Immunostimulating and Anticancer Activities of the Pectic Polysaccharide from Panax ginseng Leaves Treated with High Pressure/Enzyme Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Polysaccharide Fractions from Panax ginseng Leaves
2.2. Experimental Animals
2.3. Chemical Properties Analysis
2.4. Complement System Activation
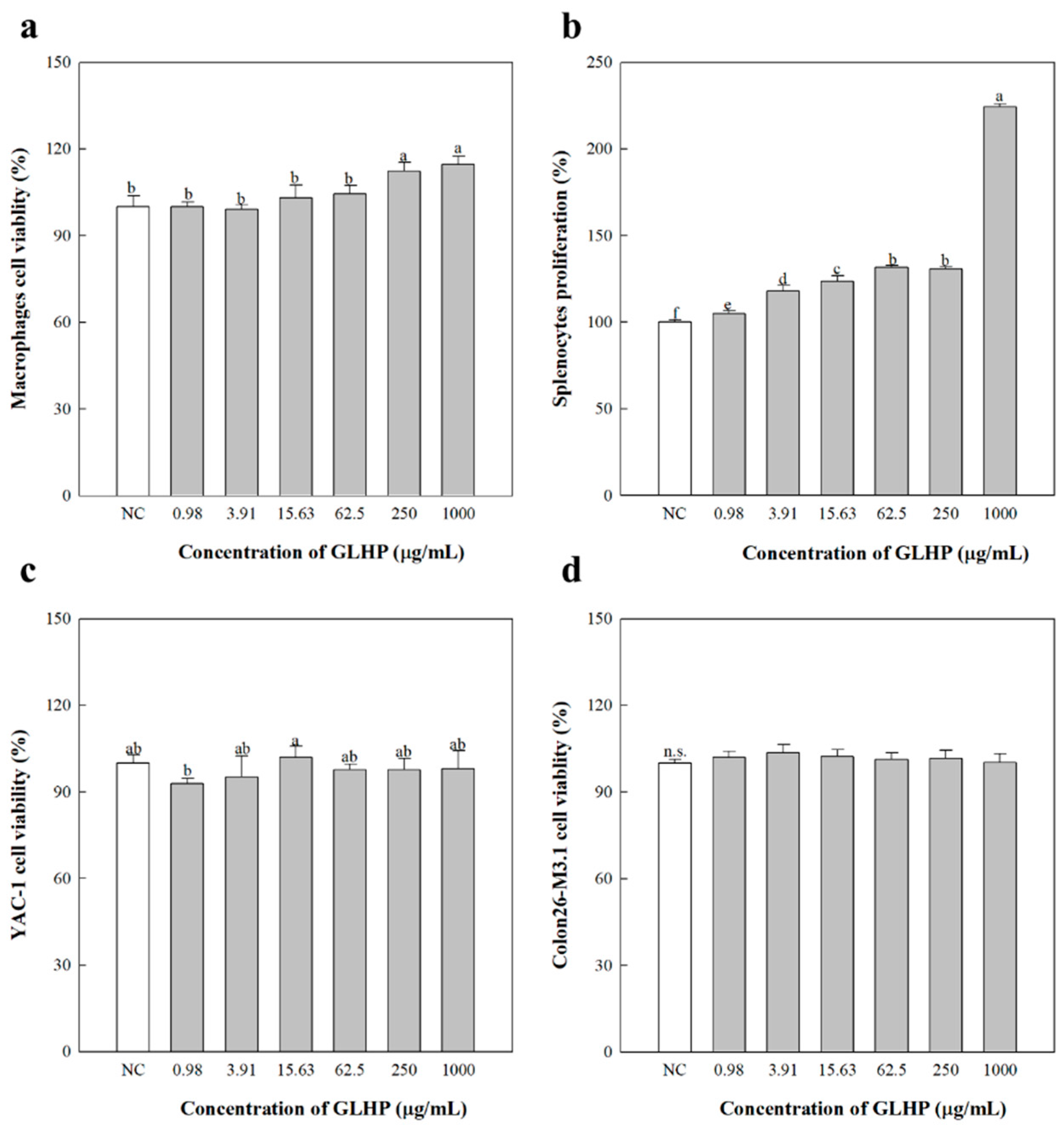
2.5. Murine Peritoneal Macrophage Activation
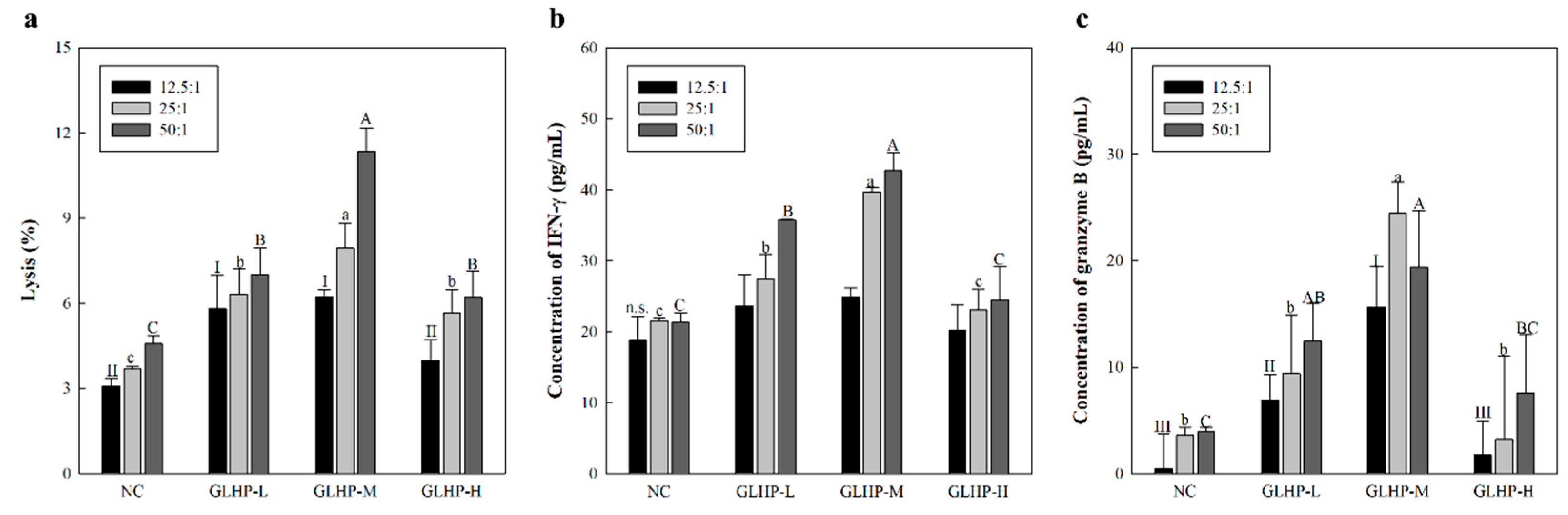
2.6. Splenic NK-Cell-Mediated Cytotoxicity Against Cancer Cell
2.7. Anticancer Metastasis Activities and NK-Cell Depleted Mice Model
2.8. Splenic Cytotoxic T Lymphocyte-Mediated Cytotoxicity Against Cancer Cells
2.9. Statistical Analysis
3. Results
3.1. Preparation and Chemical Characteristics of Active Polysaccharide from Ginseng Leaves
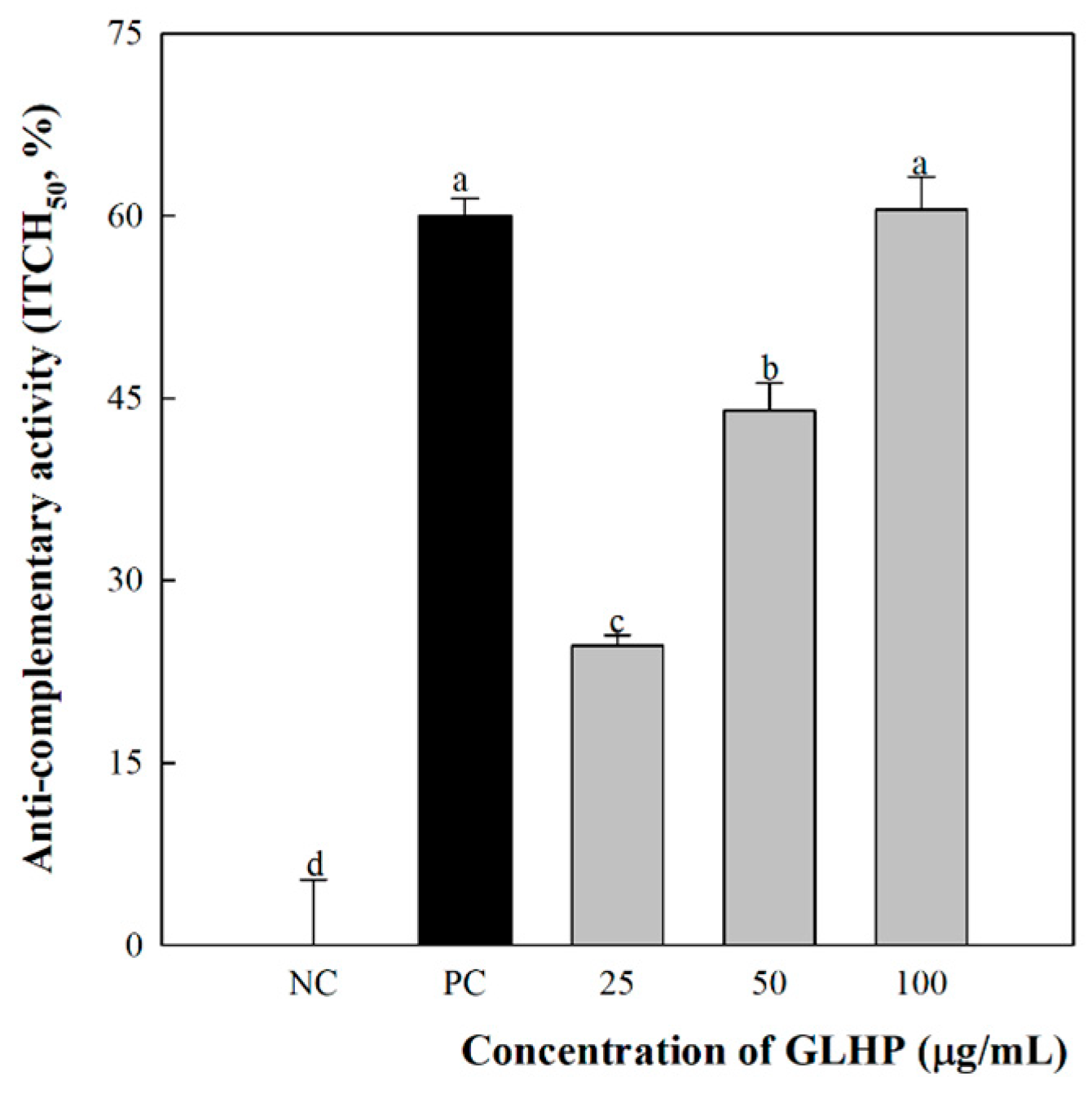
3.2. Complement System Activation by GLHP Isolated from Ginseng Leaves
3.3. Cytokine Secretion of Macrophages by GLHP Isolated from Ginseng Leaves
3.4. Cancer Cell Killing Effects Through NK Cell Stimulated by GLHP Isolated from Ginseng Leaves
3.5. Anticancer Effects of i.v. and Oral Administration of GLHP Isolated from Ginseng Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ara | Arabinose |
| A/GM | Asialo GM1/GLHP-M combination |
| A/O | Asialo GM1 only |
| CTL | Cytotoxic T lymphocyte |
| E/T | Effector/target |
| Fuc | Fucose |
| Gal | Galactose |
| GalA | Galacturonic acid |
| GLHP | Ginseng leaves high pressure- enzyme polysaccharide |
| GLHP-L | GLHP-low dose |
| GLHP-M | GLHP-medium dose |
| GLHP-H | GLHP-high dose |
| GM/O | GLHP-M only |
| GVB | Gelatin-veronal buffer |
| HG | Homogalacturonan |
| HPEM | High-pressure extraction method |
| HWEM | Hot water extraction method |
| IL | Interleukin |
| ITCH50 | Inhibition of 50% total complement hemolysis |
| i.v. | Intravenous |
| KDO | 2-keto-3-deoxy-D-manno-octonate |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| MAC | Membrane attack complex |
| MHC | Major histocompatibility complex |
| MW | Molecular weight |
| NC | Negative control |
| NHS | Normal human serum |
| NK | Natural killer |
| PMP | 1-phenyl-3-methyl-5-pyrazolone |
| PC | Positive control |
| PSK | Polysaccharide-K |
| Rha | Rhamnose |
| RG | Rhamnogalacturonan |
| RID | Refractive index detector |
| TNF | Tumor necrosis factor |
| UVD | Ultraviolet visible detector |
| Xyl | Xylose |
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef]
- Holers, V.M. Complement and its receptors: New insights into human disease. Annu. Rev. Immunol. 2014, 32, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, N.W.; Ziblat, A. Regulation of NK cell activation and effector functions by the IL-12 family of cytokines: The case of IL-27. Front. Immunol. 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Liesche, C.; Van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandström, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J. NK cells switch from granzyme B to death receptor–mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Prakash, M.D.; Munoz, M.A.; Jain, R.; Tong, P.L.; Koskinen, A.; Regner, M.; Kleifeld, O.; Ho, B.; Olson, M.; Turner, S.J. Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity 2014, 41, 960–972. [Google Scholar] [CrossRef]
- Andersen, M.H.; Schrama, D.; thor Straten, P.; Becker, J.C. Cytotoxic T cells. J. Investig. Dermatol. 2006, 126, 32–41. [Google Scholar] [CrossRef]
- Smyth, M.J.; Cretney, E.; Kelly, J.M.; Westwood, J.A.; Street, S.E.; Yagita, H.; Takeda, K.; Van Dommelen, S.L.; Degli-Esposti, M.A.; Hayakawa, Y. Activation of NK cell cytotoxicity. Mol. Immunol. 2005, 42, 501–510. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Yang, B.B.; Wang, C.-Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Bai, C.; Chen, R.; Chen, Y.; Bai, H.; Sun, H.; Li, D.; Wu, W.; Wang, Y.; Gong, M. Plant polysaccharides extracted by high pressure: A review on yields, physicochemical, structure properties, and bioactivities. Int. J. Biol. Macromol. 2024, 263, 129939. [Google Scholar] [CrossRef]
- Zou, X.; Xiao, J.; Chi, J.; Zhang, M.; Zhang, R.; Jia, X.; Mei, D.; Dong, L.; Yi, Y.; Huang, F. Physicochemical properties and prebiotic activities of polysaccharides from Zizyphus jujube based on different extraction techniques. Int. J. Biol. Macromol. 2022, 223, 663–672. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Y.; Zhou, C.; Xu, T.; Chen, X.; Wu, Q.; Zhang, K.; Li, Y.; Li, D.; Chen, Y. Effects of ultra-high pressure treatment on structure and bioactivity of polysaccharides from large leaf yellow tea. Food Chem. 2022, 387, 132862. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Shao, S.; Wang, D.; Zhao, D.; Wang, M. Recent progress in polysaccharides from Panax ginseng CA Meyer. Food Funct. 2021, 12, 494–518. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, Y.K.; Park, N.I.; Kim, C.S.; Lee, C.Y.; Park, S.U. Chemical constituents and biological activities of the berry of Panax ginseng. J. Med. Plants Res. 2010, 4, 349–353. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.-w.; Zhu, S.; Yin, H.-p.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Son, S.U.; Suh, H.J.; Shin, K.S. Characterization of a novel sulfated-rhamnoglucuronan isolated from Korean seaweed Ulva pertusa and its efficacy for treatment of inflammatory bowel disease in mice. Carbohydr. Polym. 2024, 342, 122373. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-R.; Lee, H.-S.; Cho, S.Y.; Kim, Y.-S.; Shin, K.-S. Anti-metastatic effect of polysaccharide isolated from Colocasia esculenta is exerted through immunostimulation. Int. J. Mol. Med. 2013, 31, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-U.; Kim, H.W.; Park, M.S.; Shin, K.-S. Effects of intravenous administration of polysaccharide purified from fermented barley on tumor metastasis inhibition via immunostimulating activities. Food Biosci. 2022, 49, 101833. [Google Scholar] [CrossRef]
- Choi, E.H.; Son, S.U.; Shin, K.S. Tumor inhibitory effect via immunostimulating activities of a rhamnogalacturonan-I-rich polysaccharide isolated from turmeric (Curcuma longa L.). J. Food Biochem. 2022, 46, e14362. [Google Scholar] [CrossRef]
- Chen, Z.; Tan, B.K.H.; Chan, S.H. Activation of T lymphocytes by polysaccharide–protein complex from Lycium barbarum L. Int. Immunopharmacol. 2008, 8, 1663–1671. [Google Scholar] [CrossRef]
- Moser, M. Regulation of Th1/Th2 development by antigen-presenting cells in vivo. Immunobiol. 2001, 204, 551–557. [Google Scholar] [CrossRef]
- Voragen, A.G.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Afshar-Kharghan, V. The role of the complement system in cancer. J. Clin. Investig. 2017, 127, 780–789. [Google Scholar] [CrossRef]
- Peng, Q.; Li, K.; Anderson, K.; Farrar, C.A.; Lu, B.; Smith, R.A.; Sacks, S.H.; Zhou, W. Local production and activation of complement up-regulates the allostimulatory function of dendritic cells through C3a–C3aR interaction. Blood 2008, 111, 2452–2461. [Google Scholar] [CrossRef]
- Strainic, M.G.; Liu, J.; Huang, D.; An, F.; Lalli, P.N.; Muqim, N.; Shapiro, V.S.; Dubyak, G.R.; Heeger, P.S.; Medof, M.E. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 2008, 28, 425–435. [Google Scholar] [CrossRef]
- Cassetta, L.; Pollard, J.W. Tumor-associated macrophages. Curr. Biol. 2020, 30, R246–R248. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, K.; Craig, R.A.; Stoolman, L.M.; Chang, A.E. Effects of tumor necrosis factor-α on the in vitro maturation of tumor-reactive effector T cells. Int. J. Immunother. 2000, 23, 528–535. [Google Scholar]
- Lv, Y.; Liu, Z.; Duan, X.; Cui, J.; Zhang, W.; Ma, W.; Liu, Y.; Song, X.; Fan, Y. Immunoenhancement and antioxidative damage effects of Polygonum Cillinerve polysaccharide on RAW264. 7 cells. J. Pharm. Pharmacol. 2022, 74, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liao, W.; Ren, J. Physicochemical characterization of a polysaccharide fraction from Platycladus orientalis (L.) franco and its macrophage immunomodulatory and anti-hepatitis B virus activities. J. Agric. Food Chem. 2016, 64, 5813–5823. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Son, S.U.; Lee, H.W.; Shin, K.S. Immunostimulating Activities and Anti-Cancer Efficacy of Rhamnogalacturonan-I Rich Polysaccharide Purified from Panax ginseng Leaf. Food Biosci. 2023, 53, 102618. [Google Scholar] [CrossRef]
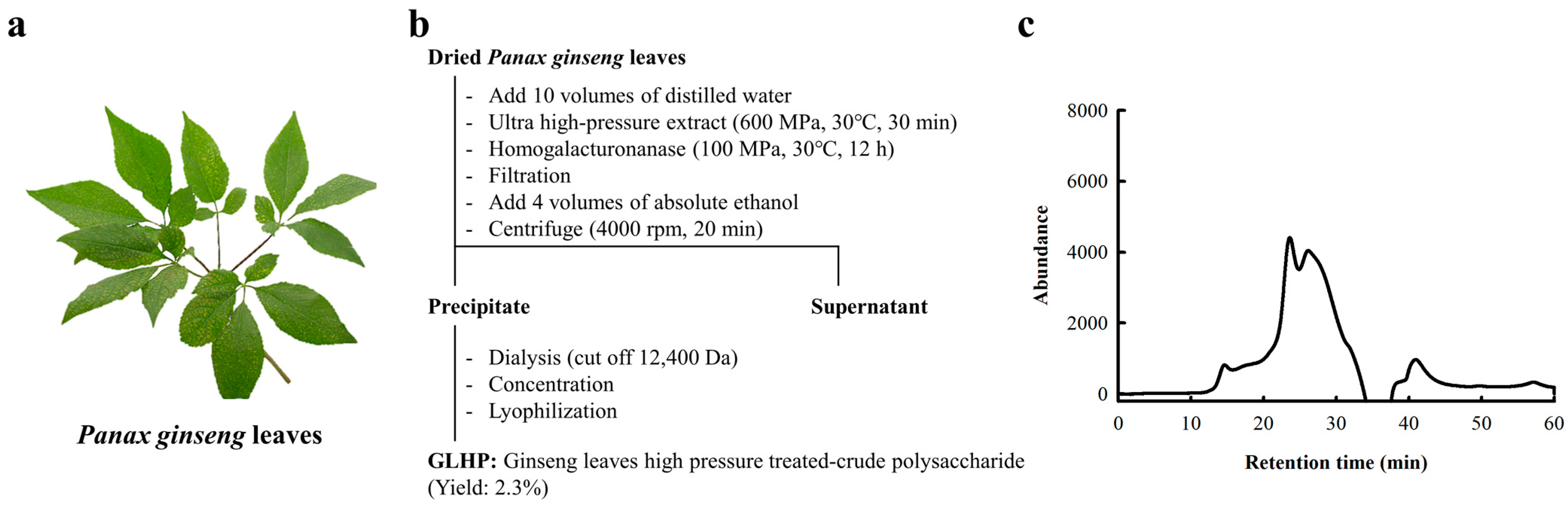



| Chemical Properties | GLHP (µg/100 µg) |
|---|---|
| Neutral sugar | 53.9 ± 0.8 |
| Uronic acid | 26.3 ± 0.4 |
| Protein | 9.1 ± 0.7 |
| KDO *-liked material | 2.3 ± 0.0 |
| Sugar Composition | GLHP (µg/100 µg) |
| Rhamnose | 7.5 ± 0.3 |
| Mannose | 7.3 ± 0.0 |
| Glucose | 16.1 ± 0.4 |
| Galactose | 19.0 ± 0.6 |
| Fucose | 2.7 ± 0.5 |
| Xylose | 1.5 ± 0.3 |
| Arabinose | 12.5 ± 0.1 |
| Glucuronic acid | 5.3 ± 0.3 |
| Galacturonic acid | 10.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.-U.; Hong, K.R.; Shin, K.-S. Immunostimulating and Anticancer Activities of the Pectic Polysaccharide from Panax ginseng Leaves Treated with High Pressure/Enzyme Process. Curr. Issues Mol. Biol. 2025, 47, 257. https://doi.org/10.3390/cimb47040257
Son S-U, Hong KR, Shin K-S. Immunostimulating and Anticancer Activities of the Pectic Polysaccharide from Panax ginseng Leaves Treated with High Pressure/Enzyme Process. Current Issues in Molecular Biology. 2025; 47(4):257. https://doi.org/10.3390/cimb47040257
Chicago/Turabian StyleSon, Seung-U, Ki Rim Hong, and Kwang-Soon Shin. 2025. "Immunostimulating and Anticancer Activities of the Pectic Polysaccharide from Panax ginseng Leaves Treated with High Pressure/Enzyme Process" Current Issues in Molecular Biology 47, no. 4: 257. https://doi.org/10.3390/cimb47040257
APA StyleSon, S.-U., Hong, K. R., & Shin, K.-S. (2025). Immunostimulating and Anticancer Activities of the Pectic Polysaccharide from Panax ginseng Leaves Treated with High Pressure/Enzyme Process. Current Issues in Molecular Biology, 47(4), 257. https://doi.org/10.3390/cimb47040257








