Associating Patient Responses with Drug Sensitivity in Non-Small Cell Lung Carcinoma Using an Immunoassay on Patient-Derived Cell Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishment of NSCLC Primary Cell Cultures
2.2. Treatments with Chemotherapeutics
2.3. Immunofluorescence Assay
- Inhibitory Effect at Maximum Dose: The percentage inhibition at the highest dose is multiplied by 0.1. If stromal cells show a greater inhibitory effect, the value is multiplied by −0.1.
- Dose for 10% Inhibition (IC10): This dose is multiplied by 0.05. If the dose required for cancer cells is higher than that for stromal cells, it is multiplied by −0.05.
- Dose for 25% Inhibition (IC25): this dose is also multiplied by 0.05, with the same adjustment made if the cancer cells require a higher dose than the stromal cells.
- Dose for 50% Inhibition (IC50): The dose is multiplied by 0.1 unless it exceeds 100 µM, in which case the factor is set to 0. If stromal cells require a lower dose, the value is multiplied by −0.1 or by −10 if the dose exceeds 100 µM.
- Dose for 75% Inhibition (IC75): The dose is multiplied by 0.05 and reduced to 0 if it exceeds 100 µM. If stromal cells require a lower dose, the value is adjusted to −0.05 or −5 if the dose exceeds 100 µM.
- Dose for 90% Inhibition (IC90): similar to IC75, the dose is multiplied by 0.05, adjusted to 0 above 100 µM, and set to −0.05 or −5 if stromal cells require a lower dose.
- Slope
- Area Under the Curve (AUC): this value is multiplied by 0.35.
- Stimulation of Cancer Cell Growth: If none of the tested doses stimulates cancer cell growth, the lowest dose is multiplied by 0.05. If growth stimulation is observed (up to 125%), the same factor is applied. Growth stimulation between 125% and 150% is multiplied by 0, and stimulation above 150% is multiplied by −0.05.
- Breakthrough Dose: If the drug has a rapid effect at lower doses but only a limited effect at higher doses, the breakthrough dose is multiplied by −0.05. Conversely, in the case of a dose-dependent effect, the dose that causes 50% inhibition is multiplied by 0.05.
- Incomplete Inhibition at Maximum Dose: If more than 25% of the cancer cells remain viable at the highest dose, the value is multiplied by −0.05. For a viability of 10% to 25% the multiplier is 0, and for less than 10%, the multiplier is 0.05.
2.4. Barnard’s Exact Test
2.5. Progression-Free Survival via Kaplan–Meier Analysis
3. Results
3.1. Clinical Parameters of NSCLC Patients
3.2. Ex Vivo Evaluation of the Sensitivity of Patient-Derived Cells to Chemotherapy and Erlotinib
3.3. Scoring of Patients’ Cell Responses to Chemotherapeutics and Erlotinib
3.4. Correlation of Drug Response Scores of Patients’ Samples with Patients’ Responses to Therapy
3.5. Progression-Free Survival Is Associated with Ex Vivo Sensitivity to Chemotherapeutics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCB1 | ATP-binding cassette transporter B1 |
| ALK | Anaplastic lymphoma kinase |
| BSA | Bovine serum albumin |
| CK8/18 | Cytokeratin (8/18) |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl sulfoxide |
| EGFR | Epidermal growth factor receptor |
| IC50 | Half-maximal inhibitory concentration |
| NSCLC | Non-small cell lung carcinoma |
| PBS | Phosphate-buffered saline |
| PDXs | Patient-derived xenografts |
| PDOs | Patient-derived organoids |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| SCLC | Small cell lung carcinoma |
| SEM | The standard error of the mean |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Zhang, Y.; Vaccarella, S.; Morgan, E.; Li, M.; Etxeberria, J.; Chokunonga, E.; Manraj, S.S.; Kamate, B.; Omonisi, A.; Bray, F. Global Variations in Lung Cancer Incidence by Histological Subtype in 2020: A Population-Based Study. Lancet Oncol. 2023, 24, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Bi, J.H.; Tuo, J.Y.; Xiao, Y.X.; Tang, D.D.; Zhou, X.H.; Jiang, Y.F.; Ji, X.W.; Tan, Y.T.; Yuan, H.Y.; Xiang, Y.B. Observed and Relative Survival Trends of Lung Cancer: A Systematic Review of Population-Based Cancer Registration Data. Thorac. Cancer 2024, 15, 142–151. [Google Scholar] [CrossRef]
- Lee, S.H. Chemotherapy for Lung Cancer in the Era of Personalized Medicine. Tuberc. Respir. Dis. 2018, 82, 179. [Google Scholar] [CrossRef]
- Wu, J.; Lin, Z. Non-Small Cell Lung Cancer Targeted Therapy: Drugs and Mechanisms of Drug Resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef]
- Peters, S.; Gettinger, S.; Johnson, M.L.; Jänne, P.A.; Garassino, M.C.; Christoph, D.; Toh, C.K.; Rizvi, N.A.; Chaft, J.E.; Costa, E.C.; et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J. Clin. Oncol. 2017, 35, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Korol, E.E.; Kayaniyil, S.; Varol, N.; Ebner, T.; Goring, S.M. Efficacy and Safety of First-Line Carboplatin-versus Cisplatin-Based Chemotherapy for Non-Small Cell Lung Cancer: A Meta-Analysis. Lung Cancer 2019, 135, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Liu, X.; Belani, C.P. Taxanes, Past, Present, and Future Impact on Non-Small Cell Lung Cancer. Anticancer. Drugs 2014, 25, 571–583. [Google Scholar] [CrossRef]
- Kenmotsu, H.; Yamamoto, N.; Yamanaka, T.; Yoshiya, K.; Takahashi, T.; Ueno, T.; Goto, K.; Daga, H.; Ikeda, N.; Sugio, K.; et al. Randomized Phase III Study of Pemetrexed Plus Cisplatin Versus Vinorelbine Plus Cisplatin for Completely Resected Stage II to IIIA Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Langer, C.J.; Gadgeel, S.M.; Borghaei, H.; Papadimitrakopoulou, V.A.; Patnaik, A.; Powell, S.F.; Gentzler, R.D.; Martins, R.G.; Stevenson, J.P.; Jalal, S.I.; et al. Carboplatin and Pemetrexed with or without Pembrolizumab for Advanced, Non-Squamous Non-Small-Cell Lung Cancer: A Randomised, Phase 2 Cohort of the Open-Label KEYNOTE-021 Study. Lancet Oncol. 2016, 17, 1497–1508. [Google Scholar] [CrossRef]
- Thorel, L.; Perréard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.B.; et al. Patient-Derived Tumor Organoids: A New Avenue for Preclinical Research and Precision Medicine in Oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef]
- Foglizzo, V.; Cocco, E.; Marchiò, S. Advanced Cellular Models for Preclinical Drug Testing: From 2D Cultures to Organ-on-a-Chip Technology. Cancers 2022, 14, 3692. [Google Scholar] [CrossRef]
- Cai, E.Y.; Garcia, J.; Liu, Y.; Vakar-Lopez, F.; Arora, S.; Nguyen, H.M.; Lakely, B.; Brown, L.; Wong, A.; Montgomery, B.; et al. A Bladder Cancer Patient-Derived Xenograft Displays Aggressive Growth Dynamics in Vivo and in Organoid Culture. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Dinić, J.; Podolski-Renić, A.; Dragoj, M.; Jovanović Stojanov, S.; Stepanović, A.; Lupšić, E.; Pajović, M.; Jovanović, M.; Petrović Rodić, D.; Marić, D.; et al. Immunofluorescence-Based Assay for High-Throughput Analysis of Multidrug Resistance Markers in Non-Small Cell Lung Carcinoma Patient-Derived Cells. Diagnostics 2023, 13, 3617. [Google Scholar] [CrossRef] [PubMed]
- Podolski-Renić, A.; Dragoj, M.; Dinić, J.; Jovanović Stojanov, S.; Lupšić, E.; Pajović, M.; Ercegovac, M.; Glumac, S.; Marić, D.; Pešić, M. Optimizing Treatment Response in Lung Squamous Cell Carcinoma by Cell-Based Functional Sensitivity Screening. Oncol. Insights 2024, 2, 18–24. [Google Scholar]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-Small-Cell Lung Cancers: A Heterogeneous Set of Diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef]
- Cobain, E.F.; Wu, Y.M.; Vats, P.; Chugh, R.; Worden, F.; Smith, D.C.; Schuetze, S.M.; Zalupski, M.M.; Sahai, V.; Alva, A.; et al. Assessment of Clinical Benefit of Integrative Genomic Profiling in Advanced Solid Tumors. JAMA Oncol. 2021, 7, 525–533. [Google Scholar] [CrossRef]
- McLaughlin, J.; Berkman, J.; Nana-Sinkam, P. Targeted Therapies in Non-Small Cell Lung Cancer: Present and Future. Fac. Rev. 2023, 12, 22. [Google Scholar] [CrossRef]
- Stinchcombe, T.E. The Use of EGFR Tyrosine Kinase Inhibitors in EGFR Wild-Type Non-Small-Cell Lung Cancer. Curr. Treat. Options Oncol. 2016, 17, 1–11. [Google Scholar] [CrossRef]
- Taurin, S.; Alzahrani, R.; Aloraibi, S.; Ashi, L.; Alharmi, R.; Hassani, N. Patient-Derived Tumor Organoids: A Preclinical Platform for Personalized Cancer Therapy. Transl. Oncol. 2025, 51, 102226. [Google Scholar] [CrossRef]
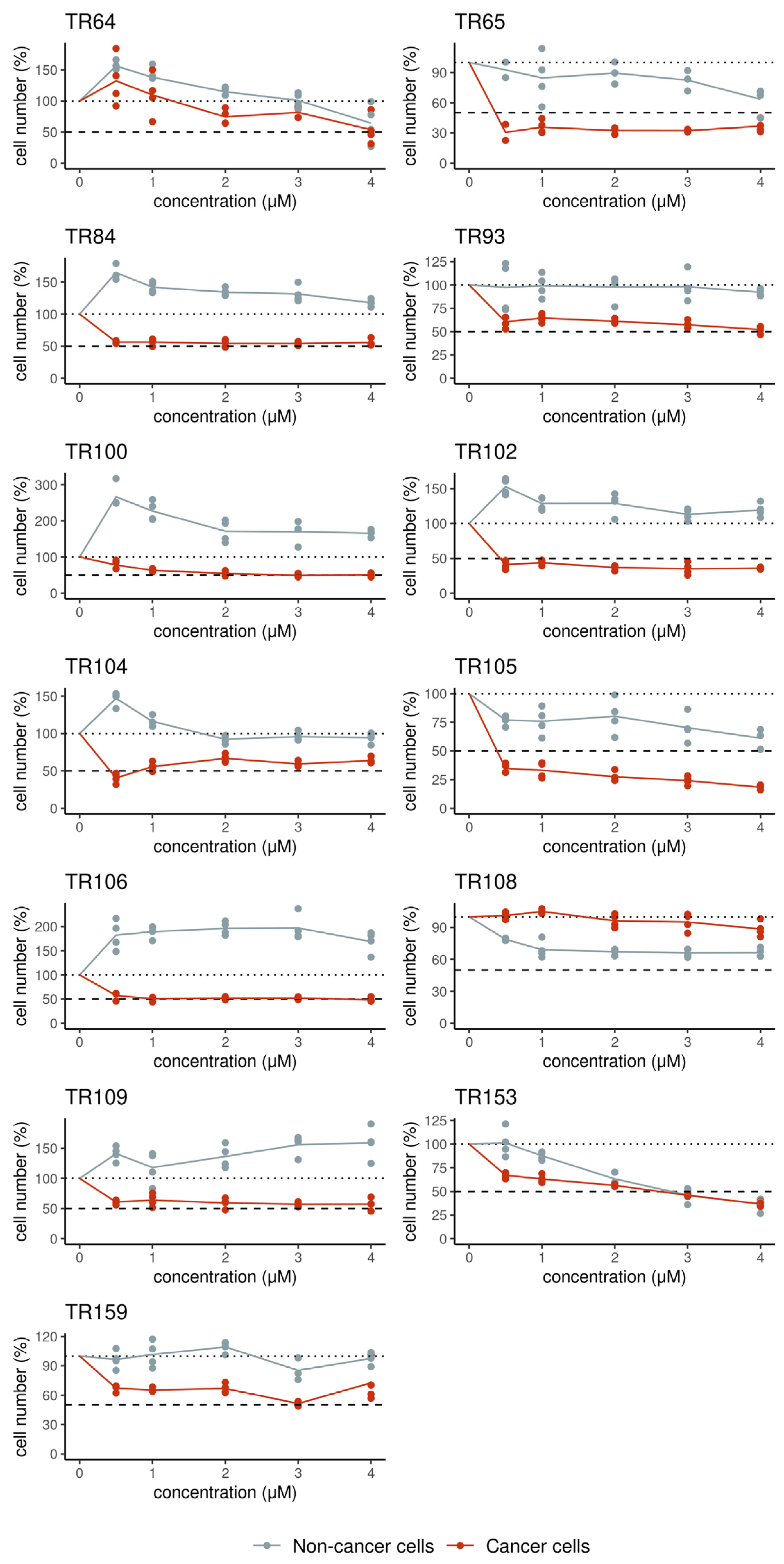
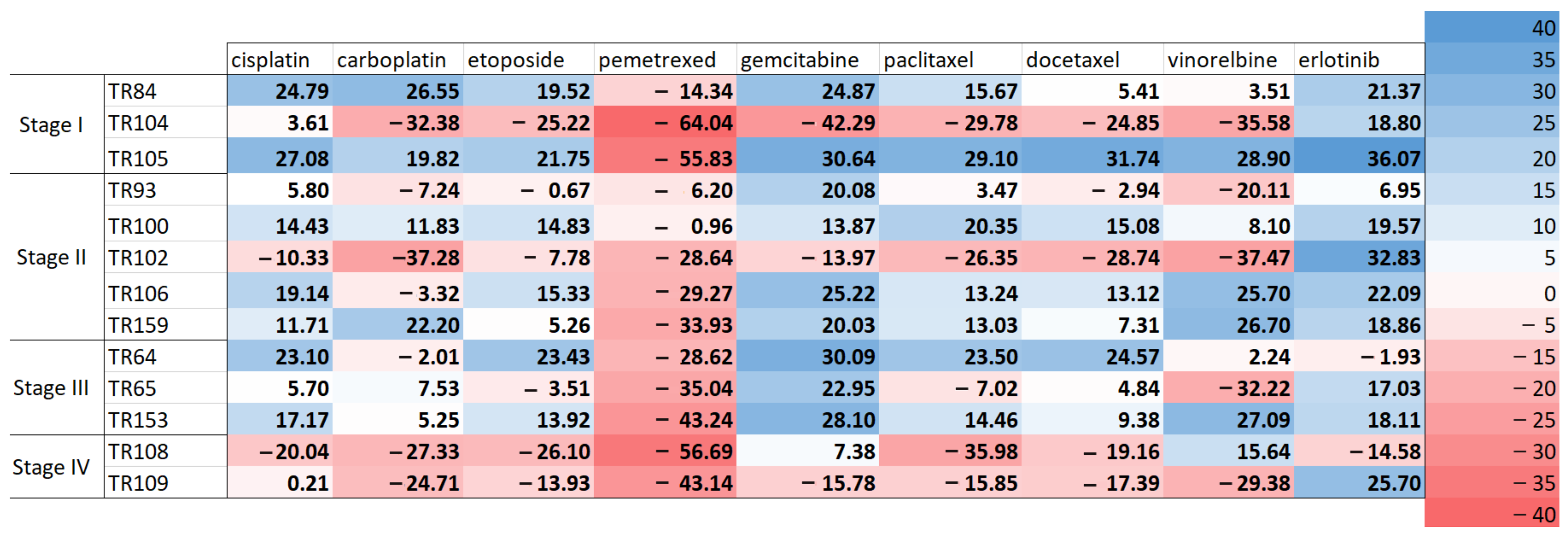
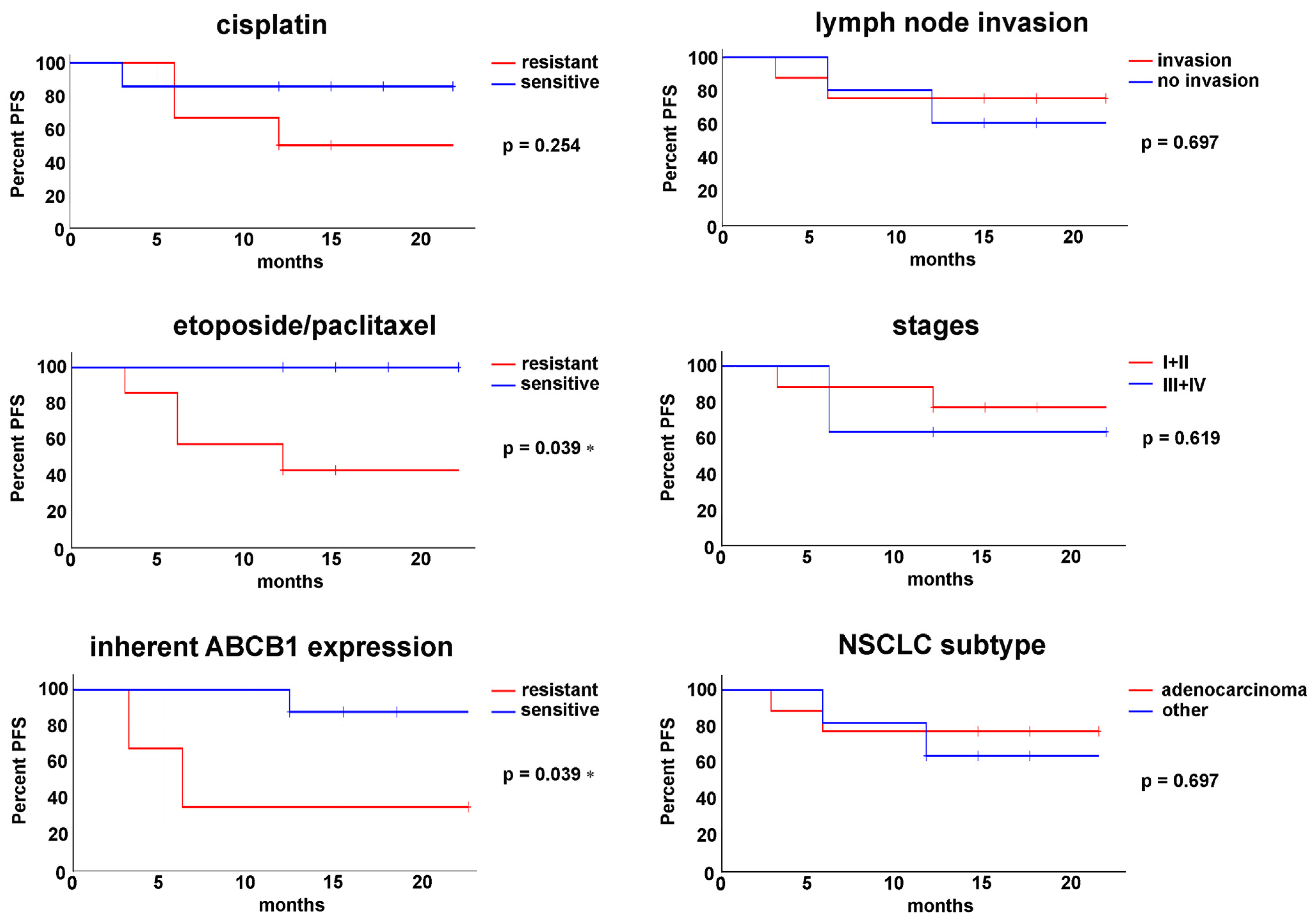
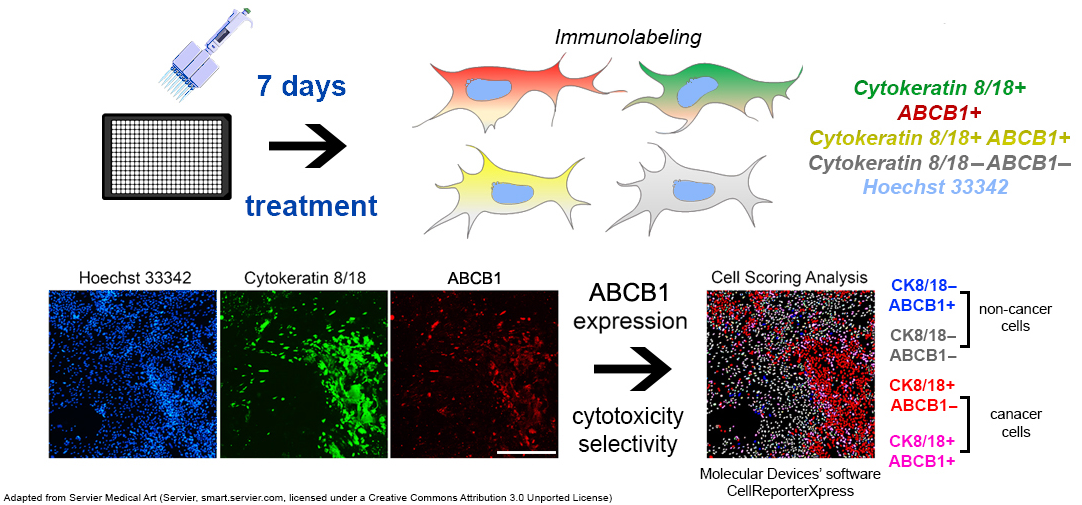
| Patient | Sex | Age | NSCLC Subtype | Lymph Node Invasion | Stage | EGFR Status | Chemotherapy | Chemotherapy After Relapse |
|---|---|---|---|---|---|---|---|---|
| TR64 | male | 71 | adenocarcinoma | N2 | IIIA | Mutation L858R | cisplatin + etoposide, 3 cycles, osimertinib | / |
| TR65 | male | 51 | large cell neuroendocrine carcinoma | N0 | IIIA | wt | cisplatin + etoposide, 3 cycles | cisplatin + etoposide, 2 cycles |
| TR84 | female | 58 | squamous cell carcinoma | N0 | IB | wt | cisplatin + etoposide, 3 cycles | / |
| TR93 | male | 72 | squamous cell carcinoma | N0 | IIA | wt | cisplatin + etoposide, 3 cycles | / |
| TR100 | male | 61 | adenocarcinoma | N0 | IIA | wt | cisplatin + etoposide, 3 cycles | / |
| TR102 | male | 55 | adenocarcinoma | N0 | IIB | wt | cisplatin + etoposide, 3 cycles | / |
| TR104 | male | 68 | adenocarcinoma | N0 | IB | wt | cisplatin + etoposide, 2 cycles | / |
| TR105 | male | 66 | squamous cell carcinoma | N0 | IB | wt | cisplatin + etoposide, 3 cycles | / |
| TR106 | female | 74 | squamous cell carcinoma | N0 | IIB | wt | cisplatin + etoposide, 3 cycles | / |
| TR108 | male | 65 | adenocarcinoma | N0 | IV | wt | cisplatin + paclitaxel, 5 cycles | cisplatin + gemcitabin, 2 cycles—chemotoxicity, proceed with atezolizumab |
| TR109 | male | 59 | adenocarcinoma | N1 | IV | wt | cisplatin + paclitaxel, 6 cycles | / |
| TR153 | female | 68 | adenocarcinoma | N2 | IIIA | wt | cisplatin + etoposide, 3 cycles | / |
| TR159 | male | 63 | adenocarcinoma | N1 | IIA | wt | / | pembrolizumab, 5 cycles |
| NSCLC Cultures | IC50 µM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Cells(CK8/18+) | Cisplatin | Carboplatin | Etoposide | Pemetrexed | Gemcitabine | Paclitaxel | Docetaxel | Vinorelbine | |||
| Stage I | TR84 | 2.11 * | 8.15 * | 2.98 * | >300 * | 2.68 * | 2.57 * | 1.85 * | 0.18 * | ||
| TR104 | 12.24 | >100 | >30 | >300 | >100 | >5 | >5 | >1 | |||
| TR105 | 2.12 | 7.51 | 4.37 | >300 | 2.77 | 0.49 | 0.49 | 0.05 | |||
| Stage II | TR93 | 9.02 | 55.27 | 20.94 | >300 | 7.37 | 2.63 | 3.71 | 0.65 | ||
| TR100 | 3.87 | 15.57 | 5.46 | 54.79 | 7.61 | 0.51 | 0.54 | 0.16 | |||
| TR102 | 16.30 | >100 | >30 | >300 | 44.37 | >5 | >5 | >1 | |||
| TR106 | 4.19 | 38.64 | 13.59 | >300 | 2.68 | 2.69 | 2.76 | 0.33 | |||
| TR159 | 6.22 | 20.60 | 11.98 | >300 | 9.79 | 1.57 | 1.58 | 0.34 | |||
| Stage III | TR64 | 6.07 * | 38.98 * | 11.92 * | 251.86 * | 4.92 * | 1.35 * | 1.56 * | 0.17 * | ||
| TR65 | 14.64 * | 22.49 * | 25.39 * | 231.47 * | 4.91 * | 1.18 * | >5 * | >1 * | |||
| TR153 | 4.95 | 30.88 | 7.35 | >300 | 5.24 | 1.31 | 1.33 | 0.17 | |||
| Stage IV | TR108 | >15 | >100 | >30 | >300 | 11.01 | >5 | >5 | >1 | ||
| TR109 | 14.85 | >100 | >30 | >300 | 63.96 | >5 | >5 | >1 | |||
| Non-Cancer Cells (CK8/18−) | Cisplatin | Carboplatin | Etoposide | Pemetrexed | Gemcitabine | Paclitaxel | Docetaxel | Vinorelbine | |||
| Stage I | TR84 | 0.79 * | 1.28 * | 1.01 * | >300 * | 0.49 * | 0.31 * | 0.18 * | 0.03 * | ||
| TR104 | 6.42 | 48.56 | 15.61 | >300 | 25.80 | 2.71 | 2.71 | 0.25 | |||
| TR105 | 1.35 | 6.32 | 2.95 | 185.80 | 0.73 | 1.54 S | 1.04 S | 0.28 S | |||
| Stage II | TR93 | 4.29 | 21.01 | 7.06 | >300 S | 0.82 | 2.03 | 1.11 | 0.28 | ||
| TR100 | 1.88 | 3.40 | 1.67 | 44.86 | 1.48 | 0.07 | 0.06 | 0.04 | |||
| TR102 | 5.18 | 38.40 | 12.30 | >300 | 7.50 | 1.76 | 2.27 | 0.13 | |||
| TR106 | 2.23 | 15.20 | 7.11 | >300 | 0.46 | 3.22 S | 2.27 | 0.53 S | |||
| TR159 | 0.73 | 17.31 | 1.58 | >300 | 1.90 | 0.29 | 0.19 | 048 S | |||
| Stage III | TR64 | 8.26 *S | 12.76 * | 18.13 *S | >300 *S | 7.06 *S | 1.95 *S | 2.73 *S | 0.18 * | ||
| TR65 | 1.21 * | 2.16 * | 2.08 * | 25.18 * | 0.35 * | 1.40 * | 0.46 * | 0.03 * | |||
| TR153 | 1.04 | 4.98 | 1.26 | >300 | 0.82 | 1.00 | 0.66 | 0.52 S | |||
| Stage IV | TR108 | 11.47 | 90.12 | >30 | >300 | 6.07 | >5 | >5 | >1 S | ||
| TR109 | 6.49 | 38.14 | 12.28 | >300 | 9.22 | 3.72 | 2.62 | 0.19 | |||
| Patient | Categorization of Ex Vivo Responses to Cisplatin * | Categorization of Ex Vivo Responses to Etoposide/Paclitaxel * | Categorization According to the Inherent Expression of the ABCB1 Transporter # | Progression-Free Survival (PFS) |
|---|---|---|---|---|
| TR64 | sensitive | sensitive | resistant | >22 months |
| TR65 | resistant | resistant | resistant | 6 months |
| TR84 | sensitive | sensitive | sensitive | >18 months |
| TR93 | resistant | resistant | sensitive | 12 months |
| TR100 | sensitive | sensitive | sensitive | >18 months |
| TR102 | resistant | resistant | sensitive | >15 months |
| TR104 | resistant | resistant | sensitive | >15 months |
| TR105 | sensitive | sensitive | sensitive | >15 months |
| TR106 | sensitive | sensitive | sensitive | >12 months |
| TR108 | resistant | resistant | no data | 6 months |
| TR109 | resistant | resistant | sensitive | >12 months |
| TR153 | sensitive | sensitive | no data | >12 months |
| TR159 | sensitive | resistant | resistant | 3 months |
| p = 0.031006 # | Ex Vivo Sensitive | Ex Vivo Resistant | Total |
|---|---|---|---|
| Clinically sensitive (relapse after 12 months from surgery) | 6 | 3 | 9 |
| Clinically resistant (relapse within 12 months from surgery) | 0 | 4 # | 4 |
| Total | 6 | 7 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolski-Renić, A.; Jovanović Stojanov, S.; Marić, D.; Dinić, J.; Dragoj, M.; Stepanović, A.; Lupšić, E.; Pajović, M.; Glumac, S.; Ercegovac, M.; et al. Associating Patient Responses with Drug Sensitivity in Non-Small Cell Lung Carcinoma Using an Immunoassay on Patient-Derived Cell Cultures. Curr. Issues Mol. Biol. 2025, 47, 281. https://doi.org/10.3390/cimb47040281
Podolski-Renić A, Jovanović Stojanov S, Marić D, Dinić J, Dragoj M, Stepanović A, Lupšić E, Pajović M, Glumac S, Ercegovac M, et al. Associating Patient Responses with Drug Sensitivity in Non-Small Cell Lung Carcinoma Using an Immunoassay on Patient-Derived Cell Cultures. Current Issues in Molecular Biology. 2025; 47(4):281. https://doi.org/10.3390/cimb47040281
Chicago/Turabian StylePodolski-Renić, Ana, Sofija Jovanović Stojanov, Dragana Marić, Jelena Dinić, Miodrag Dragoj, Ana Stepanović, Ema Lupšić, Milica Pajović, Sofija Glumac, Maja Ercegovac, and et al. 2025. "Associating Patient Responses with Drug Sensitivity in Non-Small Cell Lung Carcinoma Using an Immunoassay on Patient-Derived Cell Cultures" Current Issues in Molecular Biology 47, no. 4: 281. https://doi.org/10.3390/cimb47040281
APA StylePodolski-Renić, A., Jovanović Stojanov, S., Marić, D., Dinić, J., Dragoj, M., Stepanović, A., Lupšić, E., Pajović, M., Glumac, S., Ercegovac, M., & Pešić, M. (2025). Associating Patient Responses with Drug Sensitivity in Non-Small Cell Lung Carcinoma Using an Immunoassay on Patient-Derived Cell Cultures. Current Issues in Molecular Biology, 47(4), 281. https://doi.org/10.3390/cimb47040281


_Kim.png)






