Mapping Small Extracellular Vesicle Secretion Potential in Healthy Human Gingiva Using Spatial Transcriptomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Preprocessing
2.2. Seurat Object Creation
2.3. Data Normalization and Quality Control
2.4. Dimensional Reduction and Clustering
2.5. Differential Expression Analysis
2.6. Gene Ontology Analysis
2.7. Additional Data Visualization
2.8. Software and Hardware
3. Results
3.1. Description of Assigned Clusters
3.2. Description of Differentially Expressed Genes in Assigned Clusters
3.2.1. Description of DEGs in Cluster 0
3.2.2. Description of DEGs in Cluster 2
3.2.3. Description of DEGs in Cluster 3
3.2.4. Description of DEGs in Cluster 4
3.2.5. Description of DEGs in Cluster 5
3.2.6. Description of DEGs in Cluster 6
3.2.7. Description of DEGs in Clusters 1 and 7
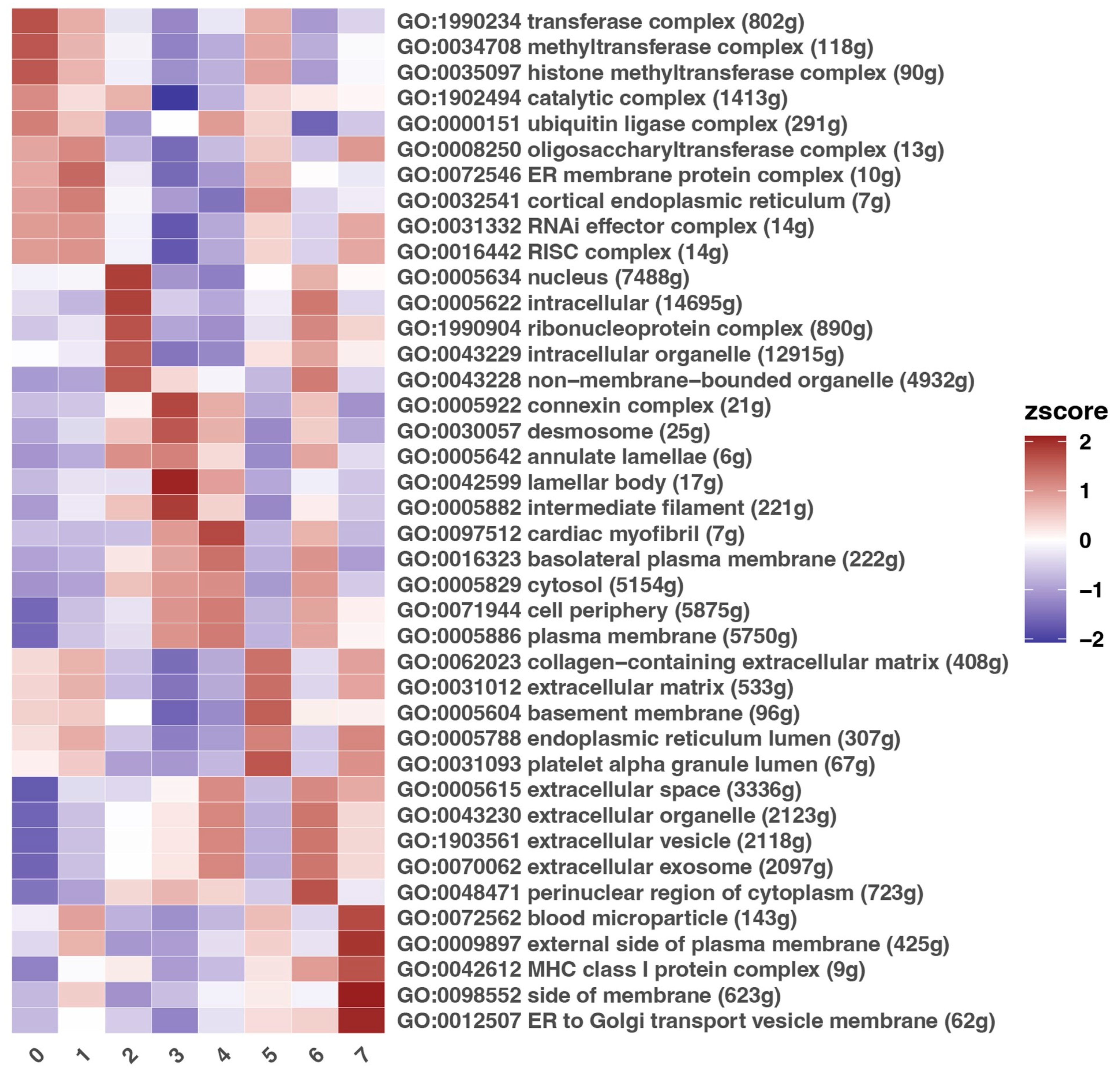
3.3. Description of Gene Ontology Processes
3.4. Description of Selected Genes with a Potential or Documented Role in sEV Biogenesis
3.4.1. Description of Clusters 4 and 6 in Context of Selected Genes
3.4.2. Description of Selected Genes That Were Not Exclusively Characteristic of Clusters 4 and 6
3.5. Summary of the Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2M | Alpha-2-Macroglobulin |
| AG | Attached gingiva |
| ALIX | Programmed Cell Death 6 Interacting Protein |
| ANGPTL7 | Angiopoietin Like 7 |
| ANXA, 2, 5, 11 | Annexin, II, V, XI |
| ATP6V0A4 | ATPase H+-Transporting V0 Subunit A4 |
| AQP5 | Aquaporin 5 |
| BAG6 | BAG Cochaperone 6 |
| CCNJL | Cyclin J-like |
| CCL14 | Chemokine C-C motif Ligand 14 |
| CCR2 | C-C Motif Chemokine Receptor 2 |
| CD247, 63, 73, 81, 82, 9 | CD247, 63, 73, 81, 82, 9 molecule |
| CERK | Ceramide Kinase |
| CHMP1A, 2A, 4B, 4C | Charged Multivesicular Body Protein 1A, 2A, 4B, 4C |
| CLCA4 | Chloride Channel Accessory 4 |
| CLTC | Clathrin Heavy Chain |
| COL11A1 | Collagen Type XI Alpha 1 Chain |
| CRC | Colorectal cancer |
| CYP24A1 | Cytochrome P450 Family 24 Subfamily A Member 1 |
| DEG | Differentially expressed gene |
| DUOXA2 | Dual Oxidase Maturation Factor 2 |
| ECM | Extracellular matrix |
| EGFR | Epidermal Growth Factor Receptor |
| ESCRT-I, -III | Endosomal Sorting Complex Required For Transport I, III |
| EVs | Extracellular vesicles |
| FG | Free gingiva |
| FGF22 | Fibroblast Growth Factor 22 |
| FLOT1 | Flotillin-1 |
| FUT6 | Fucosyltransferase 6 |
| GEO | Gene Expression Omnibus |
| GG | Gingival groove |
| GMSC | Gingival mesenchymal stem cell |
| GO | Gene Ontology |
| GZMA | Granzyme A |
| H2O2 | Hydrogen peroxide |
| H&E | Hematoxylin and eosin |
| HSP70 | Heat Shock Protein 70 (Hsp70) Family Protein |
| HSP90AA1 | Heat Shock Protein 90 Alpha Family Class A Member 1 |
| HSPA8 | Heat Shock Protein Family A (Hsp70) Member 8 |
| ICAM1 | Intercellular Adhesion Molecule 1 |
| IGHM | Immunoglobulin Heavy Constant Mu |
| IGHA1 | Immunoglobulin Heavy Constant Alpha 1 |
| IGHG2 | Immunoglobulin Heavy Constant Gamma 2 |
| IGHV3-30 | Immunoglobulin Heavy Variable 3-30 |
| IGHV6-1 | Immunoglobulin Heavy Variable 6-1 |
| IGKV | Immunoglobulin Kappa Variable Cluster |
| ILV | Intraluminal vesicle |
| ITGB3 | Integrin Subunit Beta 3 |
| JE | Junctional epithelium |
| LAMC2 | Oxidative Stress-Induced Growth Inhibitor 1 |
| LC3 | Light chain 3 |
| LCE2A, 2B, 6A | Late Cornified Envelope 2A, 2B, 6A |
| LGALS3BP | Galectin 3 Binding Protein |
| LP | Lamina propria |
| MAPK/ERK | Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase |
| MFGE8 | Milk Fat Globule EGF and Factor V/VIII Domain Containing |
| miRNA | Microribonucleic Acid |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| mRNA | Messenger Ribonucleic Acid |
| MS4A1 | Membrane Spanning 4-Domains A1 |
| MUC1 | Mucin 1 |
| MVB | Multivesicular body |
| MZB1 | Marginal Zone B and B1 Cell-Specific Protein 1 |
| NK (cells) | Natural killer |
| OE | Oral epithelium |
| OSGIN1 | Oxidative Stress-Induced Growth Inhibitor 1 |
| PCA | Principal component analysis |
| PEG3 | Paternally Expressed 3 |
| PLA2G2F | Phospholipase A2 Group IIF |
| PLD2 | Phospholipase D2 |
| PTEN | Phosphatase and Tensin Homolog |
| RAB11A, B | RAB11A, B, Member RAS Oncogene Family |
| RAB22A | RAB22A, Member RAS Oncogene Family |
| RAB27A, B | RAB27A, B, Member RAS Oncogene Family |
| RAB35 | RAB35, Member RAS Oncogene Family |
| RAB5A | RAB5A, Member RAS Oncogene Family |
| RAB7A | RAB7A, Member RAS Oncogene Family |
| RNA | Ribonucleic Acid |
| SE | Sulcular epithelium |
| SDCBP2 | Syndecan Binding Protein 2 |
| sEVs | Small extracellular vesicles |
| SMPD3 | Sphingomyelin Phosphodiesterase 3 |
| SNAP23 | Synaptosome-Associated Protein 23 |
| SNN | Shared nearest neighbor |
| SPIB | Spi-B Transcription Factor |
| STX4 | Syntaxin 4 |
| T | Tooth |
| TLR7 | Toll-like receptor 7 |
| TNFRSF9 | TNF Receptor Superfamily Member 9 |
| TP53 | Tumor Protein P53 |
| TRAJ20 | Cytochrome P450 Family 24 Subfamily A Member 1 |
| TSG101 | Tumor Susceptibility 101 |
| UMAP | Uniform Manifold Approximation and Projection |
| UNC93B1 | Unc-93 Homolog B1, TLR Signaling Regulator |
| VAMP7 | Vesicle-Associated Membrane Protein 7 |
| VPS37B, C | VPS37B, C Subunit Of ESCRT-I |
| VPS4A, B | Vacuolar Protein Sorting 4 Homolog A, B |
Appendix A
| Source | Gene Name | Citation | |||
|---|---|---|---|---|---|
| 1 | The incorporation of extracellular vesicle markers varies among vesicles with distinct surface charges, Maeda et al. | CD63 | CD81 | CD9 | [48] |
| FLOT1 | |||||
| 2 | Identification of specific markers for human pluripotent stem cell-derived small extracellular vesicles, Chen et al. | OCT4 | PODXL | OCT4 | [122] |
| 3 | Pentapartite fractionation of particles in oral fluids by differential centrifugation, Hiraga et al. | AQP5 | CD133 | CD63 | [123] |
| CD81 | CD9 | ||||
| 4 | ExoCarta: Exosome markers, Mathivanan et al. | A2M | ACLY | ACTB | [124] |
| ACTG1 | ACTN4 | AHCY | |||
| ALB | ALDOA | ANXA1 | |||
| ANXA11 | ANXA2 | ANXA4 | |||
| ANXA5 | ANXA6 | ARF1 | |||
| ATP1A1 | BSG | CCT2 | |||
| CCT3 | CCT5 | CD63 | |||
| CD81 | CDC42 | CFL1 | |||
| CLIC1 | CLTC | EEF1A1 | |||
| EEF2 | EHD4 | ENO1 | |||
| EZR | FASN | FLNA | |||
| FLOT1 | FN1 | GAPDH | |||
| GDI2 | GNAI2 | GNAS | |||
| GNB1 | GNB2 | GSN | |||
| HIST1H4A | HIST1H4B | HIST2H4A | |||
| HSP90AA1 | HSP90AB1 | HSPA1A | |||
| HSPA5 | HSPA8 | ITGA6 | |||
| ITGB1 | KPNB1 | LAMP2 | |||
| LDHA | LDHB | LGALS3BP | |||
| MFGE8 | MSN | PDCD6IP | |||
| PFN1 | PGK1 | PKM | |||
| PPIA | PRDX1 | PRDX2 | |||
| PTGFRN | PTGFRN | RAB14 | |||
| RAB1A | RAB5A | RAB5B | |||
| RAB5C | RAB7A | RAB8A | |||
| RAC1 | RAN | RAP1B | |||
| RHOA | SDCBP | SLC16A1 | |||
| SLC3A2 | STOM | TCP1 | |||
| TFRC | THBS1 | TKT | |||
| TPI1 | TSG101 | TUBA1A | |||
| TUBA1B | TUBA1C | UBA1 | |||
| VCP | YWHAB | YWHAE | |||
| YWHAG | YWHAH | YWHAQ | |||
| YWHAZ | |||||
| 5 | Differential Expression of Keratinocyte-Derived Extracellular Vesicle Mirnas Discriminate Exosomes From Apoptotic Bodies and Microvesicles, Than et al. | HSP70 | AGO2 | TSG101 | [125] |
| CD9 | CD163 | CD9 | |||
| 6 | Advances in mesenchymal stem cell exosomes: a review, Tang et al. | CD10 | CD133 | CD29 | [126] |
| CD44 | CD63 | CD73 | |||
| CD73 | CD105 | FLOT1 | |||
| ICAM1 | ALIX | EPCAM | |||
| TSG101 | CD81 | CD106 | |||
| 7 | Endosomal signalling via exosome surface TGFβ-1, Shelke et al. | TGFB1 | [127] | ||
| 8 | Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification, Choezom et al. | ATP6V1A | ATP6VE1 | [128] | |
| 9 | ITGB3-mediated uptake of small extracellular vesicles facilitates intercellular communication in breast cancer cells, Fuentes et al. | ITGB3 | [129] | ||
| 10 | Exocyst controls exosome biogenesis via Rab11a, Bai et al. | RAB11A | [130] | ||
| 11 | Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53, Schuldner et al. | BAG6 | [131] | ||
| 12 | Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release, Horbay et al. | CERK | [132] | ||
| 13 | Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles, Egea-Jimenez et al. | PLD2 | [133] | ||
| 14 | PTEN Deficiency Facilitates Exosome Secretion and Metastasis in Cholangiocarcinoma by Impairing TFEB-mediated Lysosome Biogenesis, Jiang et al. | PTEN | [134] | ||
| 15 | Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer, Lee et al. | CD8 | [135] | ||
| 16 | Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation, Lv et al. | IL6 | [136] | ||
| 17 | MUC1- C is a master regulator of MICA/B NKG2D ligand and exosome secretion in human cancer cells, Morimoto et al. | MUC1 (MUC1-C) | [50] | ||
| 18 | Embryonic signals mediate extracellular vesicle biogenesis and trafficking at the embryo–maternal interface, Guzewska et al. | VPS37B | [55] | ||
| 19 | MDA-9/Syntenin: An emerging global molecular target regulating cancer invasion and metastasis, Das et al. | SDCBP2 | [54] | ||
| 20 | The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles, Coulter et al. | CHMP1A | [137] | ||
| 21 | Identification of the SNARE complex that mediates the fusion of multivesicular bodies with the plasma membrane in exosome secretion, Liu et al. | SNAP23, VAMP7 | [138] | ||
| 22 | SMPD3-mediated extracellular vesicle biogenesis inhibits oligodendroglioma growth, Balakrishnan et al. | SMPD3 | [139] | ||
| 23 | VAMP5 and distinct sets of cognate Q-SNAREs mediate exosome release, Matsui et al. | STX4 | [140] | ||
| 24 | ALIX- and ESCRT-III–dependent sorting of tetraspanins to exosomes, Larios et al. | CHMP4B | [141] | ||
| 25 | Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A–C, Hsu et al. | RAB35 | [142] | ||
| 26 | Vps4A-mediated tumor suppression upon exosome modulation? Akrap et al. | VPS4A | [143] | ||
| 27 | p-AKT/VPS4B regulates the small extracellular vesicle size in venous malformation endothelial cells, Lai et al. | VPS4B | [144] | ||
| 28 | A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer, Sun et al. | TP53 | [145] | ||
| 29 | Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells, Honegger et al. | CHMP4C | [49] | ||
| 30 | LRRK2 secretion in exosomes is regulated by 14-3-3, Fraser et al. | LRRK2 | [146] | ||
| 31 | Rab27a and Rab27b control different steps of the exosome secretion pathway, Ostrowski et al. | RAB27A, RAB27B | [53] | ||
References
- Prabhu, S.R. Healing: Tissue Regeneration and Repair. In Textbook of General Pathology for Dental Students; Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 49–56. [Google Scholar] [CrossRef]
- Poss, K.D.; Tanaka, E.M. Hallmarks of Regeneration. Cell Stem Cell 2024, 31, 1244–1261. [Google Scholar] [CrossRef]
- Yun, M.H. Changes in Regenerative Capacity through Lifespan. Int. J. Mol. Sci. 2015, 16, 25392–25432. [Google Scholar] [CrossRef]
- Giancotti, A.; Monti, M.; Nevi, L.; Safarikia, S.; D’Ambrosio, V.; Brunelli, R.; Pajno, C.; Corno, S.; Di Donato, V.; Musella, A.; et al. Functions and the Emerging Role of the Foetal Liver into Regenerative Medicine. Cells 2019, 8, 914. [Google Scholar] [CrossRef] [PubMed]
- Glim, J.E.; Everts, V.; Niessen, F.B.; Ulrich, M.M.; Beelen, R.H.J. Extracellular Matrix Components of Oral Mucosa Differ from Skin and Resemble That of Foetal Skin. Arch. Oral Biol. 2014, 59, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Boushell, L.W.; Sturdevant, J.R. Clinical Significance of Dental Anatomy, Histology, Physiology, and Occlusion. In Sturdevant’s Art and Science of Operative Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–39. [Google Scholar] [CrossRef]
- Griffin, M.F.; Fahy, E.J.; King, M.; Guardino, N.; Chen, K.; Abbas, D.B.; Lavin, C.V.; Diaz Deleon, N.M.; Lorenz, H.P.; Longaker, M.T.; et al. Understanding Scarring in the Oral Mucosa. Adv. Wound Care 2022, 11, 537. [Google Scholar] [CrossRef]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Maier, T. Oral Microbiome in Health and Disease: Maintaining a Healthy, Balanced Ecosystem and Reversing Dysbiosis. Microorganisms 2023, 11, 1453. [Google Scholar] [CrossRef]
- Page, R.C.; Schroeder, H.E. Pathogenesis of Inflammatory Periodontal Disease. A Summary of Current Work. Lab. Investig. 1976, 34, 235–249. [Google Scholar]
- Kinane, D.F. Causation and Pathogenesis of Periodontal Disease. Periodontology 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Kinane Denis, F.; Lindhe, J. Pathogenesis of Periodontitis, 3rd ed.; Blackwell Science Inc.: Copenhagen, Denmark, 1997. [Google Scholar]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Petrovčíková, E.; Vičíková, K.; Leksa, V. Extracellular Vesicles—Biogenesis, Composition, Function, Uptake and Therapeutic Applications. Biologia 2018, 73, 437–448. [Google Scholar] [CrossRef]
- de Jong, O.G.; van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular Vesicles: Potential Roles in Regenerative Medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.S. Extracellular Vesicles in Regenerative Medicine: Potentials and Challenges. Tissue Eng. Regen. Med. 2021, 18, 479–484. [Google Scholar] [CrossRef]
- Pawlaczyk-Kamienska, T.; Torlinska-Walkowiak, N.; Borysewicz-Lewicka, M. The Relationship between Oral Hygiene Level and Gingivitis in Children. Adv. Clin. Exp. Med. 2018, 27, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, L.A.; Andreana, S.; Bush, P.J.; Cohen, R.E. Toothbrushing and Gingival Recession. Int. Dent. J. 2003, 53, 67–72. [Google Scholar] [CrossRef]
- Wang, R.; Ji, Q.; Meng, C.; Liu, H.; Fan, C.; Lipkind, S.; Wang, Z.; Xu, Q. Role of Gingival Mesenchymal Stem Cell Exosomes in Macrophage Polarization under Inflammatory Conditions. Int. Immunopharmacol. 2020, 81, 106030. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-Treated Human Gingiva-Derived MSCs Enhance M2 Macrophage Polarization and Inhibit Periodontal Bone Loss. Acta Biomater. 2021, 122, 306. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An Introduction to Spatial Transcriptomics for Biomedical Research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Ortiz, C.; Carlén, M.; Meletis, K. Spatial Transcriptomics: Molecular Maps of the Mammalian Brain. Annu. Rev. Neurosci. 2021, 44, 547–562. [Google Scholar] [CrossRef]
- Maynard, K.R.; Collado-Torres, L.; Weber, L.M.; Uytingco, C.; Barry, B.K.; Williams, S.R.; Catallini, J.L.; Tran, M.N.; Besich, Z.; Tippani, M.; et al. Transcriptome-Scale Spatial Gene Expression in the Human Dorsolateral Prefrontal Cortex. Nat. Neurosci. 2021, 24, 425–436. [Google Scholar] [CrossRef]
- Caetano, A.J.; Redhead, Y.; Karim, F.; Dhami, P.; Kannambath, S.; Nuamah, R.; Volponi, A.A.; Nibali, L.; Booth, V.; D’agostino, E.M.; et al. Spatially Resolved Transcriptomics Reveals Pro-Inflammatory Fibroblast Involved in Lymphocyte Recruitment through CXCL8 and CXCL10. eLife 2023, 12, e81525. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary Learning for Integrative, Multimodal and Scalable Single-Cell Analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hao, S.; Andersen-Nissen, E.; Mauck, W.M.; Zheng, S.; Butler, A.; Lee, M.J.; Wilk, A.J.; Darby, C.; Zager, M.; et al. Integrated Analysis of Multimodal Single-Cell Data. Cell 2021, 184, 3573. [Google Scholar] [CrossRef]
- Blatkiewicz, M.; Hryhorowicz, S.; Szyszka, M.; Suszyńska-Zajczyk, J.; Pławski, A.; Plewiński, A.; Porzionato, A.; Malendowicz, L.K.; Rucinski, M. Molecular Landscape of the Mouse Adrenal Gland and Adjacent Adipose by Spatial Transcriptomics. bioRxiv 2025, 1–21. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2; Springer Nature: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Pedersen, T.L. Patchwork: The Composer of Plots. CRAN: Contributed Packages. 2019. Available online: https://patchwork.data-imaginist.com/ (accessed on 2 February 2025).
- Schauberger, P.; Walker, A.; Braglia, L. Openxlsx: Read, Write and Edit Xlsx Files, version 4; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Bates, D.; Maechler, M.; Jagan, M. Matrix: Sparse and Dense Matrix Classes and Methods, version 0.999375-43; R Foundation for Statistical Computing: Vienna, Austria, 2010. Available online: http://cran.r-project.org/package=Matrix (accessed on 2 February 2025).
- Bengtsson, H. Various Programming Utilities [R Package R. Utils Version 2.13.0]. 2025. Available online: https://cran.r-project.org/web/packages/R.utils/index.html (accessed on 2 February 2025).
- R Core Team R: A Language and Environment for Statistical Computing|BibSonomy R Foundation for Statistical Computing 2022. Available online: https://www.r-project.org/foundation/ (accessed on 2 February 2025).
- Brizuela, M.; Winters, R. Histology, Oral Mucosa. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Ayansola, H.; Mayorga, E.J.; Jin, Y. Subepithelial Stromal Cells: Their Roles and Interactions with Intestinal Epithelial Cells during Gut Mucosal Homeostasis and Regeneration. Biomedicines 2024, 12, 668. [Google Scholar] [CrossRef]
- Ferreira, B.; Ferreira, C.; Martins, C.; Nunes, R.; das Neves, J.; Leite-Pereira, C.; Sarmento, B. Establishment of a 3D Multi-Layered in Vitro Model of Inflammatory Bowel Disease. J. Control. Release 2025, 377, 675–688. [Google Scholar] [CrossRef]
- Jacobi, A.; Loy, K.; Schmalz, A.M.; Hellsten, M.; Umemori, H.; Kerschensteiner, M.; Bareyre, F.M. FGF 22 Signaling Regulates Synapse Formation during Post-injury Remodeling of the Spinal Cord. EMBO J. 2015, 34, 1231–1243. [Google Scholar] [CrossRef]
- Shikani, A.H.; Sidhaye, V.K.; Basaraba, R.J.; Shikani, H.J.; Alqudah, M.A.; Kirk, N.; Cope, E.; Leid, J.G. Mucosal Expression of Aquaporin 5 and Epithelial Barrier Proteins in Chronic Rhinosinusitis with and without Nasal Polyps. Am. J. Otolaryngol. 2013, 35, 377. [Google Scholar] [CrossRef]
- Grasberger, H.; Gao, J.; Nagao-Kitamoto, H.; Kitamoto, S.; Zhang, M.; Kamada, N.; Eaton, K.A.; El-Zaatari, M.; Shreiner, A.B.; Merchant, J.L.; et al. Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine. Gastroenterology 2015, 149, 1849. [Google Scholar] [CrossRef]
- Fan, R.; Han, X.; Gong, Y.; He, L.; Xue, Z.; Yang, Y.; Sun, L.; Fan, D.; You, Y.; Meng, F.; et al. Alterations of Fucosyltransferase Genes and Fucosylated Glycans in Gastric Epithelial Cells Infected with Helicobacter Pylori. Pathogens 2021, 10, 168. [Google Scholar] [CrossRef]
- Cai, Y.; Ling, Y.; Huang, L.; Huang, H.; Chen, X.; Xiao, Y.; Zhu, Z.; Chen, J. C-C Motif Chemokine 14 as a Novel Potential Biomarker for Predicting the Prognosis of Epithelial Ovarian Cancer. Oncol. Lett. 2020, 19, 2875. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef]
- Garg, M.; Braunstein, G.; Koeffler, H.P. LAMC2 as a Therapeutic Target for Cancers. Expert. Opin. Ther. Targets 2014, 18, 979–982. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring Vitamin D Metabolism and Function in Cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Bergmeier, L.A.; Dutzan, N.; Smith, P.C.; Kraan, H. Editorial: Immunology of the Oral Mucosa. Front. Immunol. 2022, 13, 877209. [Google Scholar] [CrossRef]
- Flach, H.; Rosenbaum, M.; Duchniewicz, M.; Kim, S.; Zhang, S.L.; Cahalan, M.D.; Mittler, G.; Grosschedl, R. Mzb1 Protein Regulates Calcium Homeostasis, Antibody Secretion, and Integrin Activation in Innate-like B Cells. Immunity 2010, 33, 723. [Google Scholar] [CrossRef] [PubMed]
- Valapala, M.; Vishwanatha, J.K. Lipid Raft Endocytosis and Exosomal Transport Facilitate Extracellular Trafficking of Annexin A2. J. Biol. Chem. 2011, 286, 30911. [Google Scholar] [CrossRef]
- Maeda, K.; Goto, S.; Miura, K.; Saito, K.; Morita, E. The Incorporation of Extracellular Vesicle Markers Varies among Vesicles with Distinct Surface Charges. J. Biochem. 2024, 175, 299–312. [Google Scholar] [CrossRef]
- Honegger, A.; Leitz, J.; Bulkescher, J.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Silencing of Human Papillomavirus (HPV) E6/E7 Oncogene Expression Affects Both the Contents and the Amounts of Extracellular Microvesicles Released from HPV-Positive Cancer Cells. Int. J. Cancer 2013, 133, 1631–1642. [Google Scholar] [CrossRef]
- Morimoto, Y.; Yamashita, N.; Daimon, T.; Hirose, H.; Yamano, S.; Haratake, N.; Ishikawa, S.; Bhattacharya, A.; Fushimi, A.; Ahmad, R.; et al. MUC1-C Is a Master Regulator of MICA/B NKG2D Ligand and Exosome Secretion in Human Cancer Cells. J. Immunother. Cancer 2023, 11, e006238. [Google Scholar] [CrossRef]
- Gorji-bahri, G.; Moghimi, H.R.; Hashemi, A. RAB5A Is Associated with Genes Involved in Exosome Secretion: Integration of Bioinformatics Analysis and Experimental Validation. J. Cell. Biochem. 2021, 122, 425–441. [Google Scholar] [CrossRef]
- Fan, B.; Wang, L.; Wang, J. RAB22A as a Predictor of Exosome Secretion in the Progression and Relapse of Multiple Myeloma. Aging 2024, 16, 4169. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Sarkar, D.; Emdad, L.; Fisher, P.B. MDA-9/Syntenin: An Emerging Global Molecular Target Regulating Cancer Invasion and Metastasis. Adv. Cancer Res. 2019, 144, 137–191. [Google Scholar] [CrossRef] [PubMed]
- Guzewska, M.M.; Myszczynski, K.; Heifetz, Y.; Kaczmarek, M.M. Embryonic Signals Mediate Extracellular Vesicle Biogenesis and Trafficking at the Embryo–Maternal Interface. Cell Commun. Signal. 2023, 21, 210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Zhang, Y.; Zhou, W.; Zhang, X.; Peng, C.; Ji, T.; Zou, X.; Zhang, Z.; Ren, Z. Spatial Transcriptomics Reveals That Metabolic Characteristics Define the Tumor Immunosuppression Microenvironment via ICAF Transformation in Oral Squamous Cell Carcinoma. Int. J. Oral Sci. 2024, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Seubert, A.C.; Krafft, M.; Bopp, S.; Helal, M.; Bhandare, P.; Wolf, E.; Alemany, A.; Riedel, A.; Kretzschmar, K. Spatial Transcriptomics Reveals Molecular Cues Underlying the Site Specificity of the Adult Mouse Oral Mucosa and Its Stem Cell Niches. Stem Cell Rep. 2024, 19, 1706–1719. [Google Scholar] [CrossRef]
- Caetano, A.J.; Redhead, Y.; Karim, F.; Dhami, P.; Kannambath, S.; Nuamah, R.; Volponi, A.A.; Nibali, L.; Booth, V.; D’Agostino, E.M.; et al. Mapping the Spatial Dynamics of the Human Oral Mucosa in Chronic Inflammatory Disease. bioRxiv 2022, 27. [Google Scholar] [CrossRef]
- Jaber, Y.; Sarusi-Portuguez, A.; Netanely, Y.; Naamneh, R.; Yacoub, S.; Saar, O.; Drawshave, N.; Eli-Berchoer, L.; Shapiro, H.; Elinav, E.; et al. Gingival Spatial Analysis Reveals Geographic Immunological Variation in a Microbiota-Dependent and -Independent Manner. npj Biofilms Microbiomes 2024, 10, 142. [Google Scholar] [CrossRef]
- Santacroce, L.; Passarelli, P.C.; Azzolino, D.; Bottalico, L.; Charitos, I.A.; Cazzolla, A.P.; Colella, M.; Topi, S.; Godoy, F.G.; D’Addona, A. Oral Microbiota in Human Health and Disease: A Perspective. Exp. Biol. Med. 2023, 248, 1288. [Google Scholar] [CrossRef]
- Bhandari, A.; Bhatta, N. Tobacco and Its Relationship with Oral Health. JNMA J. Nepal Med. Assoc. 2021, 59, 1204. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Giudice, A.; Polizzi, A.; Troiano, G.; Merlo, E.M.; Sclafani, R.; Grosso, G.; Isola, G. A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis. Nutrients 2022, 14, 2426. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral Microbiome: Unveiling the Fundamentals. J. Oral. Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M. Interactions between Host and Oral Commensal Microorganisms Are Key Events in Health and Disease Status. Can. J. Infect. Dis. 2002, 13, 47. [Google Scholar] [CrossRef]
- Ji, S.; Choi, Y. Microbial and Host Factors That Affect Bacterial Invasion of the Gingiva. J. Dent. Res. 2020, 99, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Histological and Immunological Characteristics of the Junctional Epithelium. Jpn. Dent. Sci. Rev. 2017, 54, 59. [Google Scholar] [CrossRef]
- Dabija-Wolter, G.; Bakken, V.; Cimpan, M.R.; Johannessen, A.C.; Costea, D.E. In Vitro Reconstruction of Human Junctional and Sulcular Epithelium. J. Oral Pathol. Med. 2012, 42, 396. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Milo, R. The Distribution of Cellular Turnover in the Human Body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef]
- Vitkov, L.; Hannig, M.; Krautgartner, W.D.; Fuchs, K. Bacterial Adhesion to Sulcular Epithelium in Periodontitis. FEMS Microbiol. Lett. 2002, 211, 239–246. [Google Scholar] [CrossRef]
- Kho, H.S. Oral Epithelial MUC1 and Oral Health. Oral Dis. 2018, 24, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.S.; Yung, K.K.L. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Kullaa, A.M. Regulation of Mucin 1 Expression and Its Relationship with Oral Diseases. Arch. Oral Biol. 2020, 117, 104791. [Google Scholar] [CrossRef]
- Derrien, M.; van Passel, M.W.J.; van de Bovenkamp, J.H.B.; Schipper, R.G.; de Vos, W.M.; Dekker, J. Mucin-Bacterial Interactions in the Human Oral Cavity and Digestive Tract. Gut Microbes 2010, 1, 254. [Google Scholar] [CrossRef]
- Brockhausen, I.; Falconer, D.; Sara, S. Relationships between Bacteria and the Mucus Layer. Carbohydr. Res. 2024, 546, 109309. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wubbolts, R.W.; Bleumink-Pluym, N.M.C.; van Putten, J.P.M.; Strijbis, K. The Transmembrane Mucin Muc1 Facilitates B1-Integrin-Mediated Bacterial Invasion. mBio 2021, 12, e03491-20. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Nunes, D.P.; Troxler, R.F.; Offner, G.D. Pro-Inflammatory Cytokines up-Regulate MUC1 Gene Expression in Oral Epithelial Cells. J. Dent. Res. 2003, 82, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; McAuley, J. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front. Cell. Infect. Microbiol. 2019, 9, 452801. [Google Scholar] [CrossRef]
- Du, Y.; Li, L.L.; Chen, F. Targeting SDCBP2 in Acute Myeloid Leukemia. Cell Signal 2023, 112, 110889. [Google Scholar] [CrossRef] [PubMed]
- Majer, O.; Liu, B.; Kreuk, L.S.M.; Krogan, N.; Barton, G.M. UNC93B1 Recruits Syntenin-1 to Dampen TLR7 Signalling and Prevent Autoimmunity. Nature 2019, 575, 366–370. [Google Scholar] [CrossRef]
- Lee, K.M.; Seo, E.C.; Lee, J.H.; Kim, H.J.; Hwangbo, C. The Multifunctional Protein Syntenin-1: Regulator of Exosome Biogenesis, Cellular Function, and Tumor Progression. Int. J. Mol. Sci. 2023, 24, 9418. [Google Scholar] [CrossRef]
- Addi, C.; Presle, A.; Frémont, S.; Cuvelier, F.; Rocancourt, M.; Milin, F.; Schmutz, S.; Chamot-Rooke, J.; Douché, T.; Duchateau, M.; et al. The Flemmingsome Reveals an ESCRT-to-Membrane Coupling via ALIX/Syntenin/Syndecan-4 Required for Completion of Cytokinesis. Nat. Commun. 2020, 11, 1941. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Hwangbo, C.; Tran, P.T.; Lee, J.H. Syntenin-1-Mediated Small Extracellular Vesicles Promotes Cell Growth, Migration, and Angiogenesis by Increasing Onco-MiRNAs Secretion in Lung Cancer Cells. Cell Death Dis. 2022, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Balzano, M.; Lechat, B.; Lambaerts, K.; Egea-Jimenez, A.L.; Lembo, F.; Fares, J.; Meeussen, S.; Kügler, S.; Roebroek, A.; et al. Syntenin-Knock out Reduces Exosome Turnover and Viral Transduction. Sci. Rep. 2021, 11, 4083. [Google Scholar] [CrossRef]
- Ju, Y.; Bai, H.; Ren, L.; Zhang, L. The Role of Exosome and the ESCRT Pathway on Enveloped Virus Infection. Int. J. Mol. Sci. 2021, 22, 9060. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Wang, M.; Cao, Q.Q.; Li, Q. Chromatin Modified Protein 4C (CHMP4C) Facilitates the Malignant Development of Cervical Cancer Cells. FEBS Open Bio 2020, 10, 1295–1303. [Google Scholar] [CrossRef]
- Yu, L.; Guo, Q.; Li, Y.; Mao, M.; Liu, Z.; Li, T.; Wang, L.; Zhang, X. CHMP4C Promotes Pancreatic Cancer Progression by Inhibiting Necroptosis via the RIPK1/RIPK3/MLKL Pathway. J. Adv. Res. 2025, in press. [Google Scholar] [CrossRef]
- Moulin, C.; Crupi, M.J.F.; Ilkow, C.S.; Bell, J.C.; Boulton, S. Extracellular Vesicles and Viruses: Two Intertwined Entities. Int. J. Mol. Sci. 2023, 24, 1036. [Google Scholar] [CrossRef]
- Russell, T.; Samolej, J.; Hollinshead, M.; Smith, G.L.; Kite, J.; Elliott, G. Novel Role for ESCRT-III Component CHMP4C in the Integrity of the Endocytic Network Utilized for Herpes Simplex Virus Envelopment. mBio 2021, 12, e02183-20. [Google Scholar] [CrossRef]
- Arii, J.; Watanabe, M.; Maeda, F.; Tokai-Nishizumi, N.; Chihara, T.; Miura, M.; Maruzuru, Y.; Koyanagi, N.; Kato, A.; Kawaguchi, Y. ESCRT-III Mediates Budding across the Inner Nuclear Membrane and Regulates Its Integrity. Nat. Commun. 2018, 9, 3379. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Riley, T.; Levine, A.J. The Regulation of the Endosomal Compartment by P53 the Tumor Suppressor Gene. FEBS J. 2009, 276, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, L.; Mela, I.; Abad, M.A.; Jeyaprakash, A.A.; Edwardson, J.M.; D’Avino, P.P. Coordinated Regulation of the ESCRT-III Component CHMP4C by the Chromosomal Passenger Complex and Centralspindlin during Cytokinesis. Open Biol. 2016, 6, 160248. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Moita, C.; Van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef] [PubMed]
- Juan, T.; Fürthauer, M. Biogenesis and Function of ESCRT-Dependent Extracellular Vesicles. Semin. Cell Dev. Biol. 2018, 74, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Eastman, S.W.; Martin-Serrano, J.; Chung, W.; Zang, T.; Bieniasz, P.D. Identification of Human VPS37C, a Component of Endosomal Sorting Complex Required for Transport-I Important for Viral Budding. J. Biol. Chem. 2005, 280, 628–636. [Google Scholar] [CrossRef]
- Kolmus, K.; Erdenebat, P.; Szymanska, E.; Stewig, B.; Goryca, K.; Derezinska-Wołek, E.; Szumera-Ciećkiewicz, A.; Brewinska-Olchowik, M.; Piwocka, K.; Prochorec-Sobieszek, M.; et al. Concurrent Depletion of Vps37 Proteins Evokes ESCRT-I Destabilization and Profound Cellular Stress Responses. J. Cell Sci. 2021, 134, jcs250951. [Google Scholar] [CrossRef]
- Jiang, Q.; Yu, Y.; Ruan, H.; Luo, Y.; Guo, X. Morphological and Functional Characteristics of Human Gingival Junctional Epithelium. BMC Oral Health 2014, 14, 30. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 109543. [Google Scholar] [CrossRef]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of Exosome versus Small Ectosome Secretion Revealed by Live Intracellular Tracking of CD63 and CD9. Nat. Commun. 2021, 12, 4389. [Google Scholar] [CrossRef]
- Brosseau, C.; Colas, L.; Magnan, A.; Brouard, S. CD9 Tetraspanin: A New Pathway for the Regulation of Inflammation? Front. Immunol. 2018, 9, 2316. [Google Scholar] [CrossRef]
- Kodam, S.P.; Baghban, N.; Ullah, M. CD9 Role in Proliferation, Rejuvenation, and Therapeutic Applications. Genes Dis. 2023, 11, 101008. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Hu, Y. CD81, a New Actor in the Development of Preeclampsia. Cell. Mol. Immunol. 2021, 18, 2061. [Google Scholar] [CrossRef]
- Vences-Catalán, F.; Rajapaksa, R.; Srivastava, M.K.; Marabelle, A.; Kuo, C.C.; Levy, R.; Levy, S. Tetraspanin CD81, a Modulator of Immune Suppression in Cancer and Metastasis. Oncoimmunology 2015, 5, e1120399. [Google Scholar] [CrossRef] [PubMed]
- Pols, M.S.; Klumperman, J. Trafficking and Function of the Tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef]
- Xu, J.; Gu, J.; Pei, W.; Zhang, Y.; Wang, L.; Gao, J. The Role of Lysosomal Membrane Proteins in Autophagy and Related Diseases. FEBS J. 2024, 291, 3762–3785. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Cheerathodi, M.R.; Nkosi, D.; York, S.B.; Meckes, D.G. Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J. Virol. 2018, 92, e01969-17. [Google Scholar] [CrossRef] [PubMed]
- Toribio, V.; Yáñez-Mó, M. Tetraspanins Interweave EV Secretion, Endosomal Network Dynamics and Cellular Metabolism. Eur. J. Cell Biol. 2022, 101, 151229. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, P.; Wan, Y.; Chen, T.; Wang, Q.; Mettbach, U.; Baluška, F.; Śamaj, J.; Fang, X.; Lucas, W.J.; et al. A Membrane Microdomain-Associated Protein, Arabidopsis Flot1, Is Involved in a Clathrin-Independent Endocytic Pathway and Is Required for Seedling Development. Plant Cell 2012, 24, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sane, S.; Kim, J.E.; Yun, S.; Kim, H.J.; Jo, K.B.; Wright, J.P.; Khoshdoozmasouleh, N.; Lee, K.; Oh, H.T.; et al. Biogenesis and Delivery of Extracellular Vesicles: Harnessing the Power of EVs for Diagnostics and Therapeutics. Front. Mol. Biosci. 2023, 10, 1330400. [Google Scholar] [CrossRef]
- Lai, R.C.; Lim, S.K. Membrane Lipids Define Small Extracellular Vesicle Subtypes Secreted by Mesenchymal Stromal Cells. J. Lipid Res. 2018, 60, 318. [Google Scholar] [CrossRef]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab Family of Small GTPases: An Updated View on Their Regulation and Functions. FEBS J. 2020, 288, 36. [Google Scholar] [CrossRef] [PubMed]
- Skjeldal, F.M.; Haugen, L.H.; Mateus, D.; Frei, D.M.; Rødseth, A.V.; Hu, X.; Bakke, O. De Novo Formation of Early Endosomes during Rab5-to-Rab7a Transition. J. Cell Sci. 2021, 134, jcs254185. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zheng, X.; Chang, B.; Lin, Y.; Huang, X.; Wang, W.; Ding, S.; Zhan, W.; Wang, S.; Xiao, B.; et al. Intercellular Transfer of Activated STING Triggered by RAB22A-Mediated Non-Canonical Autophagy Promotes Antitumor Immunity. Cell Res. 2022, 32, 1086. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Song, Q.; Zhang, Y.; Wang, X.; Wang, Z. Molecular Characterization and Immune Responses of Rab5 in Large Yellow Croaker (Larimichthys crocea). Aquac. Fish. 2017, 2, 165–172. [Google Scholar] [CrossRef]
- Kong, L.; Huang, S.; Bao, Y.; Chen, Y.; Hua, C.; Gao, S. Crucial Roles of Rab22a in Endosomal Cargo Recycling. Traffic 2023, 24, 397–412. [Google Scholar] [CrossRef]
- Shan, J.; Chang, L.Y.; Li, D.J.; Wang, X.Q. Rab27b Promotes Endometriosis by Enhancing Invasiveness of ESCs and Promoting Angiogenesis. Am. J. Reprod. Immunol. 2023, 90, e13762. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, M.; Guo, Q.; Li, R.; Li, G.; Tan, S.; Li, X.; Wei, Y.; Wu, M. Annexin A2 Binds to Endosomes and Negatively Regulates TLR4-Triggered Inflammatory Responses via the TRAM-TRIF Pathway. Sci. Rep. 2015, 5, 15859. [Google Scholar] [CrossRef]
- Huang, Y.; Jia, M.; Yang, X.; Han, H.; Hou, G.; Bi, L.; Yang, Y.; Zhang, R.; Zhao, X.; Peng, C.; et al. Annexin A2: The Diversity of Pathological Effects in Tumorigenesis and Immune Response. Int. J. Cancer 2022, 151, 497–509. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Wang, Q.; Zheng, M.; Pang, L. Annexin A2 Functions Downstream of C-Jun N-terminal Kinase to Promote Skin Fibroblast Cell Migration. Mol. Med. Rep. 2017, 15, 4207–4216. [Google Scholar] [CrossRef] [PubMed]
- Grindheim, A.K.; Saraste, J.; Vedeler, A. Protein Phosphorylation and Its Role in the Regulation of Annexin A2 Function. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2515–2529. [Google Scholar] [CrossRef]
- Bai, X.; Ran, J.; Zhao, X.; Liang, Y.; Yang, X.; Xi, Y. The S100A10–AnxA2 Complex Is Associated with the Exocytosis of Hepatitis B Virus in Intrauterine Infection. Lab. Investig. 2021, 102, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, L.; Ye, T.; Zhou, J.; Niu, X.; Yuan, J.; Yuan, T.; Fu, D.; Li, H.; Li, Q.; et al. Identification of Specific Markers for Human Pluripotent Stem Cell-Derived Small Extracellular Vesicles. J. Extracell. Vesicles 2024, 13, e12409. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, C.; Yamamoto, S.; Hashimoto, S.; Kasahara, M.; Minamisawa, T.; Matsumura, S.; Katakura, A.; Yajima, Y.; Nomura, T.; Shiba, K. Pentapartite Fractionation of Particles in Oral Fluids by Differential Centrifugation. Sci. Rep. 2021, 11, 3326. [Google Scholar] [CrossRef]
- Mathivanan, S. ExoCarta: Exosome Markers. Available online: http://exocarta.org (accessed on 6 January 2025).
- Than, U.T.T.; Guanzon, D.; Broadbent, J.A.; Leavesley, D.I.; Salomon, C.; Parker, T.J. Differential Expression of Keratinocyte-Derived Extracellular Vesicle Mirnas Discriminate Exosomes from Apoptotic Bodies and Microvesicles. Front. Endocrinol. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, Y.; Li, H.J. Advances in Mesenchymal Stem Cell Exosomes: A Review. Stem Cell Res. Ther. 2021, 12, 71. [Google Scholar] [CrossRef]
- Shelke, G.V.; Yin, Y.; Jang, S.C.; Lässer, C.; Wennmalm, S.; Hoffmann, H.J.; Li, L.; Gho, Y.S.; Nilsson, J.A.; Lötvall, J. Endosomal Signalling via Exosome Surface TGFβ-1. J. Extracell. Vesicles 2019, 8, 1650458. [Google Scholar] [CrossRef] [PubMed]
- Choezom, D.; Gross, J.C. Neutral Sphingomyelinase 2 Controls Exosome Secretion by Counteracting V-ATPase-Mediated Endosome Acidification. J. Cell Sci. 2022, 135, jcs259324. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, P.; Sesé, M.; Guijarro, P.J.; Emperador, M.; Sánchez-Redondo, S.; Peinado, H.; Hümmer, S.; Cajal, S.R.Y. ITGB3-Mediated Uptake of Small Extracellular Vesicles Facilitates Intercellular Communication in Breast Cancer Cells. Nat. Commun. 2020, 11, 4261. [Google Scholar] [CrossRef]
- Bai, S.; Hou, W.; Yao, Y.; Meng, J.; Wei, Y.; Hu, F.; Hu, X.; Wu, J.; Zhang, N.; Xu, R.; et al. Exocyst Controls Exosome Biogenesis via Rab11a. Mol. Ther. Nucleic Acids 2021, 27, 535. [Google Scholar] [CrossRef] [PubMed]
- Schuldner, M.; Dörsam, B.; Shatnyeva, O.; Reiners, K.S.; Kubarenko, A.; Hansen, H.P.; Finkernagel, F.; Roth, K.; Theurich, S.; Nist, A.; et al. Exosome-Dependent Immune Surveillance at the Metastatic Niche Requires BAG6 and CBP/P300-Dependent Acetylation of P53. Theranostics 2019, 9, 6047–6062. [Google Scholar] [CrossRef]
- Horbay, R.; Hamraghani, A.; Ermini, L.; Holcik, S.; Beug, S.T.; Yeganeh, B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int. J. Mol. Sci. 2022, 23, 15317. [Google Scholar] [CrossRef] [PubMed]
- Egea-Jimenez, A.L.; Zimmermann, P. Phospholipase D and Phosphatidic Acid in the Biogenesis and Cargo Loading of Extracellular Vesicles. J. Lipid Res. 2018, 59, 1554. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.Y.; Shi, Y.Y.; Cui, X.W.; Pan, Y.F.; Lin, Y.K.; Feng, X.F.; Ding, Z.W.; Yang, C.; Tan, Y.X.; Dong, L.W.; et al. PTEN Deficiency Facilitates Exosome Secretion and Metastasis in Cholangiocarcinoma by Impairing TFEB-Mediated Lysosome Biogenesis. Gastroenterology 2023, 164, 424–438. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of Cargo Selection in Exosome Biogenesis and Its Biomedical Applications in Cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.L.; Feng, Y.; Wen, Y.; Wu, W.J.; Ni, H.F.; Li, Z.L.; Zhou, L.T.; Wang, B.; Zhang, J.D.; Crowley, S.D.; et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J. Am. Soc. Nephrol. 2018, 29, 919. [Google Scholar] [CrossRef]
- Coulter, M.E.; Dorobantu, C.M.; Lodewijk, G.A.; Delalande, F.; Cianferani, S.; Ganesh, V.S.; Smith, R.S.; Lim, E.T.; Xu, C.S.; Pang, S.; et al. The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep. 2018, 24, 973. [Google Scholar] [CrossRef]
- Liu, C.; Liu, D.; Wang, S.; Gan, L.; Yang, X.; Ma, C. Identification of the SNARE Complex That Mediates the Fusion of Multivesicular Bodies with the Plasma Membrane in Exosome Secretion. J. Extracell. Vesicles 2023, 12, 12356. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, A.; Saleh, F.; Adnani, L.; Chinchalongporn, V.; El-Sehemy, A.; Olender, T.; Chen, M.J.; Ahmad, S.T.; Prokopchuk, O.; Vasan, L.; et al. SMPD3-Mediated Extracellular Vesicle Biogenesis Inhibits Oligodendroglioma Growth. bioRxiv 2020. [Google Scholar] [CrossRef]
- Matsui, T.; Sakamaki, Y.; Hiragi, S.; Fukuda, M. VAMP5 and Distinct Sets of Cognate Q-SNAREs Mediate Exosome Release. Cell Struct. Funct. 2023, 48, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX-and ESCRT-III-Dependent Sorting of Tetraspanins to Exosomes. J. Cell Biol. 2020, 219, e201904113. [Google Scholar] [CrossRef]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.I.; Manrique-Hoyos, N.; Jung, S.Y.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.S.; et al. Regulation of Exosome Secretion by Rab35 and Its GTPase-Activating Proteins TBC1D10A–C. J. Cell Biol. 2010, 189, 223. [Google Scholar] [CrossRef]
- Akrap, I.; Thavamani, A.; Nordheim, A. Vps4A-Mediated Tumor Suppression upon Exosome Modulation? Ann. Transl. Med. 2016, 4, 180. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.Q.; Xia, H.F.; Chen, G.H.; Wang, X.-L.; Yang, J.G.; Wu, L.Z.; Zhao, Y.F.; Jia, Y.L.; Chen, G. P-AKT/VPS4B Regulates the Small Extracellular Vesicle Size in Venous Malformation Endothelial Cells. Oral Dis. 2024, 30, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, W.; Guo, Z.; Ju, Q.; Zhu, L.; Gao, J.; Zhou, L.; Liu, F.; Xu, Y.; Zhan, Q.; et al. A Novel TP53 Pathway Influences the HGS-Mediated Exosome Formation in Colorectal Cancer. Sci. Rep. 2016, 6, 28083. [Google Scholar] [CrossRef]
- Fraser, K.B.; Moehle, M.S.; Daher, J.P.L.; Webber, P.J.; Williams, J.Y.; Stewart, C.A.; Yacoubian, T.A.; Cowell, R.M.; Dokland, T.; Ye, T.; et al. LRRK2 Secretion in Exosomes Is Regulated by 14-3-3. Hum. Mol. Genet. 2013, 22, 4988–5000. [Google Scholar] [CrossRef]




| Source | Gene Name | Citation | |||
|---|---|---|---|---|---|
| 1 | Lipid Raft Endocytosis and Exosomal Transport Facilitate Extracellular Trafficking of Annexin A2, Valapala et al. | ANXA2 | [47] | ||
| 2 | Incorporating extracellular vesicle markers varies among vesicles with distinct surface charges, Maeda et al. | CD63 | CD81 | CD9 | [48] |
| FLOT1 | |||||
| 3 | Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells, Honegger et al. | CHMP4C | [49] | ||
| 4 | MUC1-C is a master regulator of MICA/B NKG2D ligand and exosome secretion in human cancer cells, Morimoto et al. | MUC1 (MUC1-C) | [50] | ||
| 5 | RAB5A is associated with genes involved in exosome secretion: Integration of bioinformatics analysis and experimental validation, Gorji-bahri et al. | RAB5A | [51] | ||
| 6 | RAB22A as a predictor of exosome secretion in the progression and relapse of multiple myeloma, Fan et al. | RAB22A | [52] | ||
| 7 | Rab27a and Rab27b control different steps of the exosome secretion pathway, Ostrowski et al. | RAB27B | [53] | ||
| 8 | MDA-9/Syntenin: An emerging global molecular target regulating cancer invasion and metastasis, Das et al. | SDCBP2 | [54] | ||
| 9 | Embryonic signals mediate extracellular vesicle biogenesis and trafficking at the embryo–maternal interface, Guzewska et al. | VPS37B | [55] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowiec, B.M.; Blatkiewicz, M.; Dyszkiewicz-Konwińska, M.; Bukowska, D.; Kempisty, B.; Ruciński, M.; Nowicki, M.; Budna-Tukan, J. Mapping Small Extracellular Vesicle Secretion Potential in Healthy Human Gingiva Using Spatial Transcriptomics. Curr. Issues Mol. Biol. 2025, 47, 256. https://doi.org/10.3390/cimb47040256
Borowiec BM, Blatkiewicz M, Dyszkiewicz-Konwińska M, Bukowska D, Kempisty B, Ruciński M, Nowicki M, Budna-Tukan J. Mapping Small Extracellular Vesicle Secretion Potential in Healthy Human Gingiva Using Spatial Transcriptomics. Current Issues in Molecular Biology. 2025; 47(4):256. https://doi.org/10.3390/cimb47040256
Chicago/Turabian StyleBorowiec, Blanka Maria, Małgorzata Blatkiewicz, Marta Dyszkiewicz-Konwińska, Dorota Bukowska, Bartosz Kempisty, Marcin Ruciński, Michał Nowicki, and Joanna Budna-Tukan. 2025. "Mapping Small Extracellular Vesicle Secretion Potential in Healthy Human Gingiva Using Spatial Transcriptomics" Current Issues in Molecular Biology 47, no. 4: 256. https://doi.org/10.3390/cimb47040256
APA StyleBorowiec, B. M., Blatkiewicz, M., Dyszkiewicz-Konwińska, M., Bukowska, D., Kempisty, B., Ruciński, M., Nowicki, M., & Budna-Tukan, J. (2025). Mapping Small Extracellular Vesicle Secretion Potential in Healthy Human Gingiva Using Spatial Transcriptomics. Current Issues in Molecular Biology, 47(4), 256. https://doi.org/10.3390/cimb47040256









