asian Pac. J. Trop. Biomed. 2012, 2, S178–S180. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Küpeli Akkol, E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef]
- Popescu, D.I.; Lengyel, E.; Apostolescu, F.G.; Soare, L.C.; Botoran, O.R.; Șuțan, N.A. Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania. Horticulturae 2022, 8, 952. [Google Scholar] [CrossRef]
- Popa, C.; Hanscam, E. Of Romans, Dacians and Romanians. Archaica 2020, 7–8, 244–251. [Google Scholar]
- Segneanu, A.-E.; Cepan, C.; Grozescu, I.; Cziple, F.; Olariu, S.; Ratiu, S.; Lazar, V.; Murariu, S.M.; Velciov, S.M.; Marti, T.D. Therapeutic Use of Some Romanian Medicinal Plants. In Pharmacognosy; Shagufta, P., Areej, A.-T., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 7. [Google Scholar] [CrossRef]
- Žuškin, E.; Lipozenčić, J.; Pucarin-Cvetković, J.; Mustajbegović, J.; Schachter, N.; Mučić-Pučić, B.; Neralić-Meniga, I. Ancient medicine-a review. Acta Dermatovenerol. Croat. 2008, 16, 149–157. [Google Scholar]
- Carciumaru, M. Plants used by traco-geto-dacians (Attempt of synthesis)(V). Thraco-Dacica 1987, 8, 171–176. [Google Scholar]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Avramescu, S.M.; Dinu-Pirvu, C.E.; Ionescu, D. Romanian aromatic and medicinal plants: From tradition to science. In Aromatic and Medicinal Plants-Back to Nature; IntechOpen: London, UK, 2017; pp. 149–173. [Google Scholar]
- Pogăcias, A. The Dacian Society–Fierce Warriors and Their Women Sources and Representations. Hiperboreea 2017, 4, 5–22. [Google Scholar]
- Melius, J.P. Herbarium; Heltai Gaspárné, Colosvár: Kolozsvár, Romania, 1578. [Google Scholar]
- Papp, N.; Birkás-Frendl, K.; Farkas, Á.; Pieroni, A. An ethnobotanical study on home gardens in a Transylvanian Hungarian Csángó village (Romania). Genet. Resour. Crop Evol. 2013, 60, 1423–1432. [Google Scholar] [CrossRef]
- Bartha, S.G.; Quave, C.L.; Balogh, L.; Papp, N. Ethnoveterinary practices of covasna county, transylvania, Romania. J. Ethnobiol. Ethnomedicine 2015, 11, 1–22. [Google Scholar] [CrossRef]
- Kóczián, G.; Pintér, I.; Szabó, L.G. Adatok a gyimesi csángók népi gyógyászatához. Gyógyszerészet 1975, 19, 226–230. [Google Scholar]
- Halászné, Z. Data to the medicinal plants usage of the Hungarians in Moldova. Gyógyszerészet 1981, 25, 361–367. [Google Scholar]
- Péntek, J.; Szabó, A. Ember és Növényvilág: Kalotaszeg Növényzete és Népi Növényismerete; Kriterion könyvkiadó: Cluj-Napoca, Romania, 1985. [Google Scholar]
- Martindale, W.; Westcott, W.W. The Extra Pharmacopoeia of Martindale and Westcott; HK Lewis: London, UK, 1925; Volume 2. [Google Scholar]
- Dörfler, H.; Roselt, G. Heilpfanzen Gestern und Heute; Urania: Leipzig, Germany; Jena, Germany; Berlin, Germany, 1988. [Google Scholar]
- Richardson, D.M. Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Žuna Pfeiffer, T.; Krstin, L.; Špoljarić Maronić, D.; Hmura, M.; Eržić, I.; Bek, N.; Stević, F. An ethnobotanical survey of useful wild plants in the north-eastern part of Croatia (Pannonian region). Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2020, 154, 463–473. [Google Scholar] [CrossRef]
- Koczian, G.; Szabo, I.; Szabo, L. Etnobotanikai adatok Kalotaszebrol. Bot. Kozlemenyek 1977, 64, 23–29. [Google Scholar]
- Butură, V. Enciclopedie de Etnobotanică Românească; Editura Științifică și Enciclopedică: Bucharest, Romania, 1979. [Google Scholar]
- Gub, J. Adatok a Nagy-Homoród és a Nagy-Küküllő közötti terület népi növényismeretéhez. Néprajzi Látóhatár 1993, 1, 95–110. [Google Scholar]
- Halász, P. Növények a Moldvai Magyarok Hagyományában és Mindennapjaiban; General Press Konyvkiado: Budapest, Hungary, 2010. [Google Scholar]
- Janaćković, P.; Gavrilović, M.; Savić, J.; Marin, P.D.; Stevanović, Z.D. Traditional knowledge on plant use from Negotin Krajina (Eastern Serbia): An ethnobotanical study. Indian J. Tradit. Knowl. 2019, 18, 25–33. [Google Scholar]
- Gunda, B. Egy kárpátaljai magyar falu ethnobotanikája. Nyíregyházi Jósa András Múzeum Évkönyve 1978, 1978, 25–41. [Google Scholar]
- Kóczián, G.; Szabó, L.G. Nagyatád Egy Kertészgazdaságának Leírása, Agrobotanikai Értékei És Géntartaléka (Description of a Garden in Nagyatád, Its Agrobotanical Values and Gene Resources). Agrártudományi Szle 1978, 1–2, 181–197. [Google Scholar]
- Matejić, J.S.; Stefanović, N.; Ivković, M.; Živanović, N.; Marin, P.D.; Džamić, A.M. Traditional uses of autochthonous medicinal and ritual plants and other remedies for health in Eastern and South-Eastern Serbia. J. Ethnopharmacol. 2020, 261, 113186. [Google Scholar] [CrossRef]
- Tucakov, J. Lečenje Biljem (Healing with Herbs); Rad: Belgrade, Serbia, 1986. [Google Scholar]
- Kóczian, G. Hagyományos Parasztgazdálkodás, Termesztett, a Gyujtögeto Gazdálkodás, Vad Növényfajainak Etnobotanikai Értékelése. (Ethnobotanical Evaluation of Traditional Farming, Cultivation, and Collecting of Wild Plant Species); Nagyatádi Kulturális és Sport Központ: Nagyatád, Hungary, 2014. [Google Scholar]
- Keszeg, V. A Mezőségi Detrehemtelep Népi Gyógyászata (Folk Medicine of the Detrehemtelep in the Field). In Népismereti Dolgozatok; Kriterion Könyvkiadó: Bucharest, Romania, 1981; pp. 97–117. [Google Scholar]
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Belda, A.; Peiró, V.; Seva, E. The Relationship between Plants Used to Sustain Finches (Fringillidae) and Uses for Human Medicine in Southeast Spain. Evid.-Based Complement. Altern. Med. 2012, 2012, 360913. [Google Scholar] [CrossRef]
- Zhu, P.; Qi, R.-Q.; Yang, Y.; Huo, W.; Zhang, Y.; He, L.; Wang, G.; Xu, J.; Zhang, F.; Yang, R.; et al. Clinical guideline for the diagnosis and treatment of cutaneous warts (2022). J. Evid.-Based Med. 2022, 15, 284–301. [Google Scholar] [CrossRef]
- Gras, A.; Serrasolses, G.; Vallès, J.; Garnatje, T. Traditional knowledge in semi-rural close to industrial areas: Ethnobotanical studies in western Gironès (Catalonia, Iberian Peninsula). J. Ethnobiol. Ethnomed. 2019, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Carrió, E.; Vallès, J. Ethnobotany of medicinal plants used in Eastern Mallorca (Balearic Islands, Mediterranean Sea). J. Ethnopharmacol. 2012, 141, 1021–1040. [Google Scholar] [CrossRef]
- Benarba, B.; Belabid, L.; Righi, K.; Bekkar, A.a.; Elouissi, M.; Khaldi, A.; Hamimed, A. Ethnobotanical study of medicinal plants used by traditional healers in Mascara (North West of Algeria). J. Ethnopharmacol. 2015, 175, 626–637. [Google Scholar] [CrossRef]
- Chermat, S.; Gharzouli, R. Ethnobotanical study of medicinal flora in the North East of Algeria-An empirical knowledge in Djebel Zdimm (Setif). J. Mater. Sci. Eng. 2015, 5, 50–59. [Google Scholar]
- Hmamouchi, M.; Hamamouchi, J.; Zouhdi, M.; Bessiere, J.M. Chemical and Antimicrobial Properties of Essential Oils of Five Moroccan Pinaceae. J. Essent. Oil Res. 2001, 13, 298–302. [Google Scholar] [CrossRef]
- Ghanmi, M.; Satrani, B.; Chaouch, A.; Aafi, A.; Abid, A.E.; Ismaili, M.R.; Farah, A. Composition chimique et activité antimicrobienne de l’essence de térébenthine du pin maritime (Pinus pinaster) et du pin d’Alep (Pinus hale- pensis) du Maroc. Acta Bot. Gall. 2007, 154, 293–300. [Google Scholar] [CrossRef]
- Bachir, R. Antibacterial potential of essential oils of the needles of Pinus halepensis against Staphylococcus aureus and Escherichia coli. J. Coast. Life Med. 2014, 2, 651–655. [Google Scholar] [CrossRef]
- Fekih, N.; Allali, H.; Merghache, S.; Chaïb, F.; Merghache, D.; El Amine, M.; Djabou, N.; Muselli, A.; Tabti, B.; Costa, J. Chemical composition and antibacterial activity of Pinus halepensis Miller growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Sadou, N.; Seridi, R.; Djahoudi, A.; Hadef, Y. Composition chimique et activité antibactérienne des Huiles Essentielles des aiguilles de Pinus halepensis Mill. du Nord est Algérien. Synthèse Rev. Sci. Technol. 2015, 30, 33–39. [Google Scholar]
- Mitić, Z.S.; Jovanović, B.; Jovanović, S.Č.; Stojanović-Radić, Z.Z.; Mihajilov-Krstev, T.; Jovanović, N.M.; Nikolić, B.M.; Marin, P.D.; Zlatković, B.K.; Stojanović, G.S. Essential oils of Pinus halepensis and P. heldreichii: Chemical composition, antimicrobial and insect larvicidal activity. Ind. Crops Prod. 2019, 140, 111702. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Al Farraj, D.A.; Salem, M.Z.M.; Elshikh, M.S.; Al-Kufaidy, R.; Alshammari, M.K.; Salem, A.Z.M. Potential impacts of Pinus halepensis Miller trees as a source of phytochemical compounds: Antibacterial activity of the cones essential oil and n-butanol extract. Agrofor. Syst. 2020, 94, 1403–1413. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Gargouri, S.; Fezzani, T.; Jamoussi, B. Chemical composition, physico-chemical properties, antifungal and herbicidal activities of Pinus halepensis Miller essential oils. Biol. Agric. Hortic. 2013, 29, 91–106. [Google Scholar] [CrossRef]
- Hamrouni, L.; Hanana, M.; Amri, I.; Romane, A.E.; Gargouri, S.; Jamoussi, B. Allelopathic effects of essential oils of Pinus halepensis Miller: Chemical composition and study of their antifungal and herbicidal activities. Arch. Phytopathol. Plant Prot. 2015, 48, 145–158. [Google Scholar] [CrossRef]
- Eryilmaz, M.; Tosun, A.; Tumen, I. Antimicrobial Activity of Some Species from Pinaceae and Cupressaceae. Turk. J. Pharm. Sci. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Al-Bazaz, H.K.; Al-jubori, I.S.; Eldalawy, R.; Nasser, N.M. Antimicrobial and Antioxidant Activity Pinus halepensis Miller. Cone Extract which grown in Iraq. Res. J. Pharm. Technol. 2018, 11, 4977–4980. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Behiry, S.I.; Ali, H.M.; EL-Hefny, M.; Salem, M.Z.M.; Ashmawy, N.A. Phytochemical Compounds of Branches from P. halepensis Oily Liquid Extract and S. terebinthifolius Essential Oil and Their Potential Antifungal Activity. Processes 2020, 8, 330. [Google Scholar] [CrossRef]
- Bouzenna, H.; Samout, N.; Amani, E.; Mbarki, S.; Tlili, Z.; Rjeibi, I.; Elfeki, A.; Talarmin, H.; Hfaiedh, N. Protective Effects of Pinus halepensis L. Essential Oil on Aspirin-induced Acute Liver and Kidney Damage in Female Wistar Albino Rats. J. Oleo Sci. 2016, 65, 701–712. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Bouaziz, M.; Giroux-Metges, M.-A.; Elfeki, A.; Talarmin, H. Cytoprotective effects of essential oil of Pinus halepensis L. against aspirin-induced toxicity in IEC-6 cells. Arch. Physiol. Biochem. 2017, 123, 364–370. [Google Scholar] [CrossRef]
- Sarri, M.; Boudjelal, A.; Hendel, N.; Sarri, D.; Benkhaled, A. Flora and ethnobotany of medicinal plants in the southeast of the capital of Hodna (Algeria). Arab. J. Med. Aromat. Plants 2015, 1, 24–30. [Google Scholar]
- Senouci, F.; Ababou, A.; Chouieb, M. Ethnobotanical Survey of the Medicinal Plants used in the Southern Mediterranean. Case Study: The Region of Bissa (Northeastern Dahra Mountains, Algeria). Pharmacogn. J. 2019, 11, 647–659. [Google Scholar] [CrossRef]
- Chohra, D.; Ferchichi, L. Ethnobotanical study of Belezma National Park (BNP) plants in Batna: East of Algeria. Acta Sci. Nat. 2019, 6, 40–54. [Google Scholar] [CrossRef]
- Nazim, B.; Houarı, T.; Ismaıl, B. Ethnobotanical Survey of Some Plants Used in Tessala Region, Algeria. Curr. Perspect. Med. Aromat. Plants 2020, 3, 25–30. [Google Scholar] [CrossRef]
- Miara, M.D.; Bendif, H.; Rebbas, K.; Rabah, B.; Hammou, M.A.; Maggi, F. Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria). J. Herb. Med. 2019, 16, 100262. [Google Scholar] [CrossRef]
- Mendelsohn, A.; Sato, T.; Subedi, A.; Wurcel, A.G. State-of-the-Art Review: Evaluation and Management of Delusional Infestation. Clin. Infect. Dis. 2024, 79, e1–e10. [Google Scholar] [CrossRef]
- Laid, B.; Rebbas, K.; Mouloud, G.; Rabah, B.; Brini, F.; Bouzerzour, H. Ethnobotanical study of medicinal plants in Jebel Messaad region (M’sila, Algeria). Glob. J. Res. Med. Plants Indig. Med. 2014, 3, 445–459. [Google Scholar]
- Moussi, M.; Filali, H.; Tazi, A.; Hakkou, F. Ethnobotanical survey of healing medicinal plants traditionally used in the main Moroccan cities. J. Pharmacogn. Phytother. 2015, 7, 164–182. [Google Scholar]
- Salhi, N.; Bouyahya, A.; Fettach, S.; Zellou, A.; Cherrah, Y. Ethnopharmacological study of medicinal plants used in the treatment of skin burns in occidental Morocco (area of Rabat). S. Afr. J. Bot. 2019, 121, 128–142. [Google Scholar] [CrossRef]
- Mouhajir, F.; Hudson, J.B.; Rejdali, M.; Towers, G.H.N. Multiple Antiviral Activities of Endemic Medicinal Plants Used by Berber Peoples of Morocco. Pharm. Biol. 2001, 39, 364–374. [Google Scholar] [CrossRef]
- El Abbouyi, A.; Filali-Ansari, N.; Khyari, P.; Loukili, H. Inventory of medicinal plants prescribed by traditional healers in El Jadida city and suburbs (Morocco). Int. J. Green Pharm. 2014, 8, 242–251. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Boubaker, H.; Msanda, F.; El Mousadik, A. An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (southwest Morocco). J. Appl. Biosci. 2014, 84, 7707–7722. [Google Scholar] [CrossRef]
- Shinde, U.A.; Phadke, A.S.; Nair, A.M.; Mungantiwar, A.A.; Dikshit, V.J.; Saraf, M.N. Membrane stabilizing activity—A possible mechanism of action for the anti-inflammatory activity of Cedrus deodara wood oil. Fitoterapia 1999, 70, 251–257. [Google Scholar] [CrossRef]
- Umapathy, E.; Ndebia, E.; Meeme, A.; Adam, B.; Menziwa, P.; Nkeh-Chungag, B.; Iputo, J. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res. 2010, 4, 789–795. [Google Scholar]
- Ghayth, R.; Jebali, J.; Ghazghazi, H.; Riguene, H.; Khouja, M.; Salem, R. Chemical composition and biological activities of Pinus halepensis Mill. Oil. Rev. Roum. De Chim. 2019, 64, 999–1006. [Google Scholar] [CrossRef]
- Kadri, N.; Khettal, B.; Adjebli, A.; Cresteil, T.; Yahiaoui-Zaidi, R.; Barragan-Montero, V.; Montero, J.-L. Antiangiogenic activity of neutral lipids, glycolipids, and phospholipids fractions of Pinus halepensis Mill. seeds. Ind. Crops Prod. 2014, 54, 6–12. [Google Scholar] [CrossRef]
- Merzouki, A.; Ed-derfoufi, F.; Molero Mesa, J. Contribution to the knowledge of Rifian traditional medicine. II: Folk medicine in Ksar Lakbir district (NW Morocco). Fitoterapia 2000, 71, 278–307. [Google Scholar] [CrossRef]
- Youbi, A.E.; Ouahidi, I.; Mansouri, L.E.; Daoudi, A.; Bousta, D. Ethnopharmacological survey of plants used for immunological diseases in four regions of Morocco. Eur. J. Med. Plants 2016, 13, 1. [Google Scholar] [CrossRef]
- Benlamdini, N.; Elhafian, M.; Rochdi, A.; Zidane, L. Étude floristique et ethnobotanique de la flore médicinale du Haut Atlas oriental (Haute Moulouya). J. Appl. Biosci. 2014, 78, 6771–6787. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Et-Touys, A.; Bakri, Y.; Dakka, N. Indigenous knowledge of the use of medicinal plants in the North-West of Morocco and their biological activities. Eur. J. Integr. Med. 2017, 13, 9–25. [Google Scholar] [CrossRef]
- Bilbis, L.S.; Shehu, R.A.; Abubakar, M.G. Hypoglycemic and hypolipidemic effects of aqueous extract of Arachis hypogaea in normal and Alloxan-induced diabetic rats. Phytomedicine 2002, 9, 553–555. [Google Scholar] [CrossRef]
- King, J.C.; Blumberg, J.; Ingwersen, L.; Jenab, M.; Tucker, K.L. Tree nuts and peanuts as components of a healthy diet. J. Nutr. 2008, 138, 1736s–1740s. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K. On the Dissemination of Acupuncture to Europe. JournalNX 2020, 6, 201–209. [Google Scholar]
- Periferakis, A. H Εμφάνιση του Βελονισμού στην Ευρώπη, 1683–1850-Ascensus Acupuncturae in Europam, 1683–1850; Akadimia of Ancient Greek and Traditional Chinese Medicine: Athens, Greece, 2021. [Google Scholar]
- Wiart, C. Ethnopharmacology of medicinal plants: Asia and the Pacific; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Bertioli, D.; Seijo, G.; Freitas, F.; Valls, J.; Leal-Bertioli, S.; Moretzsohn, M. An overview of peanut and its wild relatives. Plant Genet. Resour. 2011, 9, 134–149. [Google Scholar] [CrossRef]
- Hammons, R.O.; Herman, D.; Stalker, H.T. Chapter 1—Origin and Early History of the Peanut. In Peanuts; Stalker, H.T., F. Wilson, R., Eds.; AOCS Press: Champaign, IL, USA, 2016; pp. 1–26. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Peanut (Arachis hypogaea L.): A Prospective Legume Crop to Offer Multiple Health Benefits Under Changing Climate. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1325–1338. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Shi, M. Clinical efficacy of Groundnut leaves on insomnia treatments. Shanghai J. Trad. Chin. Med. 2001, 5, 8–10. [Google Scholar]
- Hu, P.; Fan, R.; Li, Y.; Pang, C. Studies on pharmacological action of Luohuashengzhiye extract. Chin. Trad. Pat. Med. 2001, 23, 919–920. [Google Scholar]
- Wang, Y.; Li, H.; Xu, Y.; Zhang, Y.; Xu, D.; Xiao, L.; Li, X. Clinical confirmation of preparation from the branch and leaf of peanut in treating insomnia. Shanghai J. Tradit. Chin. Med. 2001, 5, 8–10. [Google Scholar]
- Lou, H.; Yamazaki, Y.; Sasaki, T.; Uchida, M.; Tanaka, H.; Oka, S. A-type proanthocyanidins from peanut skins. Phytochemistry 1999, 51, 297–308. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, M.-K.; Kim, Y.-K.; Kim, K.-E.; Hyun, K.-Y. Attenuant effects of Hovenia dulcis extract on inflammatory orifacial pain in rats. J. Korea Acad.-Ind. Coop. Soc. 2014, 15, 5088–5094. [Google Scholar]
- Lim, S.J.; Kim, M.; Randy, A.; Nam, E.J.; Nho, C.W. Effects of Hovenia dulcis Thunb. extract and methyl vanillate on atopic dermatitis-like skin lesions and TNF-α/IFN-γ-induced chemokines production in HaCaT cells. J. Pharm. Pharmacol. 2016, 68, 1465–1479. [Google Scholar] [CrossRef]
- Park, J.-Y.; Moon, J.-Y.; Park, S.-D.; Park, W.-H.; Kim, H.; Kim, J.-E. Fruits extracts of Hovenia dulcis Thunb. suppresses lipopolysaccharide-stimulated inflammatory responses through nuclear factor-kappaB pathway in Raw 264.7 cells. Asian Pac. J. Trop. Med. 2016, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Oh, Y.-C.; Cho, W.-K.; Yim, N.-H.; Ma, J.Y. Hoveniae Semen Seu Fructus Ethanol Extract Exhibits Anti-Inflammatory Activity via MAPK, AP-1, and STAT Signaling Pathways in LPS-Stimulated RAW 264.7 and Mouse Peritoneal Macrophages. Mediat. Inflamm. 2019, 2019, 9184769. [Google Scholar] [CrossRef]
- Xu, B.-J.; Deng, Y.-Q.; Sung, C.-K. Advances in studies on bioactivity of Hovenia dulcis. Agric. Chem. Biotechnol. 2004, 47, 1–5. [Google Scholar]
- Yoshikawa, M.; Ueda, T.; Muraoka, O.; Aoyama, H.; Matsuda, H.; Shimoda, H.; Yamahara, J.; Murakami, N. Absolute stereostructures of hovenidulciosides A1 and A2, bioactive novel triterpene glycosides from hoveniae semen seu fructus, the seeds and fruit of Hovenia dulcis Thunb. Chem. Pharm. Bull. 1995, 43, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Ueda, T.; Matsuda, H.; Yamahara, J.; Murakami, N. Bioactive Saponins and Glycosides. IV. Four Methyl-Migrated 16, 17-Seco-Dammarane Triterpene Glycosides from Chinese Natural Medicine, Hoveniae Semen Seu Fructus, the Seeds and Fruit of Hovenia dulcis Thunb: Absolute Stereostructures and Inhibitory Activity on Histamine Release of Hovenidulcioseds A1, A2, B1, and B2. Chem. Pharm. Bull. 1996, 44, 1736–1743. [Google Scholar]
- Choi, C.-Y.; Cho, S.-S.; Yoon, I.-S. Hot-water extract of the branches of Hovenia dulcis Thunb.(Rhamnaceae) ameliorates low-fiber diet-induced constipation in rats. Drug Des. Dev. Ther. 2018, 12, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Cha, P.-H.; Shin, W.; Zahoor, M.; Kim, H.-Y.; Min, D.S.; Choi, K.-Y. Hovenia dulcis Thunb extract and its ingredient methyl vanillate activate wnt/β-catenin pathway and increase bone mass in growing or ovariectomized mice. PLoS ONE 2014, 9, e85546. [Google Scholar] [CrossRef]
- Kim, H.-L.; Sim, J.-E.; Choi, H.-M.; Choi, I.-Y.; Jeong, M.-Y.; Park, J.; Jung, Y.; Youn, D.-H.; Cho, J.-H.; Kim, J.-H. The AMPK pathway mediates an anti-adipogenic effect of fruits of Hovenia dulcis Thunb. Food Funct. 2014, 5, 2961–2968. [Google Scholar] [CrossRef]
- Pinto, J.T.; Oliveira, T.T.d.; Alvarenga, L.F.; Barbosa, A.S.; Pizziolo, V.R.; Costa, M.R.d. Pharmacological activity of the hydroalcoholic extract from Hovenia dulcis thunberg fruit and the flavonoid dihydromyricetin during hypercholesterolemia induced in rats. Braz. J. Pharm. Sci. 2014, 50, 727–735. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Q.; Song, X.; Yang, Q.; Kan, J. Physicochemical properties and bioactive function of Japanese grape (Hovenia dulcis) pomace insoluble dietary fibre modified by ball milling and complex enzyme treatment. Int. J. Food Sci. Technol. 2019, 54, 2363–2373. [Google Scholar] [CrossRef]
- Cha, W.S.; Oh, J.H.; Park, H.J.; Ahn, S.W.; Hong, S.Y.; Kim, N.I. Historical difference between traditional Korean medicine and traditional Chinese medicine. Neurol. Res. 2007, 29 (Suppl. 1), S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B.; Mutalik, G.; Tillu, G. Chapter 2—Evolution of Medicine. In Integrative Approaches for Health; Patwardhan, B., Mutalik, G., Tillu, G., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 27–52. [Google Scholar] [CrossRef]
- Kumar, H.; Song, S.Y.; More, S.V.; Kang, S.M.; Kim, B.W.; Kim, I.S.; Choi, D.K. Traditional Korean East Asian medicines and herbal formulations for cognitive impairment. Molecules 2013, 18, 14670–14693. [Google Scholar] [CrossRef]
- Kuete, V.; Seo, E.-J.; Krusche, B.; Oswald, M.; Wiench, B.; Schröder, S.; Greten, H.J.; Lee, I.-S.; Efferth, T. Cytotoxicity and pharmacogenomics of medicinal plants from traditional Korean medicine. Evid.-Based Complement. Altern. Med. 2013, 2013, 341724. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Park, J.Y.; Sung, A.D.; Sung, S.H. The utilization of traditional herbal medicine for treatment in traditional Korean medicine clinics. In Medicinal Plants-Use in Prevention and Treatment of Diseases; Hassan, B.A.R., Ed.; IntechOpen: London, UK, 2019; pp. 111–126. [Google Scholar]
- Sakai, K.; Yamane, T.; Saitoh, Y.; Ikawa, C.; Nishihata, T. Effect of water extracts of crude drugs in decreasing blood ethanol concentrations in rats. Chem. Pharm. Bull. 1987, 35, 4597–4604. [Google Scholar] [CrossRef]
- An, S.-W.; Kim, Y.-G.; Kim, M.-H.; Lee, B.-I.; Lee, S.-H.; Kwon, H.-I.; Hwang, B.; Lee, H.-Y. Comparison of Hepatic Detoxification activity and reducing Serum Alcohol concentration of Hovenia dulsis Thunb and Alnus japonica Steud. Korean J. Med. Crop Sci. 1999, 7, 263–268. [Google Scholar]
- Kim, M.-H.; Chung, Y.-T.; Lee, J.-H.; Park, Y.-S.; Shin, M.-K.; Kim, H.-S.; Kim, D.-H.; Lee, H.-Y. Hepatic detoxification activity and reduction of serum alcohol concentration of Hovenia dulcis Thunb from Korea and China. Korean J. Med. Crop Sci. 2000, 8, 225–233. [Google Scholar]
- Cha, B.-C.; Lee, E.-H.; Lee, E.; Park, H.-H. Activity of glutathione S-transferase and effect of alcohol decomposition on the fruit of Hovenia dulcis Thunb. Yakhak Hoeji 2004, 48, 213–217. [Google Scholar]
- Sung-Hee, K.; Jin-Yeul, M. A study on the extraction and efficacy of bioactive compound from Hovenia dulcis. KSBB J. 2006, 21, 11–15. [Google Scholar]
- Hänsel, R.; Sticher, O. Pharmakognosie-Phytopharmazie; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Chen, S.; Zhong, G.; Li, A.; Li, S.; Wu, L. Influence of Hovenia dulcis on alcohol concentration in blood and activity of alcohol dehydrogenase (ADH) of animals after drinking. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2006, 31, 1094–1096. [Google Scholar]
- Hase, K.; Ohsugi, M.; Xiong, Q.; Basnet, P.; Kadota, S.; Namba, T. Hepatoprotective effect of Hovenia dulcis Thunb. on experimental liver injuries induced by carbon tetrachloride or D-galactosamine: Lipopolysaccharide. Biol. Pharm. Bull. 1997, 20, 381–385. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Ueda, T.; Yoshizumi, S.; Ninomiya, K.; Murakami, N.; Matsuda, H.; Saito, M.; Fujii, W.; Tanaka, T. Bioactive constituents of Chinese natural medicines. III. Absolute stereostructures of new dihydroflavonols, hovenitins I, II, and III, isolated from hoveniae semen seu fructus, the seed and fruit of Hovenia dulcis THUNB.(Rhamnaceae): Inhibitory effect on alcohol-induced muscular relaxation and hepatoprotective activity. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1997, 117, 108–118. [Google Scholar]
- Ji, Y.; Lu, H. Protective effect of the water extract from Hovenia dulcis Thunb. on CCl4-induced experimental hepatic injury. Lishizhen Med. Mater. Med. Res. 2002, 13, 327–328. [Google Scholar]
- Cederbaum, A.I. Introduction: Role of lipid peroxidation and oxidative stress in alcohol toxicity. Free Radic. Biol. Med. 1989, 7, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Raju, B.S.S.; Sharma, V.; Reddanna, P.; Babu, P.P. Formation of acetaldehyde adducts of glutathione S-transferase A3 in the liver of rats administered alcohol chronically. Alcohol 2005, 35, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.; Khan, M.A.; Jan, F. Traditional medicinal and economic uses of gymnosperms of Dir Kohistan Valleys, NWFP, Pakistan. Ethnobot. Leafl. 2009, 2009, 9. [Google Scholar]
- Acar, İ.; Gül, G.; Örtel, E. Türkiye’de kızılçam ormanlarından akma reçine üretiminde asit pasta tahrik tekniğinin uygulanması esasları üzerine araştırmalar. Ege Or. Ar. En. Müd. Teknik. Bülten 1996, 5, 1–52. [Google Scholar]
- Rezzi, S.; Bighelli, A.; Castola, V.; Casanova, J. Composition and chemical variability of the oleoresin of Pinus nigra ssp. laricio from Corsica. Ind. Crops Prod. 2005, 21, 71–79. [Google Scholar] [CrossRef]
- Tumen, I.; Reunanen, M. A Comparative Study on Turpentine Oils of Oleoresins of Pinus sylvestris L. from Three Districts of Denizli. Rec. Nat. Prod. 2010, 4, 224–229. [Google Scholar]
- Varga, J.; Kocsubé, S.; Tóth, B.; Frisvad, J.C.; Perrone, G.; Susca, A.; Meijer, M.; Samson, R.A. Aspergillus brasiliensis sp. nov., a biseriate black Aspergillus species with world-wide distribution. Int. J. Syst. Evol. Microbiol. 2007, 57, 1925–1932. [Google Scholar] [CrossRef]
- Kizil, M.; Kizil, G.; Yavuz, M.; Aytekin, Ç. Antimicrobial Activity of the Tar Obtained from the Roots and Stems of Pinus brutia. Pharm. Biol. 2002, 40, 135–138. [Google Scholar] [CrossRef]
- Chammam, A.; Fillaudeau, L.; Romdhane, M.; Bouajila, J. Chemical Composition and In Vitro Bioactivities of Extracts from Cones of P. halepensis, P. brutia, and P. pinea: Insights into Pharmaceutical and Cosmetic Potential. Plants 2024, 13, 1802. [Google Scholar] [CrossRef] [PubMed]
- Coppen, J.J.W.; Robinson, J.M.; Kaushal, A.N. Composition of xylem resin from Pinus wallichiana and P. roxburghii. Phytochemistry 1988, 27, 2873–2875. [Google Scholar] [CrossRef]
- Willför, S.; Ali, M.; Karonen, M.; Reunanen, M.; Arfan, M.; Harlamow, R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung 2009, 63, 551–558. [Google Scholar] [CrossRef]
- Parihar, P.; Parihar, L.; Bohra, A. Antibacterial activity of extracts of Pinus roxburghii Sarg. Bangladesh J. Bot. 2006, 35, 85–86. [Google Scholar]
- Zafar, I.; Fatima, A.; Khan, S.; Rehman, Z.; Mehmud, S. GC-MS studies of needles essential oil of Pinus roxburghaii and their antimicrobial activity from. Electron. J. Environ. Agric. Food Chem. 2010, 9, 468–473. [Google Scholar]
- Chaudhary, A.K.; Ahmad, S.; Mazumder, A. Study of antibacterial and antifungal activity of traditional Cedrus deodara and Pinus roxburghii Sarg. CellMed 2012, 2, 37.31–37.34. [Google Scholar]
- Maimoona, A.; Naeem, I.; Shujaat, S.; Saddiqe, Z.; Mughal, T.; Mehmood, T. Comparison of Radical Scavenging Capacity of Different Extracts of Barks and Needles of Pinus roxburghii and Pinus wallichiana. Asian J. Chem. 2011, 23, 819–822. [Google Scholar]
- Kaushik, D.; Kumar, A.; Kaushik, P.; Rana, A.C. Analgesic and Anti-Inflammatory Activity of Pinus roxburghii Sarg. Adv. Pharmacol. Sci. 2012, 2012, 245431. [Google Scholar] [CrossRef]
- Kaushik, D.; Kumar, A.; Kaushik, P.; Rana, A.C. Anticonvulsant activity of alcoholic extract of bark of Pinus roxburghii Sarg. Zhong Xi Yi Jie He Xue Bao 2012, 10, 1056–1060. [Google Scholar] [CrossRef]
- Rastogi, S.; Shukla, A.; Kolhapure, S. Evaluation of the clinical efficacy and safety of RG-01 (Rumalaya gel) in the management of chronic sub-acute inflammatory joint disorder. Med. Update 2004, 12, 31–37. [Google Scholar]
- Ahmad, R.; Rao, R.A.K.; Masood, M.M. Removal and recovery of Cr (VI) from synthetic and industrial wastewater using bark of Pinus roxburghii as an adsorbent. Water Qual. Res. J. 2005, 40, 462–468. [Google Scholar] [CrossRef]
- Azmat, A.; Ahmed, K.Z.; Ahmed, M.; Tariq, B. Antinociceptive effects of poly herbal oil extract (PHOE). Pak. J. Pharmacol. 2006, 23, 1–7. [Google Scholar]
- Khan, I.; Singh, V.; Chaudhary, A.K. Hepatoprotective activity of Pinus roxburghii Sarg. wood oil against carbon tetrachloride-and ethanol-induced hepatotoxicity. Bangladesh J. Pharmacol. 2012, 7, 94–99. [Google Scholar] [CrossRef]
- Ahsan, R.; Islam, M. In vitro antibacterial screening and toxicological study of some useful plants (Cajanus cajan). Eur. J. Sci. Res. 2009, 41, 227–232. [Google Scholar]
- Zu, Y.G.; Liu, X.L.; Fu, Y.J.; Wu, N.; Kong, Y.; Wink, M. Chemical composition of the SFE-CO extracts from Cajanus cajan (L.) Huth and their antimicrobial activity in vitro and in vivo. Phytomedicine 2010, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz-Villena, A.; Parga-Lozano, C.; Moreno, E.; Areces, C.; Rey, D.; Gomez-Prieto, P. The Origin of Amerindians and the Peopling of the Americas According to HLA Genes: Admixture with Asian and Pacific People. Curr. Genom. 2010, 11, 103–114. [Google Scholar] [CrossRef]
- Chandler, R.F.; Freeman, L.; Hooper, S.N. Herbal remedies of the maritime Indians. J. Ethnopharmacol. 1979, 1, 49–68. [Google Scholar] [CrossRef]
- Arnason, T.; Hebda, R.J.; Johns, T. Use of plants for food and medicine by Native Peoples of eastern Canada. Can. J. Bot. 1981, 59, 2189–2325. [Google Scholar] [CrossRef]
- Turner, N.J.; Hebda, R.J. Contemporary use of bark for medicine by two salishan native elders of Southeast Vancouver Island, Canada. J. Ethnopharmacol. 1990, 29, 59–72. [Google Scholar] [CrossRef]
- Gottesfeld, L.M.J. Wet’Suwet’en ethnobotany: Traditional plant uses. J. Ethnobiol. 1994, 14, 185–210. [Google Scholar]
- Uprety, Y.; Asselin, H.; Dhakal, A.; Julien, N. Traditional use of medicinal plants in the boreal forest of Canada: Review and perspectives. J. Ethnobiol. Ethnomed. 2012, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.S.; Lambert, J.; Saleem, A.; Coonishish, J.; Martineau, L.C.; Cuerrier, A.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.L. Antidiabetic Activity of Extracts from Needle, Bark, and Cone of Picea glauca.: Organ-Specific Protection from Glucose Toxicity and Glucose Deprivation. Pharm. Biol. 2008, 46, 126–134. [Google Scholar] [CrossRef]
- Mooney, J. The sacred formulas of the Cherokees. In Seventh Annual Report of the Bureau of Ethnology; Powell, J.W., Ed.; Government Printing Office: Washington, DC, USA, 1891; Volume 7, pp. 301–397. [Google Scholar]
- Hamel, P.B.; Chiltoskey, M.U. Cherokee Plants and Their Uses: A 400 Year History; Herald Publishing Company: Sylva, NC, USA, 1975. [Google Scholar]
- Garrett, J.T. The Cherokee Herbal: Native Plant Medicine from the Four Directions; Simon and Schuster: Rochester, NY, USA, 2003. [Google Scholar]
- Setzer, W.N. The Phytochemistry of Cherokee Aromatic Medicinal Plants. Medicines 2018, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Kirkman, L.K. Trees of Georgia and Adjacent States; Timber Press: Portland, OR, USA, 2000. [Google Scholar]
- Radford, A.E.; Ahles, H.E.; Bell, C.R. Manual of the Vascular Flora of the Carolinas; Univ of North Carolina Press: Chapel Hill, NC, USA, 2010. [Google Scholar]
- Boag, D.; Kiceniuk, J. Protein and caloric content of lodge pole pine needles. For. Chron. 1968, 44, 28–31. [Google Scholar] [CrossRef]
- Anäs, E.; Ekman, R.; Holmbom, B. Composition of nonpolar extractives in bark of Norway spruce and Scots pine. J. Wood Chem. Technol. 1983, 3, 119–130. [Google Scholar] [CrossRef]
- Fischer, C.; Höll, W. Food reserves of Scots pine (Pinus sylvestris L.) I. Seasonal changes in the carbohydrate and fat reserves of pine needles. Trees 1991, 5, 187–195. [Google Scholar] [CrossRef]
- Nergiz, C.; Dönmez, I. Chemical composition and nutritive value of Pinus pinea L. seeds. Food Chem. 2004, 86, 365–368. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crops Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Angel, H.Z.; Priest, J.S.; Stovall, J.P.; Oswald, B.P.; Weng, Y.; Williams, H.M. Individual tree and stand-level carbon and nutrient contents across one rotation of loblolly pine plantations on a reclaimed surface mine. New For. 2019, 50, 733–753. [Google Scholar] [CrossRef]
- Dziedzinski, M.; Kobus-Cisowska, J.; Szymanowska, D.; Stuper-Szablewska, K.; Baranowska, M. Identification of polyphenols from coniferous shoots as natural antioxidants and antimicrobial compounds. Molecules 2020, 25, 3527. [Google Scholar] [CrossRef]
- Nedelea, D.-G.; Vulpe, D.E.; Viscopoleanu, G.; Radulescu, A.C.; Mihailescu, A.A.; Gradinaru, S.; Orghidan, M.; Scheau, C.; Cergan, R.; Dragosloveanu, S. Progressive Thoracolumbar Tuberculosis in a Young Male: Diagnostic, Therapeutic, and Surgical Insights. Infect. Dis. Rep. 2024, 16, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Reed, C. Complications of tuberculosis. Curr. Opin. Infect. Dis. 2014, 27, 403–410. [Google Scholar] [CrossRef]
- Nadgir, C.A.; Biswas, D.A. Antibiotic Resistance and Its Impact on Disease Management. Cureus 2023, 15, e38251. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal Drug Resistance: An Emergent Health Threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.A.; Nordstrom, M.; Maloney, P.; Rodriguez, M.; Naceanceno, K.; Gallo, G.; Mejia, R.; Hershow, R. Parasitic infections represent a significant health threat among recent immigrants in Chicago. Parasitol. Res. 2020, 119, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Intestinal Parasitic Infections in 2023. Gastroenterol. Res 2023, 16, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.A.; Pritt, B.S. The Landscape of Parasitic Infections in the United States. Mod. Pathol. 2023, 36, 100217. [Google Scholar] [CrossRef]
- Mishra, B.; Rath, S.; Mohanty, M.; Mohapatra, P.R. The Threat of Impending Pandemics: A Proactive Approach. Cureus 2023, 15, e36723. [Google Scholar] [CrossRef]
- Casadevall, A. Pandemics past, present, and future: Progress and persistent risks. J. Clin. Investig. 2024, 134, e179519. [Google Scholar] [CrossRef]
- Scott, H.F.; Greenwald, E.E.; Bajaj, L.; Davies, S.J.D.; Brou, L.; Kempe, A. The sensitivity of clinician diagnosis of sepsis in tertiary and community-based emergency settings. J. Pediatr. 2018, 195, 220–227.e221. [Google Scholar] [CrossRef] [PubMed]
- Wentowski, C.; Mewada, N.; Nielsen, N.D. Sepsis in 2018: A review. Anaesth. Intensive Care Med. 2019, 20, 6–13. [Google Scholar] [CrossRef]
- Cuesta-Montero, P.; Navarro-Martínez, J.; Yedro, M.; Galiana-Ivars, M. Sepsis and Clinical Simulation: What Is New?(and Old). J. Pers. Med. 2023, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, G.P.; Tomescu, D.R.; Pleș, L.; Panaitescu, A.M.; Dragosloveanu, Ș.; Scheau, C.; Sima, R.M.; Coman, I.S.; Grigorean, V.T.; Cochior, D. Implications of using artificial intelligence in the diagnosis of sepsis/sepsis shock. Germs 2024, 14, 77–84. [Google Scholar] [CrossRef]
- Couce, A.; Blázquez, J. Side effects of antibiotics on genetic variability. FEMS Microbiol. Rev. 2009, 33, 531–538. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.S.; Shah, S. Antifungal drugs. Side Eff. Drugs Annu. 2019, 41, 285–292. [Google Scholar]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiviral drugs: Current state of the art. J. Clin. Virol. 2001, 22, 73–89. [Google Scholar] [CrossRef]
- Abers, M.S.; Shandera, W.X.; Kass, J.S. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs 2014, 28, 131–145. [Google Scholar] [CrossRef]
- Rosenblatt, J.E. Antiparasitic agents. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1161–1174. [Google Scholar]
- Baggish, A.L.; Hill, D.R. Antiparasitic agent atovaquone. Antimicrob. Agents Chemother. 2002, 46, 1163–1173. [Google Scholar] [CrossRef]
- Kappagoda, S.; Singh, U.; Blackburn, B.G. Antiparasitic therapy. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 561–583. [Google Scholar]
- Periferakis, A.; Caruntu, A.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Caruntu, C.; Scheau, C. Availability, Toxicology and Medical Significance of Antimony. Int. J. Environ. Res. Public. Health 2022, 19, 4669. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Avellana-Zaragoza, J.A.; Martínez-Reig, M.; Mas-Bargues, C.; Inglés, M. Oxidative stress and exceptional human longevity: Systematic review. Free Radic. Biol. Med. 2020, 149, 51–63. [Google Scholar] [CrossRef]
- Spector, A. Review: Oxidative Stress and Disease. J. Ocul. Pharmacol. Ther. 2000, 16, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Shoham, A.; Hadziahmetovic, M.; Dunaief, J.L.; Mydlarski, M.B.; Schipper, H.M. Oxidative stress in diseases of the human cornea. Free Radic. Biol. Med. 2008, 45, 1047–1055. [Google Scholar] [CrossRef]
- Căruntu, C.; Boda, D.; Musat, S.; Căruntu, A.; Mandache, E. Stress-induced mast cell activation in glabrous and hairy skin. Mediat. Inflamm. 2014, 2014, 105950. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Mateo, R.; Beyer, W.N.; Spann, J.; Hoffman, D.; Ramis, A. Relationship Between Oxidative Stress, Pathology, and Behavioral Signs of Lead Poisoning in Mallards. J. Toxicol. Environ. Health Part A 2003, 66, 1371–1389. [Google Scholar] [CrossRef]
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Carding, S.R. Inflammatory bowel disease: Cause and immunobiology. Lancet 2007, 369, 1627–1640. [Google Scholar] [CrossRef]
- Sands, B.E. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology 2015, 149, 1275–1285.e1272. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of disease: Inflammatory bowel diseases. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–165. [Google Scholar]
- Păsărică, M.A.; Curcă, P.F.; Dragosloveanu, C.D.M.; Tătaru, C.I.; Manole, I.R.; Murgoi, G.E.; Grigorescu, A.C. Underlying Ciliary Body Uveal Melanoma in a Patient with Chronic Lymphocytic Leukemia Presenting for Hyphema. Diagnostics 2022, 12, 1312. [Google Scholar] [CrossRef] [PubMed]
- Păsărică, M.A.; Curcă, P.F.; Burcea, M.; Schmitzer, S.; Dragosloveanu, C.D.M.; Grigorescu, A.C. The Effects of Oncological Treatment on Redox Balance in Patients with Uveal Melanoma. Diagnostics 2023, 13, 1907. [Google Scholar] [CrossRef]
- Păsărică, M.A.; Curcă, P.F.; Dragosloveanu, C.D.M.; Grigorescu, A.C.; Nisipașu, C.I. Pathological and Molecular Diagnosis of Uveal Melanoma. Diagnostics 2024, 14, 958. [Google Scholar] [CrossRef] [PubMed]
- Caruntu, C.; Boda, D.; Constantin, C.; Caruntu, A.; Neagu, M. Catecholamines Increase in Vitro Proliferation of Murine B16F10 Melanoma Cells. Acta Endocrinol. 2014, 10, 545–558. [Google Scholar] [CrossRef]
- Zabaleta, J. Multifactorial etiology of gastric cancer. Cancer Epigenetics: Methods Protocols; Springer: Berlin/Heidelberg, Germany, 2012; pp. 411–435. [Google Scholar]
- Ion, A.; Popa, I.M.; Papagheorghe, L.M.; Lisievici, C.; Lupu, M.; Voiculescu, V.; Caruntu, C.; Boda, D. Proteomic Approaches to Biomarker Discovery in Cutaneous T-Cell Lymphoma. Dis. Markers 2016, 2016, 9602472. [Google Scholar] [CrossRef]
- Hausman, D.M. What is cancer? Perspect. Biol. Med. 2019, 62, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Scheau, C.; Constantin, C.; Neagu, M. Recent Advances in Signaling Pathways Comprehension as Carcinogenesis Triggers in Basal Cell Carcinoma. J. Clin. Med. 2020, 9, 3010. [Google Scholar] [CrossRef]
- Wan, M.-l.; Wang, Y.; Zeng, Z.; Deng, B.; Zhu, B.-s.; Cao, T.; Li, Y.-k.; Xiao, J.; Han, Q.; Wu, Q. Colorectal cancer (CRC) as a multifactorial disease and its causal correlations with multiple signaling pathways. Biosci. Rep. 2020, 40, BSR20200265. [Google Scholar] [CrossRef]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef]
- Scheau, C.; Draghici, C.; Ilie, M.A.; Lupu, M.; Solomon, I.; Tampa, M.; Georgescu, S.R.; Caruntu, A.; Constantin, C.; Neagu, M.; et al. Neuroendocrine Factors in Melanoma Pathogenesis. Cancers 2021, 13, 2277. [Google Scholar] [CrossRef]
- Caruntu, A.; Moraru, L.; Surcel, M.; Munteanu, A.; Costache, D.O.; Tanase, C.; Constantin, C.; Scheau, C.; Neagu, M.; Caruntu, C. Persistent Changes of Peripheral Blood Lymphocyte Subsets in Patients with Oral Squamous Cell Carcinoma. Healthcare 2022, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Cretu, B.; Zamfir, A.; Bucurica, S.; Scheau, A.E.; Savulescu Fiedler, I.; Caruntu, C.; Caruntu, A.; Scheau, C. Role of Cannabinoids in Oral Cancer. Int. J. Mol. Sci. 2024, 25, 969. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Paresoglou, I.; Paresoglou, N. The significance of the Lavrion mines in Greek and European Geoheritage. Eur. Geol. 2019, 48, 24–27. [Google Scholar]
- Matei, A.-M.; Caruntu, C.; Tampa, M.; Georgescu, S.R.; Matei, C.; Constantin, M.M.; Constantin, T.V.; Calina, D.; Ciubotaru, D.A.; Badarau, I.A. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl. Sci. 2021, 11, 4915. [Google Scholar] [CrossRef]
- Zhen, X.; Xie, C.; Jiang, Y.; Ai, X.; Xing, B.; Pu, K. Semiconducting Photothermal Nanoagonist for Remote-Controlled Specific Cancer Therapy. Nano Lett. 2018, 18, 1498–1505. [Google Scholar] [CrossRef]
- Bhagwat, D.A.; Swami, P.A.; Nadaf, S.J.; Choudhari, P.B.; Kumbar, V.M.; More, H.N.; Killedar, S.G.; Kawtikwar, P.S. Capsaicin Loaded Solid SNEDDS for Enhanced Bioavailability and Anticancer Activity: In-Vitro, In-Silico, and In-Vivo Characterization. J. Pharm. Sci. 2021, 110, 280–291. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Mu, Y.; Foda, M.F.; Han, H. Activation of TRPV1 by capsaicin-loaded CaCO(3) nanoparticle for tumor-specific therapy. Biomaterials 2022, 284, 121520. [Google Scholar] [CrossRef]
- Timofticiuc, I.A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2023, 15, 7. [Google Scholar] [CrossRef]
- Chae, S.; Cho, D.W. Biomaterial-based 3D bioprinting strategy for orthopedic tissue engineering. Acta Biomater. 2023, 156, 4–20. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Garofil, N.D.; Didilescu, A.C.; Caruntu, C.; Scheau, C. 3D Bioprinting in Limb Salvage Surgery. J. Funct. Biomater. 2024, 15, 383. [Google Scholar] [CrossRef]
- He, Y.; Chen, J.; Chen, Y.; Qian, H. Effect of Operating Room Nursing Management on Nosocomial Infection in Orthopedic Surgery: A Meta-Analysis. J. Healthc. Eng. 2022, 2022, 4193932. [Google Scholar] [CrossRef]
- Dragosloveanu, S.; Petre, M.A.; Capitanu, B.S.; Dragosloveanu, C.D.M.; Cergan, R.; Scheau, C. Initial Learning Curve for Robot-Assisted Total Knee Arthroplasty in a Dedicated Orthopedics Center. J. Clin. Med. 2023, 12, 6950. [Google Scholar] [CrossRef] [PubMed]
- Gaudias, J. Antibiotic prophylaxis in orthopedics-traumatology. Orthop. Traumatol. Surg. Res. 2021, 107, 102751. [Google Scholar] [CrossRef] [PubMed]
- Dragosloveanu, S.; Petre, M.-A.; Gherghe, M.E.; Nedelea, D.-G.; Scheau, C.; Cergan, R. Overall Accuracy of Radiological Digital Planning for Total Hip Arthroplasty in a Specialized Orthopaedics Hospital. J. Clin. Med. 2023, 12, 4503. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Monteiro, F.J.; Ferraz, M.P. Bioengineering Approaches to Fight against Orthopedic Biomaterials Related-Infections. Int. J. Mol. Sci. 2022, 23, 11658. [Google Scholar] [CrossRef]
- Dragosloveanu, S.; Petre, M.-A.; Gherghe, M.E.; Baz, R.O.; Cergan, R.; Scheau, C. Elbow Reconstruction with Megaprosthesis: An Effective Strategy for Salvage Surgery in Trauma Patients. Diagnostics 2024, 14, 724. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, T.; Zhang, D.; He, Q.; Du, M.; Zeng, F. Impact of negative pressure wound treatment on incidence of surgical site infection in varied orthopedic surgeries: A systematic review and meta-analysis. Int. Wound J. 2023, 20, 2334–2345. [Google Scholar] [CrossRef]
- Bahadır, B.; Atik, O.; Kanatlı, U.; Sarıkaya, B. A brief introduction to medical image processing, designing and 3D printing for orthopedic surgeons. Jt. Dis. Relat. Surg. 2023, 34, 451–454. [Google Scholar] [CrossRef]
- Dragosloveanu, Ș.; Dragosloveanu, C.D.; Cotor, D.C.; Stoica, C.I. Short vs. long intramedullary nail systems in trochanteric fractures: A randomized prospective single center study. Exp. Ther. Med. 2022, 23, 1–5. [Google Scholar] [CrossRef]
- Huang, H.K.; Aberle, D.R.; Lufkin, R.; Grant, E.G.; Hanafee, W.N.; Kangarloo, H. Advances in medical imaging. Ann. Intern. Med. 1990, 112, 203–220. [Google Scholar] [CrossRef]
- Căruntu, C.; Boda, D.; Căruntu, A.; Rotaru, M.; Baderca, F.; Zurac, S. In vivo imaging techniques for psoriatic lesions. Rom. J. Morphol. Embryol. 2014, 55, 1191–1196. [Google Scholar] [PubMed]
- Periferakis, A.; Bolocan, A.; Ion, D. A Review of Innovation in Medicine. Technol. Innov. Life Sci. 2022, 1, 42–48. [Google Scholar] [CrossRef]
- Enyedi, M.; Scheau, C.; Baz, R.O.; Didilescu, A.C. Circle of Willis: Anatomical variations of configuration. A magnetic resonance angiography study. Folia Morphol. 2023, 82, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Dragosloveanu, S.; Capitanu, B.-S.; Josanu, R.; Vulpe, D.; Cergan, R.; Scheau, C. Radiological Assessment of Coronal Plane Alignment of the Knee Phenotypes in the Romanian Population. J. Clin. Med. 2024, 13, 4223. [Google Scholar] [CrossRef]
- Nedelea, D.-G.; Vulpe, D.; Enyedi, M.; Cergan, R.; Scheau, C.; Baz, R.O.; Dragosloveanu, S. Comprehensive Approach of the Diagnosis, Treatment, and Medical Rehabilitation of Patients with Spondylolisthesis. Balneo PRM Res. J. 2024, 15, 2. [Google Scholar] [CrossRef]
- Rong, J.; Liu, Y. Advances in medical imaging techniques. BMC Methods 2024, 1, 10. [Google Scholar] [CrossRef]
- Petran, E.M.; Periferakis, A.; Troumpata, L.; Periferakis, A.-T.; Scheau, A.-E.; Badarau, I.A.; Periferakis, K.; Caruntu, A.; Savulescu-Fiedler, I.; Sima, R.-M.; et al. Capsaicin: Emerging Pharmacological and Therapeutic Insights. Curr. Issues Mol. Biol. 2024, 46, 7895–7943. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B. The effect of obesity on health outcomes. Mol. Cell. Endocrinol. 2010, 316, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.E. The adverse effects of obesity on reproduction. Reproduction 2010, 140, 343. [Google Scholar] [CrossRef]
- Lam, B.C.C.; Lim, A.Y.L.; Chan, S.L.; Yum, M.P.S.; Koh, N.S.Y.; Finkelstein, E.A. The impact of obesity: A narrative review. Singap. Med. J. 2023, 64, 163–171. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.-E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef] [PubMed]
- Neagu, M.; Constantin, C.; Surcel, M.; Munteanu, A.; Scheau, C.; Savulescu-Fiedler, I.; Caruntu, C. Diabetic neuropathy: A NRF2 disease? J. Diabetes 2024, 16, e13524. [Google Scholar] [CrossRef]
- Pouwer, F.; Nefs, G.; Nouwen, A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: A review. Endocrinol. Metab. Clin. 2013, 42, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Labib, A.-M.; Martins, A.P.; Raposo, J.F.; Torre, C. The association between polypharmacy and adverse health consequences in elderly type 2 diabetes mellitus patients; a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2019, 155, 107804. [Google Scholar]
- King, K.; Rosenthal, A. The adverse effects of diabetes on osteoarthritis: Update on clinical evidence and molecular mechanisms. Osteoarthr. Cartil. 2015, 23, 841–850. [Google Scholar] [CrossRef]
- Kubyshkin, A.; Shevandova, A.; Petrenko, V.; Fomochkina, I.; Sorokina, L.; Kucherenko, A.; Gordienko, A.; Khimich, N.; Zyablitskaya, E.; Makalish, T. Anti-inflammatory and antidiabetic effects of grape-derived stilbene concentrate in the experimental metabolic syndrome. J. Diabetes Metab. Disord. 2020, 19, 1205–1214. [Google Scholar] [CrossRef]
- Choi, S.Z.; Lee, S.O.; Jang, K.U.; Chung, S.H.; Park, S.H.; Kang, H.C.; Yang, E.Y.; Cho, H.J.; Lee, K.R. Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch. Pharmacal Res. 2005, 28, 1027–1030. [Google Scholar] [CrossRef]
- Peng, J.; Lu, C.; Luo, Y.; Su, X.; Li, S.; Ho, C.-T. Hypoglycemic effects and associated mechanisms of resveratrol and related stilbenes in diet. Food Funct. 2024, 15, 2381–2405. [Google Scholar] [CrossRef]
- Abbas, A.M. Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats. J. Physiol. Biochem. 2016, 72, 469–484. [Google Scholar] [CrossRef]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Savulescu-Fiedler, I.; Dorobantu-Lungu, L.R.; Dragosloveanu, S.; Benea, S.N.; Dragosloveanu, C.D.M.; Caruntu, A.; Scheau, A.E.; Caruntu, C.; Scheau, C. The Cross-Talk Between the Peripheral and Brain Cholesterol Metabolisms. Curr. Issues Mol. Biol. 2025, 47, 115. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Li, J.; Liu, J.; Cha, L.; Mu, J. Resveratrol lowers blood pressure in spontaneously hypertensive rats via calcium-dependent endothelial NO production. Clin. Exp. Hypertens. 2016, 38, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Milanović, M.; Milić, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic Review on Natural Antioxidant Properties of Resveratrol. Nat. Prod. Commun. 2018, 13, 1934578X1801300923. [Google Scholar] [CrossRef]
- Choi, K. A Pilot Study of the Total Cholesterol/High-Density Lipoprotein Ratio as a Prognostic Indicator of Hyperlipidemia-Related Diseases in Dogs and Cats. Curr. Issues Mol. Biol. 2024, 46, 12174–12182. [Google Scholar] [CrossRef]
- Park, J.; Pyee, J.; Park, H. Pinosylvin at a high concentration induces AMPK-mediated autophagy for preventing necrosis in bovine aortic endothelial cells. Can. J. Physiol. Pharmacol. 2014, 92, 993–999. [Google Scholar] [CrossRef]
- Kungwani, N.; Singh, P.; Kumar, V. Medicinal plants: Adjunct treatment to tuberculosis chemotherapy to prevent hepatic damage. J. Ayurveda Integr. Med. 2019, 11, 522–528. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; García-Luna y González-Rubio, M.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, S.; Mao, G.; Li, G. Clinical characteristics and the risk factors of hepatic injury in 221 children with infectious mononucleosis. Front. Pediatr. 2022, 9, 809005. [Google Scholar] [CrossRef]
- Shen, R.; Zhou, Y.; Zhang, L.; Yang, S. The value of bile acid spectrum in the evaluation of hepatic injury in children with infectious mononucleosis caused by Epstein Barr virus infection. Front. Pediatr. 2023, 11, 1109762. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, G.; Moriconi, F.; Malik, I.; Dudas, J. Physiology and pathophysiology of liver inflammation, damage and repair. J. Physiol. Pharmacol. 2008, 59, 107–117. [Google Scholar]
- Dong, V.; Nanchal, R.; Karvellas, C.J. Pathophysiology of acute liver failure. Nutr. Clin. Pract. 2020, 35, 24–29. [Google Scholar] [CrossRef]
- Pinzani, M. Pathophysiology of liver fibrosis. Dig. Dis. 2015, 33, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Tsigas, G.; Periferakis, A.-T.; Badarau, I.A.; Scheau, A.-E.; Tampa, M.; Georgescu, S.R.; Didilescu, A.C.; Scheau, C.; Caruntu, C. Antitumoral and Anti-inflammatory Roles of Somatostatin and Its Analogs in Hepatocellular Carcinoma. Anal. Cell. Pathol. 2021, 2021, 1840069. [Google Scholar] [CrossRef]
- Periferakis, A.; Tsigas, G.; Periferakis, A.-T.; Tone, C.M.; Hemes, D.A.; Periferakis, K.; Troumpata, L.; Badarau, I.A.; Scheau, C.; Caruntu, A.; et al. Agonists, Antagonists and Receptors of Somatostatin: Pathophysiological and Therapeutical Implications in Neoplasias. Curr. Issues Mol. Biol. 2024, 46, 9721–9759. [Google Scholar] [CrossRef] [PubMed]
- Scheau, A.-E.; Jurca, S.O.; Scheau, C.; Lupescu, I.G. Non-Surgical Treatment for Hepatocellular Carcinoma: What to Expect at Follow-Up Magnetic Resonance Imaging—A Pictorial Review. Appl. Sci. 2024, 14, 9159. [Google Scholar] [CrossRef]
- Roberti, M.; Pizzirani, D.; Simoni, D.; Rondanin, R.; Baruchello, R.; Bonora, C.; Buscemi, F.; Grimaudo, S.; Tolomeo, M. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J. Med. Chem. 2003, 46, 3546–3554. [Google Scholar] [CrossRef]
- Waffo-Téguo, P.; Hawthorne, M.E.; Cuendet, M.; Mérillon, J.M.; Kinghorn, A.D.; Pezzuto, J.M.; Mehta, R.G. Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera) cell cultures. Nutr. Cancer 2001, 40, 173–179. [Google Scholar] [CrossRef]
- Shagufta, A.I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018, 143, 515–531. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef]
- Mendoza, R.G. Lords of the medicine bag: Medical science and traditional practice in ancient Peru and South America. In Medicine across Cultures: History and Practice of Medicine in Non-Western Cultures; Springer: Berlin/Heidelberg, Germany, 2003; pp. 225–257. [Google Scholar]
- Elferink, J.G. The Inca healer: Empirical medical knowledge and magic in pre-Columbian Peru. Rev. De Indias 2015, 75, 323–350. [Google Scholar] [CrossRef]
- Periferakis, A. A review of obsidian source exploitation in pre-columbian south America. Bull. Geol. Soc. Greece 2019, 55, 65–108. [Google Scholar] [CrossRef]
- Guerra, F. Aztec medicine. Med. Hist. 1966, 10, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G. Finding the present in the past: Ancient Mexican medicine. Hum. Med. 2004, 2, 1–3. [Google Scholar]
- Maher, P. A review of ‘traditional’Aboriginal health beliefs. Aust. J. Rural Health 1999, 7, 229–236. [Google Scholar] [CrossRef]
- Oliver, S.J. The role of traditional medicine practice in primary health care within Aboriginal Australia: A review of the literature. J. Ethnobiol. Ethnomed. 2013, 9, 1–8. [Google Scholar] [CrossRef]
- Pieroni, A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef]
- Ramalingum, N.; Mahomoodally, M.F. The therapeutic potential of medicinal foods. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 354264. [Google Scholar] [CrossRef]
- Brain, A.; John, M. Phenolic content and antioxidant activity of selected Ugandan traditional medicinal foods. Afr. J. Food Sci. 2014, 8, 427–434. [Google Scholar]
- Tavakkoli-Kakhki, M.; Motavasselian, M.; Mosaddegh, M.; Esfahani, M.M.; Kamalinejad, M.; Nematy, M.; Eslami, S. Omega-3 and omega-6 content of medicinal foods for depressed patients: Implications from the Iranian Traditional Medicine. Avicenna J. Phytomed. 2014, 4, 225. [Google Scholar]
- Hou, Y.; Wang, X.; Zhang, Y.; Wang, S.; Meng, X. Highland mate: Edible and functional foods in traditional medicine for the prevention and treatment of hypoxia-related symptoms. Curr. Opin. Pharmacol. 2021, 60, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Garbowski, T.; Charazińska, S.; Pulikowski, K.; Wiercik, P. Application of microalgae cultivated on pine bark for the treatment of municipal wastewater in cylindrical photobioreactors. Water Environ. J. 2020, 34, 949–959. [Google Scholar] [CrossRef]
- Sousa, S.; Jiménez-Guerrero, P.; Ruiz, A.; Ratola, N.; Alves, A. Organochlorine pesticides removal from wastewater by pine bark adsorption after activated sludge treatment. Environ. Technol. 2011, 32, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.B.; Teal, J.M. The role of plants in ecologically engineered wastewater treatment systems. Ecol. Eng. 1996, 6, 137–148. [Google Scholar] [CrossRef]
- Eisenberg, D.; Soller, J.; Sakaji, R.; Olivieri, A. A methodology to evaluate water and wastewater treatment plant reliability. Water Sci. Technol. 2001, 43, 91–99. [Google Scholar] [CrossRef]
- Alasino, N.; Mussati, M.C.; Scenna, N. Wastewater treatment plant synthesis and design. Ind. Eng. Chem. Res. 2007, 46, 7497–7512. [Google Scholar] [CrossRef]
- Periferakis, A. The Yukon Gold Rush: Early Examples of the Socioeconomic and Environmental Impact of Mining. In Proceedings of the 15th International Congress of the Geological Society of Greece, Athens, Greece, 22–24 May 2019; pp. 710–711. [Google Scholar]
- Periferakis, A. The Keramos Antimonite Mines in Chios Island, Greece: Mining History and Current Situation. News Miner. 2020, 35, 5–21. [Google Scholar]
- Lombardi, G.; Lanzirotti, A.; Qualls, C.; Socola, F.; Ali, A.M.; Appenzeller, O. Five hundred years of mercury exposure and adaptation. J. Biomed. Biotechnol. 2012, 2012, 472858. [Google Scholar] [CrossRef]
- Clackett, S.P.; Porter, T.J.; Lehnherr, I. The tree-ring mercury record of Klondike gold mining at Bear Creek, central Yukon. Environ. Pollut. 2021, 268, 115777. [Google Scholar] [CrossRef]
- Strosnider, W.H.J.; Llanos López, F.S.; LaBar, J.A.; Palmer, K.J.; Nairn, R.W. Unabated acid mine drainage from Cerro Rico de Potosí, Bolivia: Uncommon constituents of concern impact the Rio Pilcomayo headwaters. Environ. Earth Sci. 2014, 71, 3223–3234. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hajyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Aghababai Beni, A. Comprehensive analysis of heavy metal soil contamination in mining Environments: Impacts, monitoring Techniques, and remediation strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Stamatis, G.; Voudouris, K.; Karefilakis, F. Groundwater Pollution by Heavy Metals in Historical Mining Area of Lavrio, Attica, Greece. Water Air Soil Pollut. 2001, 128, 61–83. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Mavrogonatos, C.; Photiades, A.; Moraiti, E.; Rieck, B.; Kolitsch, U.; Tarantola, A.; Scheffer, C.; Morin, D.; et al. The Lavrion Mines: A Unique Site of Geological and Mineralogical Heritage. Minerals 2021, 11, 76. [Google Scholar] [CrossRef]
- Antoniadis, V.; Thalassinos, G.; Levizou, E.; Wang, J.; Wang, S.-L.; Shaheen, S.M.; Rinklebe, J. Hazardous enrichment of toxic elements in soils and olives in the urban zone of Lavrio, Greece, a legacy, millennia-old silver/lead mining area and related health risk assessment. J. Hazard. Mater. 2022, 434, 128906. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Bozatzi, A.; Periferakis, A. Geodidactics and CLIL: Synthesizing a syllabus for the ELT classroom through games, stories and technology. In Proceedings of the 16th International Congress of the Geological Society of Greece, Patras, Greece, 17–19 October 2022. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Van Der Werf, H.M. Assessing the impact of pesticides on the environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Gill, H.K.; Garg, H. Pesticide: Environmental impacts and management strategies. Pestic.-Toxic. Asp. 2014, 8, 10–5772. [Google Scholar]
- Stefanidis, K.; Christopoulou, A.; Poulos, S.; Dassenakis, E.; Dimitriou, E. Nitrogen and Phosphorus Loads in Greek Rivers: Implications for Management in Compliance with the Water Framework Directive. Water 2020, 12, 1531. [Google Scholar] [CrossRef]
- Della Rossa, P.; Jannoyer, M.; Mottes, C.; Plet, J.; Bazizi, A.; Arnaud, L.; Jestin, A.; Woignier, T.; Gaude, J.-M.; Cattan, P. Linking current river pollution to historical pesticide use: Insights for territorial management? Sci. Total Environ. 2017, 574, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Kirchhelle, C. Toxic Tales—Recent Histories of Pollution, Poisoning, and Pesticides (ca. 1800–2010). NTM Z. Gesch. Wiss. Tech. Med. 2018, 26, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Cobilinschi, C.; Tincu, R.; Totan, A.; Ghiorghiu, Z.; Neagu, T.P.; Macovei, R. Thyroid dysfunction caused by organophosphate poisoning. Toxicol. Lett. 2017, 280, S169. [Google Scholar] [CrossRef]
- Cobilinschi, C.; Țincu, R.; Ungureanu, R.; Dumitru, I.; Băetu, A.; Isac, S.; Cobilinschi, C.O.; Grințescu, I.M.; Mirea, L. Toxic-Induced Nonthyroidal Illness Syndrome Induced by Acute Low-Dose Pesticides Exposure—Preliminary In Vivo Study. Toxics 2022, 10, 511. [Google Scholar] [CrossRef]
- Cobilinschi, C.; Olteanu, A.; Cobilinschi, C.; Grintescu, I.; Macovei, R.; Tudosie, M.; Serban, D.; Tincu, R. From the discovery of combat gases to insecticides and bioterrorism—A brief history. Rom. J. Mil. Med. 2022, 125, 247–252. [Google Scholar] [CrossRef]
- Franklin, M.R.; Fernandes, H.M. Identifying and overcoming the constraints that prevent the full implementation of decommissioning and remediation programs in uranium mining sites. J. Environ. Radioact. 2013, 119, 48–54. [Google Scholar] [CrossRef]
- Lima, A.T.; Mitchell, K.; O’Connell, D.W.; Verhoeven, J.; Van Cappellen, P. The legacy of surface mining: Remediation, restoration, reclamation and rehabilitation. Environ. Sci. Policy 2016, 66, 227–233. [Google Scholar] [CrossRef]
- Guzman, E.R. Canadian Financial Assurance Frameworks for the Remediation of Mining Sites: An Assessment of Ontario’s, British Columbia’s and Quebec’s Schemes and Three Potential Reform Initiatives. J. Environ. Law Pract. 2017, 31, 1–35. [Google Scholar]
- Mulligan, D.; Grigg, A.; Madsen, T.; Pearce, A.; Roe, P. Research initiatives for the remediation of land following open-cut coal mining in central Queensland. In Remediation and Management of Degraded Lands; Routledge: London, UK, 2018; pp. 25–36. [Google Scholar]
- O’Brien, R.M.; Phelan, T.J.; Smith, N.M.; Smits, K.M. Remediation in developing countries: A review of previously implemented projects and analysis of stakeholder participation efforts. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1259–1280. [Google Scholar] [CrossRef]
- Miu, B.A.; Pop, C.-E.; Crăciun, N.; Deák, G. Bringing life back into former mining sites: A mini-review on soil remediation using organic amendments. Sustainability 2022, 14, 12469. [Google Scholar] [CrossRef]
- Reichenberger, S.; Bach, M.; Skitschak, A.; Frede, H.-G. Mitigation strategies to reduce pesticide inputs into ground-and surface water and their effectiveness; a review. Sci. Total Environ. 2007, 384, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Destandau, F.; Imfeld, G.; Rozan, A. Regulation of diffuse pesticide pollution: Combining point source reduction and mitigation in stormwater wetland (Rouffach, France). Ecol. Eng. 2013, 60, 299–308. [Google Scholar] [CrossRef]
- Jin, S.; Bluemling, B.; Mol, A.P. Mitigating land pollution through pesticide packages–The case of a collection scheme in Rural China. Sci. Total Environ. 2018, 622, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Quaglia, G.; Joris, I.; Broekx, S.; Desmet, N.; Koopmans, K.; Vandaele, K.; Seuntjens, P. A spatial approach to identify priority areas for pesticide pollution mitigation. J. Environ. Manag. 2019, 246, 583–593. [Google Scholar] [CrossRef]
- Sahin, C.; Karpuzcu, M.E. Mitigation of organophosphate pesticide pollution in agricultural watersheds. Sci. Total Environ. 2020, 710, 136261. [Google Scholar] [CrossRef]
- Intisar, A.; Ramzan, A.; Sawaira, T.; Kareem, A.T.; Hussain, N.; Din, M.I.; Bilal, M.; Iqbal, H.M. Occurrence, toxic effects, and mitigation of pesticides as emerging environmental pollutants using robust nanomaterials–A review. Chemosphere 2022, 293, 133538. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Robalds, A.; Tran, H.; Mitrogiannis, D.; Giannakoudakis, D.A.; Hosseini-Bandegharaei, A.; Dotto, G. Removal of heavy metals by leaves-derived biosorbents. Environ. Chem. Lett. 2018, 17, 755–766. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Kornaros, M.; Tsigkou, K.; Brulé, M.; Koukouzas, N.; Alexopoulos, D.; Palles, D.; Kamitsos, E.; Oikonomou, G.; et al. Calcium-modified clinoptilolite as a recovery medium of phosphate and potassium from anaerobically digested olive mill wastewater. Environ. Sci. Pollut. Res. 2020, 27, 2977–2991. [Google Scholar] [CrossRef]
- Mitrogiannis, D.; Psychoyou, M.; Baziotis, I.; Mavrogonatos, C.; Koukouzas, N.; Anastopoulos, I.; Fyrillas, M.; Inglezakis, V. Phosphate removal by Ca(OH)2-treated natural minerals: Experimental and modeling studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 660, 130805. [Google Scholar] [CrossRef]
- Koitabashi, R.; Suzuki, T.; Kawazu, T.; Sakai, A.; Kuroiwa, H.; Kuroiwa, T. 1,8-Cineole inhibits root growth and DNA synthesis in the root apical meristem of Brassica campestris L. J. Plant Res. 1997, 110, 1–6. [Google Scholar] [CrossRef]
- De Feo, V.; De Simone, F.; Senatore, F. Potential allelochemicals from the essential oil of Ruta graveolens. Phytochemistry 2002, 61, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene Inhibits Growth and Induces Oxidative Stress in Roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef]
- Koutsaviti, K.; Giatropoulos, A.; Pitarokili, D.; Papachristos, D.; Michaelakis, A.; Tzakou, O. Greek Pinus essential oils: Larvicidal activity and repellency against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Sakulpanich, A.; Attrapadung, S.; Gritsanapan, W. Insecticidal activity of Stemona collinsiae root extract against Parasarcophaga ruficornis (Diptera: Sarcophagidae). Acta Trop. 2017, 173, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sakulpanich, A.; Attrapadung, S.; Gritsanapan, W. Larvicidal activity of Stemona collinsiae root extract against Musca domestica and Chrysomya megacephala. Sci. Rep. 2023, 13, 15689. [Google Scholar] [CrossRef]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides; Official Journal of the European Union: Luxembourg, 2009; pp. 71–86.
- Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC; European Parliament, Ed.; Official Journal of the European Union: Luxembourg, 2009; pp. 1–50. [Google Scholar]
- Karamfilova, E. Revision of Directive 2009/128/EC on the sustainable use of pesticides. In Briefing Implementation Appraisal; European Parliamentary Research Service PE 730; European Parliamentary Research Service: Brussels, Belgium, 2022; pp. 1–12. [Google Scholar]
- Dinu, A.; Karamfilova, E. Regulation (EC) 1107/2009 on the Placing of Plant Protection Products on the Market; European Parliamentary Research Service: Brussels, Belgium, 2018; pp. 1–70. [Google Scholar]
- Moagar-Poladian, S.; Folea, V.; Paunica, M. Competitiveness of EU member states in attracting EU funding for research and innovation. Rom. J. Econ. Forecast. 2017, 20, 150–167. [Google Scholar]
- Schebesta, H.; Candel, J.J.L. Game-changing potential of the EU’s Farm to Fork Strategy. Nat. Food 2020, 1, 586–588. [Google Scholar] [CrossRef]
- Billen, G.; Aguilera, E.; Einarsson, R.; Garnier, J.; Gingrich, S.; Grizzetti, B.; Lassaletta, L.; Le Noë, J.; Sanz-Cobena, A. Beyond the Farm to Fork Strategy: Methodology for designing a European agro-ecological future. Sci. Total Environ. 2024, 908, 168160. [Google Scholar] [CrossRef]
- Communication from The Commission to The European Parliament, The European Council, The Council, The European Economic and Social Committee and the Committee of the Regions. In The European Green Deal; European Commission: Brussels, Belgium, 2019.
- Salt, D.E.; Smith, R.; Raskin, I. Phytoremediation. Annu. Rev. Plant Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladi, S.M.; Henderson, K.L.; Coats, J.R. Phytoremediation—An overview. Crit. Rev. Plant Sci. 2005, 24, 109–122. [Google Scholar] [CrossRef]
- Assembly, G. Transforming our world: The 2030 Agenda for Sustainable Development Resolutions/Recommendations/Declarations/Decisions, A/RES/70/1; General Assembly of the United Nations: New York, NY, USA, 2015. [Google Scholar]
- Treaty on European Union; Official Journal of the European Communities: Luxembourg, 1992.
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; O J L 327; European Parliament: Strasbourg, France, 2008; pp. 1–73.
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives; 312; Official Journal of the European Union: Legislation Series, Ed.; European Parliament: Strasbourg, France, 2008; pp. 3–30. [Google Scholar]
- Directive, E. Council Directive of 21. May 1991 concerning urban waste water treatment (91/271/EEC). J. Eur. Commun. 1991, 34, 40. [Google Scholar]
- Preisner, M.; Smol, M.; Szołdrowska, D. Trends, insights and effects of the Urban Wastewater Treatment Directive (91/271/EEC) implementation in the light of the Polish coastal zone eutrophication. Environ. Manag. 2021, 67, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Coric, V.; Bojovic, A.K.; Glintic, M. Thirtieth Anniversary of the Urban Waste Water Treatment Directive 91/271/EEC: Consideration of the Need for Its Amendments. Rev. Eur. L. 2021, 23, 73. [Google Scholar]
- Sandström, E. The LIFE-Fund of the European Union as a Means to Support the Development of the Implementation of the Swedish Forest Policy. In Financial Instruments of Forest Policy; European Forest Institute, Gummerus Printing: Saarijärvi, Finland, 2002; pp. 77–86. [Google Scholar]
- de Sadeleer, N. The Principle of Subsidiarity and Its Impact on EU Environmental Law and Policy. Droit Soc. 2012, 80, 73. [Google Scholar] [CrossRef]
- Summary of the Federal Insecticide, Fungicide, and Rodenticide Act. In 7 U.S.C. §136 et seq; United States Environmental Protection Agency, (U.S. EPA) (website); 1996. Available online: https://www.epa.gov/laws-regulations/summary-federal-insecticide-fungicide-and-rodenticide-act (accessed on 12 February 2025).
- The Food Quality Protection Act (FQPA), or H.R.1627. In Public Law 104-170; The U.S. National Archives and Records Administration: College Park, MD, USA, 1996.
- The National Environmental Policy Act (NEPA). In Public Law 91-70; The U.S. National Archives and Records Administration: College Park, MD, USA, 1969.
- The Toxic Substances Control Act (TSCA). In 15 U.S.C. §2601 et seq; The U.S. National Archives and Records Administration: College Park, MD, USA, 1976.
- Agricultural Chemicals Regulation Act, Act No. 82 of 1948 (Japan), as Revised by the Act No. 53 of 2018. 2018. [Agricultural Chemicals Regulation Act—English—Japanese Law Translation]. Available online: https://www.japaneselawtranslation.go.jp/en/laws/view/3507/en (accessed on 12 February 2025).
- Act on Promotion of Organic Agriculture, Act No. 112 of 2006 (Japan), as Revised by the Act No. 105 of 2011. 2011. [Act on Promotion of Organic Agriculture—English—Japanese Law Translation]. Available online: https://www.japaneselawtranslation.go.jp/en/laws/view/3728/en (accessed on 12 February 2025).
- Basic Act on the Environment, Act No. 91 of 1993 (Japan) Domestic Law. 1993. [The Basic Environment Law—Ministry of the Environment, Government of Japan]. Available online: https://www.env.go.jp/en/laws/policy/basic/index.html (accessed on 12 February 2025).
- Framework Act on Environmental Policy, as revised by Act No. 18918. 10 June 2022. Available online: https://faolex.fao.org/docs/pdf/kor51838.pdf (accessed on 12 February 2025).
- Kim, S.; Seol, T.K. Environmental law in South Korea. Lexology. Available online: https://www.lexology.com/library/detail.aspx?g=4b3fbe13-1c55-48df-b34b-14f1dd35b0bb (accessed on 12 January 2025).
- Agrochemicals Control Act (Act No. 445 of August 28, 1957, as Last Amended by Act No. 9658 of May 8, 2009). 2009. [Agrochemicals Control Act (Act No. 445 of August 28, 1957, as last amended by Act No. 9658 of May 8, 2009), Republic of Korea, WIPO Lex]. Available online: https://www.wipo.int/wipolex/en/legislation/details/12867 (accessed on 12 February 2025).
- Consolidated Act No. 11459 of 1 June 2012, as Amended Last by Act No. 18026 of 13 April 2021. 2021. [Act on the Promotion of Environment-Friendly Agriculture and Fisheries and the Management of and Support for Organic Foods, etc.—UNEP Law and Environment Assistance Platform]. Available online: https://leap.unep.org/en/countries/kr/national-legislation/act-promotion-environment-friendly-agriculture-and-fisheries-and?fbclid=IwY2xjawJDx_JleHRuA2FlbQIxMAABHckAEC6NAN5ePGRtzw9qLTOifzu1UMmoasP1Dcq1GDRZuwUXHv3xtn8jCQ_aem_AZ0Iv7bD6rwPj-FkMSRdhA (accessed on 12 February 2025).
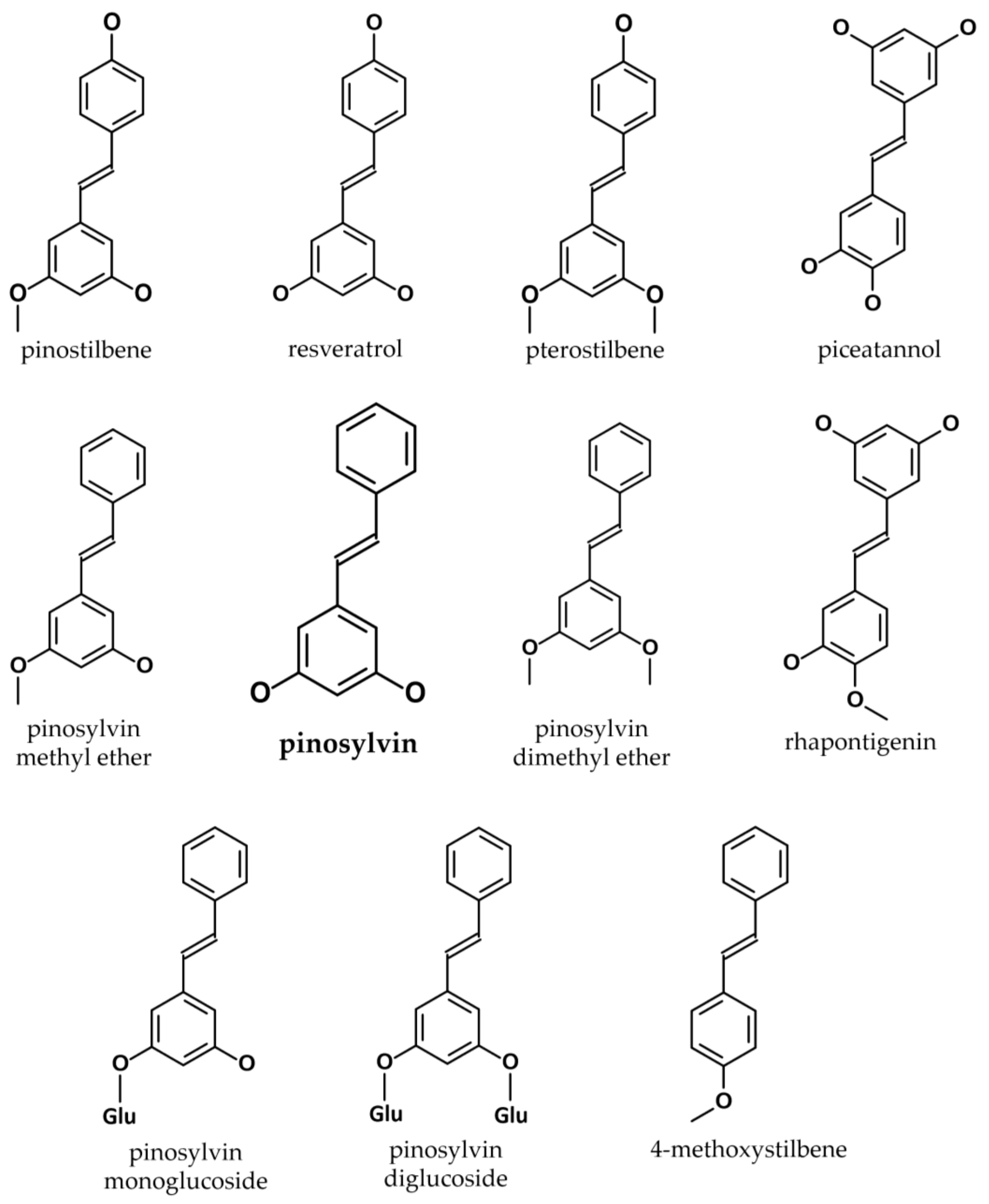
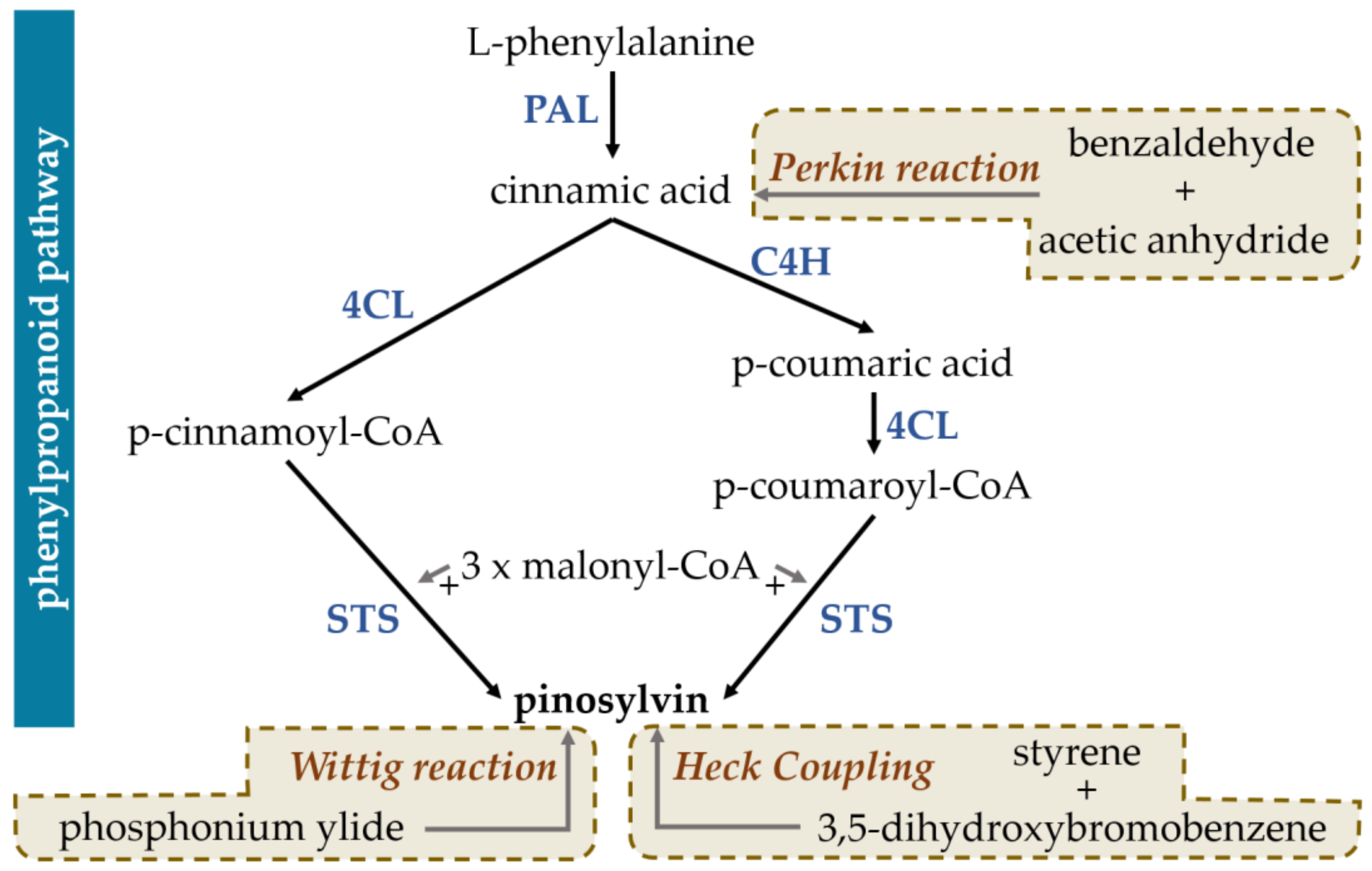

| Order | Family | Genus | Species | Reference |
|---|---|---|---|---|
| Fabales | Fabaceae | Arachis | A. hypogaea | [51] |
| Cajanus | C. cajan | [53] | ||
| Rosales | Rhamnaceae | Hovenia | H. dulcis Thunb. | [54] |
| Laurales | Lauraceae | Lindera | L. reflexa Hemsl. | [55] |
| Myrtales | Myrtaceae | Agonis | A. flexuosa | [56] |
| Pinales | Pinaceae | Picea | P. abies | [57] |
| P. glauca | [58] | |||
| Pinus | P. banksiana | [59,60,61] | ||
| P. brutia Hen. | [62] | |||
| P. caribaea | [63] | |||
| P. cembra | [64] | |||
| P. contorta | [64] | |||
| P. densiflora | [65,66] | |||
| P. halepensis Mill. | [67,68] | |||
| P. merkusii | [69] | |||
| P. nigra Arn. | [70] | |||
| P. palustris | [71] | |||
| P. pinaster | [72,73,74] | |||
| P. resinosa | [60] | |||
| P. roxburghii Sargent | [75] | |||
| P. strobus | [71] | |||
| P. sibirica | [64] | |||
| P. sylvestris | [76] | |||
| P. taeda | [77] | |||
| Pandanales | Stemonaceae | Stemona | S. cf. peirrei | [78] |
| S. collinsae | [79,80] | |||
| S. tuberosa | [81] |
| Genus | Species | Tested Substance | Extract Origin | Effectiveness | Year | Reference |
|---|---|---|---|---|---|---|
| Achromobacter | A. xylosoxidans | Pinosylvin, pinosylvin monomethyl ether | P. banksiana, P. contorta, P. resinosa, P. sylvestris | 11–20 (and more) mm 1 | 2004 | [60] |
| Arcobacter | A. butzleri | Pinosylvin | n/a (pure compound) | 128 μg/mL (MIC) | 2019 | [139] |
| Bacillus | B. cereus | Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 101 ± 6, 92 ± 4, 82 ± 3 (% of inhibition) | 2007 | [140] |
| B. coagulans | Pinosylvin, pinosylvin monomethyl ether | P. contorta, P. banksiana, P. resinosa, P. sylvestris | 16–20 (and more) mm 1 | 2004 | [60] | |
| B. subtilis | Pinosylvin | n/a (laboratory synthesis) | 64 μg/mL (MIC) | 2016 | [141] | |
| Pinosylvin | n/a (pure compound) | 99.2% 2 | 2019 | [142] | ||
| Burkholderia | B. multivorans | Pinosylvin, pinosylvin monomethyl ether | P. contorta, P. banksiana, P. resinosa, P. sylvestris | 11–20 (and more) mm 1 | 2004 | [60] |
| Campylobacter | C. coli, C. jejuni | Pinosylvin | n/a (pure compound) | 25–50 μg/mL (MIC) | 2015 | [143] |
| Pinosylvin | n/a (pure compound) | Multiple values of inhibition haloes based on experimental parameters | 2018 | [144] | ||
| Escherichia | E. coli | Pinosylvin | n/a (pure compound) | 250 μg/mL (MIC) | 2005 | [145] |
| Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 54 ± 8, 71 ± 7, 18 ± 2 (% of inhibition) | 2007 | [140] | ||
| Pinosylvin | n/a (laboratory synthesis) | 64 μg/mL (MIC) | 2016 | [141] | ||
| Pinosylvin | n/a (pure compound) | 58.9% 2 | 2019 | [142] | ||
| Listeria | L. monocytogenes | Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 62 ± 15, 100 ± 7, 64 ± 12 (% of inhibition) | 2007 | [140] |
| Pinosylvin | n/a (laboratory synthesis) | 93.2 ± 0.4 (% of inhibition at concentration of 0.5 mM) | 2013 | [146] | ||
| Pinosylvin | n/a (pure compound) | 97.9% 2 | 2019 | [142] | ||
| Proteus | P. vulgaris | Pinosylvin | n/a (laboratory synthesis) | >128 μg/mL (MIC) | 2016 | [141] |
| Pseudomonas | P. aeruginosa | Pinosylvin | n/a (laboratory synthesis) | >128 μg/mL (MIC) | 2016 | [141] |
| Pinosylvin | n/a (pure compound) | 8.9% 2 | 2019 | [142] | ||
| P. fluorescens | Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 50 ± 15, 35 ± 2, 22 ± 6 (% of inhibition) | 2007 | [140] | |
| Salmonella | S. infantis | Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 42 ± 20, 40 ± 7, 14 ± 2 (% of inhibition) | 2007 | [140] |
| S. enteritidis | Pinosylvin | n/a (laboratory synthesis) | 80.6% 2 | 2019 | [142] | |
| Staphylococcus | S. aureus | Pinosylvin | n/a (pure compound) | 250 μg/mL (MIC) | 2005 | [145] |
| Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 76 ± 2, 105 ± 12, 76 ± 4 (% of inhibition) | 2007 | [140] | ||
| Pinosylvin | n/a (laboratory synthesis) | 75 μg/mL MIC | 2013 | [146] | ||
| Pinosylvin | n/a (laboratory synthesis) | 64 μg/mL (MIC) (higher for some MRSA strains) | 2016 | [141] | ||
| Pinosylvin | A. hypogea | ≤100 μg/mL (MIC) | 2018 | [147] | ||
| Pinosylvin | n/a (pure compound) | 100% 2 | 2019 | [142] | ||
| S. epidermidis | Pinosylvin | n/a (laboratory synthesis) | 128 μg/mL (MIC) | 2016 | [141] |
| Genus | Species | Tested Substance | Extract Origin | Effectiveness | Year | Reference |
|---|---|---|---|---|---|---|
| Aspergillus | A. fumigatus | Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 14 ± 1/ 12 ± 3 1 | 2007 | [140] |
| Candida | C. albicans | Pinosylvin | n/a (pure compound) | 62.5 μg/mL (MIC) | 2005 | [145] |
| Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 85 ± 5/ 80 ± 4 1 | 2007 | [140] | ||
| Cladosporium | C. herbarum | Pinosylvin, 4′-methylpinosylvin | S. collinsae | n/a (only structurally similar stilbenoids were tested) | 2002 | [79] |
| Pinosylvin, dihydropinosylvin | S. cf. pierrei | 10mg/mL (EC50) | 2004 | [78] | ||
| Plasmopara | P. viticola | Pinosylvin, pinosylvin monomethyl ether | P. pinaster | 23, 18 μΜ (available as IC50) | 2017 | [73] |
| Penicillium | P. brevicompactum | Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 15 ± 1/ 14 ± 2 1 | 2007 | [140] |
| Saccharomyces | S. cerevisiae | Pinosylvin | n/a (pure compound) | 125 μg/mL (MIC) | 2005 | [145] |
| Pinosylvin, pinosylvin monomethyl ether, dihydropinosylvin monomethyl ether | P. strobus, P. sylvestris | 82 ± 13/ 35 ± 21 1 | 2007 | [140] |
| Compound | Plant | Tested on | Mechanism | Effect | Concentration/ Administration | Year | Reference |
|---|---|---|---|---|---|---|---|
| Pinosylvin | n/a (laboratory synthesis) | In vitro—Raji and Molt human lymphoblastoid cell lines | Inhibition of protein uptake | Antiproliferative | Direct addition in culture—15, 30 μg/ml | 1986 | [339] |
| Pinosylvin | P. sylvestris | In vitro—MCF-7 or T-47D breast cancer cells | Increase in oestrogen expression | Pro-proliferative | Dilution in ethanol and culture for 7 days—1 pM to 1 μΜ | 1996 | [344] |
| Pinosylvin | P. sylvestris | In vitro—RAW264.7 cells and HT-9 human colon cancer cells | COX-2 inhibition, free radicals scavenging and xanthine oxidase activity inhibition | Antiproliferative | n/a | 2003 | [359] |
| Pinosylvin, pinosylvin monomethyl ether, pinosylvin dimethyl ether | P. resinosa Ait. | In vitro—lung carcinoma cells A549 lung carcinoma cells, DLD-1 human colorectal adenocarcinoma cells, WS1 healthy cells | Unknown | Cytotoxic | Incubation for 48 h—different concentrations (IC50 = ~41–130 μg/mL depending on cell line; value for extract) | 2008 | [356] |
| Pinosylvin | n/a (laboratory synthesis) | In vitro—HT1080 human fibrosarcoma cells | Decreased expression of matrix metalloproteinases | Antimetastatic | Treatment of cells at almost confluency, with pinosylvin—12.5, 25 and 50 μM | 2012 | [347] |
| In vivo—mice | Downregulation of COX-2 expression, decreased ERK1/2 and Akt phosphorylation and reduced metalloproteinase expression | Intraperitoneal administration—10 mg/kg per body weight | |||||
| Pinosylvin | n/a (laboratory synthesis) | In vitro—HTC 116 human colorectal cancer cells | Src/ERK and GSK-3/β-catenin signalling suppression | Antiproliferative | Incubation for different periods—various concentrations (depending on different experimental protocols); IC50 = 48.2 μM (for 24 h) | 2013 | [340] |
| Pinosylvin methyl ether | n/a (pure compound) | In vitro—LNCaP prostate cancer cells, RWPE-1, and EP156T non-malignant cancer cells | Alteration of cell cycle-related genes, modification of steroid and cholesterol biosynthesis and androgen-signalling | Antiproliferative | Incubation over a span of 3 days (EC50 = 250 nM) | 2016 | [341] |
| Pinosylvin | n/a (pure compound) | In vitro—THP-1 and U937 human monocytic cell lines | Caspace-3 activation, flipflop of phosphatidylserine, p62 degradation and LC3-II accumulation, downregulation of AMPKa expression | Cytotoxic | Pretreatment with pinosylvin—0 to 100 μmol/L (IC50 = ~20–30 μmol/L) | 2018 | [357] |
| Pinosylvin | n/a (pure compound) | In vitro—SAS, SCC-9, and HSC-3 cells | Inhibition of MMP-2, upregulation of TIMP-2 expression, and downregulation of the ERK1/2 signalling pathway | Antiproliferative and anti-metastatic | Direct addition in culture medium—20, 40, 80 μΜ | 2019 | [263] |
| Pinosylvin | n/a (pure compound) | In vitro—NPC-039, NPC-BM and RPMI 2650 cells (nasopharyngeal carcinoma cells) | Inhibition of MMP-2, and decreased expression of MMP-2 and MMP-9; decrease in vimentin and N-cadherin and E-cadherin expression and of ZO-1 | Antimetastatic | Treatment of cells with pinosylvin—20, 40, 80 μΜ | 2021 | [348] |
| Pinosylvin | n/a (pure compound) | In vitro—ECA109 and TE1 oesophageal cancer cells | Reduction in syntaxin-6 and integrin α3 expression and enhanced vasodilator-stimulated phosphoprotein expression | Antimetastatic | Treatment of cells with pinosylvin for 24 h—0, 10, 20, 40, and 80 μM | 2023 | [354] |
| Compound | Plant | Tested on | Mechanism | Effect | Concentration and Administration | Year | Reference |
|---|---|---|---|---|---|---|---|
| Pinosylvin | n/a (pure compound) | In vitro—OGD/R-damaged PC12 cells | Upregulation of PINK1/Parkin-regulated mitophagy, upregulation of the Nrf2 pathway | Neuroprotection | Pretreatment of cells with pinosylvin for 24 h—10 μΜ | 2021 | [364] |
| In vivo—rats | Reduction in infarct size and of neural cell death | Decrease in brain function loss | Intraperitoneal injection—50 mg/kg |
| Plant | Traditional/ Ethnobotanical Uses | Medical System | Tested Actions | Region/ Populations | References | |
|---|---|---|---|---|---|---|
| Tax. Name | Common Name | |||||
| A. hypogaea | Peanut | Treatment of sleep disorders, antidiabetic, antilipidemic, weight loss, anticancer use, cardiovascular pathologies and haemorrhages, treatment of bronchitis, antibacterial | Traditional Chinese medicine, Nigerian folk medicine | Treatment of sleep disorders, antimicrobial, antioxidant | China, Nigeria/Chinese, local population | [381,382,383] |
| C. cajan | Pigeon pea | Antidiabetic, stimulant, analgesic, treatment of haemorrhage, oral pathologies, laxative, lactation induction | Bangladesh medical tradition, Trinidad and Tobacco medical tradition, traditional Chinese medicine, Ayurveda | Antimicrobial, antidyslipidaemic, antidiabetic, antioxidant, anticancer, hepatoprotective | Bangladesh, Trinidad and Tobacco, China, India | [384,385,386,387] |
| H. dulcis | Japanese raisin tree | Alcohol detoxification (perhaps hepatoprotective) | Traditional Chinese medicine, traditional Japanese medicine, traditional Korean medicine | Alcohol detoxification, hepatoprotective effect, antioxidant, antidiabetic, antimicrobial, antilipidaemic, anti-allergic, anti-inflammatory, prokinetic | China, Japan, Korea/Chinese, Japanese, Koreans | [388,389] |
| P. glauca | White spruce | Antibacterial, treatment for sore mouth and strep throat, sinusitis, haemoptysis, wound treatment, chronic pain treatment | Carrier people’s medical practices, Canadian Boreal Forest people | Antimicrobial, organoprotective, antioxidant | Canada/Carrier people, indigenous populations | [390,391,392,393] |
| P. brutia Ten. | Turkish red pine | Antitussive, antidiabetic, treatment of gastrointestinal complains, tonic | Turkish folk medicine | Antifungal, antibacterial, (potential) treatment of acute lung injury, (potential) antioxidant action, anti-inflammatory | Turkey/local population | [394,395,396,397,398,399] |
| P. densiflora Sieb. et Zucc. | Korean red pine | Antihypertensive, anti-atherosclerotic, treatment of strokes, diabetes, cancer, and balding | Traditional Korean medicine | n/a | Korea/Korean people | [400] |
| P. halepensis Mill. | Aleppo pine | Respiratory pathologies, wound treatments, anti-inflammatory, urinary problems, GI ulcers, prostate infections, infertility, antiseptic, adrenal gland stimulant, antimicrobial, toothache, baldness | Local medical traditions | Antibacterial, antifungal, antioxidant, cytoprotective, anticancer (cytotoxic), anti-coagulant, anti-haemolytic, anti-inflammatory | Italy, Spain, Algeria, Morocco/Berber people, local populations | [68] |
| P. nigra | Black pine | Colds, cough, treatment of respiratory pathologies, furuncles, warts, treatment of teeth decay, digestive complaints | Local medical traditions and Austro-Hungarian pharmacopoeia | Antimicrobial, antioxidant (weak), food supplement, antiproliferative | Romania/local people of Transylvania | [401,402,403,404] |
| P. mugo Tura | Dwarf mountain pine, scrub mountain pine, Swiss mountain pine | Expectorant | Local medical traditions | n/a | Italy | [405,406] |
| P. roxburghii Sargent | Chir pine, long-leaved pine | Antiseptic, diarrhetic, diaphoretic, tonic, vermifuge (anthelmintic), rubefacient, spasmolytic, antioxidant, anti-inflammatory, treatment for ocular and ear pathologies, ulcer treatment, bronchitis treatment, treatment of skin diseases, treatment of blood diseases, treatment of snake and scorpion bites | Ayurveda, various local medical traditions | Antibacterial, antioxidant, anticonvulsant, anti-asthmatic, analgesic activity | Himalayas, Hindu Kush/local tribes | [407,408,409,410] |
| P. sylvestris | Scots pine, Baltic pine, European red pine | Treatment of asthma, cough and respiratory complaints, treatment of rheumatisms and varicose veins | Local medical traditions and Austro-Hungarian pharmacopoeia | Antioxidant, anti-inflammatory, food supplement | Romania/local people of Transylvania | [403,404,411] |
| P. virginiana Miller | Virginia pine | Treatment of skin ulcers and sores, baths for painful joints, and treatment of cold and fever | Native American traditional medicine | n/a | Cherokee Native Americans | [412] |
| Plant Part Used | Purpose/Activity | Mechanism | Year | Reference |
|---|---|---|---|---|
| Bark of P. roxburghii | Water purification | Removal of Cr(VI) via adsorption | 2005 | [579] |
| Pine bark | Organochlorine pesticides removal | 2011 | [722] | |
| Pine bark acting as a substrate for biofilm formation | Phycoremediation | 2020 | [721] | |
| Essential oils of different Pinus spp. | Insecticidal activity | Repellent and larvicidal activity against A. albopticus mosquito larvae | 2015 | [765] |
| Ethanolic extract from S. collinsiae roots | Elimination of P. ruficornis in larval and adult stages | 2017 | [766] | |
| Ethanolic extract from S. collinsiae roots | Elimination of C. megacephala flies at the larval stage | 2023 | [767] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Periferakis, A.; Periferakis, A.-T.; Troumpata, L.; Periferakis, K.; Georgatos-Garcia, S.; Touriki, G.; Dragosloveanu, C.D.M.; Caruntu, A.; Savulescu-Fiedler, I.; Dragosloveanu, S.; et al. Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential. Curr. Issues Mol. Biol. 2025, 47, 204. https://doi.org/10.3390/cimb47030204
Periferakis A, Periferakis A-T, Troumpata L, Periferakis K, Georgatos-Garcia S, Touriki G, Dragosloveanu CDM, Caruntu A, Savulescu-Fiedler I, Dragosloveanu S, et al. Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential. Current Issues in Molecular Biology. 2025; 47(3):204. https://doi.org/10.3390/cimb47030204
Chicago/Turabian StylePeriferakis, Argyrios, Aristodemos-Theodoros Periferakis, Lamprini Troumpata, Konstantinos Periferakis, Spyrangelos Georgatos-Garcia, Georgia Touriki, Christiana Diana Maria Dragosloveanu, Ana Caruntu, Ilinca Savulescu-Fiedler, Serban Dragosloveanu, and et al. 2025. "Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential" Current Issues in Molecular Biology 47, no. 3: 204. https://doi.org/10.3390/cimb47030204
APA StylePeriferakis, A., Periferakis, A.-T., Troumpata, L., Periferakis, K., Georgatos-Garcia, S., Touriki, G., Dragosloveanu, C. D. M., Caruntu, A., Savulescu-Fiedler, I., Dragosloveanu, S., Scheau, A.-E., Badarau, I. A., Caruntu, C., & Scheau, C. (2025). Pinosylvin: A Multifunctional Stilbenoid with Antimicrobial, Antioxidant, and Anti-Inflammatory Potential. Current Issues in Molecular Biology, 47(3), 204. https://doi.org/10.3390/cimb47030204











