A New Method for Preparation of Decellularized Human Scaffolds for Facial Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
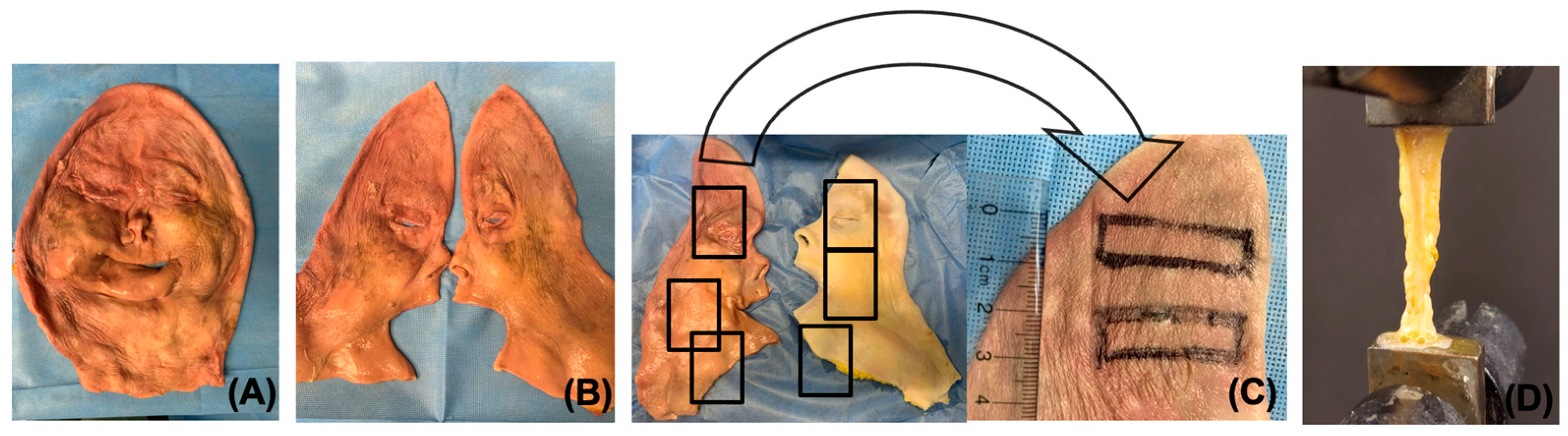
2.1. Tissue Procurement
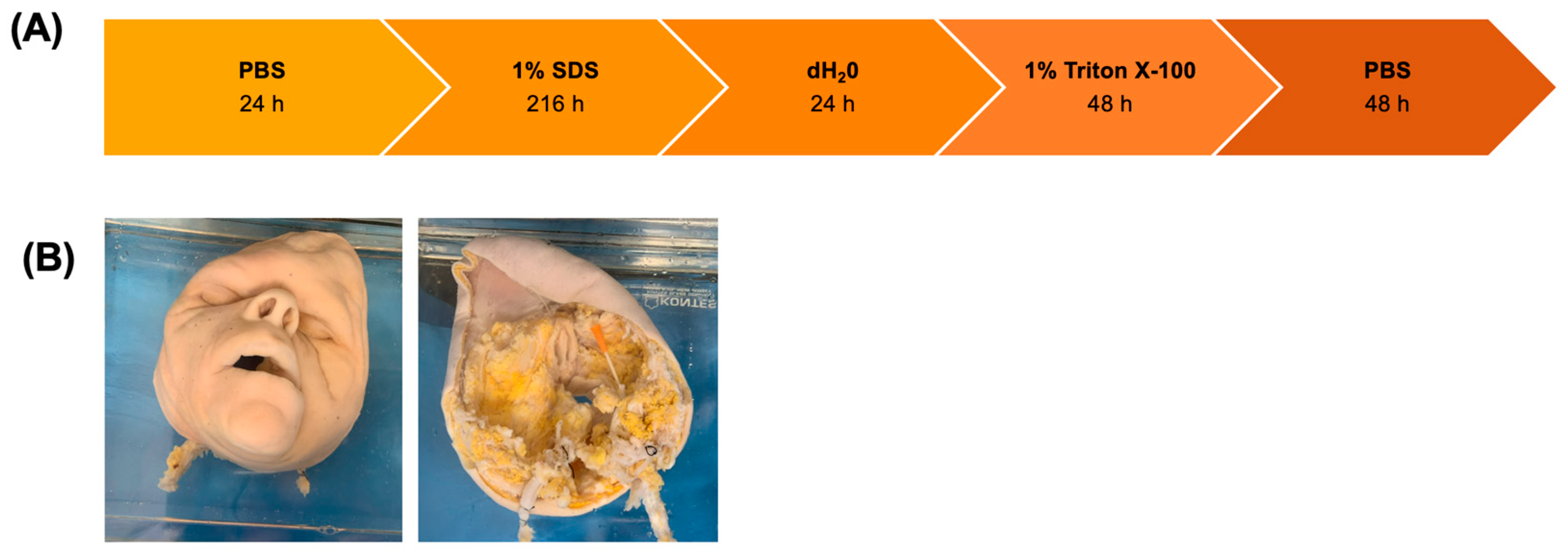
2.2. Decellularization of Facial Grafts
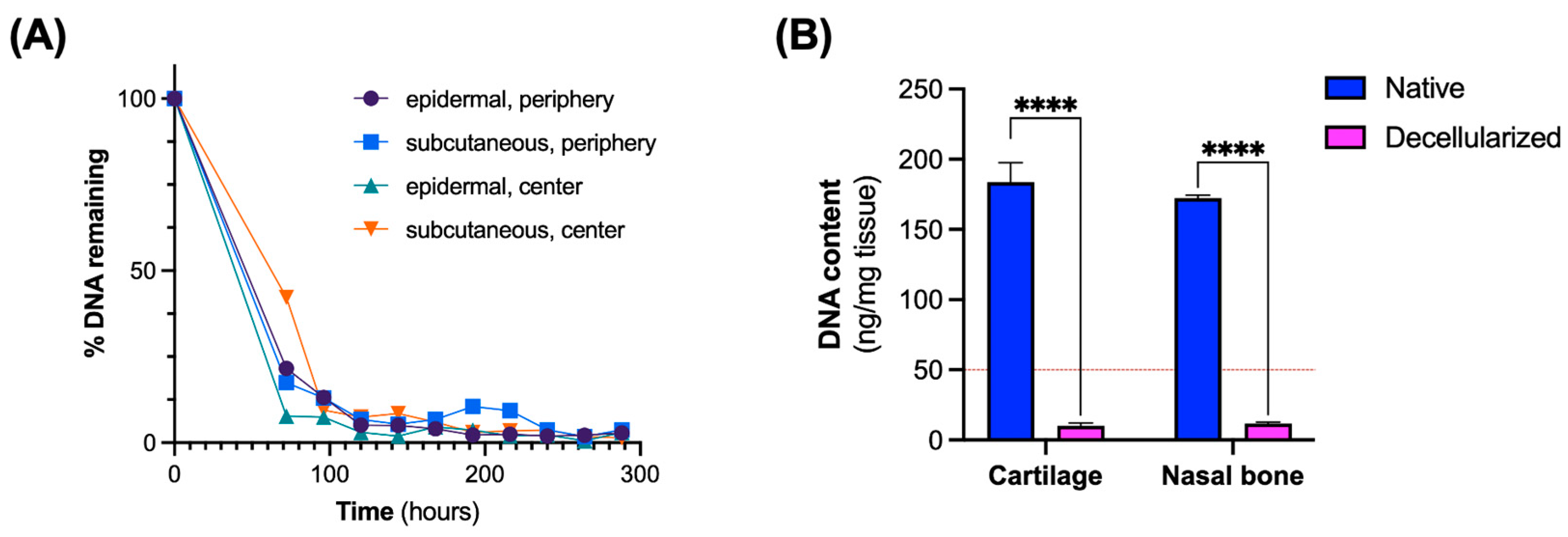
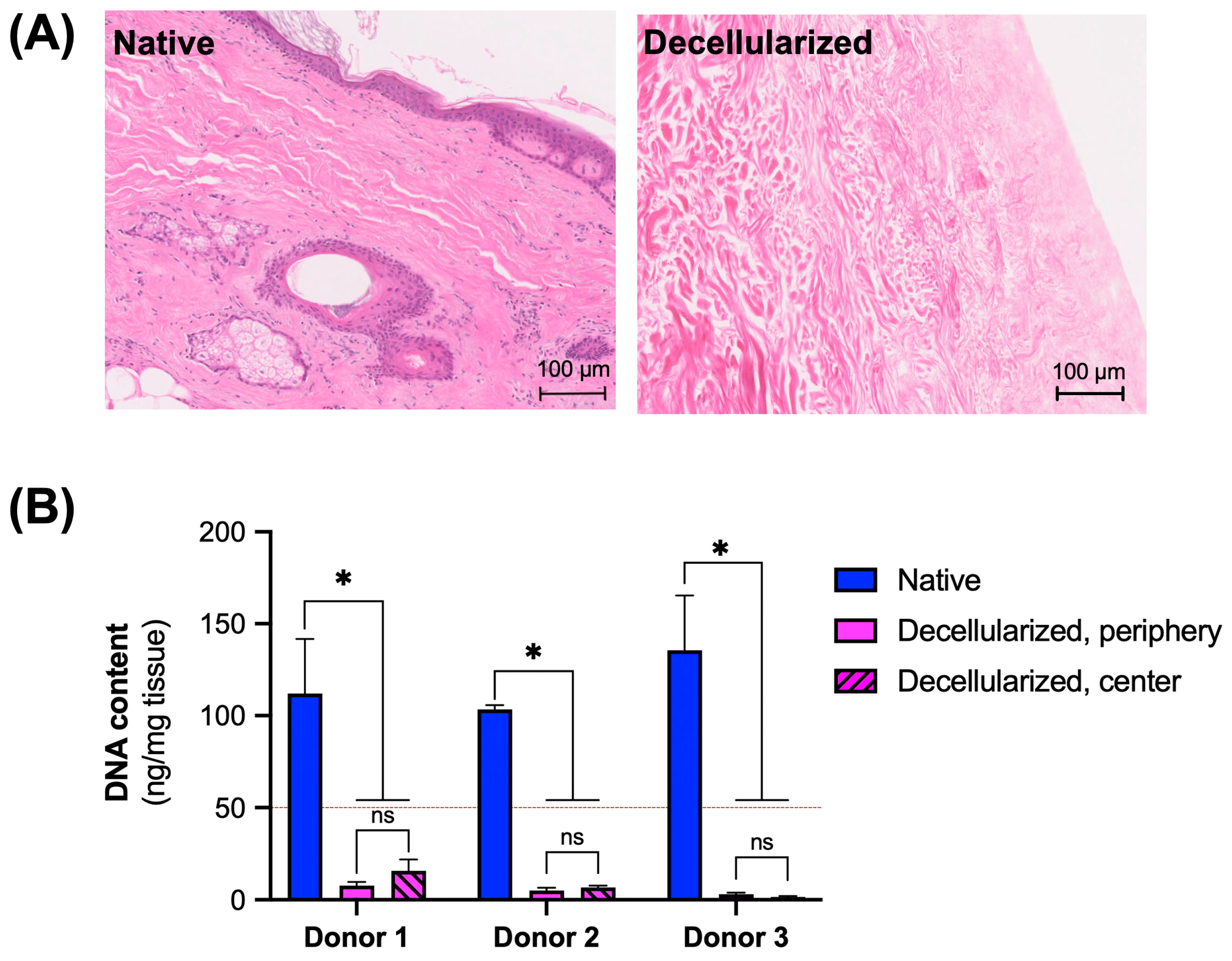
2.3. Analysis of DNA Removal
2.4. Quantification of Extracellular Matrix Components
2.5. Histological Assessment
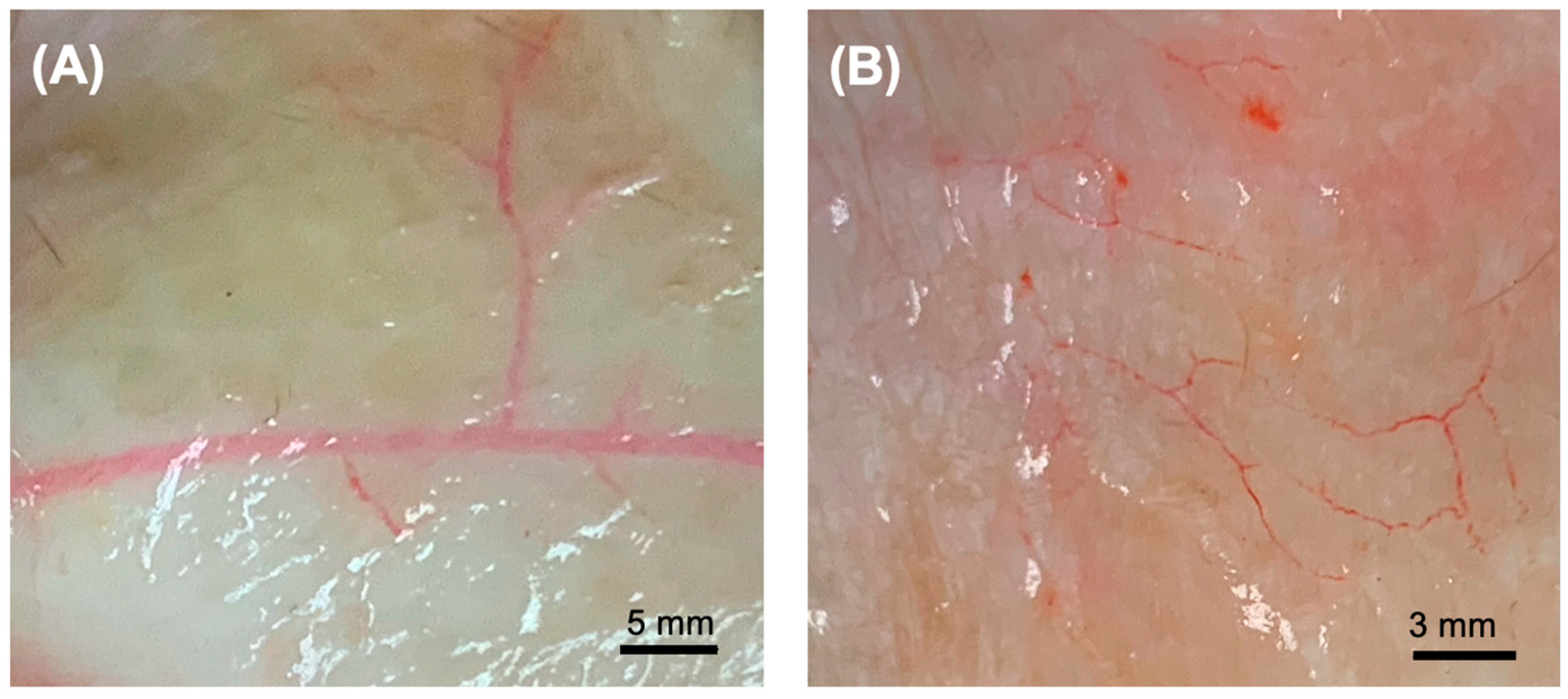
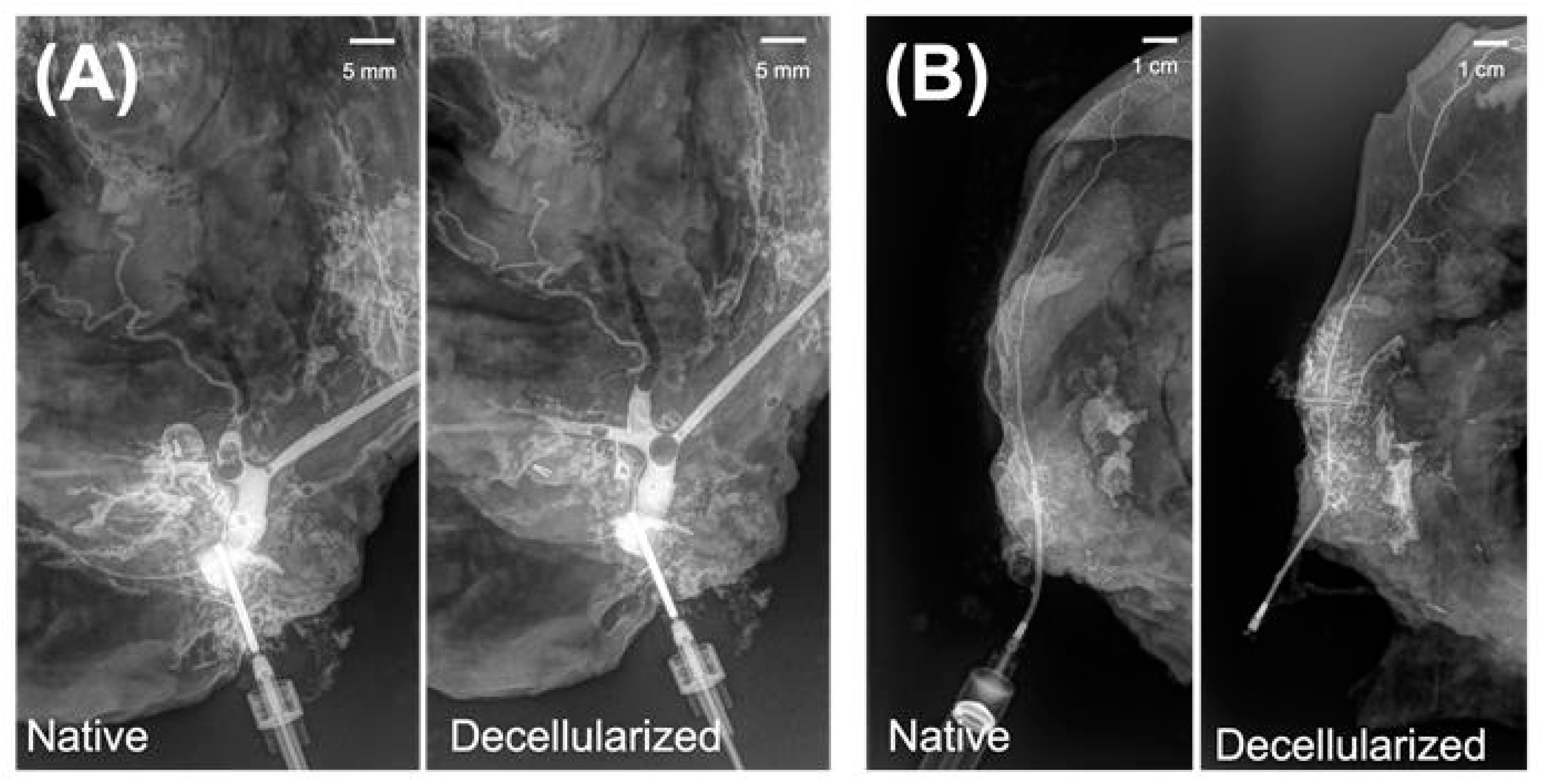
2.6. Assessment of Vascular Architecture
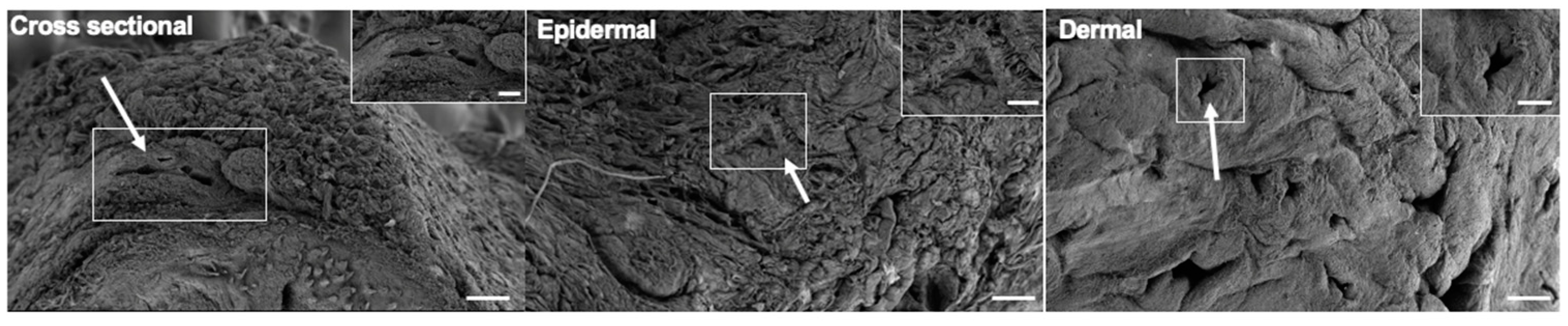
2.7. Scanning Electron Microscopy Imaging
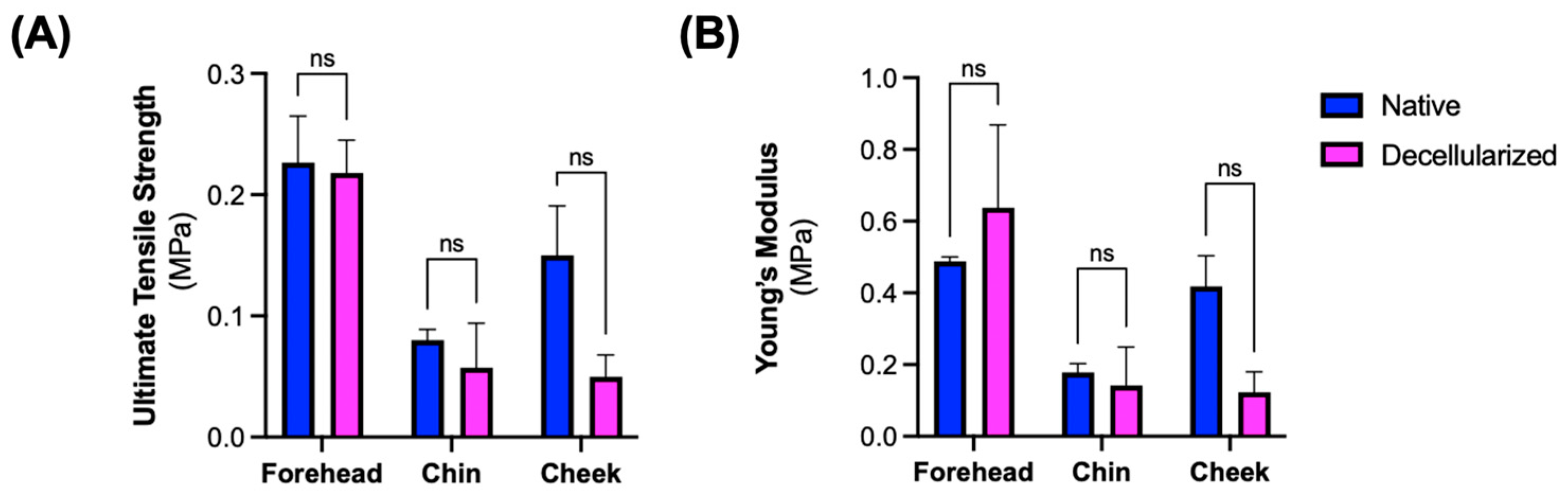
2.8. Mechanical Testing of Decellularized Grafts
2.9. In Vitro Biocompatibility of Decellularized Grafts
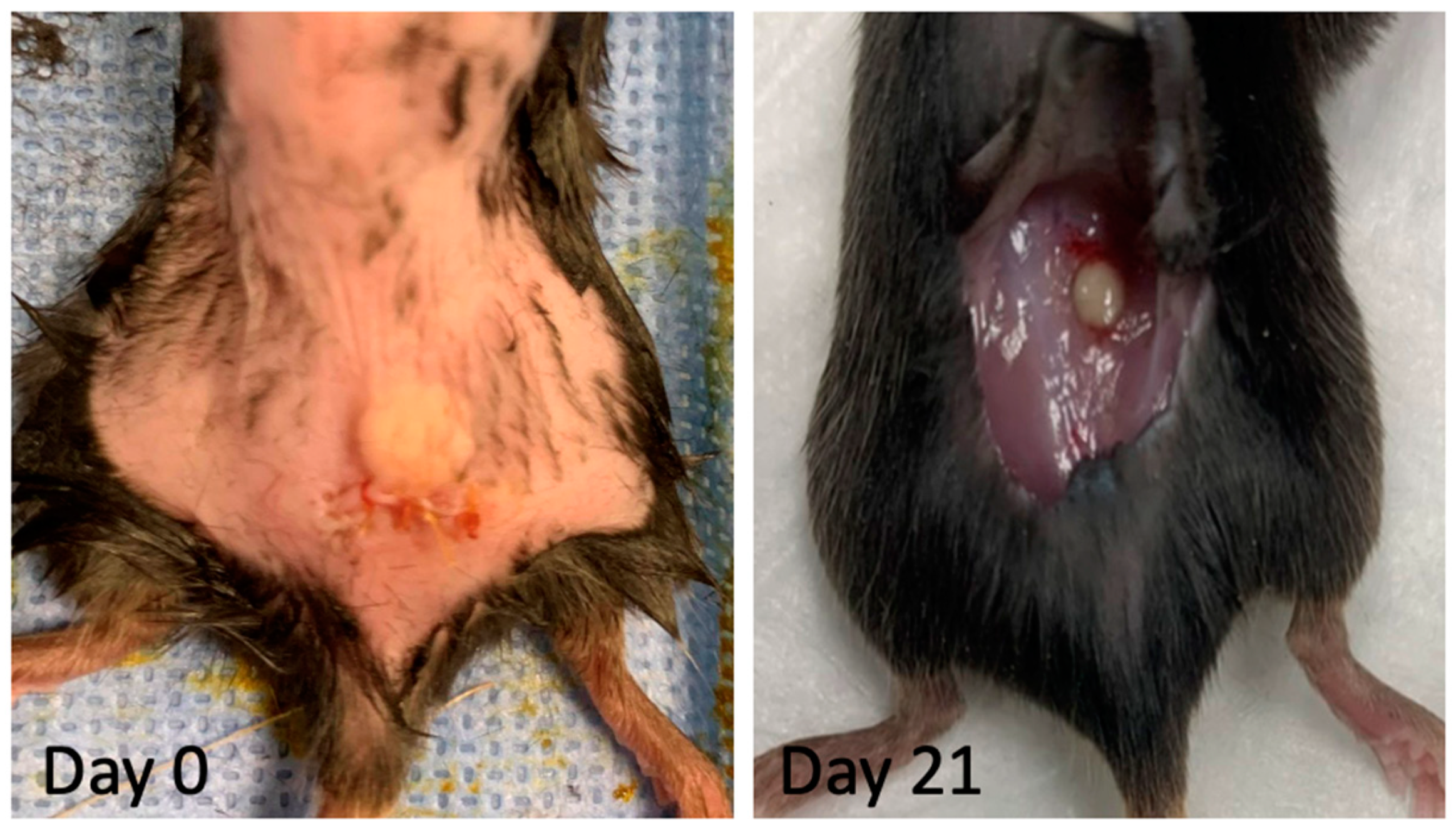
2.10. Implantation of Decellularized Grafts for Immunological Evaluation
2.11. Statistical Analysis
3. Results
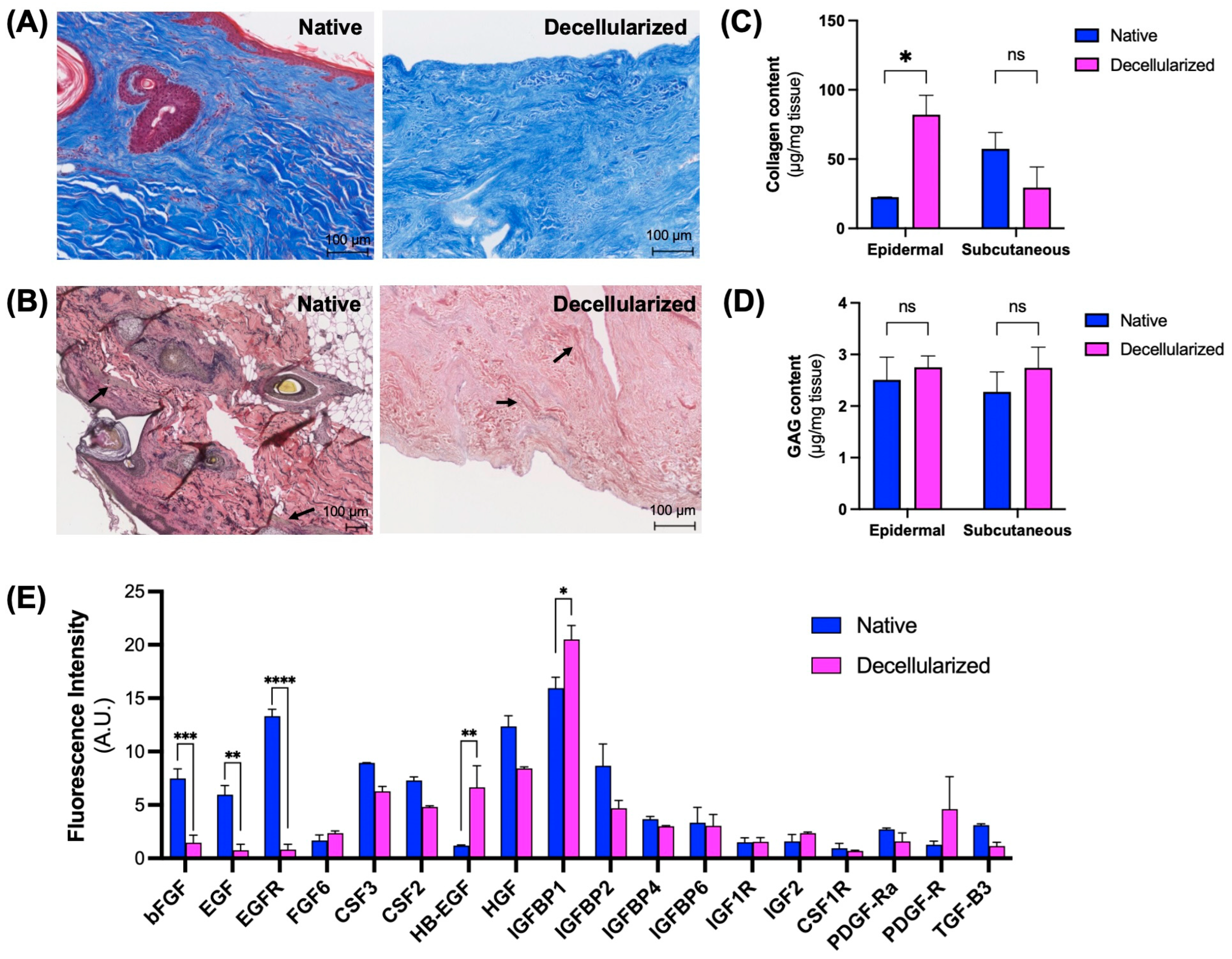
3.1. Assessment of Decellularization Efficiency
3.2. Maintenance of Extracellular Matrix (ECM) Components
3.3. Microarchitectural and Mechanical Characteristics of Decellularized Facial Grafts
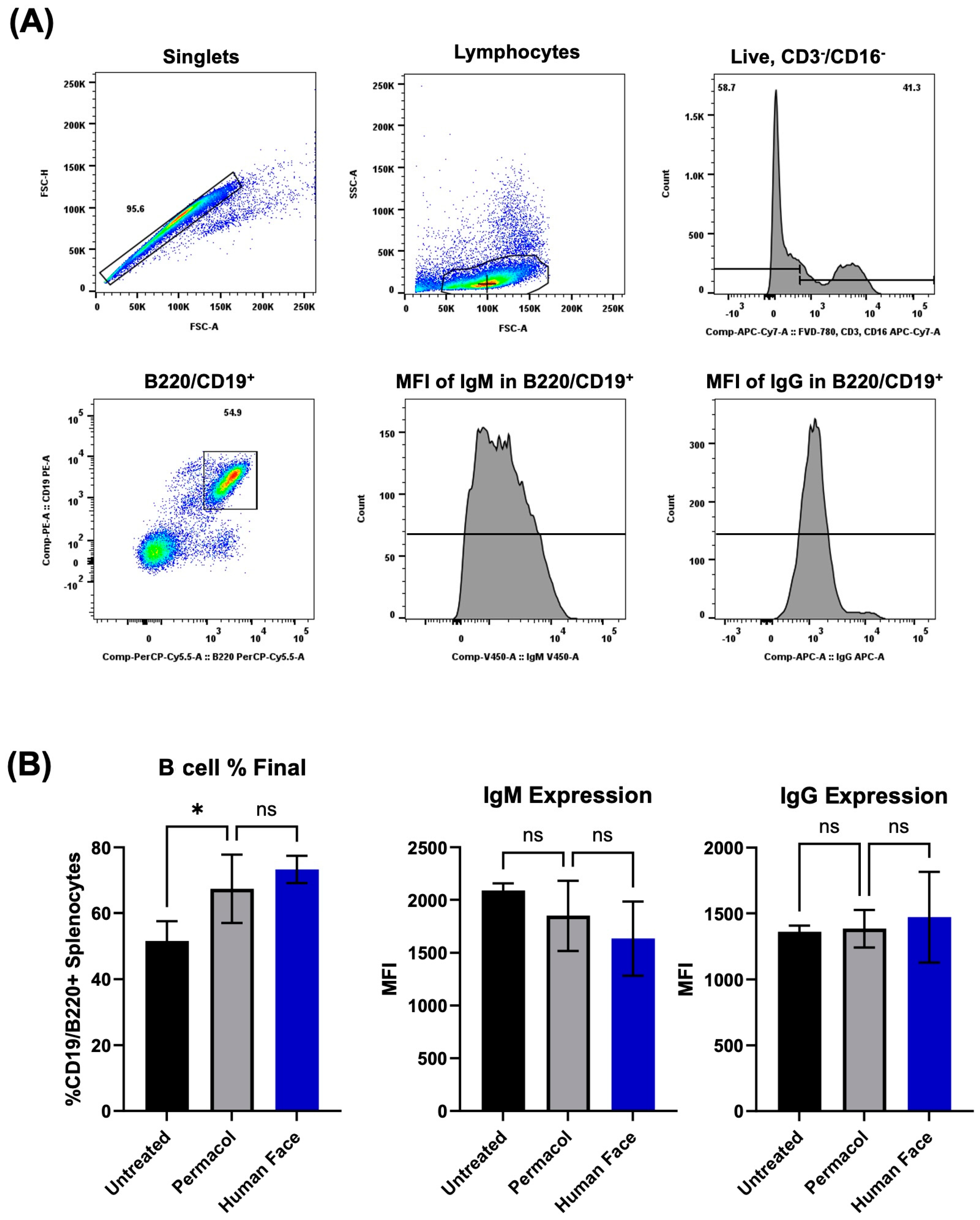
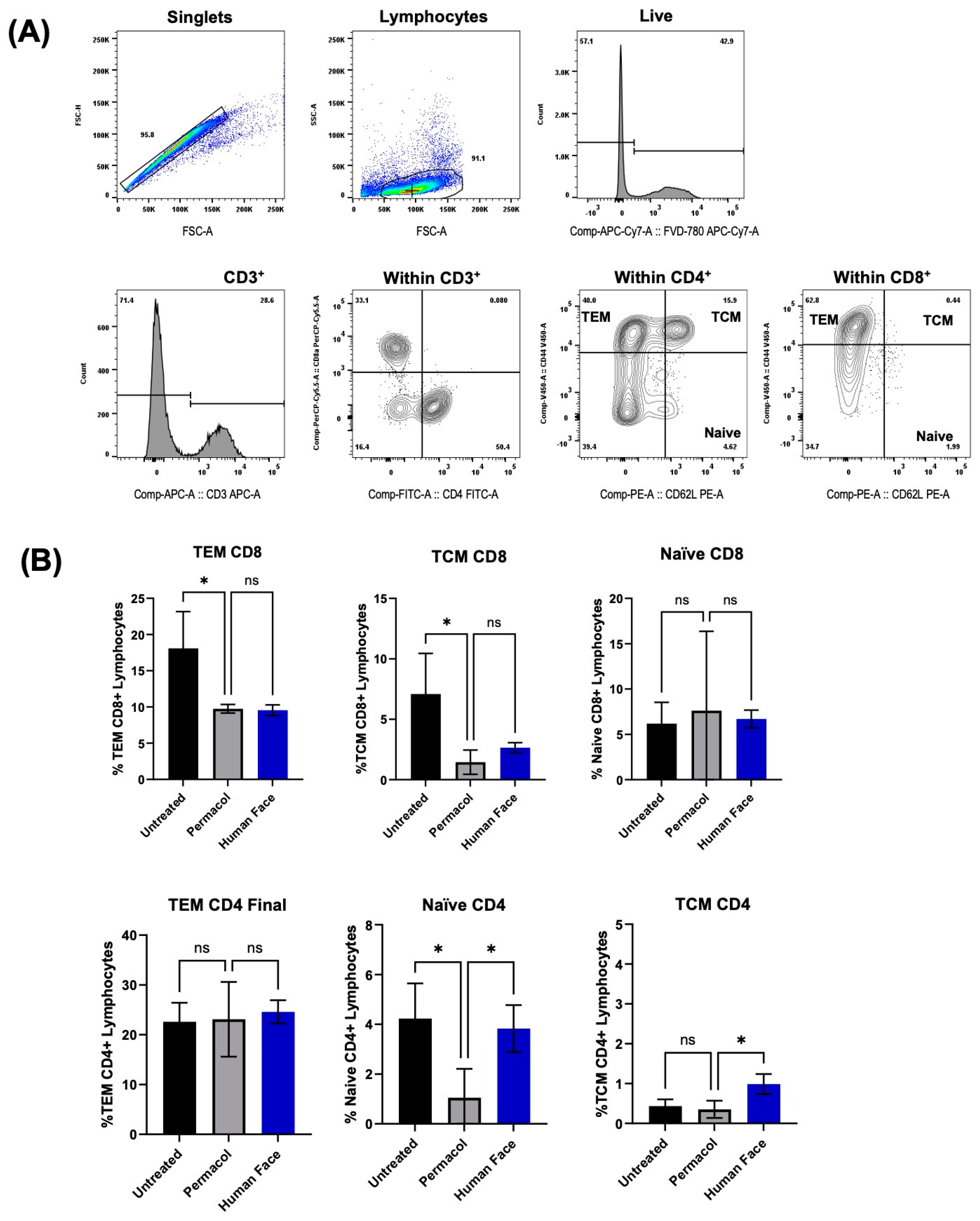
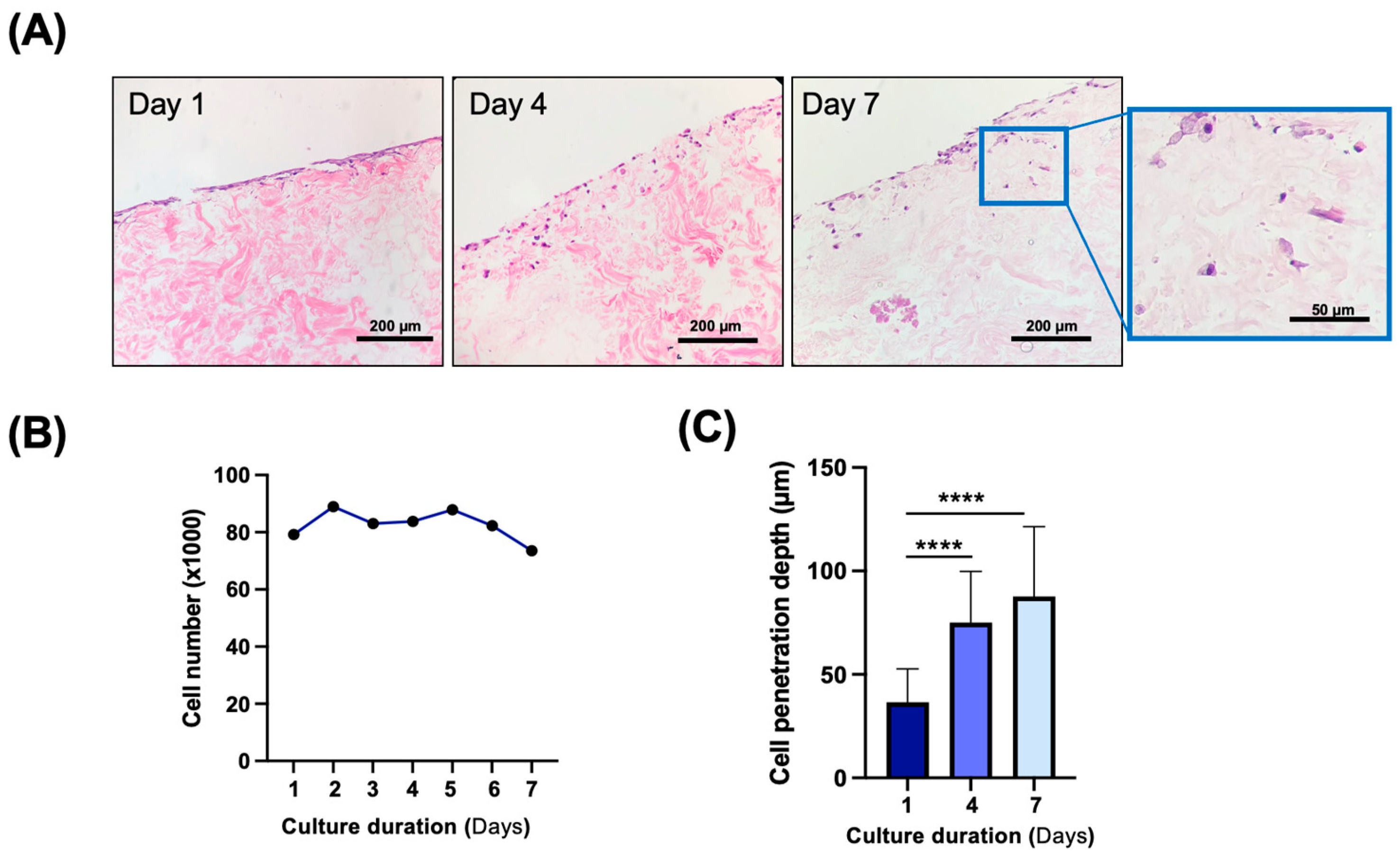
3.4. In Vitro and In Vivo Biocompatibility of Decellularized Facial Grafts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| bFGF | Basic fibroblast growth gactor |
| DAMPs | Damage-associated molecular patterns |
| dH2O | Deionized water |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| FVCA | Facial vascularized composite allotransplantation |
| GAG | Glycosaminoglycan |
| HBEGF | Heparin-binding EGF |
| H&E | Hematoxylin and Eosin |
| IGFBP1 | Insulin like growth factor mRNA binding protein 1 |
| IIAM | International Institute for the Advancement of Medicine |
| IRB | Institutional Review Board |
| MGH | Massachusetts General Hospital |
| MHC | Major histocompatibility complex |
| PBS | Phosphate-buffered saline |
| SDS | Sodium dodecyl sulfate |
| VCA | Vascularized composite allotransplantation |
Appendix A
| Donor Face Preparation | |
|---|---|
| 1 | Mark the donor face (coronal/pretragus/taking the cervical skin laterally) |
| 2 | Make incision starting from the neck to harvest the vessels at their origin |
| 3 | Localize the sternocleidomastoid muscle (SCM) |
| 4 | Expose the external jugular vein (EJV) |
| 5 | Free up the SCM laterally to expose the vessels |
| 6 | Dissect the external carotid and the internal jugular vein (TLF trunk) |
| 7 | Locate the division of the digastric muscle and hypoglossal |
| 8 | Section the posterior belly of the digastric muscle |
| 9 | Localize the hypoglossal nerve (source of nerve graft if needed) |
| 10 | Expose the facial nerve at its origin (retro auricular) to obtain the maximum length |
| 11 | +/− Transect the external auditory canal (more length for the facial nerve) and dissect the maxillary artery |
| 12 | Ligate the posterior maxillary artery |
| 13 | Make incision of the scalp (coronal incision) |
| 14 | Raise the scalp in a sus periosteal plane up to 1 cm before the appearance of the supra orbital nerve and then in a subperiosteal plane |
| 15 | Cut the supraorbital nerveas close to the bony foramen |
| 16 | Localize the Levator palpabrae superioris (LPS) muscle (with the eyeball in place++ to facilitate the location of the LPS), test it, and tag it using a stitch |
| 17 | Perform an enucleation and keep the maximum of conjunctiva (circular incision) |
| 18 | Cut off the superior eyelid horizontally |
| 19 | Cut off the inferior eyelid (with the maximum amount of conjunctiva): dissect through the inferior fornix to just inside the inferior orbital rim, which will allow dissection through the inferior orbital fat and lower lid retractors, preserving the palpebral conjunctiva |
| 20 | Localize and cut the inferior orbital nerve |
| 21 | Raise the parotid and expose the Mandible |
| 22 | Stay on the top of the masseter muscle (safe plane) |
| 23 | Identify the buccal fat pad at the anterior border of the masseter. The buccal fat pad is left in place |
| 24 | Finish up the dissection to the orbit and the zygoma |
| 25 | Perform osteotomy of the nasal bone and continue laterally to include part of the maxillary bone |
| 26 | Cut off the nasal septum longitudinally |
| 27 | Cut off the superior mucosa gingiva |
| 28 | Cut off the inferior mucosa gingiva |
| 29 | Cut off the mental nerve as close to the bony foramen |
| 30 | Connect the planes in the middle to connect to the oral cavity (on top of the sternohyoid and omohyoid superior muscle +/− anterior jugular vein) |
| 31 | Ligate the internal/external jugular vein/external carotid as far as possible |
| 32 | Detach entirely the face |
| 33 | Wash the free flap with heparin saline and check the integrity of the facial artery |
| 34 | Place the face in an organ preservation solution (static cold storage) |
References
- Devauchelle, B.; Badet, L.; Lengele, B.; Morelon, E.; Testelin, S.; Michallet, M.; D’Hauthuille, C.; Dubernard, J.M. First human face allograft: Early report. Lancet 2006, 368, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Dubernard, J.M.; Lengele, B.; Morelon, E.; Testelin, S.; Badet, L.; Moure, C.; Beziat, J.L.; Dakpe, S.; Kanitakis, J.; D’Hauthuille, C.; et al. Outcomes 18 months after the first human partial face transplantation. N. Engl. J. Med. 2007, 357, 2451–2460. [Google Scholar] [CrossRef]
- Morelon, E.; Petruzzo, P.; Kanitakis, J. Chronic rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.Y.; Lellouch, A.G.; Rosales, I.A.; Geoghegan, L.; Gama, A.R.; Colvin, R.B.; Lantieri, L.A.; Randolph, M.A.; Cetrulo, C.L., Jr. Graft vasculopathy of vascularized composite allografts in humans: A literature review and retrospective study. Transpl. Int. 2019, 32, 831–838. [Google Scholar] [CrossRef]
- Giatsidis, G.; Sinha, I.; Pomahac, B. Reflections on a Decade of Face Transplantation. Ann. Surg. 2017, 265, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Lantieri, L.; Cholley, B.; Lemogne, C.; Guillemain, R.; Ortonne, N.; Grimbert, P.; Thervet, E.; Lellouch, A.G. First human facial retransplantation: 30-month follow-up. Lancet 2020, 396, 1758–1765. [Google Scholar] [CrossRef]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef]
- Uygun, B.E.; Yarmush, M.L.; Uygun, K. Application of whole-organ tissue engineering in hepatology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 738–744. [Google Scholar] [CrossRef]
- Lupon, E.; Lellouch, A.G.; Acun, A.; Andrews, A.R.; Oganesyan, R.; Goutard, M.; Taveau, C.B.; Lantieri, L.A.; Cetrulo, C.L.; Uygun, B.E. Engineering Vascularized Composite Allografts Using Natural Scaffolds: A Systematic Review. Tissue Eng. Part B Rev. 2022, 28, 677–693. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef]
- Livesey, S.A.; Herndon, D.N.; Hollyoak, M.A.; Atkinson, Y.H.; Nag, A. Transplanted acellular allograft dermal matrix. Potential as a template for the reconstruction of viable dermis. Transplantation 1995, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Guo, Q.; Huang, J.; Zhang, L.; Yao, J.; Yang, F.; Wang, S.; Xu, W.; Wang, A.; et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials 2008, 29, 2378–2387. [Google Scholar] [CrossRef]
- Rajab, T.K.; O’Malley, T.J.; Tchantchaleishvili, V. Decellularized scaffolds for tissue engineering: Current status and future perspective. Artif. Organs 2020, 44, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Duisit, J.; Maistriaux, L.; Taddeo, A.; Orlando, G.; Joris, V.; Coche, E.; Behets, C.; Lerut, J.; Dessy, C.; Cossu, G.; et al. Bioengineering a Human Face Graft: The Matrix of Identity. Ann. Surg. 2017, 266, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Jank, B.J.; Goverman, J.; Guyette, J.P.; Charest, J.M.; Randolph, M.; Gaudette, G.R.; Gershlak, J.R.; Purschke, M.; Javorsky, E.; Nazarian, R.M.; et al. Creation of a Bioengineered Skin Flap Scaffold with a Perfusable Vascular Pedicle. Tissue Eng. Part A 2017, 23, 696–707. [Google Scholar] [CrossRef]
- Oganesyan, R.V.; Lellouch, A.G.; Acun, A.; Lupon, E.; Taveau, C.B.; Burlage, L.C.; Lantieri, L.A.; Randolph, M.A.; Cetrulo, C.L., Jr.; Uygun, B.E. Acellular Nipple Scaffold Development, Characterization, and Preliminary Biocompatibility Assessment in a Swine Model. Plast. Reconstr. Surg. 2023, 151, 618e–629e. [Google Scholar] [CrossRef]
- Lupon, E.; Acun, A.; Taveau, C.B.; Oganesyan, R.; Lancia, H.H.; Andrews, A.R.; Randolph, M.A.; Cetrulo, C.L., Jr.; Lellouch, A.G.; Uygun, B.E. Optimized Decellularization of a Porcine Fasciocutaneaous Flap. Bioengineering 2024, 11, 321. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Intravaia, J.T.; Sathe, V.M.; Kumbar, S.G.; Nukavarapu, S.P. Decellularized extracellular matrix biomaterials for regenerative therapies: Advances, challenges and clinical prospects. Bioact. Mater. 2024, 32, 98–123. [Google Scholar] [CrossRef]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized Scaffolds for Skin Repair and Regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Sarmin, A.M.; Connelly, J.T. Fabrication of Human Skin Equivalents Using Decellularized Extracellular Matrix. Curr. Protoc. 2022, 2, e393. [Google Scholar] [CrossRef]
- Rosadas, M.; Silva, I.V.; Costa, J.B.; Ribeiro, V.P.; Oliveira, A.L. Decellularized dermal matrices: Unleashing the potential in tissue engineering and regenerative medicine. Front. Mater. 2024, 10, 1285948. [Google Scholar] [CrossRef]
- Akita, S.; Akino, K.; Hirano, A. Basic Fibroblast Growth Factor in Scarless Wound Healing. Adv. Wound Care (New Rochelle) 2013, 2, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Mazumder, B.; Rajkonwar, J.; Pathak, M.P.; Patowary, P.; Chattopadhyay, P. bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci. Rep. 2021, 11, 3357. [Google Scholar] [CrossRef] [PubMed]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The epidermal growth factor receptor system in skin repair and inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef]
- Holcmann, M.; Sibilia, M. Mechanisms underlying skin disorders induced by EGFR inhibitors. Mol. Cell. Oncol. 2015, 2, e1004969. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The distribution and functions of immunoglobulin isotypes. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Zou, Y.-R.; Grimaldi, C.; Diamond, B. B Cells. In Kelley and Firestein’s Textbook of Rheumatology, 10th ed.; Firestein, G.S., Budd, R.C., Gabriel, S.E., McInnes, I.B., O’Dell, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Kasravi, M.; Ahmadi, A.; Babajani, A.; Mazloomnejad, R.; Hatamnejad, M.R.; Shariatzadeh, S.; Bahrami, S.; Niknejad, H. Immunogenicity of decellularized extracellular matrix scaffolds: A bottleneck in tissue engineering and regenerative medicine. Biomater. Res. 2023, 27, 10. [Google Scholar] [CrossRef]
- Massaro, M.S.; Palek, R.; Rosendorf, J.; Cervenkova, L.; Liska, V.; Moulisova, V. Decellularized xenogeneic scaffolds in transplantation and tissue engineering: Immunogenicity versus positive cell stimulation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112203. [Google Scholar] [CrossRef]
- Sano, H.; Orbay, H.; Terashi, H.; Hyakusoku, H.; Ogawa, R. Acellular adipose matrix as a natural scaffold for tissue engineering. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 99–106. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Tan, Y.; Landford, W.N.; Garza, M.; Suarez, A.; Zhou, Z.; Coon, D. Complete Human Penile Scaffold for Composite Tissue Engineering: Organ Decellularization and Characterization. Sci. Rep. 2019, 9, 16368. [Google Scholar] [CrossRef] [PubMed]
- Gerli, M.F.M.; Guyette, J.P.; Evangelista-Leite, D.; Ghoshhajra, B.B.; Ott, H.C. Perfusion decellularization of a human limb: A novel platform for composite tissue engineering and reconstructive surgery. PLoS ONE 2018, 13, e0191497. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupon, E.; Acun, A.; Andrews, A.R.; Oganesyan, R.; Lancia, H.H.; Lantieri, L.; Randolph, M.A.; Cetrulo, C.L., Jr.; Lellouch, A.G.; Uygun, B.E. A New Method for Preparation of Decellularized Human Scaffolds for Facial Reconstruction. Curr. Issues Mol. Biol. 2025, 47, 275. https://doi.org/10.3390/cimb47040275
Lupon E, Acun A, Andrews AR, Oganesyan R, Lancia HH, Lantieri L, Randolph MA, Cetrulo CL Jr., Lellouch AG, Uygun BE. A New Method for Preparation of Decellularized Human Scaffolds for Facial Reconstruction. Current Issues in Molecular Biology. 2025; 47(4):275. https://doi.org/10.3390/cimb47040275
Chicago/Turabian StyleLupon, Elise, Aylin Acun, Alec R. Andrews, Ruben Oganesyan, Hyshem H. Lancia, Laurent Lantieri, Mark A. Randolph, Curtis L. Cetrulo, Jr., Alexandre G. Lellouch, and Basak E. Uygun. 2025. "A New Method for Preparation of Decellularized Human Scaffolds for Facial Reconstruction" Current Issues in Molecular Biology 47, no. 4: 275. https://doi.org/10.3390/cimb47040275
APA StyleLupon, E., Acun, A., Andrews, A. R., Oganesyan, R., Lancia, H. H., Lantieri, L., Randolph, M. A., Cetrulo, C. L., Jr., Lellouch, A. G., & Uygun, B. E. (2025). A New Method for Preparation of Decellularized Human Scaffolds for Facial Reconstruction. Current Issues in Molecular Biology, 47(4), 275. https://doi.org/10.3390/cimb47040275






