Cytokine Therapy in Bladder Cancer: Mechanisms, Efficacy, and Future Prospects
Abstract
1. Introduction
2. Materials and Methods
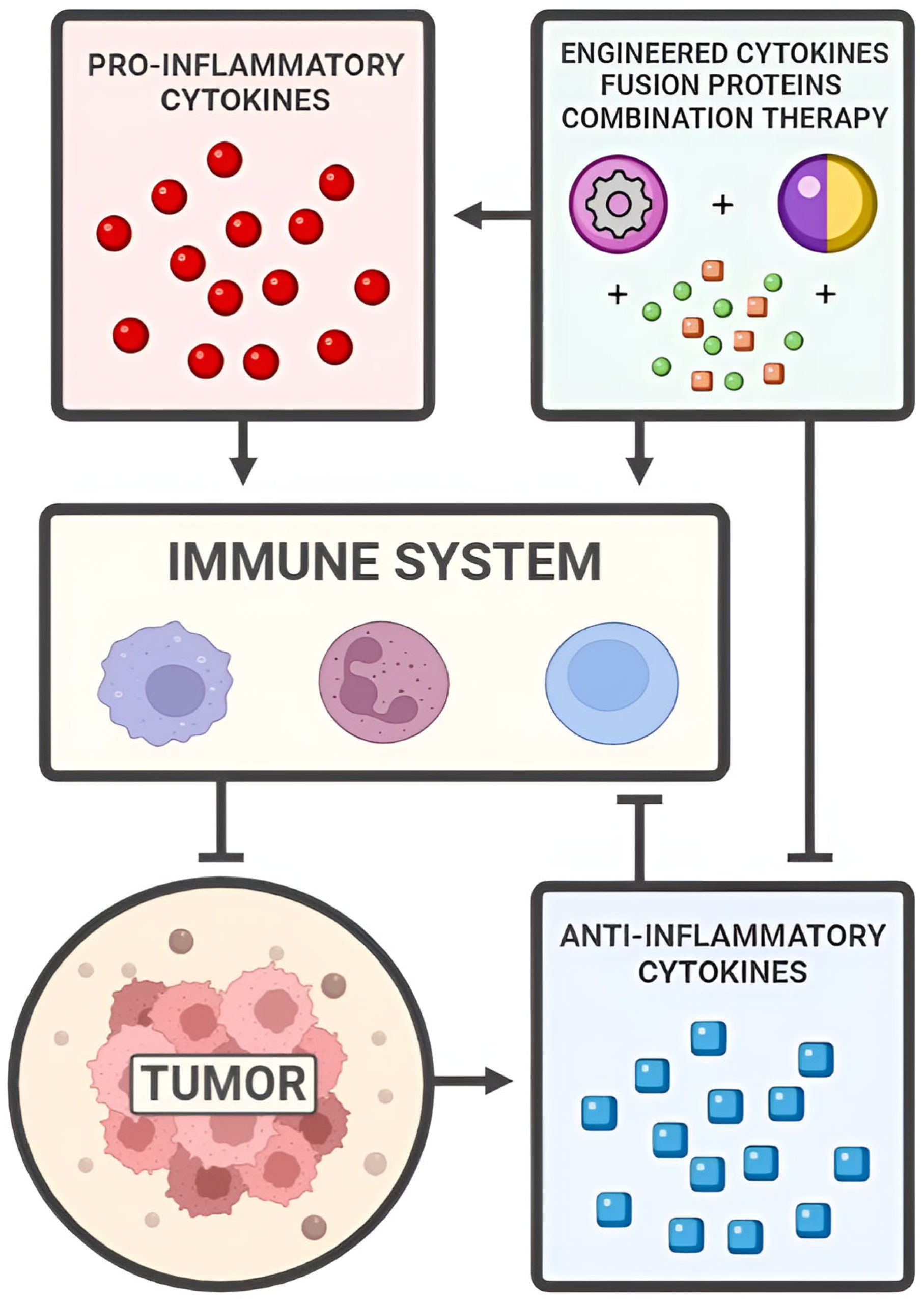
3. Pro-Inflammatory Cytokines
3.1. IL-2
3.2. IL-8
3.3. IL-12
4. Anti-Inflammatory Cytokines
4.1. TGF-β
4.2. IL-10
4.3. IL-4
5. Engineered Cytokines, Fusion Proteins, and Combination Therapies
5.1. EGF
5.2. CD40
5.3. IL-15
5.4. PD-1
5.5. IL-2 and IL-12
5.6. Gene Therapy
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| APC | Antigen-presenting cell |
| BCG | Bacillus Calmette–Guérin |
| CAR T | Chimeric antigen receptor T cell |
| CTLs | Cytotoxic T cells |
| CD40 | Cluster of differentiation 40 |
| CD40L | CD40 ligand |
| DV | Disitamab vedotin |
| EGF | Epidermal growth factor |
| EMT | Epithelial–mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| FAK | Focal adhesion kinase |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| HER2 | Human epidermal growth factor receptor 2 |
| ICB | Immune checkpoint blockade |
| IFN | Interferon |
| IL | Interleukin |
| IL-2 | Interleukin-2 |
| IL-2c | IL-2 complex |
| JAK | Janus kinase |
| MIBC | Muscle-invasive bladder cancer |
| MAPK | Mitogen-activated protein kinase |
| MMAE | Monomethyl auristatin E |
| NMIBC | Non-muscle-invasive bladder cancer |
| NK cells | Natural killer cells |
| ORR | Objective response rate |
| PAMP | Pathogen-associated molecular pattern |
| PD-1 | Programmed death receptor-1 |
| PD-L1 | Programmed death-ligand 1 |
| PI3K | Phosphoinositide 3-kinase |
| RAS | Rat Sarcoma |
| RBCEVs | Red blood cell-derived extracellular vesicles |
| rh-IL-15 | Recombinant human IL-15 |
| STAT | Signal transducer and activator of transcription |
| TAMs | Tumor-associated macrophages |
| T-DM1 | Trastuzumab emtansine |
| TGF-β | Transforming growth factor-β |
| Th1 | Type 1 T helper cells |
| Th2 | Type 2 T helper cells |
| TME | Tumor microenvironment |
| TNFs | Tumor necrosis factors |
| Tregs | Regulatory T cells |
| UC | Urothelial cancer |
References
- National Cancer Institute. Cancer of the Urinary Bladder–Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 26 February 2025).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA. Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Shobeiri, P.; Azadnajafabad, S.; Masinaei, M.; Rezaei, N.; Ghanbari, A.; Rezaei, N.; Rouhifard, M.; Shahin, S.; Rashidi, M.-M.; et al. A Global, Regional, and National Survey on Burden and Quality of Care Index (QCI) of Bladder Cancer: The Global Burden of Disease Study 1990–2019. PLoS ONE 2022, 17, e0275574. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Abern, M.; Agarwal, N.; Bangs, R.; Buyyounouski, M.K.; Chan, K.; Chang, S.S.; Chang, P.; Friedlander, T.; et al. NCCN Guidelines® Insights: Bladder Cancer, Version 3.2024. J. Natl. Compr. Cancer Netw. JNCCN 2024, 22, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, D.; Matsumoto, K.; Shiba, I.; Harano, T.; Okuda, S.; Mori, K.; Hirano, S.; Kitajima, K.; Ikeda, M.; Iwamura, M. Diagnostic Potential of Circulating Tumor Cells, Urinary MicroRNA, and Urinary Cell-Free DNA for Bladder Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 9148. [Google Scholar] [CrossRef] [PubMed]
- Wraith, D.C. The Future of Immunotherapy: A 20-Year Perspective. Front. Immunol. 2017, 8, 1668. [Google Scholar] [CrossRef]
- Naran, K.; Nundalall, T.; Chetty, S.; Barth, S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018, 9, 3158. [Google Scholar] [CrossRef]
- Definition of Immunotherapy–NCI Dictionary of Cancer Terms—NCI. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immunotherapy (accessed on 23 February 2025).
- Kazemi, T.; Younesi, V.; Jadidi-Niaragh, F.; Yousefi, M. Immunotherapeutic Approaches for Cancer Therapy: An Updated Review. Artif. Cells Nanomed. Biotechnol. 2016, 44, 769–779. [Google Scholar] [CrossRef]
- Brody, J.; Kohrt, H.; Marabelle, A.; Levy, R. Active and Passive Immunotherapy for Lymphoma: Proving Principles and Improving Results. J. Clin. Oncol. 2011, 29, 1864–1875. [Google Scholar] [CrossRef]
- Vaillant, J.A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Liao, W.; Lin, J.-X.; Leonard, W.J. IL-2 Family Cytokines: New Insights into the Complex Roles of IL-2 as a Broad Regulator of T Helper Cell Differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljkovic, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Dinarello, C.A. Proinflammatory Cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Wang, K.S.; Frank, D.A.; Ritz, J. Interleukin-2 Enhances the Response of Natural Killer Cells to Interleukin-12 through up-Regulation of the Interleukin-12 Receptor and STAT4. Blood 2000, 95, 3183–3190. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Lee, H.-J.; Chang, J.-E. Inflammatory Cytokine: An Attractive Target for Cancer Treatment. Biomedicines 2022, 10, 2116. [Google Scholar] [CrossRef]
- Abbas, A.K. The Surprising Story of IL-2. Am. J. Pathol. 2020, 190, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Brand, D.; Zheng, S.G. Targeting IL-2: An Unexpected Effect in Treating Immunological Diseases. Signal Transduct. Target. Ther. 2018, 3, 2. [Google Scholar] [CrossRef]
- Green, J.; Fuge, O.; Allchorne, P.; Vasdev, N. Immunotherapy for Bladder Cancer. Res. Rep. Urol. 2015, 4, 65. [Google Scholar] [CrossRef]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the Treatment of Bladder Cancer-Current Understanding and the Prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, X.; O’Donnell, M.A. Role of Th1 and Th2 Cytokines in BCG-Induced IFN-γ Production: Cytokine Promotion and Simulation of BCG Effect. Cytokine 2003, 21, 17–26. [Google Scholar] [CrossRef]
- Reyes, R.M.; Deng, Y.; Zhang, D.; Ji, N.; Mukherjee, N.; Wheeler, K.; Gupta, H.B.; Padron, A.S.; Kancharla, A.; Zhang, C.; et al. CD122-Directed Interleukin-2 Treatment Mechanisms in Bladder Cancer Differ from αPD-L1 and Include Tissue-Selective Γδ T Cell Activation. J. Immunother. Cancer 2021, 9, e002051. [Google Scholar] [CrossRef]
- Tang, A.; Harding, F. The Challenges and Molecular Approaches Surrounding Interleukin-2-Based Therapeutics in Cancer. Cytokine X 2019, 1, 100001. [Google Scholar] [CrossRef]
- Sharma, M.; Khong, H.; Fa’ak, F.; Bentebibel, S.-E.; Janssen, L.M.E.; Chesson, B.C.; Creasy, C.A.; Forget, M.-A.; Kahn, L.M.S.; Pazdrak, B.; et al. Bempegaldesleukin Selectively Depletes Intratumoral Tregs and Potentiates T Cell-Mediated Cancer Therapy. Nat. Commun. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Siefker-Radtke, A.O.; Cho, D.C.; Diab, A.; Sznol, M.; Bilen, M.A.; Balar, A.V.; Grignani, G.; Puente, E.; Tang, L.; Chien, D.; et al. Bempegaldesleukin plus Nivolumab in First-Line Metastatic Urothelial Carcinoma: Results from PIVOT-02. Eur. Urol. 2022, 82, 365–373. [Google Scholar] [CrossRef]
- Bosschieter, J.; Nieuwenhuijzen, J.A.; Hentschel, A.; Vis, A.N.; Lissenberg-Witte, B.I.; den Otter, W.; van Moorselaar, R.J.A. Value of a Marker Lesion in Non-Muscle-Invasive Bladder Cancer Patients Treated with Interleukin-2 Instillations: A Randomized Controlled Multicentre Trial. Urol. Int. 2019, 102, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An Evolving Chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Reis, S.T.; Leite, K.R.M.; Piovesan, L.F.; Pontes-Junior, J.; Viana, N.I.; Abe, D.K.; Crippa, A.; Moura, C.M.; Adonias, S.P.; Srougi, M.; et al. Increased Expression of MMP-9 and IL-8 Are Correlated with Poor Prognosis of Bladder Cancer. BMC Urol. 2012, 12, 18. [Google Scholar] [CrossRef]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of Chemokines in Tumor Growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cong, X.; Gao, H.; Lan, X.; Li, Z.; Wang, W.; Song, S.; Wang, Y.; Li, C.; Zhang, H.; et al. Tumor-Associated Neutrophils Induce EMT by IL-17a to Promote Migration and Invasion in Gastric Cancer Cells. J. Exp. Clin. Cancer Res. 2019, 38, 6. [Google Scholar] [CrossRef]
- Waugh, D.J.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, H.; Chang, H.; Feng, Z.; Zhang, C.; Yang, X. The Interaction of Interleukin-8 and PTEN Inactivation Promotes the Malignant Progression of Head and Neck Squamous Cell Carcinoma via the STAT3 Pathway. Cell Death Dis. 2020, 11, 405. [Google Scholar] [CrossRef]
- Jing, W.; Wang, G.; Cui, Z.; Li, X.; Zeng, S.; Jiang, X.; Li, W.; Han, B.; Xing, N.; Zhao, Y.; et al. Tumor–Neutrophil Cross Talk Orchestrates the Tumor Microenvironment to Determine the Bladder Cancer Progression. Proc. Natl. Acad. Sci. USA 2024, 121, e2312855121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Jin, P.; Liu, J.; Li, S.; Liu, W.; Xi, S. HER2 Overexpression Triggers the IL-8 to Promote Arsenic-Induced EMT and Stem Cell-like Phenotypes in Human Bladder Epithelial Cells. Ecotoxicol. Environ. Saf. 2021, 208, 111693. [Google Scholar] [CrossRef]
- VandenBussche, C.J.; Heaney, C.D.; Kates, M.; Hooks, J.J.; Baloga, K.; Sokoll, L.; Rosenthal, D.; Detrick, B. Urinary IL-6 and IL-8 as Predictive Markers in Bladder Urothelial Carcinoma: A Pilot Study. Cancer Cytopathol. 2024, 132, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Smith, S.G.; Koppolu, B.P.; Ravindranathan, S.; Kurtz, S.L.; Yang, L.; Katz, M.D.; Zaharoff, D.A. Intravesical Chitosan/Interleukin-12 Immunotherapy Induces Tumor-Specific Systemic Immunity against Murine Bladder Cancer. Cancer Immunol. Immunother. 2015, 64, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Zaharoff, D.A.; Hoffman, B.S.; Hooper, H.B.; Benjamin, C.J., Jr.; Khurana, K.K.; Hance, K.W.; Rogers, C.J.; Pinto, P.A.; Schlom, J.; Greiner, J.W. Intravesical Immunotherapy of Superficial Bladder Cancer with Chitosan/Interleukin-12. Cancer Res. 2009, 69, 6192–6199. [Google Scholar] [CrossRef]
- Marchenko, I.; Trushina, D. Local Drug Delivery in Bladder Cancer: Advances of Nano/Micro/Macro-Scale Drug Delivery Systems. Pharmaceutics 2023, 15, 2724. [Google Scholar] [CrossRef]
- Veranič, P.; Erman, A.; Kerec-Kos, M.; Bogataj, M.; Mrhar, A.; Jezernik, K. Rapid Differentiation of Superficial Urothelial Cells after Chitosan-Induced Desquamation. Histochem. Cell Biol. 2009, 131, 129–139. [Google Scholar] [CrossRef]
- Greiner, J.W.; Morillon, Y.M.; Schlom, J. NHS-IL12, a Tumor-Targeting Immunocytokine. Immunotargets Ther. 2021, 10, 155–169. [Google Scholar] [CrossRef]
- Wu, Z.; Li, W.; Tan, M.; How, F.Y.X.; Sadhasivan, H.; Mahendran, R.; Wu, Q.; Chiong, E.; Le, M.T.N. IL-12 Minicircle Delivery via Extracellular Vesicles as Immunotherapy for Bladder Cancer. Cell Prolif. 2025, 58, e13739. [Google Scholar] [CrossRef]
- Strauss, J.; Deville, J.-L.; Sznol, M.; Ravaud, A.; Maruzzo, M.; Pachynski, R.K.; Gourdin, T.S.; Maio, M.; Dirix, L.; Schlom, J.; et al. First-in-Human Phase Ib Trial of M9241 (NHS-IL12) plus Avelumab in Patients with Advanced Solid Tumors, Including Dose Expansion in Patients with Advanced Urothelial Carcinoma. J. Immunother. Cancer 2023, 11, e005813. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.M.; McCulloch, T.R.; Alim, L.; Omer, N.; Mehdi, A.M.; Tuong, Z.K.; Bonfim-Melo, A.; Chung, E.; Nicol, A.; Simpson, F.; et al. TGF-β Signalling Limits Effector Function Capacity of NK Cell Anti-Tumour Immunity in Human Bladder Cancer. eBioMedicine 2024, 104, 105176. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, T.; Yan, L.; Jin, T.; Zhang, R.; Chen, B.; Feng, J.; Duan, T.; Xiang, Y.; Zhang, M.; et al. The Inflammatory Microenvironment and the Urinary Microbiome in the Initiation and Progression of Bladder Cancer. Genes Dis. 2020, 8, 781–797. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting Cytokine and Chemokine Signaling Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Yeung, T.-L.; Huang, H.; Wegener, A.A.; Saha, S.; Toister-Achituv, M.; Jenkins, M.H.; Chiu, L.-Y.; Lazorchak, A.; Tarcic, O.; et al. Colocalized Targeting of TGF-β and PD-L1 by Bintrafusp Alfa Elicits Distinct Antitumor Responses. J. Immunother. Cancer 2022, 10, e004122. [Google Scholar] [CrossRef]
- Wu, F.; Weigel, K.J.; Zhou, H.; Wang, X.-J. Paradoxical Roles of TGF-β Signaling in Suppressing and Promoting Squamous Cell Carcinoma. Acta Biochim. Biophys. Sin. 2018, 50, 98–105. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β Signaling in the Tumor Metabolic Microenvironment and Targeted Therapies. J. Hematol. Oncol.J Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, S.; Vahabirad, M.; Seghatoleslam, A.; Sadeghi, E.; Kiani, R.; Ghasemi, H. Prognostic Value of TGF-β Expression in Bladder Cancer: A Systematic Review and Meta-Analysis. Urol. Res. Pract. 2024, 50, 148–153. [Google Scholar] [CrossRef]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical Development of Galunisertib (LY2157299 Monohydrate), a Small Molecule Inhibitor of Transforming Growth Factor-Beta Signaling Pathway. Drug Des. Devel. Ther. 2015, 9, 4479–4499. [Google Scholar] [CrossRef]
- Alsaffar, R.M.; Ali, S.; Rashid, S.; Rashid, S.M.; Majid, S.; Rehman, M.U. Immunomodulation: An Immune Regulatory Mechanism in Carcinoma Therapeutics. Int. Immunopharmacol. 2021, 99, 107984. [Google Scholar] [CrossRef]
- Melisi, D.; Oh, D.-Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and Activity of the TGFβ Receptor I Kinase Inhibitor Galunisertib plus the Anti-PD-L1 Antibody Durvalumab in Metastatic Pancreatic Cancer. J. Immunother. Cancer 2021, 9, e002068. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Lind, H.; Gameiro, S.R.; Jochems, C.; Donahue, R.N.; Strauss, J.; Gulley, J.L.; Palena, C.; Schlom, J. Dual Targeting of TGF-β and PD-L1 via a Bifunctional Anti-PD-L1/TGF-βRII Agent: Status of Preclinical and Clinical Advances. J. Immunother. Cancer 2020, 8, e000433. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.R.; Strauss, J.; Gulley, J.L.; Schlom, J. Preclinical and Clinical Studies of Bintrafusp Alfa, a Novel Bifunctional Anti-PD-L1/TGFβRII Agent: Current Status. Exp. Biol. Med. 2022, 247, 1124–1134. [Google Scholar] [CrossRef]
- Tschernia, N.P.; Gulley, J.L. Tumor in the Crossfire: Inhibiting TGF-β to Enhance Cancer Immunotherapy. Biodrugs 2022, 36, 153–180. [Google Scholar] [CrossRef]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2019, 11, a028548. [Google Scholar] [CrossRef]
- Dennis, K.L.; Blatner, N.R.; Gounari, F.; Khazaie, K. Current Status of IL-10 and Regulatory T-Cells in Cancer. Curr. Opin. Oncol. 2013, 25, 637–645. [Google Scholar] [CrossRef]
- Smith, L.K.; Boukhaled, G.M.; Condotta, S.A.; Mazouz, S.; Guthmiller, J.J.; Vijay, R.; Butler, N.S.; Bruneau, J.; Shoukry, N.H.; Krawczyk, C.M.; et al. Interleukin-10 Directly Inhibits CD8+ T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity 2018, 48, 299–312.e5. [Google Scholar] [CrossRef] [PubMed]
- Mirlekar, B. Tumor Promoting Roles of IL-10, TGF-β, IL-4, and IL-35: Its Implications in Cancer Immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef] [PubMed]
- Salkeni, M.A.; Naing, A. Interleukin-10 in Cancer Immunotherapy from Bench to Bedside. Trends Cancer 2023, 9, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Infante, J.R.; Papadopoulos, K.P.; Chan, I.H.; Shen, C.; Ratti, N.P.; Rojo, B.; Autio, K.A.; Wong, D.J.; Patel, M.R.; et al. PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8+ T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell 2018, 34, 775–791.e3. [Google Scholar] [CrossRef]
- Ni, G.; Zhang, L.; Yang, X.; Li, H.; Ma, B.; Walton, S.; Wu, X.; Yuan, J.; Wang, T.; Liu, X. Targeting Interleukin-10 Signalling for Cancer Immunotherapy, a Promising and Complicated Task. Hum. Vaccines Immunother. 2020, 16, 2328. [Google Scholar] [CrossRef]
- Naing, A.; Wong, D.J.; Infante, J.R.; Korn, W.M.; Aljumaily, R.; Papadopoulos, K.P.; Autio, K.A.; Pant, S.; Bauer, T.M.; Drakaki, A.; et al. Pegilodecakin Combined with Pembrolizumab or Nivolumab for Patients with Advanced Solid Tumours (IVY): A Multicentre, Multicohort, Open-Label, Phase 1b Trial. Lancet Oncol. 2019, 20, 1544–1555. [Google Scholar] [CrossRef]
- Joshi, B.; Leland, P.; Lababidi, S.; Varrichio, F.; Puri, R. Interleukin-4 Receptor Alpha Overexpression in Human Bladder Cancer Correlates with the Pathological Grade and Stage of the Disease. Cancer Med. 2014, 3, 1615–1628. [Google Scholar] [CrossRef]
- Chapoval, S.; Dasgupta, P.; Dorsey, N.J.; Keegan, A.D. Regulation of the T Helper Cell Type 2 (Th2)/T Regulatory Cell (Treg) Balance by IL-4 and STAT6. J. Leukoc. Biol. 2010, 87, 1011–1018. [Google Scholar] [CrossRef]
- Kwaśniak, K.; Czarnik-Kwaśniak, J.; Maziarz, A.; Aebisher, D.; Zielińska, K.; Karczmarek-Borowska, B.; Tabarkiewicz, J. Scientific Reports Concerning the Impact of Interleukin 4, Interleukin 10 and Transforming Growth Factor β on Cancer Cells. Cent.-Eur. J. Immunol. 2019, 44, 190–200. [Google Scholar] [CrossRef]
- Guo, S.; Wang, L.; Bu, D.; Liu, F. Tumors in the Setting of Dupilumab Use: A Review of the Literature. World Allergy Organ. J. 2024, 18, 101006. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.E.; Myers, K.V.; Krueger, T.E.G.; Brennen, W.N.; Amend, S.R.; Pienta, K.J. Targeting Interleukin 4 Receptor Alpha on Tumor-Associated Macrophages Reduces the pro-Tumor Macrophage Phenotype. Neoplasia 2022, 32, 100830. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, R.M. Cytokine Combinations: Therapeutic Use in Patients with Advanced Renal Cell Carcinoma. Semin. Oncol. 2000, 27, 204–212. [Google Scholar]
- Kang, S.; Mansurov, A.; Kurtanich, T.; Chun, H.R.; Slezak, A.J.; Volpatti, L.R.; Chang, K.; Wang, T.; Alpar, A.T.; Refvik, K.C.; et al. Engineered IL-7 Synergizes with IL-12 Immunotherapy to Prevent T Cell Exhaustion and Promote Memory without Exacerbating Toxicity. Sci. Adv. 2023, 9, eadh9879. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Bernards, R. Rational Combinations of Targeted Cancer Therapies: Background, Advances and Challenges. Nat. Rev. Drug Discov. 2023, 22, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, Y.; Bi, J.; Huang, Y.; Cheng, Y.; Li, Y.; Wu, Y.; Cao, G.; Tian, Z. The Use of Supercytokines, Immunocytokines, Engager Cytokines, and Other Synthetic Cytokines in Immunotherapy. Cell. Mol. Immunol. 2022, 19, 192–209. [Google Scholar] [CrossRef]
- Fabilane, C.S.; Stephenson, A.C.; Leonard, E.K.; VanDyke, D.; Spangler, J.B. Cytokine/Antibody Fusion Protein Design and Evaluation. Curr. Protoc. 2024, 4, e1061. [Google Scholar] [CrossRef]
- Yu, K.; Liu, C.; Kim, B.-G.; Lee, D.-Y. Synthetic Fusion Protein Design and Applications. Biotechnol. Adv. 2015, 33, 155–164. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Fu, Y.-X. Facts and Hopes on Chimeric Cytokine Agents for Cancer Immunotherapy. Clin. Cancer Res. 2024, 30, 2025–2038. [Google Scholar] [CrossRef]
- Young, P.A.; Morrison, S.L.; Timmerman, J.M. Antibody-Cytokine Fusion Proteins for Treatment of Cancer: Engineering Cytokines for Improved Efficacy and Safety. Semin. Oncol. 2014, 41, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2–Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.L.; Shin, S.J.; Bae, W.K.; Lee, H.J.; Byun, J.H.; Choi, Y.J.; Youk, J.; Ock, C.Y.; Kim, S.; et al. Phase II Study of a Trastuzumab Biosimilar in Combination with Paclitaxel for HER2-Positive Recurrent or Metastatic Urothelial Carcinoma: KCSG GU18-18. ESMO Open 2023, 8, 101588. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.G.E.; Rüschoff, J.; Lolkema, M.; Tabernero, J.; Gianni, L.; Voest, E.; de Groot, D.J.A.; Castellano, D.; Erb, G.; Naab, J.; et al. Phase II Study (KAMELEON) of Single-agent T-DM1 in Patients with HER2-positive Advanced Urothelial Bladder Cancer or Pancreatic Cancer/Cholangiocarcinoma. Cancer Med. 2023, 12, 12071–12083. [Google Scholar] [CrossRef]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular Basis and Therapeutic Implications of CD40/CD40L Immune Checkpoint. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef]
- Garris, C.S.; Wong, J.L.; Ravetch, J.V.; Knorr, D.A. Intravesical Dendritic Cell Targeting with Fc-Enhanced CD40 Agonistic Antibodies Induces Durable Bladder Cancer Immunity. Sci. Transl. Med. 2021, 13, eabd1346. [Google Scholar] [CrossRef]
- Wong, J.L.; Smith, P.; Angulo-Lozano, J.; Ranti, D.; Bochner, B.H.; Sfakianos, J.P.; Horowitz, A.; Ravetch, J.V.; Knorr, D.A. IL-15 Synergizes with CD40 Agonist Antibodies to Induce Durable Immunity against Bladder Cancer. Proc. Natl. Acad. Sci. USA 2023, 120, e2306782120. [Google Scholar] [CrossRef]
- Tran, B.; Voskoboynik, M.; Bendell, J.; Gutierrez, M.; Lemech, C.; Day, D.; Frentzas, S.; Garrido-Laguna, I.; Standifer, N.; Wang, F.; et al. A Phase 1 Study of the CD40 Agonist MEDI5083 in Combination with Durvalumab in Patients with Advanced Solid Tumors. Immunotherapy 2024, 16, 759–774. [Google Scholar] [CrossRef]
- Lui, G.; Minnar, C.M.; Soon-Shiong, P.; Schlom, J.; Gameiro, S.R. Exploiting an Interleukin-15 Heterodimeric Agonist (N803) for Effective Immunotherapy of Solid Malignancies. Cells 2023, 12, 1611. [Google Scholar] [CrossRef]
- Rosser, C.J.; Tikhonenkov, S.; Nix, J.W.; Chan, O.T.M.; Ianculescu, I.; Reddy, S.; Soon-Shiong, P. Safety, Tolerability, and Long-Term Clinical Outcomes of an IL-15 Analogue (N-803) Admixed with Bacillus Calmette-Guérin (BCG) for the Treatment of Bladder Cancer. Oncoimmunology 2021, 10, 1912885. [Google Scholar] [CrossRef]
- Chamie, K.; Chang, S.S.; Kramolowsky, E.; Gonzalgo, M.L.; Agarwal, P.K.; Bassett, J.C.; Bjurlin, M.; Cher, M.L.; Clark, W.; Cowan, B.E.; et al. IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. NEJM Evid. 2022, 2, EVIDoa2200167. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727. [Google Scholar] [PubMed]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging Connectivity of Programmed Cell Death Pathways and Its Physiological Implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory Mechanisms of PD-1/PD-L1 in Cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, Y.; Li, S. Programmed Death Receptor (PD-)1/PD-Ligand (L)1 in Urological Cancers: The “All-around Warrior” in Immunotherapy. Mol. Cancer 2024, 23, 183. [Google Scholar] [CrossRef]
- Constantinidou, A.; Alifieris, C.; Trafalis, D.T. Targeting Programmed Cell Death -1 (PD-1) and Ligand (PD-L1): A New Era in Cancer Active Immunotherapy. Pharmacol. Ther. 2019, 194, 84–106. [Google Scholar] [CrossRef]
- Lee, D.; Cho, M.; Kim, E.; Seo, Y.; Cha, J.-H. PD-L1: From Cancer Immunotherapy to Therapeutic Implications in Multiple Disorders. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 4235–4255. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, A.; Sun, Z.; Liang, Y.; Ye, J.; Qiao, J.; Li, B.; Fu, Y.-X. Selective Delivery of Low-Affinity IL-2 to PD-1+ T Cells Rejuvenates Antitumor Immunity with Reduced Toxicity. J. Clin. Investig. 2022, 132, e153604. [Google Scholar] [CrossRef]
- Holcomb, E.A.; Zou, W. A Forced Marriage of IL-2 and PD-1 Antibody Nurtures Tumor-Infiltrating T Cells. J. Clin. Investig. 2022, 132, e156628. [Google Scholar] [CrossRef]
- Rahimi Kalateh Shah Mohammad, G.; Ghahremanloo, A.; Soltani, A.; Fathi, E.; Hashemy, S.I. Cytokines as Potential Combination Agents with PD-1/PD-L1 Blockade for Cancer Treatment. J. Cell. Physiol. 2020, 235, 5449–5460. [Google Scholar] [CrossRef] [PubMed]
- Overwijk, W.W.; Tagliaferri, M.A.; Zalevsky, J. Engineering IL-2 to Give New Life to T Cell Immunotherapy. Annu. Rev. Med. 2021, 72, 281–311. [Google Scholar] [CrossRef]
- Ullrich, K.A.-M.; Schulze, L.L.; Paap, E.-M.; Müller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An Update on Functional Activities and Implications for Disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [CrossRef]
- Horton, B.L.; D’Souza, A.D.; Zagorulya, M.; McCreery, C.V.; Abhiraman, G.C.; Picton, L.; Sheen, A.; Agarwal, Y.; Momin, N.; Wittrup, K.D.; et al. Overcoming Lung Cancer Immunotherapy Resistance by Combining Nontoxic Variants of IL-12 and IL-2. JCI Insight 2023, 8, e172728. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.K.; Rakhra, K.; Meetze, K.A.; Li, B.; Momin, N.; Chang, J.Y.H.; Wittrup, K.D.; Baeuerle, P.A.; Michaelson, J.S. CLN-617 Retains IL2 and IL12 in Injected Tumors to Drive Robust and Systemic Immune-Mediated Antitumor Activity. Cancer Immunol. Res. 2024, 12, 1022–1038. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.A.; Luo, Y.I.; Hunter, S.E.; Chen, X.; Hayes, L.L.; Clinton, S.K. The Essential Role of Interferon-γ During Interleukin-12 Therapy for Murine Transitional Cell Carcinoma of the Bladder. J. Urol. 2004, 171, 1336–1342. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Swartout, B.G.; Hörig, H.; Lubensky, I. Combination Interleukin-2 and Interleukin-12 Induces Severe Gastrointestinal Toxicity and Epithelial Cell Apoptosis in Mice. Cytokine 2002, 17, 43–52. [Google Scholar] [CrossRef]
- Belete, T.M. The Current Status of Gene Therapy for the Treatment of Cancer. Biol. Targets Ther. 2021, 15, 67–77. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef]
- Malmström, P.-U.; Loskog, A.S.I.; Lindqvist, C.A.; Mangsbo, S.M.; Fransson, M.; Wanders, A.; Gårdmark, T.; Tötterman, T.H. AdCD40L Immunogene Therapy for Bladder Carcinoma—The First Phase I/IIa Trial. Clin. Cancer Res. 2010, 16, 3279–3287. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA Drug Delivery Using Red Blood Cell Extracellular Vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
| Cytokine Therapy | Molecule | Cytokine/Physiological Mechanism | Treatment Results | Treatment Risks | References |
|---|---|---|---|---|---|
| Pro-Inflammatory | IL-2 | Promotes T cell proliferation and differentiation | Combined with BCG, enhances immune activation; IL-2 complexes reduce tumor size; BEMPEG conjunction therapy reduces tumor size | High doses: toxic; low doses: promotes immunosuppression | [18,19,20,21,22,23,24,25,26,27] |
| IL-8 | Activates neutrophils | Genistein suppressed IL-8; elevated IL-8 levels may be a biomarker | IL-8 promotes tumor angiogenesis and metastasis | [28,29,30,31,32,33,34,35,36] | |
| IL-12 | Promotes IFN-γ production, increases NK cell activity, promotes Th1 differentiation, stimulates inflammation | CS/IL-12 resulted in 88% long-term survival; RBCEV delivery reduced tumor growth; M9241 and avelumab had little effect | Dose-dependent toxicity | [37,38,39,40,41,42,43,44] | |
| Anti-Inflammatory | TGF-β | Regulates cell proliferation, suppresses immune cells, promotes angiogenesis | Galunisertib slowed tumor growth; Bintrafusp Alfa targeted cancer cells | Aids in cancer spread by suppressing immune cells; Galunisertib: nausea, fatigue, hematological issues; Bintrafusp Alfa: itching, skin rashes, decreased appetite | [51,52,53,54,55,56,57,58,59,60,61,62] |
| IL-10 | Blocks inflammatory cytokine expression, activates signaling pathways | Anti-tumor effects in small amounts; Pegilodecakin mimics natural IL-10 and increases T cell proliferation | Leads to tumor resistance and suppression of cytotoxic T and NK cell production, promoting immune evasion; Pegilodecakin: red blood cell hemophagocytosis, fever, fatigue | [63,64,65,66,67,68,69,70] | |
| IL-4 | Reduces inflammation, suppresses cytotoxic T cells | Dupilumab reduces the pro-tumor phenotype of TAMs by blocking IL-4 signaling | Promotes metastasis, immune evasion, and tumor growth | [71,72,73,74] | |
| Engineered Cytokine/Fusion Protein/Combination Therapy | EGF | Activates intracellular signaling pathways, promoting cell growth | DV achieved 50.5% ORR; trastuzumab-pkrb and paclitaxel achieved 48.1% ORR; T-DMI achieved 38.5% ORR | Peripheral neuropathy, neutropenia, and leukopenia | [85,86,87,88] |
| CD40 | Initiates innate and adaptive immune responses | 2141-V11 supercytokine showed greater anti-tumor capabilities through enhanced CD8+ T cell response; 2141-V11 and IL-15 further enhanced tumor reduction and T cell activation | Systemic toxicity such as thrombocytopenia and transaminitis | [89,90,91,92] | |
| IL-15 | Acts as an anti-apoptotic factor for T cells and a stimulator of memory T cells | N803 prolonged the stimulation of CTLs and NK cells and had significant anti-tumor activity; N803 and BCG achieved 100% survival at 24 months | No evidence of systemic toxicity | [4,93,94,95] | |
| PD-1 | Prevents excessive immune activation by inducing T cell exhaustion | Combination with IL-2 demonstrated immunosuppression and improved treatment outcomes | Long-term efficacy is limited | [96,97,98,99,100,101,102,103,104] | |
| IL-2 and IL-12 | Modulate immune responses and promote inflammation | Increased IFN-γ production, T cell cytotoxicity, and tumor cell elimination | Severe gastrointestinal toxicity and epithelial cell apoptosis | [105,106,107,108,109,110] | |
| Gene Therapy | Enhances the body’s ability to recognize and eliminate tumor cells | AdCD40L gene successfully transferred and increased T cells and decreased tumor load; IL-12-encoding plasmids loaded on RBCEVs showed fivefold increase in IL-12 expression | No observable toxicity | [43,111,112,113,114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyler, H.J.; Bruton, L.G.; Maher, A.J.; Yu, D.A.; Shely, N.W.; Wakefield, M.R.; Fang, Y. Cytokine Therapy in Bladder Cancer: Mechanisms, Efficacy, and Future Prospects. Curr. Issues Mol. Biol. 2025, 47, 278. https://doi.org/10.3390/cimb47040278
Oyler HJ, Bruton LG, Maher AJ, Yu DA, Shely NW, Wakefield MR, Fang Y. Cytokine Therapy in Bladder Cancer: Mechanisms, Efficacy, and Future Prospects. Current Issues in Molecular Biology. 2025; 47(4):278. https://doi.org/10.3390/cimb47040278
Chicago/Turabian StyleOyler, Hayden J., Layne G. Bruton, Austin J. Maher, Darien A. Yu, Nicholas W. Shely, Mark R. Wakefield, and Yujiang Fang. 2025. "Cytokine Therapy in Bladder Cancer: Mechanisms, Efficacy, and Future Prospects" Current Issues in Molecular Biology 47, no. 4: 278. https://doi.org/10.3390/cimb47040278
APA StyleOyler, H. J., Bruton, L. G., Maher, A. J., Yu, D. A., Shely, N. W., Wakefield, M. R., & Fang, Y. (2025). Cytokine Therapy in Bladder Cancer: Mechanisms, Efficacy, and Future Prospects. Current Issues in Molecular Biology, 47(4), 278. https://doi.org/10.3390/cimb47040278






