Rutin Ameliorates BHBA-Induced Inflammation and Lipid Accumulation in Calf Hepatocytes Through NF-κB Signaling Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Calf Primary Hepatocyte Culture
2.2. BHBA and RT Preparation
2.3. Experimental Design
2.4. Cell Viability
2.5. Detection of Inflammation Indicators
2.6. TG, TC Determination, and Oil Red O Staining
2.7. Real-Time Quantitative PCR Assay
2.8. Protein Extraction and Western Blotting Assay
2.9. Statistical Analysis
3. Results
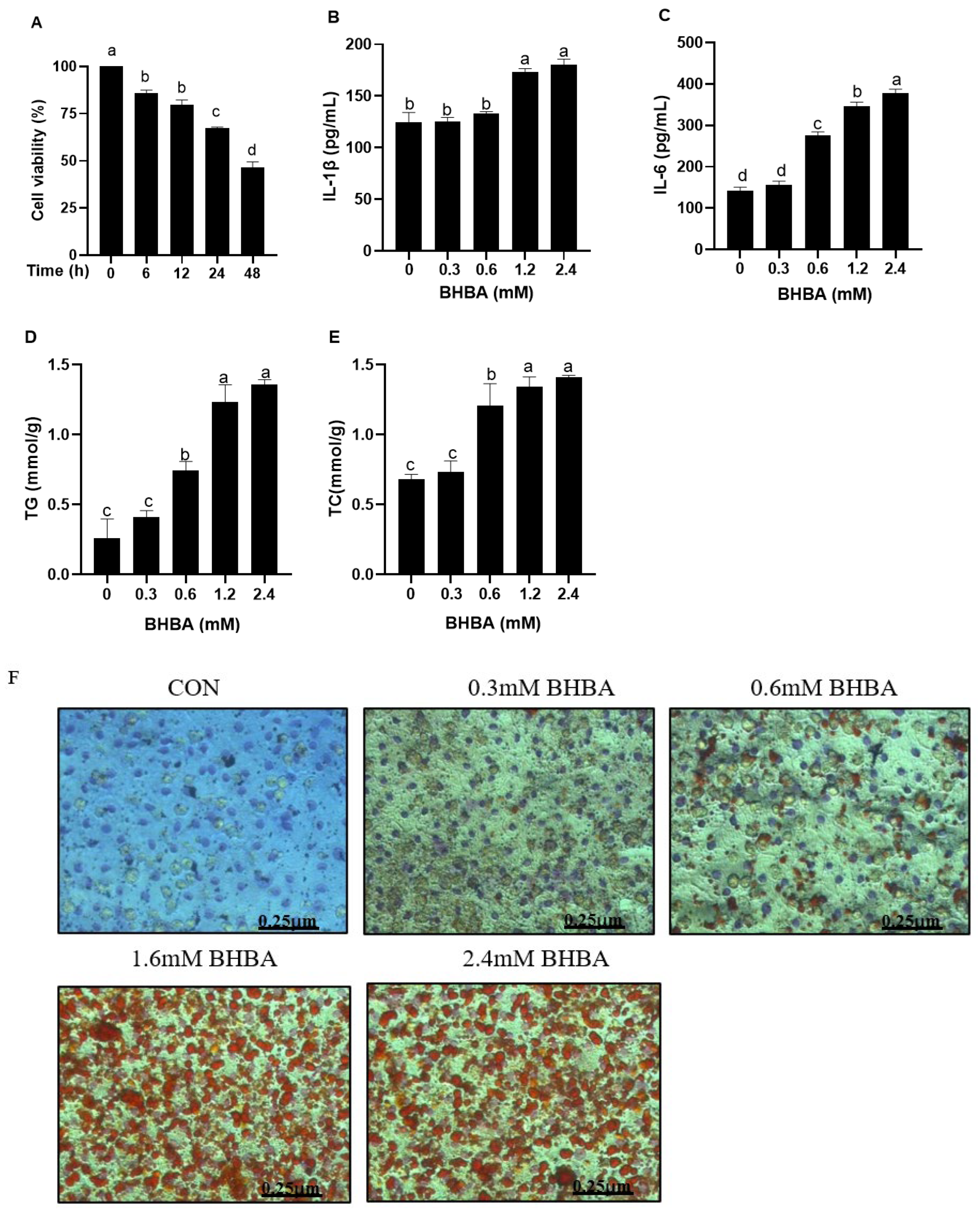
3.1. Effect of BHBA on Cell Viability and Inflammatory Indices in Calf Hepatocytes
3.2. Effect of BHBA on Lipid Accumulation in Calf Hepatocytes
3.3. Effect of BHBA on Inflammatory Factors in Hepatocytes and the Intervention of RT
3.4. Effect of RT on BHBA-Induced Lipid Accumulation in Hepatocytes
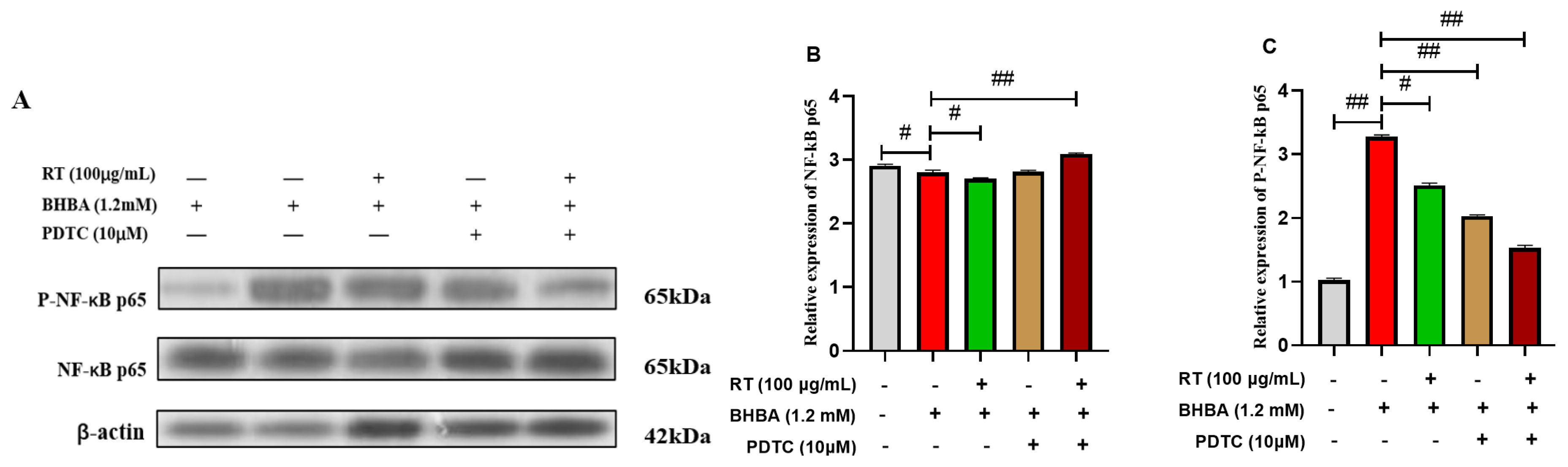
3.5. Effect of RT on the NF-κB Signaling Pathway
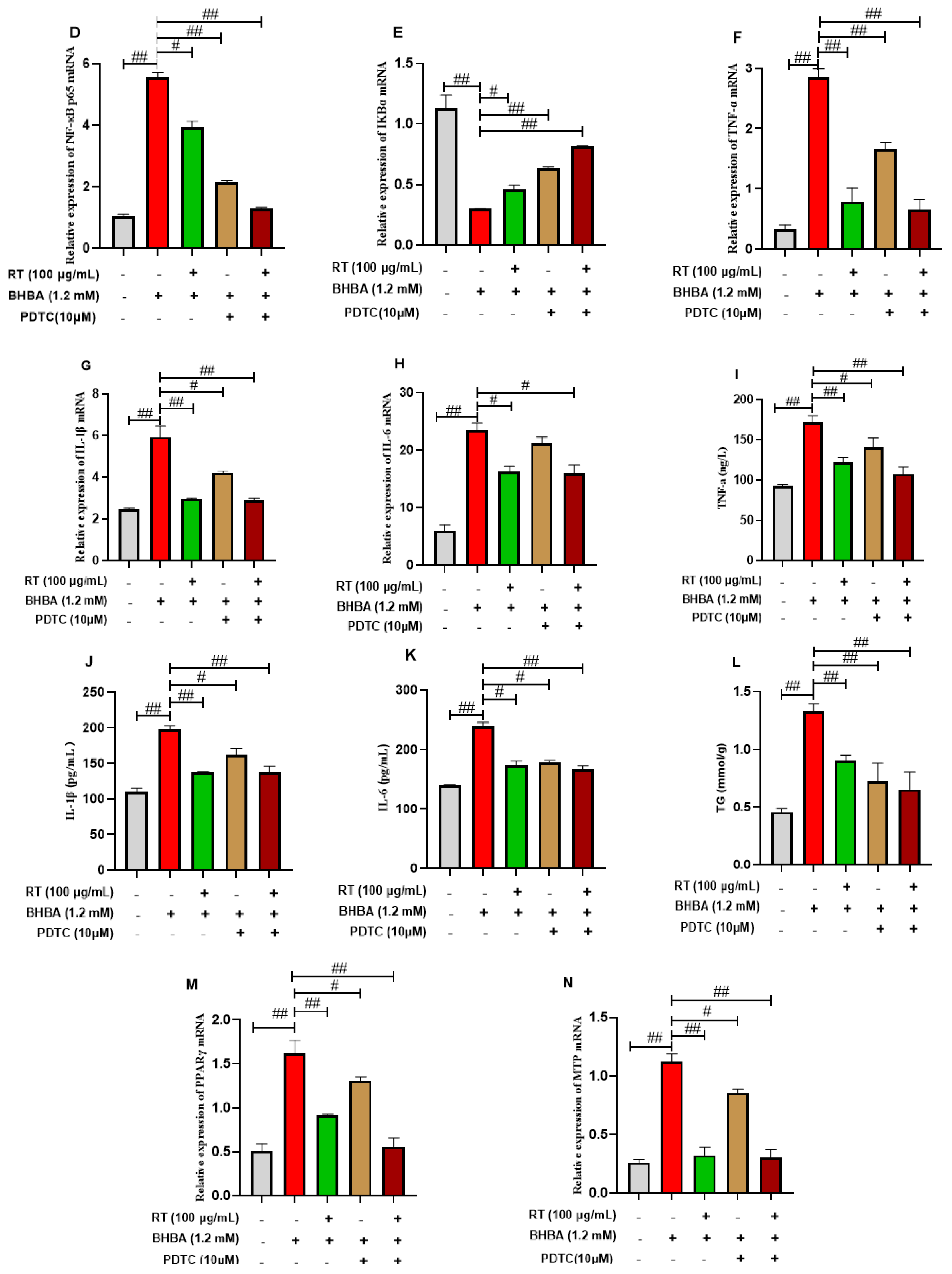
3.6. The Expressions of Pro-Inflammatory Factors in Hepatocytes
3.7. The Expressions of Lipid Metabolism Genes in Hepatocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melendez, P.; Serrano, V.M. Update on ketosis in dairy cattle with major emphasis on subclinical ketosis and abdominal adiposity. Vet. Med. Sci. 2024, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kok, A.; Burgers, E.E.A.; Bruckmaier, R.M.; Goselink, R.M.A.; Gross, J.J.; Kemp, B.; Lam, J.G.M.; Minuti, A.; Saccenti, E.; et al. Time profiles of energy balance in dairy cows in association with metabolic status, inflammatory status, and disease. J. Dairy Sci. 2024, 11, 9960–9977. [Google Scholar] [CrossRef] [PubMed]
- Yeshambel, M. Negative energy balance and its implication on productive and reproductive performance of early lactating dairy cows: Review paper. J. Appl. Anim. Res. 2023, 51, 220–229. [Google Scholar]
- Wang, X.; Zhang, C.; Li, R. Down-regulation of miR-29 improves lipid metabolism in fatty liver of dairy cows. Anim. Biotechnol. 2024, 35, 2396414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Cui, Y.Z.; Indugu, N.; Loor, J.J.; Jiang, Q.M.; Yu, Z.T.; Baker, L.D.; Pitta, D.P.; Deng, Z.J.; Xu, C. Integrated meta-omics analyses reveal a role of ruminal microorganisms in ketone body accumulation and ketosis in lactating dairy cows. J. Dairy Sci. 2023, 106, 4906–4917. [Google Scholar] [CrossRef]
- Pravin, B.; Nanaware, V.; Ashwini, B.; Wondmie, G.F.; Jardan, Y.A.B.; Bourhia, M. Assessing the antioxidant properties of naringin and rutin and investigating their oxidative DNA damage effects in breast cancer. Sci. Rep. 2024, 14, 15314. [Google Scholar] [CrossRef]
- Fatemeh, F.; Toktam, S.; Aristidis, T.; Mehrdad, I.; Rezaee, R. Rutin: A pain-relieving flavonoid. Inflammopharmacology 2025, 1, 1289–1301. [Google Scholar]
- Zhang, H.Z.; Shi, H.; Li, X.; Zhou, S.; Song, X.; Ma, N.; Meng, M.; Chang, G.; Shen, X. Quercetin alleviates LPS/iE-DAP-induced liver injury by suppressing ferroptosis via regulating ferritinophagy and intracellular iron efflux. Redox Biol. 2025, 81, 103557. [Google Scholar] [CrossRef]
- Duan, H.; Wang, F.; Wang, K.; Yang, S.; Zhang, R.; Xue, C.; Xiao, L. Quercetin ameliorates oxidative stress-induced apoptosis of granulosa cells in dairy cow follicular cysts by activating autophagy via the SIRT1/ROS/AMPK signaling pathway. J. Anim. Sci. Biotechnol. 2024, 15, 119. [Google Scholar] [CrossRef]
- Dai, D.; Dong, C.; Kong, F.; Wang, S.; Wang, S.; Wang, W.; Li, S. Dietary supplementation of Scutellariae radix flavonoid extract improves lactation performance in dairy cows by regulating gastrointestinal microbes, antioxidant capacity and immune function. Anim. Nutr. 2025, 20, 499–508. [Google Scholar] [CrossRef]
- Niederberger, E.; Geisslinger, G. Analysis of NF-κB signaling pathways by proteomic approaches. Expert Rev. Proteom. 2010, 7, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.A.; Wang, Q.Q.; Wang, G.G.; Zhang, Q.Q.; Guo, Y.P.; Su, X.; Tang, Y.; Koci, M.; Zhang, J.Y.; Ma, Q.G.; et al. Rutin, a natural flavonoid glycoside, ameliorates zearalenone induced liver inflammation via inhibiting lipopolysaccharide gut leakage and NF-κB signaling pathway in mice. Food Chem. Toxicol. 2024, 191, 12312. [Google Scholar] [CrossRef] [PubMed]
- He, Q.Y.; Hu, H.; Zhao, K.K. Investigating the anti-inflammatory effects of rutin in carbon tetrachloride-induced hepatotoxicity: Role of TLR4/MyD88/NFκB signaling pathway modulation. Pharmacogn. Mag. 2024, 20, 107–115. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, L.; He, C.; Wang, C.; Lv, Y.L.; Li, H.M. Rutin-activated adipose tissue thermogenesis is correlated with increased intestinal short-chain fatty acid levels. Phytotherapy Res. 2022, 36, 2495–2510. [Google Scholar] [CrossRef]
- Du, X.Y.; Zhu, Z.; Peng, Y.; Cui, Q.; Zhang, Z.; Shi, Y.; Guan, X.; Sha, T.; Shen, Y.; Yang, X.; et al. High concentrations of fatty acids and beta-hydroxybutyrate impair the growth hormone-mediated hepatic JAK2-STAT5 pathway in clinically ketotic cows. J. Dairy Sci. 2018, 101, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.; Yang, W.; Loor, J.J.; Liang, Y.; Wang, S.; Zhao, Y.; Guo, H.; Ma, X.; Yu, L.; et al. Mitochondrial dysfunction and endoplasmic reticulum stress in calf hepatocytes are associated with fatty acid-induced ORAI calcium release-activated cal-cium modulator 1 signaling. J. Dairy Sci. 2020, 103, 11945–11956. [Google Scholar] [CrossRef]
- Jenkins, N.T.; Peña, G.; Risco, C.; Barbosa, C.C.; Vieira, N.A.; Galvão, K.N. Utility of inline milk fat and protein ratio to diagnose subclinical ketosis and to assign propylene glycol treatment in lactating dairy cows. Can. Vet. J. 2015, 56, 850–854. [Google Scholar] [PubMed]
- Prashant, T.; Nitish, M.; Jasmine, C.; Sonia, K.; Akash, J. Unveiling the substantial role of rutin in the management of drug-induced nephropathy using network pharmacology and molecular docking. Int. Immunopharmacol. 2025, 1, 146. [Google Scholar]
- Zhou, S.; Chen, M.; Meng, M.; Ma, N.; Xie, W.; Shen, X.; Li, Z.; Chang, G. Subclinical ketosis leads to lipid metabolism disorder by downregulating the expression of acetyl-coenzyme A acetyltransferase 2 in dairy cows. J. Dairy Sci. 2023, 106, 9892–9909. [Google Scholar] [CrossRef]
- Bzdęga, W.; Żywno, H.; Kołakowski, A.; Kurzyna, P.F.; Harasim, S.E.; Chabowski, A.; Konstantynowicz, N.K. Coumestrol as a new substance that may diminish lipid precursors of the inflammation in steatotic primary rat hepatocytes. Biochimie 2022, 204, 101. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Li, D.; Li, Y.; Song, Y.; Deng, Q.; Wang, J.; Zhang, Y.; Ding, H.; Yin, L.; et al. β-Hydroxybutyrate activates the NF-κB signaling pathway to promote the expression of pro-inflammatory factors in calf hepatocytes. Cell Physiol. Biochem. 2014, 33, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Cheng, X.; Wu, X.; Yoo, J.Y.; Cheng, C.; Guo, J.Y.; Mo, X.; Ru, P.; Hurwitz, B.; Kim, S.H.; et al. Inhibition of SOAT1 Suppresses Glioblastoma Growth via Blocking SREBP-1-Mediated Lipogenesis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5337–5348. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.J.; Schwarz, F.J.; Eder, K.; van Dorland, H.A.; Bruckmaier, R.M. Liver fat content and lipid metabolism in dairy cows during early lactation and during a mid-lactation feed restriction. J. Dairy Sci. 2013, 96, 5008–5017. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, N.; Meng, M.; Chang, G.; Shen, X. Lentinan ameliorates β-hydroxybutyrate-induced lipid metabolism disorder in bovine hepatocytes by upregulating the expression of acetyl-coenzyme a acetyltransferase 2. J. Agric. Food Chem. 2024, 72, 17392–17404. [Google Scholar] [CrossRef] [PubMed]
- Elshafey, B.G.; Elfadadny, A.; Metwally, S.; Saleh, A.G.; Ragab, R.F.; Hamada, R.; Mandour, A.S.; Hendawy, A.O.; Alkazmi, L.; Ogaly, H.A.; et al. Association between biochemical parameters and ultrasonographic measurement for the assessment of hepatic lipidosis in dairy cows. Ital. J. Anim. Sci. 2023, 22, 136–147. [Google Scholar] [CrossRef]
- Deng, Q.H.; Liu, G.W.; Liu, L.; Zhang, Y.M.; Yin, L.H.; Shi, X.X.; Wang, J.G.; Yuan, X.; Sun, G.Q.; Li, Y.; et al. BHBA influences bovine hepatic lipid metabolism via AMPK signaling pathway. J. Cell. Biochem. 2015, 116, 1070. [Google Scholar] [CrossRef]
- Li, C.M.; Huang, J.P.; Chen, X.X.; Yan, Y.; Li, L.; Zhao, W.G. Transcriptome analysis reveals that nefa and β-hydroxybutyrate induce oxidative stress and inflammatory response in bovine mammary epithelial cells. Metabolites 2022, 11, 1060. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Ileriturk, M.; Gur, C. Rutin protects rat liver and kidney from sodium valproate-induce damage by attenuating oxidative stress, ER stress, inflammation, apoptosis and autophagy. Mol. Biol. Rep. 2022, 49, 6063–6074. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Pan, R. Sodium rutin extends lifespan and health span in mice including positive impacts on liver health. Br. J. Pharmacol. 2022, 179, 1825–1838. [Google Scholar] [CrossRef]
- Li, L.Z. Interventional Effects of Rutin on LPS-Induced Inflammatory Injury of Rumen Epithelial Cells and Its Mechanism. Ph.D. Thesis, Northeast Agricultural University, Harbin, China, 2023. [Google Scholar]
- Luo, Y.G. Effects of rutin on the growth of mammary epithelial cells of dairy cows cultured in vitro. Chin. J. Anim. Husb. 2019, 55, 96–99. [Google Scholar]
- James, O.O.; Divine, O.O.; Yetunde, O.O.; Osarugue, C.L.; Precious, O.; Oritseweyinmi, T.; Paul, A.A.; Gbemisayo, A.A.; Solomon, U. Ginseng exhibits adaptogenic-like activity in mice exposed to hypoxic-anoxic stress through activation of antioxidant/BDNF protective mechanisms and inhibition of pro-inflammatory cytokines/NF-KB signaling pathways. Pharmacol. Res. Mod. Chin. Med. 2025, 1, 14. [Google Scholar]
- Wu, W.J.; Cheng, Z.; Nan, Y.; Gang, P.; Wang, Y.H. L-selectin promotes migration, invasion and inflammatory response of fibroblast-like synoviocytes in rheumatoid arthritis via NF-kB signaling pathway. In Inflammation; Springer: Berlin/Heidelberg, Germany, 2025; prepublish; pp. 1–13. [Google Scholar]
- Zou, D.; Liu, R.; Shi, S.; Du, J.; Tian, M.; Wang, X.; Hou, M.; Duan, Z.; Ma, Y. BHBA regulates the expressions of lipid synthesis and oxidation genes in sheep hepatocytes through the AMPK pathway. Res. Vet. Sci. 2021, 140, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Liu, D.J.; Liu, M.X. The protective effect of NF-κB signaling pathway inhibitor PDTC on mice with chronic atrophic gastritis. Scand. J. Gastroenterol. 2021, 56, 1131–1139. [Google Scholar]
- Song, Y.; Huang, Q.; Zhang, Z. Separate administration of ammonium pyrrolidinedithiocarbamate and phorbol myristate acetate at early and late stages decreases secondary brain injury following intracerebral haemorrhage in rats via the NF-kappaB pathway. Folia Neuropathol. 2020, 58, 166–175. [Google Scholar] [CrossRef]
- Xu, T.; Liu, J.; Li, X.R.; Yu, Y.; Luo, X.; Zheng, X.; Cheng, Y.; Yu, P.Q.; Liu, Y. The mTOR/NF-kappaB pathway mediates neuroinflammation and synaptic plasticity in diabetic encephalopathy. Mol. Neurobiol. 2021, 58, 3848–3862. [Google Scholar] [CrossRef]
- Jiang, M.; Lv, Z.; Huang, Y.; Cheng, Z.; Meng, Z.; Yang, T.; Yan, Q.; Lin, M.; Zhan, K.; Zhao, G. Quercetin alleviates lipopolysaccharide-induced inflammatory response in bovine mammary epithelial cells by suppressing TLR4/NF-κB signaling pathway. Front. Vet. Sci. 2022, 9, 915726. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, J.; Yuan, T.; Yang, C.; Zhou, Q.; Shaukat, A.; Deng, G.; Wang, X. Luteolin alleviates inflammation induced by staphylococcus aureus in bovine mammary epithelial cells by attenuating NF-κB and MAPK activation. Vet. Sci. 2025, 12, 96. [Google Scholar] [CrossRef]
- Liu, S.; Adewole, D.; Yu, L.; Sid, V.; Wang, B.O.K.; Yang, C. Rutin attenuates inflammatory responses induced by lipopolysaccharide in an in vitro mouse muscle cell (C2C12) model. Poult. Sci. 2019, 98, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Validation of the Efficacy of Quercetin on Mouse Mammary Gland and Mammary Epithelial Cell Inflammation Model. PhD Thesis, Gansu Agricultural University, Lanzhou, China, 2023. [Google Scholar]
- Jin, F.D.; Zang, T.; Zhang, Z. Protective effect of rutin on oxidative stress injury of hepatocytes and its mechanism. J. Jilin Univ. 2020, 46, 1117–1123. [Google Scholar]
- Liu, Y.; Sun, Z.; Dong, R.; Liu, P.; Zhang, X.; Li, Y.; Lai, X.; Cheong, H.F.; Wu, Y.; Wang, Y.; et al. Rutin ameliorated lipid metabolism dysfunction of diabetic NAFLD via AMPK/SREBP1 pathway. Phytomedicine 2024, 126, 155437. [Google Scholar] [CrossRef]
- Prince, P.; Stanely, M.; Kannan, N.K. Protective effect of rutin on lipids, lipoproteins, lipid metabolizing enzymes and glycoproteins in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2010, 58, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; He, X.; Yang, B.; Wang, H.; Shan, X.; Li, C.; Sun, D.; Wu, R. LPS-induced reduction of triglyceride synthesis and secretion in dairy cow mammary epithelial cells via decreased SREBP1 expression and activity. J. Dairy Res. 2018, 85, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shin, D.J.; Osborne, T.F. A simple promoter containing two Sp1 sites controls the expression of sterol-regulatory-element-binding protein 1a (SREBP-1a). Biochem. J. 2005, 386, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Anahita, A.; Arta, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar]

| Gene | Accession No. | Primer Sequence (5′ to 3′) | Length (bp) |
|---|---|---|---|
| ACTB | NM_280979 | F: GCAAATGCTTCTAGGCGGAC | 203 |
| R: ATGCTCGATCCAACCGACTG | |||
| IKBα | NM_348923 | F: TGCAGGCCACCAACTACAAT | 203 |
| R: GACATCAGCCCCACACTTCA | |||
| NF-κB p65 | NM_508233 | F: CCAGACCAACAACAACCCCT | 241 |
| R: CAGGAAGATCTCATCCCCGC | |||
| TNF-α | NM_497021 | F: TGCCTTGCTCAGATGTGTT | 181 |
| R: GAGCGGAGGTTCAGTGATGT | |||
| IL-1β | NM_370871 | F: AAGCCACCCAGGGATCCTAT | 199 |
| R: CCATCTCCCATGGAACCGAG | |||
| IL-6 | NM_378232 | F: TGCAGGCCACCAACTACAAT | 203 |
| R: GACATCAGCCCCACACTTCA | |||
| PPARγ | NM_497014 | F: GCCCCAGGTGGTGGTGGA | 281 |
| R: GTAGGAAGTCTGCCGAGAGC | |||
| MTP | NM_532992 | F: GAGGGTGGATTTACGACCC | 238 |
| R: GATGGCTGCAACCTGCTTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Zhao, H.; Gao, M.; Hu, H.; Li, D. Rutin Ameliorates BHBA-Induced Inflammation and Lipid Accumulation in Calf Hepatocytes Through NF-κB Signaling Pathway. Curr. Issues Mol. Biol. 2025, 47, 274. https://doi.org/10.3390/cimb47040274
Yang K, Zhao H, Gao M, Hu H, Li D. Rutin Ameliorates BHBA-Induced Inflammation and Lipid Accumulation in Calf Hepatocytes Through NF-κB Signaling Pathway. Current Issues in Molecular Biology. 2025; 47(4):274. https://doi.org/10.3390/cimb47040274
Chicago/Turabian StyleYang, Kun, Haixia Zhao, Min Gao, Honglian Hu, and Dabiao Li. 2025. "Rutin Ameliorates BHBA-Induced Inflammation and Lipid Accumulation in Calf Hepatocytes Through NF-κB Signaling Pathway" Current Issues in Molecular Biology 47, no. 4: 274. https://doi.org/10.3390/cimb47040274
APA StyleYang, K., Zhao, H., Gao, M., Hu, H., & Li, D. (2025). Rutin Ameliorates BHBA-Induced Inflammation and Lipid Accumulation in Calf Hepatocytes Through NF-κB Signaling Pathway. Current Issues in Molecular Biology, 47(4), 274. https://doi.org/10.3390/cimb47040274






