Ameloblastic Carcinoma: A 40-Year Scoping Review of the Literature
Abstract
:1. Introduction
- Malignant ameloblastoma.
- Ameloblastic carcinoma (occurs de novo, ex ameloblastoma or ex odontogenic cyst).
- Non-keratinizing.
- Keratinizing.
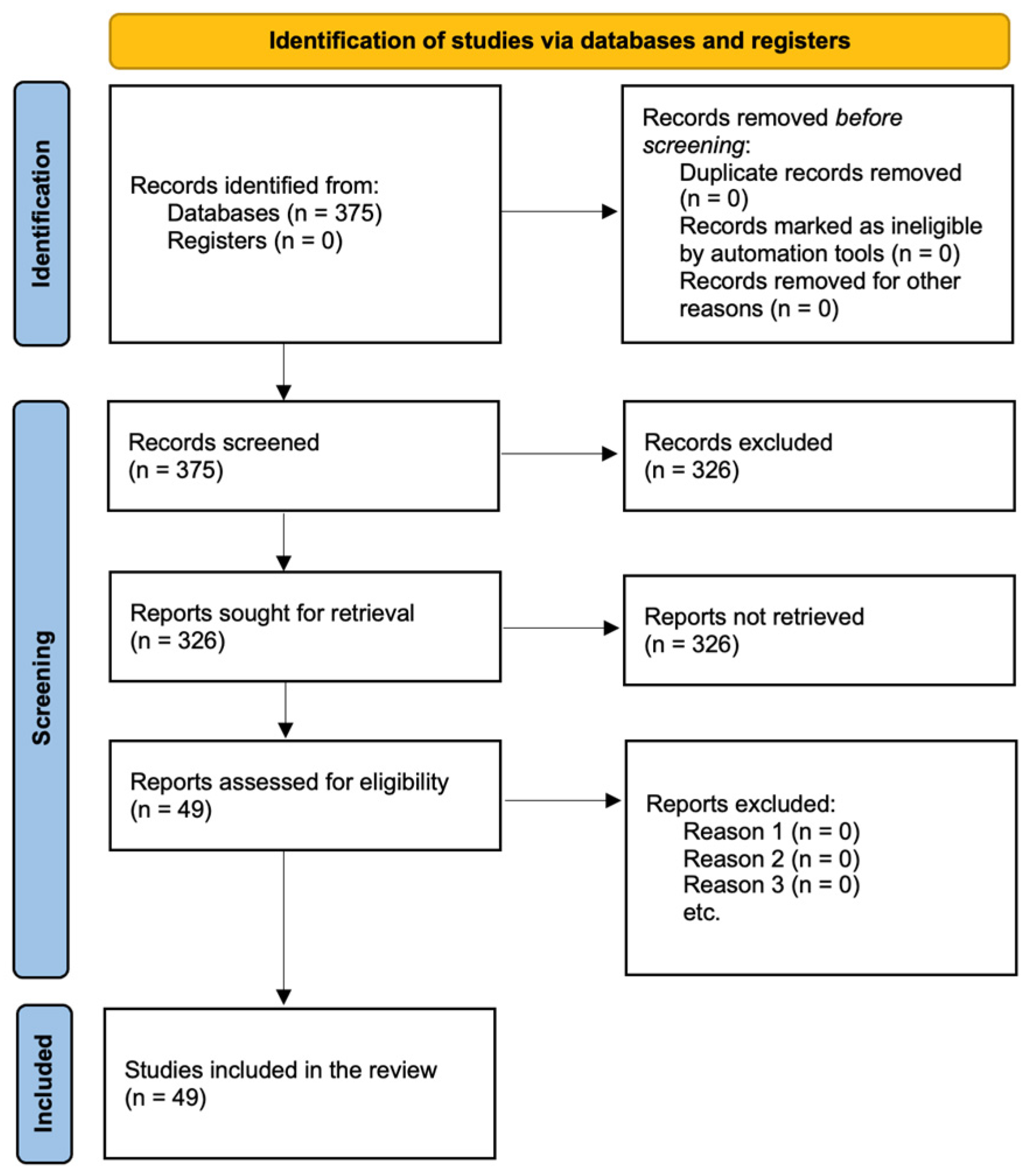
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria and Study Selection
- (1)
- P: patients diagnosed with ameloblastic carcinoma from 1984 to 2024.
- (2)
- I: patients who underwent standard treatments, such as surgery or adjuvant treatments.
- (3)
- C: patients who did not undergo any type of treatment.
- (4)
- O: studies that reported outcomes and/or complications of ameloblastic carcinoma with a minimum follow-up of six months.
2.3. Data Extraction and Quality Assessment
3. Results
3.1. Epidemiology
3.2. Clinical Features
3.3. Radiologic Features
3.4. Histological and Immunohistochemical Features
3.5. Molecular Mechanisms and Pathophysiology
3.6. Genetics
3.7. Differential Diagnosis
3.8. Clinical Course and Treatment
- In total, 29.41% of AC arising from mandible determines distant metastases and precisely lung 54.54%, lymph nodes 38.2%, bone 18.18%, pleura 11%, liver 7.2%, skull base 7.2%, brain 3.6%, kidney 3.6%, disease relapse 1.8%, parotid 1.8%, spleen 1.8%, diaphragm 1.8%, ilium 1.8%, mediastinum 1.8%, and extensive metastatic disease 34.54%.
- Overall, 25.97% of AC arising from maxilla determines distant metastases and precisely lung 40%, lymph nodes 30%, bone 20%, skull base 10%, disease relapse 10%, brain 5%, orbit 5%, pterygomaxillary fossa 5%, mandible 5%, small intestine 5%, and extensive metastatic disease 25%.
- In total, 33.33% of AC arising from the skull base determines intracranial metastases.
3.9. Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Servato, J.P.S.; Prieto-Oliveira, P.; Faria, P.R.; Loyola, A.M.; Cardoso, S.V. Odontogenic tumours: 240 cases diagnosed over 31 years at a Brazilian university and a review of international literature. Int. J. Oral Maxillofac. Surg. 2013, 42, 288–293. [Google Scholar] [PubMed]
- Martinez, M.M.; Mosqueda-Taylor, A.; Carlos, R.; Delgado-Azanero, W.; Almeida, O.P. Malignant odontogenic tumours: A multicentric Latin American study of 25 cases. Oral Dis. 2014, 20, 380–385. [Google Scholar]
- Rizzitelli, A.; Smoll, N.R.; Chae, M.P.; Rozen, W.M.; Hunter-Smith, D.J. Incidence and overall survival of malignant ameloblastoma. PLoS ONE 2015, 10, e0117789. [Google Scholar]
- Slootweg, P.J.; Müller, H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg. Oral Med. Oral Pathol. 1984, 57, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Soluk-Tekkesin, M.; Wright, J.M. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2022 (5th) Edition. Turk. Patoloji Derg. 2022, 38, 168–184. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Goldenberg, D.; Sciubba, J.; Koch, W.; Tufano, R.P. Malignant odontogenic tumors: A 22-year experience. Laryngoscope 2004, 114, 1770–1774. [Google Scholar] [CrossRef]
- Fonseca, F.P.; de Almeida, O.P.; Vargas, P.A.; Gonçalves FJúnior Corrêa Pontes, F.S.; Rebelo Pontes, H.A. Ameloblastic carcinoma (secondary type) with extensive squamous differentiation areas and dedifferentiated regions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, e154–e161. [Google Scholar] [CrossRef]
- Loyola, A.M.; Cardoso, S.V.; de Faria, P.R.; Servato, J.P.; Eisenberg, A.L.; Dias, F.L.; Accioly, M.T.; Gomes, C.C.; Gomez, R.S.; Souza, S.O.; et al. Ameloblastic carcinoma: A Brazilian collaborative study of 17 cases. Histopathology 2016, 69, 687–701. [Google Scholar] [CrossRef]
- Fomete, B.; Adebayo, E.T.; Ayuba, G.I.; Okeke, U.A. Ameloblastic carcinoma of the maxilla: A report of two cases and a review of the literature. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 43–46. [Google Scholar] [CrossRef]
- Aoki, T.; Akiba, T.; Kondo, Y.; Sasaki, M.; Kajiwara, H.; Ota, Y. The use of radiation therapy in the definitive management of ameloblastic carcinoma: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Fahradyan, A.; Odono, L.; Hammoudeh, J.A.; Howell, L.K. Ameloblastic Carcinoma In Situ: Review of Literature and a Case Presentation in a Pediatric Patient. Cleft Palate Craniofac. J. 2019, 56, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Soyele, O.O.; Adebiyi, K.E.; Adesina, O.M.; Ladeji, A.M.; Aborisade, A.; Olatunji, A.; Adeola, H.A. Ameloblastic carcinoma: A clinicopathologic analysis of cases seen in a Nigerian Teaching Hospital and review of literature. Pan Afr. Med. J. 2018, 31, 208. [Google Scholar] [CrossRef]
- Yamagata, K.; Ishikawa, H.; Saito, T.; Bukawa, H. Proton Beam Therapy for Ameloblastic Carcinoma of the Maxilla: Report of a Rare Case. J. Oral Maxillofac. Surg. 2019, 77, e1–e227. [Google Scholar] [CrossRef] [PubMed]
- Kosanwat, T.; Poomsawat, S.; Juengsomjit, R. Ameloblastic carcinoma ex ameloblastoma of the maxilla. J. Oral Maxillofac. Pathol. 2019, 23 (Suppl. S1), 58–62. [Google Scholar] [CrossRef]
- Landeen, K.; Spanos, W.C.; Powell, S. A Rare Presentation of Ameloblastic Carcinoma of the Sinus Cavity and Skull Base. Cureus 2019, 11, e6265. [Google Scholar] [CrossRef]
- Deng, L.; Wang, R.; Yang, M.; Li, W.; Zou, L. Ameloblastic carcinoma: Clinicopathological analysis of 18 cases and a systematic review. Head Neck 2019, 41, 4191–4198. [Google Scholar] [CrossRef]
- Takayama, K.; Nakamura, T.; Takada, A.; Kato, T.; Sakuma, H.; Mitsudo, K.; Fuwa, N.; Murakami, M. Proton beam therapy combined with retrograde intra-arterial infusion chemotherapy for an extremely rapid growing recurrent ameloblastic carcinoma: A case report. Mol. Clin. Oncol. 2020, 13, 34. [Google Scholar]
- Cho, B.H.; Jung, Y.H.; Hwang, J.J. Ameloblastic carcinoma of the mandible: A case report. Imaging Sci. Dent. 2020, 50, 359–363. [Google Scholar] [CrossRef]
- Ismail, T.; Haumer, A.; Lunger, A.; Osinga, R.; Kaempfen, A.; Saxer, F.; Wixmerten, A.; Miot, S.; Thieringer, F.; Beinemann, J.; et al. Case Report: Reconstruction of a Large Maxillary Defect With an Engineered, Vascularized, Prefabricated Bone Graft. Front. Oncol. 2021, 11, 775136. [Google Scholar] [CrossRef]
- Hoehnle, N.S.; Beutler, B.D.; Ulanja, M.B.; Rastegarpour, A. Ameloblastic carcinoma with hepatic metastases: A case report and review of ameloblastomic carcinoma. J. Clin. Imaging Sci. 2022, 12, 58. [Google Scholar] [CrossRef]
- Chen, I.Y.; Giampoli, E.J.; Zhang, D. Ameloblastic Carcinoma of the Maxilla: A Rare Case Report and Review of Literature from 1948 to 2021. Int. J. Surg. Pathol. 2023, 31, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Ogane, S.; Fujii, A.; Suzuki, T.; Hashimoto, K.; Hashimoto, S.; Takano, M.; Katakura, A.; Nomura, T. Ameloblastic carcinoma of the mandible: A case report. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 17. [Google Scholar] [CrossRef] [PubMed]
- Hurník, P.; Putnová, B.M.; Ševčíková, T.; Hrubá, E.; Putnová, I.; Škarda, J.; Havel, M.; Res, O.; Cvek, J.; Buchtová, M.; et al. Metastasising ameloblastoma or ameloblastic carcinoma? A case report with mutation analyses. BMC Oral Health 2023, 23, 563. [Google Scholar] [CrossRef]
- Barca, I.; Ferragina, F.; Kallaverja, E.; Cristofaro, M.G. A rare case of secondary ameloblastic carcinoma in a young Man. Ann. Ital. Chir. 2023, 93, S2239253X23039385. [Google Scholar] [PubMed]
- Dorner, L.; Sear, A.J.; Smith, G.T. A case of ameloblastic carcinoma with pulmonary metastases. Br. J. Oral Maxillofac. Surg. 1988, 26, 503–510. [Google Scholar] [CrossRef]
- Bruce, R.A.; Jackson, I.T. Ameloblastic carcinoma. Report of an aggressive case and review of the literature. J. Craniomaxillofac. Surg. 1991, 19, 267–271. [Google Scholar] [CrossRef]
- Simko, E.J.; Brannon, R.B.; Eibling, D.E. Ameloblastic carcinoma of the mandible. Head Neck 1998, 20, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Infante-Cossio, P.; Hernandez-Guisado, J.M.; Fernandez-Machin, P.; Garcia-Perla, A.; Rollon-Mayordomo, A.; Gutierrez-Perez, J.L. Ameloblastic carcinoma of the maxilla: A report of 3 cases. J. Craniomaxillofac. Surg. 1998, 26, 159–162. [Google Scholar] [CrossRef]
- Kawauchi, S.; Hayatsu, Y.; Takahashi, M.; Furuya, T.; Oga, A.; Niwa, S.; Sasaki, K. Spindle-cell ameloblastic carcinoma: A case report with immunohistochemical, ultrastructural, and comparative genomic hybridization analyses. Oncol. Rep. 2003, 10, 31–34. [Google Scholar]
- Datta, R.; Winston, J.S.; Diaz-Reyes, G.; Loree, T.R.; Myers, L.; Kuriakose, M.A.; Rigual, N.R.; Hicks, W.L., Jr. Ameloblastic carcinoma: Report of an aggressive case with multiple bony metastases. Am. J. Otolaryngol. 2003, 24, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Oginni, F.O.; Ugboko, V.I.; Owotade, J.F.; Adebiyi, K.E. Ameloblastic carcinoma of the jaws. A report of three Nigerian cases. Odontostomatol. Trop. 2003, 26, 19–22. [Google Scholar]
- Hall, J.M.; Weathers, D.R.; Unni, K.K. Ameloblastic carcinoma: An analysis of 14 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 799–807. [Google Scholar] [CrossRef]
- Benlyazid, A.; Lacroix-Triki, M.; Aziza, R.; Gomez-Brouchet, A.; Guichard, M.; Sarini, J. Ameloblastic carcinoma of the maxilla: Case report and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, e17–e24. [Google Scholar] [CrossRef] [PubMed]
- Ram, H.; Mohammad, S.; Husain, N.; Gupta, P.N. Ameloblastic carcinoma. J. Maxillofac. Oral Surg. 2010, 9, 415–419. [Google Scholar] [CrossRef]
- Lucca, M.; D’Innocenzo, R.; Kraus, J.A.; Gagari, E.; Hall, J.; Shastri, K. Ameloblastic carcinoma of the maxilla: A report of 2 cases. J. Oral Maxillofac. Surg. 2010, 68, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, J.V.; Nikolic, Z.S.; Boricic, I.V.; Tacevic, Z.D.; Tomanovic, N.R.; Drcic, L.J.; Novkovic, M.D. Total mandibular reconstruction after resection of rare “honeycomb-like” ameloblastic carcinoma—A case report. J. Craniomaxillofac. Surg. 2010, 38, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Horváth, A.; Horváth, E.; Popşor, S. Mandibular ameloblastic carcinoma in a young patient. Rom. J. Morphol. Embryol. 2012, 53, 179–183. [Google Scholar]
- Pirklbauer, K.; Kozakowski, N.; Russmueller, G.; Ewers, R.; Klug, C. Manifestation of an ameloblastic carcinoma ten years after follicular cyst enucleation in the mandibular ramus. J. Craniomaxillofac. Surg. 2012, 40, 362–365. [Google Scholar] [CrossRef]
- Kamath, V.V.; Satelur, K.; Yerlagudda, K. Spindle cell variant of ameloblastic carcinoma arising from an unicystic amelobastoma: Report of a rare case. Dent. Res. J. 2012, 9, 328–333. [Google Scholar]
- Yoshioka, Y.; Toratani, S.; Ogawa, I.; Okamoto, T. Ameloblastic carcinoma, secondary type, of the mandible: A case report. J. Oral Maxillofac. Surg. 2013, 71, e58–e62. [Google Scholar] [CrossRef] [PubMed]
- Kar, I.B.; Subramanyam, R.V.; Mishra, N.; Singh, A.K. Ameloblastic carcinoma: A clinicopathologic dilemma—Report of two cases with total review of literature from 1984 to 2012. Ann. Maxillofac. Surg. 2014, 4, 70–77. [Google Scholar] [PubMed]
- Li, J.; Du, H.; Li, P.; Zhang, J.; Tian, W.; Tang, W. Ameloblastic carcinoma: An analysis of 12 cases with a review of the literature. Oncol. Lett. 2014, 8, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Nobusawa, A.; Sano, T.; Yokoo, S.; Oyama, T. Ameloblastic carcinoma developing in preexisting ameloblastoma with a mutation of the p53 gene: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, e146–e150. [Google Scholar] [CrossRef]
- Kikuta, S.; Furukawa, Y.; Hino, K.; Nakamura, M.; Kusukawa, J. Huge ameloblastic carcinoma of the mandible with metastases treated in several different ways. Br. J. Oral Maxillofac. Surg. 2019, 57, 182–184. [Google Scholar] [CrossRef]
- Mahmoud, S.A.M.; Amer, H.W.; Mohamed, S.I. Primary ameloblastic carcinoma: Literature review with case series. Pol. J. Pathol. 2018, 69, 243–253. [Google Scholar] [CrossRef]
- Sancheti, S.; Somal, P.K.; Sarkar, S. Ameloblastic carcinoma: A diagnostic dilemma. Indian. J. Pathol. Microbiol. 2019, 62, 501–503. [Google Scholar] [CrossRef]
- Yukimori, A.; Tsuchiya, M.; Wada, A.; Michi, Y.; Kayamori, K.; Sakamoto, K.; Ikeda, T. Genetic and histopathological analysis of a case of primary intraosseous carcinoma, NOS with features of both ameloblastic carcinoma and squamous cell carcinoma. World J. Surg. Oncol. 2020, 18, 45. [Google Scholar] [CrossRef]
- Tarle, M.; Müller, D.; Tarle, A.; Blivajs, I.; Aljinović Ratković, N.; Knežević, P. Challenges in the diagnostics and treatment of ectopic ameloblastic carcinoma: A case report. Croat. Med. J. 2020, 61, 271–275. [Google Scholar] [CrossRef]
- Vu, N.B.; Le, N.T.; Chaisuparat, R.; Thunyakitpisal, P.; Tran, N.M. Ameloblastic Carcinoma in a 2-Year-Old Child: A Case Report and Review of the Literature. Case Rep. Dent. 2020, 2020, 4072890. [Google Scholar] [CrossRef]
- Behtaj, M.; Alawi, F.; Shanti, R.M.; Cannady, S.; Zhang, P.J.; Modi, M.; Lamzabi, I. Report of a Rare Case of Spindle Cell Ameloblastic Carcinoma and the Diagnostic Utility of Immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, A.S.; Narang, R.S.; Nagi, R.S. Ameloblastic carcinoma: A case report and evaluation. J. Oral Maxillofac. Pathol. 2022, 26 (Suppl. S1), S63–S67. [Google Scholar] [CrossRef]
- Schuch, L.F.; Dummel, C.; Ribeiro, J.T.; Zieger, R.A.; Carrard, V.C.; Bittencourt, R.; Martins, M.A.T.; Martins, M.D. Diagnosis, Treatment, and Total Rehabilitation of a Secondary Type Ameloblastic Carcinoma. Int. J. Surg. Pathol. 2023, 31, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Tanaka, A.; Kimura, M.; Ieda, S.; Wada, T.; Matsumura, T.; Kurose, A. Spindle Cell Variant of Ameloblastic Carcinoma: Another Example in a Japanese Male. Case Rep. Dent. 2023, 2023, 8755637. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, P.; Mallick, S.; Upadhyay, A.D.; Rath, G.K. Pattern of care and impact of prognostic factors in the outcome of ameloblastic carcinoma: A systematic review and individual patient data analysis of 199 cases. Eur. Arch. Otorhinolaryngol. 2017, 274, 3803–3810. [Google Scholar]
- Robinson, L.; Abreu, L.G.; Fonseca, F.P.; Hunter, K.D.; Ambele, M.A.; van Heerden, W.F.P. Ameloblastic carcinoma: A systematic review. J. Oral Pathol. Med. 2024, 53, 174–181. [Google Scholar] [CrossRef]
- Ferragina, F.; Sottile, A.R.; Cristofaro, M.G. Unusual presentation of pulmonary adenocarcinoma metastases in the mandibular condyle: A case report. Int. J. Surg. Case Rep. 2023, 113, 109058. [Google Scholar] [CrossRef]
- Saito, T.; Iida, A.; Kobayashi, T.; Ohnuki, H.; Narimatsu, K.; Ohnishi, M. Secondary peripheral ameloblastic carcinoma of the palate: A case report and literature review. J. Oral Maxillofac. Surg. Med. Pathol. 2016, 28, 429–433. [Google Scholar]
- Corio, R.L.; Goldblatt, L.I.; Edwards, P.A.; Hartman, K.S. Ameloblastic carcinoma: A clinicopathologic study and assessment of eight cases. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 570–576. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jo, B.C.; Shin, W.J.; Cho, Y.A.; Lee, J.I.; Hong, S.P.; Hong, S.D. Comparative immunohistochemical study of ameloblastoma and ameloblastic carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 767–776. [Google Scholar]
- Lei, Y.; Jaradat, J.M.; Owosho, A.; Adebiyi, K.E.; Lybrand, K.S.; Neville, B.W.; Müller, S.; Bilodeau, E.A. Evaluation of SOX2 as a potential marker for ameloblastic carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 608–616.e1. [Google Scholar] [CrossRef]
- Khojasteh, A.; Khodayari, A.; Rahimi, F.; Ghaderian, M.H.; Jafarian, M.; Nayebi, A.; Akbarzadeh Najar, R.; Tabatabayipanah, A.; Jahangirnia, A. Hypermethylation of p16 tumor-suppressor gene in ameloblastic carcinoma, ameloblastoma, and dental follicles. J. Oral Maxillofac. Surg. 2013, 71, 62–65. [Google Scholar] [CrossRef]
- Gomes, I.P.; Bastos, V.C.; Guimarães, L.M.; Gomes, C.C. The molecular basis of odontogenic cysts and tumours. J. Oral Pathol. Med. 2023, 52, 351–356. [Google Scholar] [CrossRef]
- Dutra, S.N.; Pires, F.R.; Armada, L.; Azevedo, R.S. Immunoexpression of Wnt/β-catenin signaling pathway proteins in ameloblastoma and calcifying cystic odontogenic tumor. J. Clin. Exp. Dent. 2017, 9, e136–e140. [Google Scholar] [CrossRef] [PubMed]
- Gondak, R.O.; Mariano, F.V.; de Sousa, S.F.; Siqueira, E.C.; Díaz, K.P.; Martins, L.A.L.; Altemani, A.; Mosqueda-Taylor, A.; Gomez, R.S.; Gomes, C.C. CTNNB1 and APC mutations in odontogenic carcinoma with dentinoid. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, e249–e256. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Devi, A.; Kamboj, M.; Narwal, A. Localization of beta catenin across the domain of odontogenic lesions: A systematic review. J. Oral Pathol. Med. 2023, 52, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh, M.; Hamblin, M.R.; Pournaghi-Azar, F.; Vakili Saatloo, M.; Kouhsoltani, M.; Vahed, N. Ki-67 expression as a diagnostic biomarker in odontogenic cysts and tumors: A systematic review and meta-analysis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 66–75. [Google Scholar] [CrossRef]
- Robinson, L.; Smit, C.; van Heerden, M.B.; Moolla, H.; Afrogheh, A.H.; Opperman, J.F.; Ambele, M.A.; van Heerden, W.F.P. Surrogate Immunohistochemical Markers of Proliferation and Embryonic Stem Cells in Distinguishing Ameloblastoma from Ameloblastic Carcinoma. Head Neck Pathol. 2024, 18, 92. [Google Scholar] [CrossRef]
- Jot, K.; Nayyar, V.; Bhatt, K.; Mishra, D. Ameloblastic carcinoma of mandible with remarkably high Ki-67 proliferative index. Oral Oncol. 2022, 134, 106122. [Google Scholar] [CrossRef]
- Carreón-Burciaga, R.G.; González-González, R.; Molina-Frechero, N.; Bologna-Molina, R. Immunoexpression of Ki-67, MCM2, and MCM3 in Ameloblastoma and Ameloblastic Carcinoma and Their Correlations with Clinical and Histopathological Patterns. Dis. Markers 2015, 2015, 683087. [Google Scholar] [CrossRef]
- García-Muñoz, A.; Rodríguez, M.A.; Licéaga-Escalera, C.; Licéaga-Reyes, R.; Carreón-Burciaga, R.G.; González-González, R.; Bologna-Molina, R. Expression of the transcription factor PITX2 in ameloblastic carcinoma. Arch. Oral Biol. 2015, 60, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Hii, E.P.W.; Ramanathan, A.; Pandarathodiyil, A.K.; Wong, G.R.; Sekhar, E.V.S.; Binti Talib, R.; Zaini, Z.M.; Zain, R.B. Homeobox Genes in Odontogenic Lesions: A Scoping Review. Head. Neck Pathol. 2023, 17, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Tanaka, F.; Morita, H.; Hiraki, A.; Hashimoto, S. Hypoxia-induced HIF-1α and ZEB1 are critical for the malignant transformation of ameloblastoma via TGF-β-dependent EMT. Cancer Med. 2019, 8, 7822–7832. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Chaurasia, S.; Maddheshiya, N.; Dhungel, D. Diagnostic markers for odontogenic tumors: An insight: A review. Discov. Oncol. 2024, 15, 558. [Google Scholar] [CrossRef]
- Phattarataratip, E.; Sappayatosok, K. The Significance of Relative Claudin Expression in Odontogenic Tumors. Head Neck Pathol. 2020, 14, 480–488. [Google Scholar] [CrossRef]
- de Souza Andrade, E.S.; Miguel, M.C.; de Almeida Freitas, R.; Pereira Pinto, L.; Batista de Souza, L. Immunoexpression of integrins in ameloblastoma, adenomatoid odontogenic tumor, and human tooth germs. Int. J. Surg. Pathol. 2008, 16, 277–285. [Google Scholar] [CrossRef]
- Al-Otaibi, O.; Khounganian, R.; Anil, S.; Rajendran, R. Syndecan-1 (CD 138) surface expression marks cell type and differentiation in ameloblastoma, keratocystic odontogenic tumor, and dentigerous cyst. J. Oral Pathol. Med. 2013, 42, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yiannis, C.; Mascolo, M.; Mignogna, M.D.; Varricchio, S.; Natella, V.; De Rosa, G.; Lo Giudice, R.; Galletti, C.; Paolini, R.; Celentano, A. Expression Profile of Stemness Markers CD138, Nestin and Alpha-SMA in Ameloblastic Tumours. Int. J. Environ. Res. Public Health 2021, 18, 3899. [Google Scholar] [CrossRef]
- Mori, M.; Yamada, T.; Doi, T.; Ohmura, H.; Takai, Y.; Shrestha, P. Expression of tenascin in odontogenic tumours. Eur. J. Cancer B Oral Oncol. 1995, 31, 275–279. [Google Scholar] [CrossRef]
- Nagai, N.; Yamachika, E.; Nishijima, K.; Inoue, M.; Shin, H.I.; Suh, M.S.; Nagatsuka, H. Immunohistochemical demonstration of tenascin and fibronectin in odontogenic tumours and human fetal tooth germs. Eur. J. Cancer B Oral Oncol. 1994, 30, 191–195. [Google Scholar] [CrossRef]
- Ghazi, N.; Saghravanian, N.; Mirhashemi, M.; Ardakani, A.A. Evaluation of Tenascin Expression in Ameloblastoma, Odontogenic Keratocyst, and Dentigerous Cyst by Immunohistochemistry. Adv. Biomed. Res. 2023, 12, 66. [Google Scholar] [CrossRef]
- de Medeiros, A.M.; Nonaka, C.F.; Galvão, H.C.; de Souza, L.B.; Freitas Rde, A. Expression of extracellular matrix proteins in ameloblastomas and adenomatoid odontogenic tumors. Eur. Arch. Otorhinolaryngol. 2010, 267, 303–310. [Google Scholar] [CrossRef]
- Sánchez-Romero, C.; Carreón-Burciaga, R.; Gónzalez-Gónzalez, R.; Villarroel-Dorrego, M.; Molina-Frechero, N.; Bologna-Molina, R. Perilipin 1 and adipophilin immunoexpression suggests the presence of lipid droplets in tooth germ, ameloblastoma, and ameloblastic carcinoma. J. Oral Pathol. Med. 2021, 50, 708–715. [Google Scholar] [CrossRef]
- Juuri, E.; Isaksson, S.; Jussila, M.; Heikinheimo, K.; Thesleff, I. Expression of the stem cell marker, SOX2, in ameloblastoma and dental epithelium. Eur. J. Oral Sci. 2013, 121, 509–516. [Google Scholar] [CrossRef]
- Kalogirou, E.M.; Lekakis, G.; Petroulias, A.; Chavdoulas, K.; Zogopoulos, V.L.; Michalopoulos, I.; Tosios, K.I. The Stem Cell Expression Profile of Odontogenic Tumors and Cysts: A Systematic Review and Meta-Analysis. Genes 2023, 14, 1735. [Google Scholar] [CrossRef]
- Pereira, T.; Shetty, S.J.; Punjabi, V.; Vidhale, R.G.; Gotmare, S.S.; Kamath, P. Immunohistochemical expression of SOX2 in OKC and ameloblastoma: A comparative study. J. Oral Maxillofac. Pathol. 2023, 27, 685–692. [Google Scholar] [CrossRef]
- Silva, B.S.; Silva, L.R.; Lima, K.L.; Dos Santos, A.C.; Oliveira, A.C.; Dezzen-Gomide, A.C.; Batista, A.C.; Yamamoto-Silva, F.P. SOX2 and BCL-2 Expressions in Odontogenic Keratocyst and Ameloblastoma. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e283–e290. [Google Scholar] [CrossRef]
- Sobhy, A.M.; Fouad, H.M.A.; Riad, S.M.; Zaitoun, I.M. Evaluation of SOX2 as a potential stem cell marker in benign and malignant odontogenic tumors. Alex. Dent. J. 2019, 44, 99–105. [Google Scholar] [CrossRef]
- Hasan, S.; Nagdy, S.; Mohsen, M. Prognostic significance of SOX2 and GPC3 in ameloblastoma and its malignant counterpart (Ameloblastic Carcinoma). J. Solid Tumors 2021, 11, 1. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Mohanty, N.; Mishra, S.; Gopinath, D.; Panda, S.; Anil, S. Differential Expression of Immunohistochemical Markers in Ameloblastoma & Ameloblastic Carcinoma: A Systematic Review and Meta-analysis of observational studies. F1000Research 2024, 13, 557. [Google Scholar] [CrossRef]
- Tornesello, M.L. TP53 mutations in cancer: Molecular features and therapeutic opportunities (Review). Int. J. Mol. Med. 2025, 55, 7. [Google Scholar] [CrossRef]
- Nagi, R.; Sahu, S.; Rakesh, N. Molecular and genetic aspects in the etiopathogenesis of ameloblastoma: An update. J. Oral Maxillofac. Pathol. 2016, 20, 497–504. [Google Scholar] [CrossRef]
- Nodit, L.; Barnes, L.; Childers, E.; Finkelstein, S.; Swalsky, P.; Hunt, J. Allelic loss of tumor suppressor genes in ameloblastic tumors. Mod. Pathol. 2004, 17, 1062–1067. [Google Scholar] [CrossRef]
- Abiko, Y.; Nagayasu, H.; Takeshima, M.; Yamazaki, M.; Nishimura, M.; Kusano, K.; Kitajo, H.; Saitoh, M.; Kawakami, T.; Chiba, I.; et al. Ameloblastic carcinoma ex ameloblastoma: Report of a case-possible involvement of CpG island hypermethylation of the p16 gene in malignant transformation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 72–76. [Google Scholar] [CrossRef]
- Aldahash, F. Systematic review and meta-analysis of the expression of p53 in the odontogenic lesions. J. Oral Maxillofac. Pathol. 2023, 27, 168–172. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, L.; Jia, Y.X.; Chen, W.H. BRAF V600E expression in ameloblastomas, ameloblastic carcinomas and cysts. Shanghai Kou Qiang Yi Xue 2023, 32, 630–634. [Google Scholar]
- Diniz, M.G.; Gomes, C.C.; Guimarães, B.V.; Castro, W.H.; Lacerda, J.C.; Cardoso, S.V.; de Faria, P.R.; Dias, F.L.; Eisenberg, A.L.; Loyola, A.M.; et al. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol. 2015, 36, 5649–5653. [Google Scholar] [CrossRef]
- González-González, R.; López-Verdín, S.; Lavalle-Carrasco, J.; Molina-Frechero, N.; Isiordia-Espinoza, M.; Carreón-Burciaga, R.G.; Bologna-Molina, R. Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review. World J. Clin. Oncol. 2020, 11, 31–42. [Google Scholar] [CrossRef]
- Kurppa, K.J.; Catón, J.; Morgan, P.R.; Ristimäki, A.; Ruhin, B.; Kellokoski, J.; Elenius, K.; Heikinheimo, K. High frequency of BRAF V600E mutations in ameloblastoma. J. Pathol. 2014, 232, 492–498. [Google Scholar] [CrossRef]
- Sweeney, R.T.; McClary, A.C.; Myers, B.R.; Biscocho, J.; Neahring, L.; Kwei, K.A.; Qu, K.; Gong, X.; Ng, T.; Jones, C.D.; et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat. Genet. 2014, 46, 722–725. [Google Scholar] [CrossRef]
- Bologna-Molina, R.; Schuch, L.; Magliocca, K.; van Heerden, W.; Robinson, L.; Bilodeau, E.A.; Hussaini, H.M.; Soluk-Tekkesin, M.; Adisa, A.O.; Tilakaratne, W.M.; et al. Targeted therapies in ameloblastomas and amelobastic carcinoma-A systematic review. Oral Dis. 2024, 30, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Nakashiro, K.I.; Sawatani, Y.; Hasegawa, T.; Kamimura, R.; Izumi, S.; Komiyama, Y.; Fukumoto, C.; Yagisawa, S.; Yaguchi, E.; et al. Whole Exome Sequencing of SMO, BRAF, PTCH1 and GNAS in Odontogenic Diseases. In Vivo 2020, 34, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Betz, B.L. Ameloblastoma: A Review of Recent Molecular Pathogenetic Discoveries. Biomark. Cancer 2015, 7 (Suppl. S2), 19–24. [Google Scholar] [CrossRef] [PubMed]
- Gültekin, S.E.; Aziz, R.; Heydt, C.; Sengüven, B.; Zöller, J.; Safi, A.F.; Kreppel, M.; Buettner, R. The landscape of genetic alterations in ameloblastomas relates to clinical features. Virchows Arch. 2018, 472, 807–814. [Google Scholar] [CrossRef]
- Albanese, M.; Nocini, P.F.; Fior, A.; Rizzato, A.; Cristofaro, M.G.; Sancassani, G.; Procacci, P. Mandibular reconstruction using fresh frozen bone allograft after conservative enucleation of a mandibular odontogenic myxoma. J. Craniofac. Surg. 2012, 23, 831–835. [Google Scholar] [CrossRef]
- Novembre, D.; Giofrè, E.; Barca, I.; Ferragina, F.; Cristofaro, M.G. A rare case of mandibular dentinogenic ghost cell tumor: Histopathological, clinical and surgical management. J. Oral Maxillofac. Pathol. 2021, 25, 206. [Google Scholar] [CrossRef]
- Niu, Z.; Li, Y.; Chen, W.; Zhao, J.; Zheng, H.; Deng, Q.; Zha, Z.; Zhu, H.; Sun, Q.; Su, L. Study on clinical and biological characteristics of ameloblastic carcinoma. Orphanet J. Rare Dis. 2020, 15, 316. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Martins-Chaves, R.R.; Pontes, F.S.C.; Fonseca, F.P.; Gomez, R.S.; Pontes, H.A.R. Comparison of survival outcomes between ameloblastic carcinoma and metastasizing ameloblastoma: A systematic review. J. Oral Pathol. Med. 2022, 51, 603–610. [Google Scholar] [CrossRef]
- França, D.C.; Moreira JMJr DEAguiar, S.M.; DECarvalhos, A.A.; Goiato, M.C. Ameloblastic carcinoma of the maxilla: A case report. Oncol. Lett. 2012, 4, 1297–1300. [Google Scholar] [CrossRef]
- Routray, S.; Majumdar, S. Ameloblastic carcinoma: Sometimes a challenge. J. Oral Maxillofac. Pathol. 2012, 16, 156–158. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Werning, J.W.; Kaye, F.J.; Mendenhall, W.M. Treatment of ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur. Arch. Otorhinolaryngol. 2016, 273, 3293–3297. [Google Scholar] [CrossRef]
- Takahashi, Y.; Bandoh, N.; Miyamoto, A.; Kamada, H. Single-Fraction Helical Tomotherapy for Ameloblastic Carcinoma. J. Oral Maxillofac. Surg. 2016, 74, 302–306. [Google Scholar] [CrossRef]
- Jensen, A.D.; Ecker, S.; Ellerbrock, M.; Nikoghosyan, A.; Debus, J.; Münter, M.W. Carbon ion therapy for ameloblastic carcinoma. Radiat. Oncol. 2011, 6, 13. [Google Scholar]
- Oh, K.Y.; Hong, S.D.; Yoon, H.J. Tumor immune microenvironment in odontogenic carcinomas: Evaluation of the therapeutic potential of immune checkpoint blockade. J. Oral Pathol. Med. 2024, 53, 217–225. [Google Scholar] [CrossRef]
- Nocini, R.; Vianini, M.; Girolami, I.; Calabrese, L.; Scarpa, A.; Martini, M.; Morbini, P.; Marletta, S.; Brunelli, M.; Molteni, G.; et al. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin. Exp. Dent. Res. 2022, 8, 690–698. [Google Scholar] [CrossRef]
- Uzawa, N.; Suzuki, M.; Miura, C.; Tomomatsu, N.; Izumo, T.; Harada, K. Primary ameloblastic carcinoma of the maxilla: A case report and literature review. Oncol. Lett. 2015, 9, 459–467. [Google Scholar]
- Bedi, R.S.; Chugh, A.; Pasricha, N. Ameloblastic carcinoma of maxilla. Natl. J. Maxillofac. Surg. 2012, 3, 70–74. [Google Scholar] [CrossRef]
- Phattarataratip, E.; Panitkul, T.; Khodkaew, W.; Anupuntanun, P.; Jaroonvechatam, J.; Pitarangsikul, S. Expression of SOX2 and OCT4 in odontogenic cysts and tumors. Head Face Med. 2021, 17, 29. [Google Scholar] [CrossRef]
| No. | Year | Author | Ref. | Age | Sex | Site of Involvement | Metastases |
|---|---|---|---|---|---|---|---|
| 1 | 1984 | Slootweg and Muller et al. | [4] | 23 | F | Mandible | - |
| 2 | 2004 | Goldenberg et al. | [7] | 60 | F | Mandible | Yes (brain) |
| 3 | 2016 | Fonseca et al. | [8] | 27 | F | Mandible | - |
| 4 | 2016 | Loyola et al. | [9] | 38 | M | Mandible | - |
| 5 | 2016 | Loyola et al. | [9] | 36 | M | Mandible | - |
| 6 | 2016 | Loyola et al. | [9] | 60 | M | Mandible | - |
| 7 | 2016 | Fomete et al. | [10] | 55 | M | Maxilla | - |
| 8 | 2016 | Fomete et al. | [10] | 32 | M | Maxilla | - |
| 9 | 2018 | Aoki T. et al. | [11] | 80 | M | Maxilla | Yes (lymph nodes) |
| 10 | 2018 | Fahradyan A. et al. | [12] | 15 | M | Mandible | - |
| 11 | 2018 | Soyele O.O et al. | [13] | 44 | M | Maxilla | - |
| 12 | 2018 | Soyele O.O et al. | [13] | 54 | M | Mandible | - |
| 13 | 2018 | Soyele O.O et al. | [13] | 47 | M | Mandible | - |
| 14 | 2018 | Soyele O.O et al. | [13] | 32 | M | Mandible | - |
| 15 | 2018 | Soyele O.O et al. | [13] | 46 | M | Mandible | - |
| 16 | 2018 | Soyele O.O et al. | [13] | 36 | F | Mandible | - |
| 17 | 2018 | Yamagata K. et al. | [14] | 70 | F | Maxilla | Yes (pterygomaxillary fossa) |
| 18 | 2019 | Kosanwat T. et al. | [15] | 46 | F | Maxilla | Yes (left orbit) |
| 19 | 2019 | Landeen K. et al. | [16] | 27 | F | Maxilla | Yes (skull base) |
| 20 | 2019 | Deng L. et al. | [17] | 36 | M | Mandible | - |
| 21 | 2019 | Deng L. et al. | [17] | 40 | F | Mandible | - |
| 22 | 2019 | Deng L. et al. | [17] | 47 | M | Maxilla | - |
| 23 | 2019 | Deng L. et al. | [17] | 61 | M | Mandible | - |
| 24 | 2019 | Deng L. et al. | [17] | 40 | M | Mandible | - |
| 25 | 2019 | Deng L. et al. | [17] | 39 | F | Mandible | - |
| 26 | 2019 | Deng L. et al. | [17] | 42 | M | Mandible | - |
| 27 | 2019 | Deng L. et al. | [17] | 46 | M | Mandible | - |
| 28 | 2019 | Deng L. et al. | [17] | 32 | M | Mandible | - |
| 29 | 2020 | Takayama K. et al. | [18] | 71 | M | Maxilla | - |
| 30 | 2020 | Cho et al. | [19] | 45 | M | Mandible | - |
| 31 | 2021 | Ismail et al. | [20] | 39 | F | Maxilla | - |
| 32 | 2022 | Hoehnle et al. | [21] | 31 | M | Maxilla | Local disease progression |
| 33 | 2023 | Chen et al. | [22] | 68 | M | Maxilla | - |
| 34 | 2023 | Ogane et al. | [23] | 72 | F | Mandible | - |
| 35 | 2023 | Hurnik et al. | [24] | - | M | Mandible | Yes (lymph nodes, orbit, lungs) |
| 36 | 2023 | Barca et al. | [25] | 33 | M | Mandible | Yes (lymph nodes, infratemporal fossa, orbit) |
| No. | Year | Author | Ref. | Age | Sex | Site of Involvement | Metastases |
|---|---|---|---|---|---|---|---|
| 1 | 1984 | Slootweg and Muller et al. | [4] | 75 | M | Mandible | - |
| 2 | 1988 | Dorner et al. | [26] | 84 | M | Mandible | Yes (lung) |
| 3 | 1991 | Bruce and Jackson et al. | [27] | 57 | M | Mandible | Yes (lung) |
| 4 | 1998 | Simko et al. | [28] | 64 | F | Mandible | Yes (lung) |
| 5 | 1998 | Infante-Cossio et al. | [29] | 69 | F | Maxilla | - |
| 6 | 1998 | Infante-Cossio et al. | [29] | 77 | M | Maxilla | Yes (brain) |
| 7 | 1998 | Infante-Cossio et al. | [29] | 64 | M | Maxilla | - |
| 8 | 2003 | Kawauchi S et al. | [30] | 67 | M | Mandible | - |
| 9 | 2003 | Datta et al. | [31] | 22 | M | Mandible | Yes (bone) |
| 10 | 2003 | Oginni et al. | [32] | 65 | M | Mandible | - |
| 11 | 2007 | Hall et al. | [33] | 27 | M | Mandible | - |
| 12 | 2007 | Hall et al. | [33] | 43 | F | Mandible | Yes (lung, liver) |
| 13 | 2007 | Hall et al. | [33] | 50 | M | Mandible | - |
| 14 | 2007 | Hall et al. | [33] | 49 | M | Mandible | - |
| 15 | 2007 | Hall et al. | [33] | 53 | F | Mandible | - |
| 16 | 2007 | Hall et al. | [33] | 59 | M | Mandible | - |
| 17 | 2007 | Hall et al. | [33] | 75 | M | Maxilla | - |
| 18 | 2007 | Hall et al. | [33] | 17 | F | Mandible | - |
| 19 | 2007 | Hall et al. | [33] | 63 | M | Maxilla | - |
| 20 | 2007 | Hall et al. | [33] | 31 | M | Mandible | - |
| 21 | 2007 | Hall et al. | [33] | 15 | M | Maxilla | - |
| 22 | 2007 | Hall et al. | [33] | 16 | M | Maxilla | Yes (lymph nodes) |
| 23 | 2007 | Hall et al. | [33] | 7 | F | Maxilla | - |
| 24 | 2007 | Hall et al. | [33] | 52 | M | Maxilla | - |
| 25 | 2007 | Benlyazid et al. | [34] | 90 | M | Maxilla | - |
| 26 | 2010 | Ram et al. | [35] | 21 | M | Mandible | - |
| 27 | 2010 | Lucca et al. | [36] | 73 | M | Maxilla | - |
| 28 | 2010 | Lucca et al. | [36] | 69 | M | Maxilla | - |
| 29 | 2010 | Jeremic et al. | [37] | 58 | M | Mandible | Yes (lymph nodes, lung) |
| 30 | 2012 | Horvath et al. | [38] | 8 | F | Mandible | Yes (lung, bone) |
| 31 | 2012 | Pirklbauer et al. | [39] | 86 | M | Mandible | Yes (lung, skull) |
| 32 | 2012 | Kamath et al. | [40] | 75 | M | Mandible | - |
| 33 | 2013 | Yoshioka et al. | [41] | 17 | M | Mandible | Yes (lung, skull) |
| 34 | 2014 | Kar et al. | [42] | 70 | M | Mandible | Yes (lymph nodes) |
| 35 | 2014 | Li et al. | [43] | 75 | M | Mandible | Yes (lung) |
| 36 | 2014 | Nobusawa et al. | [44] | 84 | F | Maxilla | Yes (liver) |
| 37 | 2016 | Loyola et al. | [8] | 21 | M | Mandible | - |
| 38 | 2018 | Kikuta S. et al. | [45] | 62 | M | Mandible | Yes (lungs, liver kidney) |
| 39 | 2018 | Mahmoud M.A.S et al. | [46] | 29 | F | Mandible | - |
| 40 | 2018 | Mahmoud M.A.S et al. | [46] | 62 | M | Maxilla | - |
| 41 | 2018 | Soyele O.O et al. | [13] | 34 | M | Mandible | - |
| 42 | 2018 | Soyele O.O et al. | [13] | 5 | F | Maxilla | - |
| 43 | 2018 | Soyele O.O et al. | [13] | 49 | M | Mandible | - |
| 44 | 2018 | Soyele O.O et al. | [13] | 60 | F | Maxilla | - |
| 45 | 2018 | Soyele O.O et al. | [13] | 32 | M | Mandible | - |
| 46 | 2018 | Soyele O.O et al. | [13] | 24 | F | Mandible | - |
| 47 | 2018 | Soyele O.O et al. | [13] | 16 | F | Mandible | - |
| 48 | 2019 | Deng L. et al. | [17] | 30 | M | Mandible | - |
| 49 | 2019 | Deng L. et al. | [17] | 35 | M | Mandible | - |
| 50 | 2019 | Deng L. et al. | [17] | 75 | M | Mandible | Yes (lung) |
| 51 | 2019 | Deng L. et al. | [17] | 57 | M | Maxilla | - |
| 52 | 2019 | Deng L. et al. | [17] | 25 | F | Mandible | - |
| 53 | 2019 | Deng L. et al. | [17] | 62 | F | Maxilla | Yes (lymph nodes) |
| 54 | 2019 | Deng L. et al. | [17] | 48 | M | Mandible | - |
| 55 | 2019 | Deng L. et al. | [17] | 72 | M | Maxilla | Yes (lymph nodes) |
| 56 | 2019 | Deng L. et al. | [17] | 54 | M | Mandible | - |
| 57 | 2019 | Sancheti S. et al. | [47] | 31 | M | Mandible | - |
| 58 | 2020 | Yukimori A. et al. | [48] | 26 | M | Mandible | -- |
| 59 | 2020 | Tarle M. et al. | [49] | 64 | M | Maxilla | Yes (brain) |
| 60 | 2020 | Vu et al. | [50] | 2 | F | Mandible | - |
| 61 | 2021 | Behtaj et al. | [51] | 20 | F | - | - |
| 62 | 2022 | Manchanda et al. | [52] | 55 | M | Mandible | - |
| 63 | 2023 | Schuch et al. | [53] | 23 | F | Maxilla | - |
| 64 | 2023 | Harada et al. | [54] | 76 | M | Mandible | Spine |
| Name | Molecular Mechanisms | Pathophysiology |
|---|---|---|
| Wnt/β-Catenin pathway | Cell proliferation, differentiation, and survival, and epithelial–mesenchymal interactions. | Cell proliferation, invasion, and apoptosis resistance. |
| Ki-67 | Cell proliferation. | Cell proliferation and invasion |
| PITX2 | Cell proliferation, differentiation, and survival. | Cell proliferation, migration, invasion, and tumor growth. |
| CD147 | Transmembrane receptor involved in cell adhesion. | Cancer progression and promotes tumor invasion and metastasis. |
| Integrins a3β1 and a5β1 | Mediate cell–cell interactions, together with the extracellular matrix, are critical for cell adhesion, signaling, migration, and tissue organization. | Local invasion and metastasis. |
| syndecan-1 | Cell proliferation, differentiation, and survival. | Tumor progression and development. |
| Tenascin-C and Tenascin-W | Tissue development, wound healing, and immune responses. | Tumor progression, invasion, and extracellular matrix remodeling, metastasis, and resistance to therapy. |
| Perilipin | Lipid metabolism, primarily regulating fat storage in adipocytes | Invasion of surrounding bone and soft tissues. |
| SOX2 | Tooth development. | Aggressive tumor behavior, recurrence, and a poor prognosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristofaro, M.G.; Barca, I.; Sottile, A.R.; Ferragina, F. Ameloblastic Carcinoma: A 40-Year Scoping Review of the Literature. Curr. Issues Mol. Biol. 2025, 47, 261. https://doi.org/10.3390/cimb47040261
Cristofaro MG, Barca I, Sottile AR, Ferragina F. Ameloblastic Carcinoma: A 40-Year Scoping Review of the Literature. Current Issues in Molecular Biology. 2025; 47(4):261. https://doi.org/10.3390/cimb47040261
Chicago/Turabian StyleCristofaro, Maria Giulia, Ida Barca, Angelo R. Sottile, and Francesco Ferragina. 2025. "Ameloblastic Carcinoma: A 40-Year Scoping Review of the Literature" Current Issues in Molecular Biology 47, no. 4: 261. https://doi.org/10.3390/cimb47040261
APA StyleCristofaro, M. G., Barca, I., Sottile, A. R., & Ferragina, F. (2025). Ameloblastic Carcinoma: A 40-Year Scoping Review of the Literature. Current Issues in Molecular Biology, 47(4), 261. https://doi.org/10.3390/cimb47040261








