The Inflammatory Link of Rheumatoid Arthritis and Thrombosis: Pathogenic Molecular Circuits and Treatment Approaches
Abstract
1. Introduction
2. Materials and Methods
3. Brief Thrombosis Concept, Risk Factors, Mechanisms, and Treatment Approaches
3.1. General Profile of Thrombosis
3.2. Introduction of Thrombosis Concepts: Deep Vein Thrombosis
3.3. Deep Vein Thrombosis and Risk Factors
3.4. Molecular Mechanisms of Thrombosis and Thrombo-Inflammation in Thromboembolism
3.5. Blood Cells and Role of Platelets in Thrombosis
3.6. Brief Introduction to Antiplatelet and Anticoagulant Drugs in the Treatment of Thrombosis Incidence Linked to Rheumatoid Arthritis
3.6.1. Action of Antiplatelet Drugs
3.6.2. Action of Anticoagulant Drugs
4. Rheumatoid Arthritis Epidemiology, Mechanisms and Complications, Thrombosis Connection, and Treatment Approaches
4.1. General Profile of Rheumatoid Arthritis
Epidemiology of Rheumatoid Arthritis
4.2. Mechanisms and Complications of Rheumatoid Arthritis
4.3. Complications of Thrombosis Involvement in Rheumatoid Arthritis
4.3.1. Endothelial Injury
4.3.2. Hypercoagulability
4.3.3. Venous Stasis
4.4. Role of Platelet-Activating Factor, Adenosine Diphosphate, Thromboxane A2, and Thrombin Mediators Associated with Thrombosis Involvement in Rheumatoid Arthritis
4.4.1. Role of PAF
4.4.2. Role of Thrombin
4.4.3. Role of ADP
4.4.4. Role of TXA2
4.5. Role of Anticoagulant, Antiplatelet, and Antithrombotic Therapy in Rheumatoid Arthritis with Thrombosis Incident
4.6. Conventional and Current Treatment Approaches for Rheumatoid Arthritis
4.6.1. Conventional Treatment Approaches
4.6.2. Current and Newly Emerged Treatment Approaches
4.6.3. Surgery-Related Treatment Approaches
5. Limitations, Future Perspectives, and Potential Treatment Solutions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W. Rheumatoid Arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, B.; Raadsen, R.; Nurmohamed, M.T. Cardiovascular Disease Risk in Rheumatoid Arthritis Anno 2022. J. Clin. Med. 2022, 11, 2704. [Google Scholar] [CrossRef]
- Entezami, P.; Fox, D.A.; Clapham, P.J.; Chung, K.C. Historical Perspective on the Etiology of Rheumatoid Arthritis. Hand Clin. 2011, 27, 1–10. [Google Scholar] [CrossRef]
- Gilbert, B.T.P.; Lamacchia, C. Predicting the Onset of Rheumatoid Arthritis. Jt. Bone Spine 2023, 90, 105556. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, M.; Fernandez-Lao, C.; Martin-Martin, L.; Gonzalez-Santos, A.; Lopez-Garzon, M.; Ortiz-Comino, L.; Lozano-Lozano, M. Therapeutic Benefits of Balneotherapy on Quality of Life of Patients with Rheumatoid Arthritis: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13216. [Google Scholar] [CrossRef]
- Fraser, K.J. Anglo-French Contributions to the Recognition of Rheumatoid Arthritis. Ann. Rheum. Dis. 1982, 41, 335–343. [Google Scholar] [CrossRef]
- Tsoucalas, G.; Sgantzos, M. Primary Asthenic Gout by Augustin-Jacob Landre-Beauvais in 1800: Is This the First Description of Rheumatoid Arthritis? Mediterr. J. Rheumatol. 2017, 28, 223–226. [Google Scholar] [CrossRef]
- Conn, D.L. The Story Behind the Use of Glucocorticoids in the Treatment of Rheumatoid Arthritis. Semin. Arthritis Rheum. 2021, 51, 15–19. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel Treatment Strategies in Rheumatoid Arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Tornero Molina, J.; Hernández-Cruz, B.; Corominas, H. Initial Treatment with Biological Therapy in Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 48. [Google Scholar] [CrossRef]
- Anton, M.-L.; Cardoneanu, A.; Burlui, A.M.; Mihai, I.R.; Richter, P.; Bratoiu, I.; Macovei, L.A.; Rezus, E. The Lung in Rheumatoid Arthritis—Friend or Enemy? Int. J. Mol. Sci. 2024, 25, 6460. [Google Scholar] [CrossRef] [PubMed]
- Makol, A.; Crowson, C.S.; Wetter, D.A.; Sokumbi, O.; Matteson, E.L.; Warrington, K.J. Vasculitis Associated with Rheumatoid Arthritis: A Case–Control Study. Rheumatology 2014, 53, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, A.; Cassone, G.; Luppi, F.; Atienza-Mateo, B.; Cavazza, A.; Sverzellati, N.; González-Gay, M.A.; Salvarani, C.; Sebastiani, M. Rheumatoid Arthritis Related Interstitial Lung Disease. Expert Rev. Clin. Immunol. 2021, 17, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Jagpal, A.; Navarro-Millán, I. Cardiovascular Co-Morbidity in Patients with Rheumatoid Arthritis: A Narrative Review of Risk Factors, Cardiovascular Risk Assessment and Treatment. BMC Rheumatol. 2018, 2, 10. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yang, H.-Y.; Lai, J.-H. Anti-Citrullinated Protein Antibodies in Patients with Rheumatoid Arthritis: Biological Effects and Mechanisms of Immunopathogenesis. Int. J. Mol. Sci. 2020, 21, 4015. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Yang, H.-Y.; Luo, S.-F.; Lai, J.-H. From Rheumatoid Factor to Anti-Citrullinated Protein Antibodies and Anti-Carbamylated Protein Antibodies for Diagnosis and Prognosis Prediction in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 686. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Omair, M.A.; Alkhelb, S.A.; Ezzat, S.E.; Boudal, A.M.; Bedaiwi, M.K.; Almaghlouth, I. Venous Thromboembolism in Rheumatoid Arthritis: The Added Effect of Disease Activity to Traditional Risk Factors. Open Access Rheumatol. Res. Rev. 2022, 14, 231. [Google Scholar] [CrossRef]
- Favalli, E.G.; Biggioggero, M.; Crotti, C.; Becciolini, A.; Raimondo, M.G.; Meroni, P.L. Sex and Management of Rheumatoid Arthritis. Clin. Rev. Allerg Immunol. 2019, 56, 333–345. [Google Scholar] [CrossRef]
- Demeter, F.; Bihari, G.; Vadicsku, D.; Sinkovits, G.; Kajdácsi, E.; Horváth, L.; Réti, M.; Müller, V.; Iványi, Z.; Gál, J.; et al. Anti-Inflammatory Cytokine Profiles in Thrombotic Thrombocytopenic Purpura—Differences Compared to COVID-19. Int. J. Mol. Sci. 2024, 25, 10007. [Google Scholar] [CrossRef]
- Bararu Bojan, I.; Dobreanu, S.; Vladeanu, M.C.; Ciocoiu, M.; Badescu, C.; Plesoianu, C.; Filip, N.; Iliescu, D.; Frasinariu, O.; Bojan, A.; et al. The Etiology of the Thrombotic Phenomena Involved in the Process of Coronary Artery Disease—What Is the Role of Thrombophilic Genes in the Development of This Pathology? Int. J. Mol. Sci. 2024, 25, 5228. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Keyßer, G. Lifestyle Factors and Their Influence on Rheumatoid Arthritis: A Narrative Review. J. Clin. Med. 2022, 11, 7179. [Google Scholar] [CrossRef] [PubMed]
- Radić, M.; Vlak, I.; Vučković, M.; Radić, J.; Bešić, E.; Vlak, T. Association between Nutritional Status, Lifestyle Habits, and Disease Activity in Dalmatian Patients with Rheumatoid Arthritis. Nutrients 2023, 15, 1738. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.E.; Battaglia, R.; Ursino, M.; Lucca, L.F.; Quintieri, M.; Vatrano, M.; Tonin, P.; Cerasa, A. Prevalence and Risk Factors of Deep Venous Thrombosis in Intensive Inpatient Neurorehabilitation Unit. Healthcare 2024, 12, 936. [Google Scholar] [CrossRef]
- Mangiafico, M.; Costanzo, L. Superficial Venous Thrombosis: A Comprehensive Review. Healthcare 2024, 12, 500. [Google Scholar] [CrossRef]
- Tsoupras, A.; Adamantidi, T.; Finos, M.A.; Philippopoulos, A.; Detopoulou, P.; Tsopoki, I.; Kynatidou, M.; Demopoulos, C.A. Re-Assessing the Role of Platelet Activating Factor and Its Inflammatory Signaling and Inhibitors in Cancer and Anti-Cancer Strategies. FBL 2024, 29, 345. [Google Scholar] [CrossRef]
- Pastori, D.; Cormaci, V.M.; Marucci, S.; Franchino, G.; Del Sole, F.; Capozza, A.; Fallarino, A.; Corso, C.; Valeriani, E.; Menichelli, D.; et al. A Comprehensive Review of Risk Factors for Venous Thromboembolism: From Epidemiology to Pathophysiology. Int. J. Mol. Sci. 2023, 24, 3169. [Google Scholar] [CrossRef]
- Oancea, A.; Maștaleru, A.; Abdulan, I.M.; Costache, A.D.; Zota, M.I.; Negru, R.; Moisă, Ș.; Trandafir, L.M.; Leon, M.M. Challenges of a Patient with Thromboembolism. Reports 2023, 6, 39. [Google Scholar] [CrossRef]
- Franczyk, B.; Gluba-Brzózka, A.; Ławiński, J.; Rysz-Górzyńska, M.; Rysz, J. Metabolomic Profile in Venous Thromboembolism (VTE). Metabolites 2021, 11, 495. [Google Scholar] [CrossRef]
- Salavati, M.; Arabshomali, A.; Nouranian, S.; Shariat-Madar, Z. Overview of Venous Thromboembolism and Emerging Therapeutic Technologies Based on Nanocarriers-Mediated Drug Delivery Systems. Molecules 2024, 29, 4883. [Google Scholar] [CrossRef]
- Jannati, S.; Patnaik, R.; Banerjee, Y. Beyond Anticoagulation: A Comprehensive Review of Non-Vitamin K Oral Anticoagulants (NOACs) in Inflammation and Protease-Activated Receptor Signaling. Int. J. Mol. Sci. 2024, 25, 8727. [Google Scholar] [CrossRef]
- Kaufmann, C.C.; Muthspiel, M.; Lunzer, L.; Pogran, E.; Zweiker, D.; Burger, A.L.; Wojta, J.; Huber, K. Antiplatelet Therapy and Anticoagulation before, during, and after Acute Coronary Syndrome. J. Clin. Med. 2024, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Bedeković, D.; Bošnjak, I.; Šarić, S.; Kirner, D.; Novak, S. Role of Inflammatory Cytokines in Rheumatoid Arthritis and Development of Atherosclerosis: A Review. Medicina 2023, 59, 1550. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Kirichenko, T.V.; Beloyartsev, D.F.; Churov, A.V.; Kovyanova, T.I.; Starodubtseva, I.A.; Sukhorukov, V.N.; Antonov, S.A.; Orekhov, A.N. Rheumatoid Arthritis: What Inflammation Do We Face? J. Mol. Pathol. 2024, 5, 454–465. [Google Scholar] [CrossRef]
- Popescu, D.; Rezus, E.; Badescu, M.C.; Dima, N.; Seritean Isac, P.N.; Dragoi, I.-T.; Rezus, C. Cardiovascular Risk Assessment in Rheumatoid Arthritis: Accelerated Atherosclerosis, New Biomarkers, and the Effects of Biological Therapy. Life 2023, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.-M. Current Therapeutic Options in the Treatment of Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef]
- Ketfi, C.; Boutigny, A.; Mohamedi, N.; Bouajil, S.; Magnan, B.; Amah, G.; Dillinger, J.-G. Risk of Venous Thromboembolism in Rheumatoid Arthritis. Jt. Bone Spine 2021, 88, 105122. [Google Scholar] [CrossRef]
- Miele, C.; Mennitti, C.; Gentile, A.; Veneruso, I.; Scarano, C.; Vastola, A.; La Monica, I.; Uomo, F.; Iafusco, F.; Capasso, F.; et al. Thrombosis and Thrombotic Risk in Athletes. J. Clin. Med. 2024, 13, 4881. [Google Scholar] [CrossRef]
- Schulman, S.; Makatsariya, A.; Khizroeva, J.; Bitsadze, V.; Kapanadze, D. The Basic Principles of Pathophysiology of Venous Thrombosis. Int. J. Mol. Sci. 2024, 25, 11447. [Google Scholar] [CrossRef]
- Gollamudi, J.; Sartain, S.E.; Navaei, A.H.; Aneja, S.; Kaur Dhawan, P.; Tran, D.; Joshi, J.; Gidudu, J.; Gollamudi, J.; Chiappini, E.; et al. Thrombosis and Thromboembolism: Brighton Collaboration Case Definition and Guidelines for Data Collection, Analysis, and Presentation of Immunization Safety Data. Vaccine 2022, 40, 6431–6444. [Google Scholar] [CrossRef] [PubMed]
- Vesa, Ş.C.; Chira, R.; Vlaicu, S.I.; Pașca, S.; Crișan, S.; Trifa, A.; Buzoianu, A.D. Systemic and Local Factors’ Influence on the Topological Differences in Deep Vein Thrombosis. Medicina 2019, 55, 691. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Gordon, A.E.; Henke, P.K. Resolution of Deep Venous Thrombosis: Proposed Immune Paradigms. Int. J. Mol. Sci. 2020, 21, 2080. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-T.; Tsai, S.-E.; Chen, Y.-C.; Yang, S.-F.; Yeh, H.-W.; Wang, B.-Y.; Yeh, L.-T.; Shih, N.-C.; Wang, Y.-H.; Chen, Y.-Y.; et al. Deep Venous Thrombosis and Risk of Consequent Sepsis Event: A Retrospective Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 7879. [Google Scholar] [CrossRef]
- Thomas, S.E.; Weinberg, I.; Schainfeld, R.M.; Rosenfield, K.; Parmar, G.M. Diagnosis of Pulmonary Embolism: A Review of Evidence-Based Approaches. J. Clin. Med. 2024, 13, 3722. [Google Scholar] [CrossRef] [PubMed]
- Peracaula, M.; Sebastian, L.; Francisco, I.; Vilaplana, M.B.; Rodríguez-Chiaradía, D.A.; Tura-Ceide, O. Decoding Pulmonary Embolism: Pathophysiology, Diagnosis, and Treatment. Biomedicines 2024, 12, 1936. [Google Scholar] [CrossRef]
- Cavallino, C.; Franzino, M.; Abdirashid, M.; Maltese, L.; Bacci, E.; Rametta, F.; Ugo, F. Novel Challenges and Therapeutic Options for Pulmonary Embolism and Deep Vein Thrombosis. J. Pers. Med. 2024, 14, 885. [Google Scholar] [CrossRef]
- Cordeanu, E.-M.; Lambach, H.; Heitz, M.; Di Cesare, J.; Mirea, C.; Faller, A.-M.; Cavaro, A.-C.; Frantz, A.-S.; Gaertner, S.; Schini-Kerth, V.; et al. Pulmonary Embolism and Coexisting Deep Vein Thrombosis: A Detrimental Association? J. Clin. Med. 2019, 8, 899. [Google Scholar] [CrossRef]
- Benincasa, G.; Costa, D.; Infante, T.; Lucchese, R.; Donatelli, F.; Napoli, C. Interplay between Genetics and Epigenetics in Modulating the Risk of Venous Thromboembolism: A New Challenge for Personalized Therapy. Thromb. Res. 2019, 177, 145–153. [Google Scholar] [CrossRef]
- ten Cate, V.; Rapp, S.; Schulz, A.; Robles, A.P.; Jurk, K.; Koeck, T.; Espinola-Klein, C.; Halank, M.; Seyfarth, H.-J.; Beutel, M.E.; et al. Circulating microRNAs Predict Recurrence and Death Following Venous Thromboembolism. J. Thromb. Haemost. 2023, 21, 2797–2810. [Google Scholar] [CrossRef]
- Kenneweg, F.; Hobohm, L.; Bang, C.; Gupta, S.K.; Xiao, K.; Thum, S.; Ten Cate, V.; Rapp, S.; Hasenfuß, G.; Wild, P.; et al. Circulating miR-Let7a Levels Predict Future Diagnosis of Chronic Thromboembolic Pulmonary Hypertension. Sci. Rep. 2024, 14, 4514. [Google Scholar] [CrossRef] [PubMed]
- Bocea, B.-A.; Catrina, B.-I.; Roman, M.-D.; Ion, N.C.I.; Fleaca, S.R.; Mohor, C.-I.; Raluca, A.O.; Moga, S.-I.; Mihaila, R.G. Incidence of Subclinical Deep Vein Thrombosis after Total Hip and Knee Arthroplasty Is Not Correlated with Number of Tranexamic Acid Doses. J. Clin. Med. 2024, 13, 3834. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Stevenson, H.C.S.; Yin, L.; Zhang, K.; Islam, Z.H.; Marcum, W.A.; Johnston, C.; Hoyt, N.; Kent, E.W.; et al. A Quantitative Method for the Evaluation of Deep Vein Thrombosis in a Murine Model Using Three-Dimensional Ultrasound Imaging. Biomedicines 2024, 12, 200. [Google Scholar] [CrossRef]
- Santana, D.C.; Emara, A.K.; Orr, M.N.; Klika, A.K.; Higuera, C.A.; Krebs, V.E.; Molloy, R.M.; Piuzzi, N.S. An Update on Venous Thromboembolism Rates and Prophylaxis in Hip and Knee Arthroplasty in 2020. Medicina 2020, 56, 416. [Google Scholar] [CrossRef] [PubMed]
- Anghel, L.; Tudurachi, B.-S.; Leonte, A.; Sascău, R.A.; Zota, I.M.; Bazyani, A.; Tinică, G.; Stătescu, C. The Challenge of High Coronary Thrombotic Events in Patients with ST-Segment Elevation Myocardial Infarction and COVID-19. J. Clin. Med. 2022, 11, 6542. [Google Scholar] [CrossRef]
- Malik, A.; Ha, N.B.; Barnes, G.D. Choice and Duration of Anticoagulation for Venous Thromboembolism. J. Clin. Med. 2024, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, M.; Camporese, G.; Di Micco, P.; Martini, R.; Ageno, W.; Prandoni, P. Treatment of Superficial Vein Thrombosis: Recent Advances, Unmet Needs and Future Directions. Healthcare 2024, 12, 1517. [Google Scholar] [CrossRef]
- Hvas, C.L.; Larsen, J.B. The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 14179. [Google Scholar] [CrossRef]
- Koami, H.; Sakamoto, Y.; Hirota, Y.; Sasaki, A.; Ogawa, H.; Furukawa, Y.; Matsuoka, A.; Shinada, K.; Nakayama, K.; Sakurai, R.; et al. Effect of Hypofibrinolysis on Clinical Outcomes of Patients with Septic Disseminated Intravascular Coagulation. Thromb. Res. 2025, 245, 109235. [Google Scholar] [CrossRef]
- Eichinger, S.; Traby, L.; Heinze, G.; Kollars, M.; Eischer, L.; Kyrle, P.A. Hypofibrinolysis and the Risk of Recurrent Venous Thromboembolism: A Prospective Cohort Study. Blood 2011, 118, 3331. [Google Scholar] [CrossRef]
- Hügle, T.; Nasi, S.; Ehirchiou, D.; Omoumi, P.; So, A.; Busso, N. Fibrin Deposition Associates with Cartilage Degeneration in Arthritis. eBioMedicine 2022, 81, 104081. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet Activation and Prothrombotic Mediators at the Nexus of Inflammation and Atherosclerosis: Potential Role of Antiplatelet Agents. Blood Rev. 2021, 45, 100694. [Google Scholar] [CrossRef] [PubMed]
- Morath, O.; Hoffmann, J.; Schilling, K.; Hochhaus, A.; Rachow, T.; Lang, S.M. Venous and Arterial Thromboembolism in Lung Cancer Patients: A Retrospective Analysis. J. Clin. Med. 2024, 13, 3773. [Google Scholar] [CrossRef]
- Xu, Y.; Carrier, M.; Kimpton, M. Arterial Thrombosis in Patients with Cancer. Cancers 2024, 16, 2238. [Google Scholar] [CrossRef]
- Kuijpers, M.J.E.; Heemskerk, J.W.M.; Jurk, K. Molecular Mechanisms of Hemostasis, Thrombosis and Thrombo-Inflammation. Int. J. Mol. Sci. 2022, 23, 5825. [Google Scholar] [CrossRef]
- Navarro, S.; Stegner, D.; Nieswandt, B.; Heemskerk, J.W.M.; Kuijpers, M.J.E. Temporal Roles of Platelet and Coagulation Pathways in Collagen- and Tissue Factor-Induced Thrombus Formation. Int. J. Mol. Sci. 2022, 23, 358. [Google Scholar] [CrossRef]
- Rocans, R.P.; Zarins, J.; Bine, E.; Mahauri, I.; Deksnis, R.; Citovica, M.; Donina, S.; Vanags, I.; Gravelsina, S.; Vilmane, A.; et al. Von Willebrand Factor Antigen, Biomarkers of Inflammation, and Microvascular Flap Thrombosis in Reconstructive Surgery. J. Clin. Med. 2024, 13, 5411. [Google Scholar] [CrossRef] [PubMed]
- Babkina, A.S.; Ostrova, I.V.; Yadgarov, M.Y.; Kuzovlev, A.N.; Grechko, A.V.; Volkov, A.V.; Golubev, A.M. The Role of Von Willebrand Factor in the Pathogenesis of Pulmonary Vascular Thrombosis in COVID-19. Viruses 2022, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-C.; Wilkie, L.; Kurosawa, T.A.; Dobromylskyj, M.; Priestnall, S.L.; Luis Fuentes, V.; Connolly, D.J. Immunohistological Evaluation of Von Willebrand Factor in the Left Atrial Endocardium and Atrial Thrombi from Cats with Cardiomyopathy. Animals 2021, 11, 1240. [Google Scholar] [CrossRef]
- Colonne, C.K.; Favaloro, E.J.; Pasalic, L. The Intriguing Connections between von Willebrand Factor, ADAMTS13 and Cancer. Healthcare 2022, 10, 557. [Google Scholar] [CrossRef]
- Castelli, R.; Berzuini, A.; Manetti, R.; Delitala, A.P.; Castro, D.; Sanna, G.; Sircana, M.C.; Profili, N.I.; Bartoli, A.; La Cava, L.; et al. ADAMTS13, von Willebrand Factor, Platelet Microparticles, Factor VIII, and Impact of Somatic Mutations in the Pathogenesis of Splanchnic Vein Thrombosis Associated with BCR-ABL-Negative Myeloproliferative Neoplasms. Life 2024, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.M.; Thomas, K.A.; Rassam, R.M.G.; Mihalko, E.P.; Daniel, C.; Sullenger, B.A.; Spinella, P.C.; Nimjee, S.M. Dose-Dependent Von Willebrand Factor Inhibition by Aptamer BB-031 Correlates with Thrombolysis in a Microfluidic Model of Arterial Occlusion. Pharmaceuticals 2022, 15, 1450. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.E. Von Willebrand Factor, ADAMTS13, and Thrombotic Thrombocytopenic Purpura. Blood 2008, 112, 11–18. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Al-Koussa, H.; AlZaim, I.; El-Sabban, M.E. Pathophysiology of Coagulation and Emerging Roles for Extracellular Vesicles in Coagulation Cascades and Disorders. J. Clin. Med. 2022, 11, 4932. [Google Scholar] [CrossRef]
- De Pablo-Moreno, J.A.; Serrano, L.J.; Revuelta, L.; Sánchez, M.J.; Liras, A. The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V. Int. J. Mol. Sci. 2022, 23, 8283. [Google Scholar] [CrossRef]
- Wilhelm, G.; Mertowska, P.; Mertowski, S.; Przysucha, A.; Strużyna, J.; Grywalska, E.; Torres, K. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int. J. Mol. Sci. 2023, 24, 12563. [Google Scholar] [CrossRef]
- Muttiah, B.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles. Biomedicines 2024, 12, 1850. [Google Scholar] [CrossRef]
- Richman, T.R.; Ermer, J.A.; Baker, J.; Siira, S.J.; Kile, B.T.; Linden, M.D.; Rackham, O.; Filipovska, A. Mitochondrial Gene Expression Is Required for Platelet Function and Blood Clotting. Cell Rep. 2023, 42, 113312. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial Cells and Coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Ng, J.Y.; D’Souza, M.; Hutani, F.; Choi, P. Management of Heparin-Induced Thrombocytopenia: A Contemporary Review. J. Clin. Med. 2024, 13, 4686. [Google Scholar] [CrossRef] [PubMed]
- Kyrle, P.A.; Eichinger, S. Deep Vein Thrombosis. Lancet 2005, 365, 1163–1174. [Google Scholar] [CrossRef]
- Zang, L.; Zhu, H.; Wang, K.; Liu, Y.; Yu, F.; Zhao, W. Not Just Anticoagulation—New and Old Applications of Heparin. Molecules 2022, 27, 6968. [Google Scholar] [CrossRef]
- Jiang, S.Z.; To, J.L.; Hughes, M.R.; McNagny, K.M.; Kim, H. Platelet Signaling at the Nexus of Innate Immunity and Rheumatoid Arthritis. Front. Immunol. 2022, 13, 977828. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, P.; Sharma, C.; Osmolovskiy, A. Fibrin and Fibrinolytic Enzyme Cascade in Thrombosis: Unravelling the Role. Life 2023, 13, 2196. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Jia, X.; Li, R.; Chen, J.; Liu, X.; Song, B.; Zhong, S.; Qi, Y. Anticoagulant and Fibrinolytic Properties of Two Heparinoid Compounds Prepared from Shrimp Waste. Foods 2023, 12, 66. [Google Scholar] [CrossRef]
- Jourdi, G.; Lordkipanidzé, M.; Philippe, A.; Bachelot-Loza, C.; Gaussem, P. Current and Novel Antiplatelet Therapies for the Treatment of Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 13079. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, V.; Sperandeo, L.; Gerardi, D.; Di Fazio, A.; Stabile, E. Antiplatelet Therapy for Elderly Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 4229. [Google Scholar] [CrossRef]
- Virk, H.U.H.; Escobar, J.; Rodriguez, M.; Bates, E.R.; Khalid, U.; Jneid, H.; Birnbaum, Y.; Levine, G.N.; Smith, S.C.; Krittanawong, C. Dual Antiplatelet Therapy: A Concise Review for Clinicians. Life 2023, 13, 1580. [Google Scholar] [CrossRef]
- Sohn, M.; Lim, S. The Role of Cilostazol, a Phosphodiesterase-3 Inhibitor, in the Development of Atherosclerosis and Vascular Biology: A Review with Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 2593. [Google Scholar] [CrossRef]
- Armstrong, P.C.J.; Leadbeater, P.D.; Chan, M.V.; Kirkby, N.S.; Jakubowski, J.A.; Mitchell, J.A.; Warner, T.D. In the Presence of Strong P2Y12 Receptor Blockade, Aspirin Provides Little Additional Inhibition of Platelet Aggregation. J. Thromb. Haemost. 2011, 9, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Andò, G.; De Santis, G.A.; Greco, A.; Pistelli, L.; Francaviglia, B.; Capodanno, D.; De Caterina, R.; Capranzano, P. P2Y12 Inhibitor or Aspirin Following Dual Antiplatelet Therapy After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2022, 15, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Gragnano, F.; Cao, D.; Pirondini, L.; Franzone, A.; Kim, H.-S.; von Scheidt, M.; Pettersen, A.-Å.R.; Zhao, Q.; Woodward, M.; Chiarito, M.; et al. P2Y12 Inhibitor or Aspirin Monotherapy for Secondary Prevention of Coronary Events. J. Am. Coll. Cardiol. 2023, 82, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Shadick, D.N.A.; Karlson, D.E.W.; Cook, D.N.R.; Maher, D.N.E.; Buring, D.J.E.; Lee, D.I.-M. Low-Dose Aspirin in the Primary Prevention of Rheumatoid Arthritis: The Women’s Health Study. Arthritis Care Res. 2010, 62, 545. [Google Scholar] [CrossRef]
- Lin, C.-R.; Tsai, S.H.L.; Wang, C.; Lee, C.-L.; Hung, S.-W.; Ting, Y.-T.; Hung, Y.C. Willow Bark (Salix Spp.) Used for Pain Relief in Arthritis: A Meta-Analysis of Randomized Controlled Trials. Life 2023, 13, 2058. [Google Scholar] [CrossRef]
- Nappi, F. P2Y12 Receptor Inhibitor for Antiaggregant Therapies: From Molecular Pathway to Clinical Application. Int. J. Mol. Sci. 2024, 25, 7575. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, F.-L.; Li, S.-C. Efficacy and Safety of Thrombin-Receptor Antagonist (Atopaxar and Vorapaxar) in Patients with Acute Coronary Syndrome or Coronary Artery Disease—A Meta-Analysis of Randomized Controlled Trials. Value Health Reg. Issues 2015, 6, 22–32. [Google Scholar] [CrossRef]
- Lucena, F.; McDougall, J.J. Protease Activated Receptors and Arthritis. Int. J. Mol. Sci. 2021, 22, 9352. [Google Scholar] [CrossRef]
- Wallace, E.L.; Smyth, S.S. Targeting Platelet Thrombin Receptor Signaling to Prevent Thrombosis. Pharmaceuticals 2013, 6, 915–928. [Google Scholar] [CrossRef]
- Jastrzebski, S.; Kalinowski, J.; Mun, S.; Shin, B.; Adapala, N.S.; Jacome-Galarza, C.E.; Mirza, F.; Aguila, H.L.; Drissi, H.; Sanjay, A.; et al. Protease-Activated Receptor 1 Deletion Causes Enhanced Osteoclastogenesis in Response to Inflammatory Signals through a Notch2-Dependent Mechanism. J. Immunol. 2019, 203, 105–116. [Google Scholar] [CrossRef]
- Billi, A.C.; Ludwig, J.E.; Fritz, Y.; Rozic, R.; Swindell, W.R.; Tsoi, L.C.; Gruzska, D.; Abdollahi-Roodsaz, S.; Xing, X.; Diaconu, D.; et al. KLK6 Expression in Skin Induces PAR1-Mediated Psoriasiform Dermatitis and Inflammatory Joint Disease. J. Clin. Investig. 2020, 130, 3151–3157. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Crosbie, L.; Song, H.-J.; Zhang, X.; Horgan, G.; Duttaroy, A.K. A Randomised Controlled Trial Comparing a Dietary Antiplatelet, the Water-Soluble Tomato Extract Fruitflow, with 75 Mg Aspirin in Healthy Subjects. Eur. J. Clin. Nutr. 2017, 71, 723–730. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, N.; Duss, R.; Duttaroy, A.K. Dietary Antiplatelets: A New Perspective on the Health Benefits of the Water-Soluble Tomato Concentrate Fruitflow®. Nutrients 2021, 13, 2184. [Google Scholar] [CrossRef] [PubMed]
- Duttaroy, A.K. Functional Foods in Preventing Human Blood Platelet Hyperactivity-Mediated Diseases—An Updated Review. Nutrients 2024, 16, 3717. [Google Scholar] [CrossRef]
- Golanski, J.; Szymanska, P.; Rozalski, M. Effects of Omega-3 Polyunsaturated Fatty Acids and Their Metabolites on Haemostasis—Current Perspectives in Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 2394. [Google Scholar] [CrossRef]
- Reiner, M.F.; Bertschi, D.A.; Werlen, L.; Wiencierz, A.; Aeschbacher, S.; Lee, P.; Rodondi, N.; Moutzouri, E.; Bonati, L.; Reichlin, T.; et al. Omega-3 Fatty Acids and Markers of Thrombosis in Patients with Atrial Fibrillation. Nutrients 2024, 16, 178. [Google Scholar] [CrossRef]
- Lu, L.W.; Quek, S.-Y.; Lu, S.-P.; Chen, J.-H. Potential Benefits of Omega-3 Polyunsaturated Fatty Acids (N3PUFAs) on Cardiovascular Health Associated with COVID-19: An Update for 2023. Metabolites 2023, 13, 630. [Google Scholar] [CrossRef]
- Koutsaliaris, I.K.; Pantazi, D.; Tsouka, A.N.; Argyropoulou, O.; Tellis, C.C.; Tselepis, A.D. Differential Effect of Omega-3 Fatty Acids on Platelet Inhibition by Antiplatelet Drugs In Vitro. Int. J. Mol. Sci. 2024, 25, 10136. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Rangarajan, S.; Orujyan, D.; Rangchaikul, P.; Radwan, M.M. Critical Role of Inflammation and Specialized Pro-Resolving Mediators in the Pathogenesis of Atherosclerosis. Biomedicines 2022, 10, 2829. [Google Scholar] [CrossRef]
- Irún, P.; Carrera-Lasfuentes, P.; Sánchez-Luengo, M.; Belio, Ú.; Domper-Arnal, M.J.; Higuera, G.A.; Hawkins, M.; de la Rosa, X.; Lanas, A. Pharmacokinetics and Changes in Lipid Mediator Profiling after Consumption of Specialized Pro-Resolving Lipid-Mediator-Enriched Marine Oil in Healthy Subjects. Int. J. Mol. Sci. 2023, 24, 16143. [Google Scholar] [CrossRef] [PubMed]
- Díaz del Campo, L.S.; Rodrigues-Díez, R.; Salaices, M.; Briones, A.M.; García-Redondo, A.B. Specialized Pro-Resolving Lipid Mediators: New Therapeutic Approaches for Vascular Remodeling. Int. J. Mol. Sci. 2022, 23, 3592. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized Pro-Resolving Mediators in Neuroinflammation: Overview of Studies and Perspectives of Clinical Applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef]
- Sousa, A.B.; Barbosa, J.N. The Use of Specialized Pro-Resolving Mediators in Biomaterial-Based Immunomodulation. J. Funct. Biomater. 2023, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Gomez, E.A.; Jouvene, C.C. Utility of the Specialized Pro-Resolving Mediators as Diagnostic and Prognostic Biomarkers in Disease. Biomolecules 2022, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. Molecular Pharmacology of Inflammation Resolution in Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 4808. [Google Scholar] [CrossRef]
- Tang, S.; Wan, M.; Huang, W.; Stanton, R.C.; Xu, Y. Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediat. Inflamm. 2018, 2018, 2380319. [Google Scholar] [CrossRef]
- Yang, S.; Zheng, Y.; Hou, X. Lipoxin A4 Restores Oxidative Stress-Induced Vascular Endothelial Cell Injury and Thrombosis-Related Factor Expression by Its Receptor-Mediated Activation of Nrf2-HO-1 Axis. Cell. Signal. 2019, 60, 146–153. [Google Scholar] [CrossRef]
- Undas, A.; Gissel, M.; Kwasny-Krochin, B.; Gluszko, P.; Mann, K.G.; Brummel-Ziedins, K.E. Thrombin Generation in Rheumatoid Arthritis: Dependence on Plasma Factor Composition. Thromb. Haemost. 2010, 104, 224. [Google Scholar] [CrossRef]
- Boos, C.J.; Hinton, A.; Lip, G.Y.H. Ximelagatran: A Clinical Perspective. Eur. J. Intern. Med. 2005, 16, 267–278. [Google Scholar] [CrossRef]
- Ansell, J. Factor Xa or Thrombin: Is Factor Xa a Better Target? J. Thromb. Haemost. 2007, 5, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Poenou, G.; Heestermans, M.; Lafaie, L.; Accassat, S.; Moulin, N.; Rodière, A.; Petit, B.; Duvillard, C.; Mismetti, P.; Bertoletti, L. Inhibition of Factor XI: A New Era in the Treatment of Venous Thromboembolism in Cancer Patients? Int. J. Mol. Sci. 2023, 24, 14433. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Szewczyk, E.M.; Gwozdzinski, K. Factors Affecting the Formation and Treatment of Thrombosis by Natural and Synthetic Compounds. Int. J. Mol. Sci. 2020, 21, 7975. [Google Scholar] [CrossRef]
- Umurungi, J.; Ferrando, F.; Cilloni, D.; Sivera, P. Cerebral Vein Thrombosis and Direct Oral Anticoagulants: A Review. J. Clin. Med. 2024, 13, 4730. [Google Scholar] [CrossRef]
- Lucà, F.; Oliva, F.; Abrignani, M.G.; Di Fusco, S.A.; Parrini, I.; Canale, M.L.; Giubilato, S.; Cornara, S.; Nesti, M.; Rao, C.M.; et al. Management of Patients Treated with Direct Oral Anticoagulants in Clinical Practice and Challenging Scenarios. J. Clin. Med. 2023, 12, 5955. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.; Jourdi, G.; Gendron, N.; Helley, D.; Gaussem, P.; Darnige, L. Recent Advances in Anticoagulant Treatment of Immune Thrombosis: A Focus on Direct Oral Anticoagulants in Heparin-Induced Thrombocytopenia and Anti-Phospholipid Syndrome. Int. J. Mol. Sci. 2022, 23, 93. [Google Scholar] [CrossRef]
- Semb, A.G.; Rollefstad, S.; Sexton, J.; Ikdahl, E.; Crowson, C.S.; van Riel, P.; Kitas, G.; Graham, I.; Kerola, A.M.; Athanasios Karpouzas, G.; et al. Oral Anticoagulant Treatment in Rheumatoid Arthritis Patients with Atrial Fibrillation Results of an International Audit. IJC Heart Vasc. 2022, 42, 101117. [Google Scholar] [CrossRef]
- Kellermair, L.; Zeller, M.W.G.; Kulyk, C.; Tomasits, J.; von Oertzen, T.J.; Vosko, M.R. Dabigatran in Cerebral Sinus Vein Thrombosis and Thrombophilia. Life 2022, 12, 970. [Google Scholar] [CrossRef]
- Santini, P.; Mosoni, C.; D’Errico, A.; Porceddu, E.; Lupascu, A.; Valeriani, E.; Tondi, P.; Pola, R.; Porfidia, A. Low-Dose Rivaroxaban to Prevent Recurrences of Venous Thromboembolism in Cancer: A Real-Life Experience with a Focus on Female Patients. J. Clin. Med. 2023, 12, 6427. [Google Scholar] [CrossRef]
- Bortoluzzi, C.; Bernardi, E.; Camporese, G.; Noventa, F.; Ceccato, D.; Tonello, C.; Vo Hong, N.; Campello, E.; Simion, C.; Imbalzano, E.; et al. Safety and Efficacy of Rivaroxaban for Extended-Phase Anticoagulation of Patients with Unprovoked or Recurrent Venous Thromboembolism: Real-Life Data from the MAC Project. Life 2022, 12, 1657. [Google Scholar] [CrossRef]
- Alshaya, O.A.; Korayem, G.B.; Al Yami, M.S.; Qudayr, A.H.; Althewaibi, S.; Fetyani, L.; Alshehri, S.; Alnashmi, F.; Albasseet, M.; Alshehri, L.; et al. Comparative Effectiveness of Apixaban and Rivaroxaban Lead-in Dosing in VTE Treatment: Observational Multicenter Real-World Study. J. Clin. Med. 2023, 12, 199. [Google Scholar] [CrossRef]
- Dalmau Llorca, M.R.; Aguilar Martín, C.; Carrasco-Querol, N.; Hernández Rojas, Z.; Forcadell Drago, E.; Rodríguez Cumplido, D.; Pepió Vilaubí, J.M.; Castro Blanco, E.; Gonçalves, A.Q.; Fernández-Sáez, J. Oral Anticoagulant Adequacy in Non-Valvular Atrial Fibrillation in Primary Care: A Cross-Sectional Study Using Real-World Data (Fantas-TIC Study). Int. J. Environ. Res. Public Health 2021, 18, 2244. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Gohel, M.; Baekgaard, N.; Bauersachs, R.; Bellmunt-Montoya, S.; Black, S.A.; ten Cate-Hoek, A.J.; Elalamy, I.; Enzmann, F.K.; Geroulakos, G.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 9–82. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.E.; Abdel-Reheim, M.A.; Morsy, M.A.; El-Daly, M.; Atwa, G.M.K.; Yahya, G.; Cavalu, S.; Saber, S.; Ahmed Gaafar, A.G. Ameliorative Effect of Dabigatran on CFA-Induced Rheumatoid Arthritis via Modulating Kallikrein-Kinin System in Rats. Int. J. Mol. Sci. 2022, 23, 10297. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Bottino, R.; D’Andrea, A.; Russo, V. Direct Oral Anticoagulants for Stroke Prevention in Special Populations: Beyond the Clinical Trials. Biomedicines 2023, 11, 131. [Google Scholar] [CrossRef]
- Kessler, A.; Kolben, Y.; Puris, G.; Ellis, M.; Alperin, M.; Simovich, V.; Lerman Shivek, H.; Muszkat, M.; Maaravi, Y.; Biton, Y. Direct Oral Anticoagulants in Special Patient Populations. J. Clin. Med. 2024, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Elango, K.; Javaid, A.; Khetarpal, B.K.; Ramalingam, S.; Kolandaivel, K.P.; Gunasekaran, K.; Ahsan, C. The Effects of Warfarin and Direct Oral Anticoagulants on Systemic Vascular Calcification: A Review. Cells 2021, 10, 773. [Google Scholar] [CrossRef]
- Karakasis, P.; Ktenopoulos, N.; Pamporis, K.; Sagris, M.; Soulaidopoulos, S.; Gerogianni, M.; Leontsinis, I.; Giannakoulas, G.; Tousoulis, D.; Fragakis, N.; et al. Efficacy and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients (BMI ≥ 30 Kg/M2) with Atrial Fibrillation or Venous Thromboembolism: An Updated Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 3784. [Google Scholar] [CrossRef]
- Oberemok, V.V.; Andreeva, O.; Laikova, K.; Alieva, E.; Temirova, Z. Rheumatoid Arthritis Has Won the Battle but Not the War: How Many Joints Will We Save Tomorrow? Medicina 2023, 59, 1853. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Azuma, C.; Uehara, F.; Toma, T.; Higa, K.; Yabiku, H.; Matsuura, N.; Inoue, Y.; Sunami, H. Management of Rheumatoid Arthritis: Possibilities and Challenges of Mesenchymal Stromal/Stem Cell-Based Therapies. Cells 2023, 12, 1905. [Google Scholar] [CrossRef]
- Choi, H.K.; Rho, Y.-H.; Zhu, Y.; Cea-Soriano, L.; Aviña-Zubieta, J.A.; Zhang, Y. The Risk of Pulmonary Embolism and Deep Vein Thrombosis in Rheumatoid Arthritis: A UK Population-Based Outpatient Cohort Study. Ann. Rheum. Dis. 2013, 72, 1182–1187. [Google Scholar] [CrossRef]
- Singh, S.; Tiwary, N.; Sharma, N.; Behl, T.; Antil, A.; Anwer, M.K.; Ramniwas, S.; Sachdeva, M.; Elossaily, G.M.; Gulati, M.; et al. Integrating Nanotechnological Advancements of Disease-Modifying Anti-Rheumatic Drugs into Rheumatoid Arthritis Management. Pharmaceuticals 2024, 17, 248. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Plewa, P.; Bratborska, A.W.; Bakinowska, E.; Pawlik, A. JAK Inhibitors in Rheumatoid Arthritis: Immunomodulatory Properties and Clinical Efficacy. Int. J. Mol. Sci. 2024, 25, 8327. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Li Gobbi, F.; Damiani, A.; Russo, E.; Guiducci, S.; Manfredi, M.; Lari, B.; Grossi, V.; Infantino, M. Real-Life Comparison of Four JAK Inhibitors in Rheumatoid Arthritis (ELECTRA-i Study). J. Clin. Med. 2024, 13, 1821. [Google Scholar] [CrossRef] [PubMed]
- Venetsanopoulou, A.I.; Alamanos, Y.; Voulgari, P.V.; Drosos, A.A. Epidemiology and Risk Factors for Rheumatoid Arthritis Development. Mediterr. J. Rheumatol. 2023, 34, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Floudas, A.; Canavan, M.; McGarry, T.; Mullan, R.; Nagpal, S.; Veale, D.J.; Fearon, U. ACPA Status Correlates with Differential Immune Profile in Patients with Rheumatoid Arthritis. Cells 2021, 10, 647. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid Arthritis: Pathological Mechanisms and Modern Pharmacologic Therapies. Bone Res. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Li, K.; Wang, M.; Zhao, L.; Liu, Y.; Zhang, X. ACPA-Negative Rheumatoid Arthritis: From Immune Mechanisms to Clinical Translation. eBioMedicine 2022, 83, 104233. [Google Scholar] [CrossRef]
- Seegobin, S.D.; Ma, M.H.Y.; Dahanayake, C.; Cope, A.P.; Scott, D.L.; Lewis, C.M.; Scott, I.C. ACPA-Positive and ACPA-Negative Rheumatoid Arthritis Differ in Their Requirements for Combination DMARDs and Corticosteroids: Secondary Analysis of a Randomized Controlled Trial. Arthritis Res. Ther. 2014, 16, R13. [Google Scholar] [CrossRef]
- Cunningham, K.Y.; Hur, B.; Gupta, V.K.; Arment, C.A.; Wright, K.A.; Mason, T.G.; Peterson, L.S.; Bekele, D.I.; Schaffer, D.E.; Bailey, M.L.; et al. Patients with ACPA-Positive and ACPA-Negative Rheumatoid Arthritis Show Different Serological Autoantibody Repertoires and Autoantibody Associations with Disease Activity. Sci. Rep. 2023, 13, 5360. [Google Scholar] [CrossRef]
- Curran, A.M.; Naik, P.; Giles, J.T.; Darrah, E. PAD Enzymes in Rheumatoid Arthritis: Pathogenic Effectors and Autoimmune Targets. Nat. Rev. Rheumatol. 2020, 16, 301–315. [Google Scholar] [CrossRef]
- Kurowska, W.; Slowinska, I.; Krogulec, Z.; Syrowka, P.; Maslinski, W. Antibodies to Citrullinated Proteins (ACPA) Associate with Markers of Osteoclast Activation and Bone Destruction in the Bone Marrow of Patients with Rheumatoid Arthritis. J. Clin. Med. 2021, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, I.; Rovero, P.; Hansen, P.R.; Frederiksen, J.L.; Houen, G.; Trier, N.H. Reactivity of Rheumatoid Arthritis-Associated Citrulline-Dependent Antibodies to Epstein-Barr Virus Nuclear Antigen1-3. Antibodies 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Abbasifard, M.; Imani, D.; Bagheri-Hosseinabadi, Z. PTPN22 Gene Polymorphism and Susceptibility to Rheumatoid Arthritis (RA): Updated Systematic Review and Meta-Analysis. J. Gene Med. 2020, 22, e3204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Huang, C.-M.; Liu, T.-Y.; Wu, N.; Chan, C.-J.; Shih, P.-Y.; Chen, H.-H.; Chen, S.-Y.; Tsai, F.-J. Effects of Human Leukocyte Antigen DRB1 Genetic Polymorphism on Anti-Cyclic Citrullinated Peptide (ANTI-CCP) and Rheumatoid Factor (RF) Expression in Rheumatoid Arthritis (RA) Patients. Int. J. Mol. Sci. 2023, 24, 12036. [Google Scholar] [CrossRef]
- De Stefano, L.; D’Onofrio, B.; Manzo, A.; Montecucco, C.; Bugatti, S. The Genetic, Environmental, and Immunopathological Complexity of Autoantibody-Negative Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 12386. [Google Scholar] [CrossRef]
- Semb, A.G.; Ikdahl, E.; Wibetoe, G.; Crowson, C.; Rollefstad, S. Atherosclerotic Cardiovascular Disease Prevention in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2020, 16, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, N.; Avina-Galindo, A.M.; Zheng, Y.; Lacaille, D.; Esdaile, J.M.; Choi, H.K.; Aviña-Zubieta, J.A. The Risk and Trend of Pulmonary Embolism and Deep Vein Thrombosis in Rheumatoid Arthritis: A General Population-Based Study. Rheumatology 2021, 60, 188–195. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Malsagova, K.A.; Kopylov, A.T.; Rudnev, V.R.; Karateev, D.E.; Markelova, E.I.; Luchikhina, E.L.; Borisova, E.E.; Kaysheva, A.L. Determination of Heterogeneous Proteomic and Metabolomic Response in Anti-TNF and Anti-IL-6 Treatment of Patients with Rheumatoid Arthritis. Life 2023, 13, 596. [Google Scholar] [CrossRef]
- Pandolfi, F.; Franza, L.; Carusi, V.; Altamura, S.; Andriollo, G.; Nucera, E. Interleukin-6 in Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 5238. [Google Scholar] [CrossRef]
- Beyene, R.T.; Kavalukas, S.L.; Barbul, A. Intra-Abdominal Adhesions: Anatomy, Physiology, Pathophysiology, and Treatment. Curr. Probl. Surg. 2015, 52, 271–319. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Muscoli, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Paone, S.; Ilari, S.; Mollace, R.; Palma, E.; Mollace, V. Endothelial Dysfunction and Extra-Articular Neurological Manifestations in Rheumatoid Arthritis. Biomolecules 2021, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Komici, K.; Perna, A.; Rocca, A.; Bencivenga, L.; Rengo, G.; Guerra, G. Endothelial Progenitor Cells and Rheumatoid Arthritis: Response to Endothelial Dysfunction and Clinical Evidences. Int. J. Mol. Sci. 2021, 22, 13675. [Google Scholar] [CrossRef]
- O’Hehir, Z.D.; Lynch, T.; O’Neill, S.; March, L.; Xue, M. Endothelial Protein C Receptor and Its Impact on Rheumatic Disease. J. Clin. Med. 2024, 13, 2030. [Google Scholar] [CrossRef]
- Peshkova, A.D.; Evdokimova, T.A.; Sibgatullin, T.B.; Ataullakhanov, F.I.; Litvinov, R.I.; Weisel, J.W. Accelerated Spatial Fibrin Growth and Impaired Contraction of Blood Clots in Patients with Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 9434. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.B.; Pippen, A.M.M.; Greenberg, C.S. Extravascular Fibrin Formation and Dissolution in Synovial Tissue of Patients with Osteoarthritis and Rheumatoid Arthritis. Arthritis Rheum. 1991, 34, 996–1005. [Google Scholar] [CrossRef]
- Zacharski, L.R.; Brown, F.E.; Memoli, V.A.; Kisiel, W.; Kudryk, B.J.; Rousseau, S.M.; Hunt, J.A.; Dunwiddie, C.; Nutt, E.M. Pathways of Coagulation Activation in Situ in Rheumatoid Synovial Tissue. Clin. Immunol. Immunopathol. 1992, 63, 155–162. [Google Scholar] [CrossRef]
- Hernández-Gilsoul, T.; Atisha-Fregoso, Y.; Vargas-Ruíz, A.G.; Rivero-Sigarroa, E.; Dominguez-Cherit, G.; Ñamendys-Silva, S.A. Pulmonary Hypertension Secondary to Hyperviscosity in a Patient with Rheumatoid Arthritis and Acquired von Willebrand Disease: A Case Report. J. Med. Case Rep. 2013, 7, 232. [Google Scholar] [CrossRef][Green Version]
- Rooney, T.; Scherzer, R.; Shigenaga, J.K.; Graf, J.; Imboden, J.B.; Grunfeld, C. Levels of Plasma Fibrinogen Are Elevated in Well-Controlled Rheumatoid Arthritis. Rheumatology 2011, 50, 1458–1465. [Google Scholar] [CrossRef]
- Gertz, M.A. Acute Hyperviscosity: Syndromes and Management. Blood 2018, 132, 1379–1385. [Google Scholar] [CrossRef]
- Krieger, E.; van Der Loo, B.; Amann-Vesti, B.R.; Rousson, V.; Koppensteiner, R. C-Reactive Protein and Red Cell Aggregation Correlate with Late Venous Function after Acute Deep Venous Thrombosis. J. Vasc. Surg. 2004, 40, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Mines, I.; Al-Abayechi, A.; Kaur, S.; Nooruddin, Z. Hyperviscosity Syndrome in Undifferentiated Connective Tissue Disease: A Diagnostic and Therapeutic Challenge. Cureus 2024, 16, e55399. [Google Scholar] [CrossRef]
- Caraba, A.; Stancu, O.; Crișan, V.; Georgescu, D. Anti TNF-Alpha Treatment Improves Microvascular Endothelial Dysfunction in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2024, 25, 9925. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, L.J.S.; Nunes-Souza, V.; Goulart, M.O.F.; Rabelo, L.A. Oxidative Stress in Rheumatoid Arthritis: What the Future Might Hold Regarding Novel Biomarkers and Add-On Therapies. Oxidative Med. Cell. Longev. 2019, 2019, 7536805. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Nasir, B.; Haq, I.U.; Kim, S.J. Oxidative Stress, Consequences and ROS Mediated Cellular Signaling in Rheumatoid Arthritis. Chem.-Biol. Interact. 2018, 281, 121–136. [Google Scholar] [CrossRef]
- Bordy, R.; Totoson, P.; Prati, C.; Marie, C.; Wendling, D.; Demougeot, C. Microvascular Endothelial Dysfunction in Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2018, 14, 404–420. [Google Scholar] [CrossRef]
- Philippoteaux, C.; Deprez, V.; Nottez, A.; Cailliau, E.; Houvenagel, E.; Deprez, X.; Philippe, P.; Pascart, T.; Flipo, R.-M.; Goëb, V.; et al. Characteristics of Patients Treated with JAK Inhibitors in Rheumatoid Arthritis before versus after VTE Risk Warnings. J. Clin. Med. 2023, 12, 207. [Google Scholar] [CrossRef]
- Chen, C.-P.; Kung, P.-T.; Chou, W.-Y.; Tsai, W.-C. Effect of Introducing Biologics to Patients with Rheumatoid Arthritis on the Risk of Venous Thromboembolism: A Nationwide Cohort Study. Sci. Rep. 2021, 11, 17009. [Google Scholar] [CrossRef]
- Conforti, A.; Berardicurti, O.; Pavlych, V.; Di Cola, I.; Cipriani, P.; Ruscitti, P. Incidence of Venous Thromboembolism in Rheumatoid Arthritis, Results from a “Real-Life” Cohort and an Appraisal of Available Literature. Medicine 2021, 100, e26953. [Google Scholar] [CrossRef]
- Mahler, B.; Moșteanu, M.I.; Bobocea, R.; Negoescu, I.; Mircea, L.F.; Tudor, A.; Bogdan, M.T.; Croitoru, A.; Marghescu, A.S. Multiple Pulmonary Involvement in the Rapidly Progressive Evolution of Rheumatoid Arthritis. Diagnostics 2024, 14, 2175. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of Incident Cardiovascular Events in Patients with Rheumatoid Arthritis: A Meta-Analysis of Observational Studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z. Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases. Antibodies 2018, 7, 8. [Google Scholar] [CrossRef]
- González-Sierra, M.; Romo-Cordero, A.; Quevedo-Abeledo, J.C.; Quevedo-Rodríguez, A.; Gómez-Bernal, F.; de Vera-González, A.; López-Mejías, R.; Martín-González, C.; González-Gay, M.Á.; Ferraz-Amaro, I. Mean Platelet Volume in a Series of 315 Patients with Rheumatoid Arthritis: Relationship with Disease Characteristics, Including Subclinical Atherosclerosis and Cardiovascular Comorbidity. Diagnostics 2023, 13, 3208. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi, M.; Choi, J.Y.; Kim, J.Y.; Kim, J.Y.; Song, J.-S.; Ivashkiv, L.B.; Lee, E.Y. Implication of the Association of Fibrinogen Citrullination and Osteoclastogenesis in Bone Destruction in Rheumatoid Arthritis. Cells 2020, 9, 2720. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, C.A. Forty Years Since the Structural Elucidation of Platelet-Activating Factor (PAF): Historical, Current, and Future Research Perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef] [PubMed]
- Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Demopoulos, C.A. Protective Effect of Olive Oil Microconstituents in Atherosclerosis: Emphasis on PAF Implicated Atherosclerosis Theory. Biomolecules 2023, 13, 700. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Sethi, R.; Shah, S.; Jafri, K.; Duran, J.; Chang, Y.; Soni, C.; Wollocko, H. The Roles of Platelet-Activating Factor and Magnesium in Pathophysiology of Hypertension, Atherogenesis, Cardiovascular Disease, Stroke and Aging. Cardiogenetics 2022, 12, 49–62. [Google Scholar] [CrossRef]
- Lordan, R.; Zabetakis, I.; Tsoupras, A. Inflammation and Chronic Diseases: The Polar Lipid Link. Proceedings 2020, 70, 70. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. The Potential Role of Dietary Platelet-Activating Factor Inhibitors in Cancer Prevention and Treatment. Adv. Nutr. 2019, 10, 148–164. [Google Scholar] [CrossRef]
- Adamantidi, T.; Maris, G.; Altantsidou, P.; Tsoupras, A. Anti-Inflammatory Benefits of Vitamin D and Its Analogues against Glomerulosclerosis and Kidney Diseases. Sclerosis 2024, 2, 217–265. [Google Scholar] [CrossRef]
- Reznichenko, A.; Korstanje, R. The Role of Platelet-Activating Factor in Mesangial Pathophysiology. Am. J. Pathol. 2015, 185, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Kumar, K.; Shah, N. PAF Physiology in Target Organ Systems—A Deep Dive to Understand the PAF Mystery in Pathogenesis of Disease. Hearts 2021, 2, 551–560. [Google Scholar] [CrossRef]
- Liu, Y.; Shields, L.B.E.; Gao, Z.; Wang, Y.; Zhang, Y.P.; Chu, T.; Zhu, Q.; Shields, C.B.; Cai, J. Current Understanding of Platelet-Activating Factor Signaling in Central Nervous System Diseases. Mol. Neurobiol. 2017, 54, 5563–5572. [Google Scholar] [CrossRef]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef]
- Cheng, Q.; Wu, H.; Du, Y. The Roles of Small-Molecule Inflammatory Mediators in Rheumatoid Arthritis. Scand. J. Immunol. 2021, 93, e12982. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Invited Review: The Anti-Inflammatory Properties of Dairy Lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- Poutzalis, S.; Lordan, R.; Nasopoulou, C.; Zabetakis, I. Phospholipids of Goat and Sheep Origin: Structural and Functional Studies. Small Rumin. Res. 2018, 167, 39–47. [Google Scholar] [CrossRef]
- Jackson, C.M.; Esnouf, P.; Duewer, D.L. Thrombin: An Approach to Developing a Higher-Order Reference Material and Reference Measurement Procedure for Substance Identity, Amount, and Biological Activities. J. Res. Natl. Inst. Stand. Technol. 2020, 125, 125021. [Google Scholar] [CrossRef]
- Ogdie, A.; Kay McGill, N.; Shin, D.B.; Takeshita, J.; Jon Love, T.; Noe, M.H.; Chiesa Fuxench, Z.C.; Choi, H.K.; Mehta, N.N.; Gelfand, J.M. Risk of Venous Thromboembolism in Patients with Psoriatic Arthritis, Psoriasis and Rheumatoid Arthritis: A General Population-Based Cohort Study. Eur. Heart J. 2018, 39, 3608–3614. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Gong, M.; Gu, Y.; Zhang, H.; Dong, B.; Guo, Q.; Pang, X.; Xiang, Q.; He, X.; et al. Risk of Venous Thromboembolism with Janus Kinase Inhibitors in Inflammatory Immune Diseases: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1189389. [Google Scholar] [CrossRef]
- Kern, A.; Balog, A.; Dulic, S.; Barabás, E.; Kiszelák, M.; Vásárhelyi, B. Alterations of the Thrombin Generation Profile in Rheumatoid Arthritis. J. Thromb. Thrombolysis 2016, 41, 359–364. [Google Scholar] [CrossRef][Green Version]
- Entsie, P.; Kang, Y.; Amoafo, E.B.; Schöneberg, T.; Liverani, E. The Signaling Pathway of the ADP Receptor P2Y12 in the Immune System: Recent Discoveries and New Challenges. Int. J. Mol. Sci. 2023, 24, 6709. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The Etiology of Rheumatoid Arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Huang, Y.; Huang, R.-Y.; Chen, X.-M.; Zhou, Y.-Y.; Yu, W.-L.; Chu, Y.-L.; Huang, Q.-C. Determination of Role of Thromboxane A2 in Rheumatoid Arthritis. Discov. Med. 2015, 19, 23–32. [Google Scholar] [PubMed]
- Huang, Q.-C.; Huang, R.-Y. The Cyclooxygenase-2/Thromboxane A2 Pathway: A Bridge from Rheumatoid Arthritis to Lung Cancer? Cancer Lett. 2014, 354, 28–32. [Google Scholar] [CrossRef]
- Kaneda, K.; Yamashita, Y.; Morimoto, T.; Chatani, R.; Nishimoto, Y.; Ikeda, N.; Kobayashi, Y.; Ikeda, S.; Kim, K.; Inoko, M.; et al. Anticoagulation Strategies and Long-Term Recurrence in Patients with Venous Thromboembolism in the Era of Direct Oral Anticoagulants. Eur. J. Intern. Med. 2023, 118, 59–72. [Google Scholar] [CrossRef]
- Sun, Z.; Hesler, B.D.; Makarova, N.; Dalton, J.E.; Doan, M.; Moraska, A.; De Oliveira, G.; Turan, A. The Association Between Rheumatoid Arthritis and Adverse Postoperative Outcomes: A Retrospective Analysis. Anesth. Analg. 2016, 122, 1887–1893. [Google Scholar] [CrossRef]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 Guidelines for Management of Venous Thromboembolism: Prevention of Venous Thromboembolism in Surgical Hospitalized Patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; Nelson, M.E.; Wells, P.S.; Gould, M.K.; Dentali, F.; et al. Antithrombotic Therapy for VTE Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e419S–e496S. [Google Scholar] [CrossRef]
- Weitz, J.I.; Lensing, A.W.A.; Prins, M.H.; Bauersachs, R.; Beyer-Westendorf, J.; Bounameaux, H.; Brighton, T.A.; Cohen, A.T.; Davidson, B.L.; Decousus, H.; et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 2017, 376, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Ellis, A.; Shaffer, N.; Gurwitz, J.; Chandramohan, A.; Saulino, J.; Ishak, A.; Okubanjo, T.; Michota, F.; Hylek, E.; et al. Comparative Effectiveness of Venous Thromboembolism Prophylaxis Options for the Patient Undergoing Total Hip and Knee Replacement: A Network Meta-Analysis. J. Thromb. Haemost. 2017, 15, 284–294. [Google Scholar] [CrossRef]
- Gu, Z.-C.; Wei, A.-H.; Zhang, C.; Wang, X.-H.; Zhang, L.; Shen, L.; Li, Z.; Pan, M.-M.; Liu, X.-Y.; Pu, J.; et al. Risk of Major Gastrointestinal Bleeding With New vs Conventional Oral Anticoagulants: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2020, 18, 792–799.e61. [Google Scholar] [CrossRef]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Porcari, A.; Raskob, G.E.; Weitz, J.I. AMPLIFY-EXT Investigators Apixaban for Extended Treatment of Venous Thromboembolism. N. Engl. J. Med. 2013, 368, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ben Mrid, R.; Bouchmaa, N.; Ainani, H.; El Fatimy, R.; Malka, G.; Mazini, L. Anti-Rheumatoid Drugs Advancements: New Insights into the Molecular Treatment of Rheumatoid Arthritis. Biomed. Pharmacother. 2022, 151, 113126. [Google Scholar] [CrossRef] [PubMed]
- Bashir, U.; Singh, G.; Bhatia, A. Rheumatoid Arthritis-Recent Advances in Pathogenesis and the Anti-Inflammatory Effect of Plant-Derived COX Inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 5363–5385. [Google Scholar] [CrossRef]
- Khalil, N.A.; Ahmed, E.M.; Tharwat, T.; Mahmoud, Z. NSAIDs between Past and Present; a Long Journey towards an Ideal COX-2 Inhibitor Lead. RSC Adv. 2024, 14, 30647–30661. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.-E. Recent Development on COX-2 Inhibitors as Promising Anti-Inflammatory Agents: The Past 10 Years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Barrowman, J.; Wilson, M. Drugs Affecting Coagulation. Anaesth. Intensive Care Med. 2024, 25, 714–722. [Google Scholar] [CrossRef]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical Treatment of Osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef]
- Patrono, C. Cardiovascular Effects of Cyclooxygenase-2 Inhibitors: A Mechanistic and Clinical Perspective. Br. J. Clin. Pharmacol. 2016, 82, 957–964. [Google Scholar] [CrossRef] [PubMed]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef]
- Attia, J.Z.; Mansour, H.S. Perioperative Duloxetine and Etoricoxibto Improve Postoperative Pain after Lumbar Laminectomy: A Randomized, Double-Blind, Controlled Study. BMC Anesth. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Gumułka, P.; Dąbrowska, M.; Starek, M. Stability Study of Selected Coxibs Used in the Treatment of Rheumatoid Diseases in Various Drug Combinations. Processes 2023, 11, 2605. [Google Scholar] [CrossRef]
- Godbole, S.; Solomon, J.L.; Johnson, M.; Srivastava, A.; Carsons, S.E.; Belilos, E.; De Leon, J.; Reiss, A.B. Treating Cardiovascular Disease in the Inflammatory Setting of Rheumatoid Arthritis: An Ongoing Challenge. Biomedicines 2024, 12, 1608. [Google Scholar] [CrossRef]
- Higgins, P.D.R.; Skup, M.; Mulani, P.M.; Lin, J.; Chao, J. Increased Risk of Venous Thromboembolic Events With Corticosteroid vs Biologic Therapy for Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2015, 13, 316–321. [Google Scholar] [CrossRef]
- Stuijver, D.J.F.; Majoor, C.J.; van Zaane, B.; Souverein, P.C.; de Boer, A.; Dekkers, O.M.; Büller, H.R.; Gerdes, V.E.A. Use of Oral Glucocorticoids and the Risk of Pulmonary Embolism: A Population-Based Case-Control Study. Chest 2013, 143, 1337–1342. [Google Scholar] [CrossRef]
- Pietschmann, P.; Butylina, M.; Kerschan-Schindl, K.; Sipos, W. Mechanisms of Systemic Osteoporosis in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 8740. [Google Scholar] [CrossRef]
- Ruyssen-Witrand, A.; Brusq, C.; Masson, M.; Bongard, V.; Salliot, C.; Poiroux, L.; Nguyen, M.; Roux, C.H.; Richez, C.; Saraux, A.; et al. Comparison of Two Strategies of Glucocorticoid Withdrawal in Patients with Rheumatoid Arthritis in Low Disease Activity (STAR): A Randomised, Placebo- Controlled, Double-Blind Trial. Ann. Rheum. Dis. 2025, 84, 49–59. [Google Scholar] [CrossRef]
- Onuora, S. Tapering csDMARDs Leads to More RA Flares. Nat. Rev. Rheumatol. 2021, 17, 378. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, F.R.; Garufi, C.; Mancuso, S.; Ceccarelli, F.; Truglia, S.; Conti, F. Tapering and Discontinuation of Glucocorticoids in Patients with Rheumatoid Arthritis Treated with Tofacitinib. Sci. Rep. 2023, 13, 15537. [Google Scholar] [CrossRef]
- Padjen, I.; Crnogaj, M.R.; Anić, B. Conventional Disease-Modifying Agents in Rheumatoid Arthritis—A Review of Their Current Use and Role in Treatment Algorithms. Reumatologia 2020, 58, 390–400. [Google Scholar] [CrossRef]
- Skácelová, M.; Nekvindová, L.; Mann, H.; Závada, J.; Křístková, Z.; Vencovský, J.; Pavelka, K.; Horák, P.; the ATTRA Registry. The Beneficial Effect of csDMARDs Co-Medication on Drug Persistence of First-Line TNF Inhibitor in Rheumatoid Arthritis Patients: Data from Czech ATTRA Registry. Rheumatol. Int. 2022, 42, 803–814. [Google Scholar] [CrossRef]
- Majorczyk, E.; Mazurek-Mochol, M.; Pawlik, A.; Kuśnierczyk, P. Clinical Factors and the Outcome of Treatment with Methotrexate in Rheumatoid Arthritis: Role of Rheumatoid Factor, Erosive Disease and High Level of Erythrocyte Sedimentation Rate. J. Clin. Med. 2022, 11, 6078. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Balsa Criado, A.; Mongey, A.-B.; Avouac, J.; Marotte, H.; Mueller, R.B. How to Get the Most from Methotrexate (MTX) Treatment for Your Rheumatoid Arthritis Patient?—MTX in the Treat-to-Target Strategy. J. Clin. Med. 2019, 8, 515. [Google Scholar] [CrossRef]
- da Silva, J.C.; Mariz, H.A.; Júnior, L.F.d.R.; Oliveira, P.S.S.d.; Dantas, A.T.; Duarte, A.L.B.P.; Pitta, I.d.R.; Galdino, S.L.; Pitta, M.G.d.R. Hydroxychloroquine Decreases Th17-Related Cytokines in Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients. Clinics 2013, 68, 766. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J. Classification of Hydroxychloroquine Retinopathy: A Literature Review and Proposal for Revision. Diagnostics 2024, 14, 1803. [Google Scholar] [CrossRef]
- Hill, S.; Frey, N. Conventional Disease-Modifying Antirheumatic Drugs for the Treatment of Rheumatoid Arthritis; CADTH Health Technology Review; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2021.
- Bhavsar, S.V.; Movahedi, M.; Cesta, A.; Pope, J.E.; Bombardier, C. Retention of Triple Therapy with Methotrexate, Sulfasalazine, and Hydroxychloroquine Compared to Combination Methotrexate and Leflunomide in Rheumatoid Arthritis. Jt. Bone Spine 2024, 91, 105732. [Google Scholar] [CrossRef]
- Saquib, M.; Agnihotri, P.; Monu, X.; Biswas, S. Exogenous miRNA: A Perspective Role as Therapeutic in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Petrov, D.; Teodorescu, D.-S.; Buliga-Finis, O.N.; Ouatu, A.; Tudorancea, I.; Rezus, E.; Rezus, C. MicroRNAs (miRNAs) in Cardiovascular Complications of Rheumatoid Arthritis (RA): What Is New? Int. J. Mol. Sci. 2022, 23, 5254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Xie, H.; Hong, F.; Yang, S. Role of miRNAs in Rheumatoid Arthritis Therapy. Cells 2023, 12, 1749. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, B.; Song, Y.-K.; Oh, Y.-J.; Lee, E.B.; Kim, I.-W.; Oh, J.M. Association of Tumor Necrosis Factor Inhibitors with the Risk of Nontuberculous Mycobacterial Infection in Patients with Rheumatoid Arthritis: A Nationwide Cohort Study. J. Clin. Med. 2023, 12, 6998. [Google Scholar] [CrossRef]
- Imam, A.A. Anti-TNF Alpha and Risk of Lymphoma in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Medicina 2024, 60, 1156. [Google Scholar] [CrossRef]
- Wysocki, T.; Paradowska-Gorycka, A. Pharmacogenomics of Anti-TNF Treatment Response Marks a New Era of Tailored Rheumatoid Arthritis Therapy. Int. J. Mol. Sci. 2022, 23, 2366. [Google Scholar] [CrossRef]
- Muth, K.N.; Rech, J.; Losch, F.O.; Hoerning, A. Reversing the Inflammatory Process—25 Years of Tumor Necrosis Factor-α Inhibitors. J. Clin. Med. 2023, 12, 5039. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, N.; Wang, Z.; Su, J.; Yang, J.; Han, J.; Zhao, Y. Microneedle-Assisted Transdermal Delivery of Etanercept for Rheumatoid Arthritis Treatment. Pharmaceutics 2019, 11, 235. [Google Scholar] [CrossRef]
- Lim, H.; Lee, S.H.; Lee, H.T.; Lee, J.U.; Son, J.Y.; Shin, W.; Heo, Y.-S. Structural Biology of the TNFα Antagonists Used in the Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 768. [Google Scholar] [CrossRef]
- Kotyla, P.J. Bimodal Function of Anti-TNF Treatment: Shall We Be Concerned about Anti-TNF Treatment in Patients with Rheumatoid Arthritis and Heart Failure? Int. J. Mol. Sci. 2018, 19, 1739. [Google Scholar] [CrossRef]
- Pesce, B.; Ribeiro, C.H.; Larrondo, M.; Ramos, V.; Soto, L.; Catalán, D.; Aguillón, J.C. TNF-α Affects Signature Cytokines of Th1 and Th17 T Cell Subsets through Differential Actions on TNFR1 and TNFR2. Int. J. Mol. Sci. 2022, 23, 9306. [Google Scholar] [CrossRef] [PubMed]
- Koehm, M.; Foldenauer, A.C.; Rossmanith, T.; Alten, R.; Aringer, M.; Backhaus, M.; Burmester, G.R.; Feist, E.; Kellner, H.; Krueger, K.; et al. Effectiveness of Different Rituximab Doses Combined with Leflunomide in the Treatment or Retreatment of Rheumatoid Arthritis: Part 2 of a Randomized, Placebo-Controlled, Investigator-Initiated Clinical Trial (AMARA). J. Clin. Med. 2022, 11, 7316. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.G.; Dumitras, I.; Giurcaneanu, C.; Berghi, O.; Radaschin, D.S.; Vivisenco, C.I.; Popescu, M.N.; Beiu, C. Mechanisms of Resistance to Rituximab Used for the Treatment of Autoimmune Blistering Diseases. Life 2024, 14, 1223. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Thompson, L.; Giles, J.T.; Bathon, J.M.; Salmon, J.E.; Beaulieu, A.D.; Codding, C.E.; Carlson, T.H.; Delles, C.; Lee, J.S.; et al. Effect of Interleukin-6 Receptor Blockade on Surrogates of Vascular Risk in Rheumatoid Arthritis: MEASURE, a Randomised, Placebo-Controlled Study. Ann. Rheum. Dis. 2015, 74, 694–702. [Google Scholar] [CrossRef]
- Gualtierotti, R.; Ingegnoli, F.; Griffini, S.; Grovetti, E.; Meroni, P.L.; Cugno, M. Prothrombotic Biomarkers in Patients with Rheumatoid Arthritis: The Beneficial Effect of IL-6 Receptor Blockade. Clin. Exp. Rheumatol. 2016, 34, 451–458. [Google Scholar]
- Imamura, H.; Momohara, S.; Yano, K.; Sakuma, Y.; Nakayama, M.; Tobimatsu, H.; Ikari, K. Tocilizumab Treatment in Patients with Rheumatoid Arthritis Is Associated with Reduced Fibrinogen Levels and Increased Blood Loss after Total Knee Arthroplasty. Mod. Rheumatol. 2018, 28, 976–980. [Google Scholar] [CrossRef]
- Goldman, A.; Galper, B.-E.L.; Druyan, A.; Grossman, C.; Sharif, K.; Shechtman, L.; Moshkovits, Y.; Lahat, A.; Ben-Zvi, I. Adverse Cardiovascular Events in Rheumatoid Arthritis Patients Treated with JAK Inhibitors: An Analysis of Postmarketing Spontaneous Safety Reports. Semin. Arthritis Rheum. 2024, 67, 152461. [Google Scholar] [CrossRef]
- Yoshida, S.; Miyata, M.; Suzuki, E.; Kanno, T.; Sumichika, Y.; Saito, K.; Matsumoto, H.; Temmoku, J.; Fujita, Y.; Matsuoka, N.; et al. Incidence Rates of Infections in Rheumatoid Arthritis Patients Treated with Janus Kinase or Interleukin-6 Inhibitors: Results of a Retrospective, Multicenter Cohort Study. J. Clin. Med. 2024, 13, 3000. [Google Scholar] [CrossRef]
- Angelini, J.; Talotta, R.; Roncato, R.; Fornasier, G.; Barbiero, G.; Dal Cin, L.; Brancati, S.; Scaglione, F. JAK-Inhibitors for the Treatment of Rheumatoid Arthritis: A Focus on the Present and an Outlook on the Future. Biomolecules 2020, 10, 1002. [Google Scholar] [CrossRef]
- Wollenhaupt, J.; Lee, E.-B.; Curtis, J.R.; Silverfield, J.; Terry, K.; Soma, K.; Mojcik, C.; DeMasi, R.; Strengholt, S.; Kwok, K.; et al. Safety and Efficacy of Tofacitinib for up to 9.5 Years in the Treatment of Rheumatoid Arthritis: Final Results of a Global, Open-Label, Long-Term Extension Study. Arthritis Res. Ther. 2019, 21, 89. [Google Scholar] [CrossRef]
- Harigai, M. Growing Evidence of the Safety of JAK Inhibitors in Patients with Rheumatoid Arthritis. Rheumatology 2019, 58, i34–i42. [Google Scholar] [CrossRef]
- Harrington, R.; Harkins, P.; Conway, R. Janus Kinase Inhibitors in Rheumatoid Arthritis: An Update on the Efficacy and Safety of Tofacitinib, Baricitinib and Upadacitinib. J. Clin. Med. 2023, 12, 6690. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2018, 27, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.C.; Pushman, A.G. Current Concepts in the Management of the Rheumatoid Hand. J. Hand Surg. 2011, 36, 736–747. [Google Scholar] [CrossRef]
- Schindler, M.; Puchner, S.; Reinhard, J.; Leiss, F.; Windhager, R.; Lass, R. Recurrence-Free Survival after Synovectomy and Subsequent Radiosynoviorthesis in Patients with Synovitis of the Knee—A Retrospective Data Analysis. J. Clin. Med. 2024, 13, 601. [Google Scholar] [CrossRef] [PubMed]
- Rynecki, N.D.; Shankar, D.S.; Morgan, A.M.; Kouk, S.; Youm, T. Outcomes of Hip Arthroscopy in Patients with Systemic Inflammatory Diseases: A Matched Cohort 5-Year Follow-Up Study. Surgeries 2023, 4, 511–521. [Google Scholar] [CrossRef]
- Ortega-Avila, A.B.; Moreno-Velasco, A.; Cervera-Garvi, P.; Martinez-Rico, M.; Chicharro-Luna, E.; Gijon-Noqueron, G. Surgical Treatment for the Ankle and Foot in Patients with Rheumatoid Arthritis: A Systematic Review. J. Clin. Med. 2020, 9, 42. [Google Scholar] [CrossRef]
- Takakubo, Y.; Wanezaki, Y.; Oki, H.; Naganuma, Y.; Shibuya, J.; Honma, R.; Suzuki, A.; Satake, H.; Takagi, M. Forefoot Deformities in Patients with Rheumatoid Arthritis: Mid- to Long-Term Result of Joint-Preserving Surgery in Comparison with Resection Arthroplasty. Int. J. Environ. Res. Public Health 2021, 18, 11257. [Google Scholar] [CrossRef]
- Lee, H.; Ishikawa, H.; Shibuya, T.; Takai, C.; Nemoto, T.; Nomura, Y.; Abe, A.; Otani, H.; Ito, S.; Nakazono, K.; et al. The Combination of Modified Mitchell’s Osteotomy and Shortening Oblique Osteotomy for Patients with Rheumatoid Arthritis: An Analysis of Changes in Plantar Pressure Distribution. Int. J. Environ. Res. Public Health 2021, 18, 9948. [Google Scholar] [CrossRef]
- Matsumoto, T.; Maenohara, Y.; Chang, S.H.; Ono, K.; Omata, Y.; Hirose, J.; Tanaka, S. Outcomes of Scarf and Akin Osteotomy with Intra-Articular Stepwise Lateral Soft Tissue Release for Correcting Hallux Valgus Deformity in Rheumatoid Arthritis. Int. J. Environ. Res. Public Health 2021, 18, 10667. [Google Scholar] [CrossRef]
- Lesman, J.; Wojna, J.; Szkutnik, P.; Tomasik, B.; Domżalski, M.; Łaganowski, P. Silicone Arthroplasty as an Alternative to Arthrodesis in the Metatarsophalangeal Degenerative Disease of Hallux Valgus—A 5-Year Observational Study. J. Clin. Med. 2024, 13, 3677. [Google Scholar] [CrossRef] [PubMed]
- Perticarini, L.; Andriollo, L.; Righini, R.; Sangaletti, R.; Benazzo, F. Trends in Hip Arthroplasty Cementation: Insights from an Italian Registry of 142,113 Patients. Prosthesis 2024, 6, 1329–1339. [Google Scholar] [CrossRef]
- de Souza, D.N.; Lorentz, N.A.; Charalambous, L.; Galetta, M.; Petrilli, C.; Rozell, J.C. Comprehensive Pain Management in Total Joint Arthroplasty: A Review of Contemporary Approaches. J. Clin. Med. 2024, 13, 6819. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Bianchi, L.; Di Martino, A.; Faldini, C.; Ursini, F. Is Total Joint Arthroplasty an Effective and Safe Option for Psoriatic Arthritis Patients? A Scoping Review. J. Clin. Med. 2024, 13, 5552. [Google Scholar] [CrossRef]
- Yano, K.; Ikari, K.; Tobimatsu, H.; Tominaga, A.; Okazaki, K. Joint-Preserving Surgery for Forefoot Deformities in Patients with Rheumatoid Arthritis: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 4093. [Google Scholar] [CrossRef]
- Wang, J.; He, L.; Li, W.; Lv, S. A Role of IL-17 in Rheumatoid Arthritis Patients Complicated with Atherosclerosis. Front. Pharmacol. 2022, 13, 828933. [Google Scholar] [CrossRef]
- Cafaro, G.; Petito, E.; Bistoni, O.; Falcinelli, E.; Cipriani, S.; Borghi, M.C.; Bonifacio, A.F.; Giglio, E.; Alunno, A.; Perricone, C.; et al. Methotrexate Improves Endothelial Function in Early Rheumatoid Arthritis Patients after 3 Months of Treatment. Arthritis. Res. Ther. 2022, 24, 236. [Google Scholar] [CrossRef]
- Gualtierotti, R.; Ingegnoli, F.; Boscolo, M.; Griffini, S.; Grovetti, E.; Cugno, M. Tocilizumab Effects on Coagulation Factor XIII in Patients with Rheumatoid Arthritis. Adv. Ther. 2019, 36, 3494–3502. [Google Scholar] [CrossRef]
- Ancuța, C.; Chirieac, R.; Ancuța, E.; Țănculescu, O.; Solomon, S.M.; Fătu, A.M.; Doloca, A.; Iordache, C. Exploring the Role of Interleukin-6 Receptor Inhibitor Tocilizumab in Patients with Active Rheumatoid Arthritis and Periodontal Disease. J. Clin. Med. 2021, 10, 878. [Google Scholar] [CrossRef]
- Sánchez-Piedra, C.; Sueiro-Delgado, D.; García-González, J.; Ros-Vilamajo, I.; Prior-Español, A.; Moreno-Ramos, M.J.; Garcia-Magallon, B.; Calvo-Gutiérrez, J.; Perez-Vera, Y.; Martín-Domenech, R.; et al. Changes in the Use Patterns of bDMARDs in Patients with Rheumatic Diseases over the Past 13 Years. Sci. Rep. 2021, 11, 15051. [Google Scholar] [CrossRef]
- Benjamin, O.; Goyal, A.; Lappin, S.L. Disease-Modifying Antirheumatic Drugs (DMARD). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Radić, M.; Đogaš, H.; Vrkić, K.; Gelemanović, A.; Marinović, I.; Perković, D.; Nazlić, J.; Radić, J.; Krstulović, D.M.; Meštrović, J. Prescription Trends of Biologic DMARDs in Treating Rheumatologic Diseases: Changes of Medication Availability in COVID-19. J. Pers. Med. 2023, 13, 1199. [Google Scholar] [CrossRef]
- Parolini, C. The Role of Marine N-3 Polyunsaturated Fatty Acids in Inflammatory-Based Disease: The Case of Rheumatoid Arthritis. Mar. Drugs 2024, 22, 17. [Google Scholar] [CrossRef]
- Ponkilainen, V.; Karjalainen, T.V.; Kuitunen, I.; Uimonen, M.; Johnston, R.V.; Saarinen, A.; Whittle, S.L.; Avery, J.C.; Glennon, V.; Grobler, L.; et al. Perioperative Use of Disease Modifying Anti‐rheumatic Drugs (DMARDs) in People with Inflammatory Arthritis. Cochrane Database Syst. Rev. 2022, 2022, CD015096. [Google Scholar] [CrossRef]
- Suto, T.; Okamura, K.; Sakane, H.; Okura, C.; Kaneko, T.; Chikuda, H. The Impact of bDMARDs on Postoperative Complications in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Medicine 2023, 102, e36132. [Google Scholar] [CrossRef]
- Mondillo, G.; Colosimo, S.; Perrotta, A.; Frattolillo, V.; Gicchino, M.F. Unveiling Artificial Intelligence’s Power: Precision, Personalization, and Progress in Rheumatology. J. Clin. Med. 2024, 13, 6559. [Google Scholar] [CrossRef]
- Batko, B.; Rolska-Wójcik, P.; Władysiuk, M. Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. Int. J. Environ. Res. Public Health 2019, 16, 2966. [Google Scholar] [CrossRef]
- Esbensen, B.A.; Kennedy, N.; Brodin, N. Prevention and Adherence in Rheumatic and Musculoskeletal Disease. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101525. [Google Scholar] [CrossRef]
- Benucci, M.; Bardelli, M.; Cazzato, M.; Bartoli, F.; Damiani, A.; Li Gobbi, F.; Bandinelli, F.; Panaccione, A.; Di Cato, L.; Niccoli, L.; et al. Efficacy and Safety of Filgotinib in Rheumatoid Arthritis Patients Aged over and under 65 Years (ENANTIA-65). J. Pers. Med. 2024, 14, 712. [Google Scholar] [CrossRef]
- Ikari, Y.; Isozaki, T.; Tsubokura, Y.; Kasama, T. Peficitinib Inhibits the Chemotactic Activity of Monocytes via Proinflammatory Cytokine Production in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. Cells 2019, 8, 561. [Google Scholar] [CrossRef]
- Murayama, M.A.; Shimizu, J.; Miyabe, C.; Yudo, K.; Miyabe, Y. Chemokines and Chemokine Receptors as Promising Targets in Rheumatoid Arthritis. Front. Immunol. 2023, 14, 1100869. [Google Scholar] [CrossRef]
- Deviatkin, A.A.; Vakulenko, Y.A.; Akhmadishina, L.V.; Tarasov, V.V.; Beloukhova, M.I.; Zamyatnin, A.A., Jr.; Lukashev, A.N. Emerging Concepts and Challenges in Rheumatoid Arthritis Gene Therapy. Biomedicines 2020, 8, 9. [Google Scholar] [CrossRef]
- Taylor, P.C.; Weinblatt, M.E.; McInnes, I.B.; Atsumi, T.; Strand, V.; Takeuchi, T.; Bracher, M.; Brooks, D.; Davies, J.; Goode, C.; et al. Anti-GM-CSF Otilimab versus Sarilumab or Placebo in Patients with Rheumatoid Arthritis and Inadequate Response to Targeted Therapies: A Phase III Randomised Trial (contRAst 3). Ann. Rheum. Dis. 2023, 82, 1527–1537. [Google Scholar] [CrossRef]
- Guccione, M.; Ettari, R.; Taliani, S.; Da Settimo, F.; Zappalà, M.; Grasso, S. G-Protein-Coupled Receptor Kinase 2 (GRK2) Inhibitors: Current Trends and Future Perspectives. J. Med. Chem. 2016, 59, 9277–9294. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Wu, L.; Zhang, M.; Hu, S.; Wang, R.; Han, Y.; Wu, Y.; Zhang, L.; Wang, X.; et al. Paroxetine Alleviates T Lymphocyte Activation and Infiltration to Joints of Collagen-Induced Arthritis. Sci. Rep. 2017, 7, 45364. [Google Scholar] [CrossRef]
- Hara, K.; Togashi, M. Successful Treatment of Multiple Sclerosis with Refractory Rheumatoid Arthritis Using Ofatumumab: A Case Report. Clin. Exp. Neuroimmunol. 2024, 15, 122–125. [Google Scholar] [CrossRef]
- Khodadust, F.; Ezdoglian, A.; Steinz, M.M.; van Beijnum, J.R.; Zwezerijnen, G.J.C.; Jansen, G.; Tas, S.W.; van der Laken, C.J. Systematic Review: Targeted Molecular Imaging of Angiogenesis and Its Mediators in Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Queiro, R.; Aurrecoechea, E.; Castro, S.A.; Blanco, I.V.; Brandy-Garcia, A.; Linge, R. Interleukin-17–Targeted Treatment in Patients with Spondyloarthritis and Associated Cardiometabolic Risk Profile. Front. Immunol. 2023, 14, 1203372. [Google Scholar] [CrossRef]
- Zhao, S.S.; Riley, D.; Austin, P.; Hernandez, G.; Alam, U. Pos0231 Comparative Safety of Jak Inhibitors Versus Tnf or Il-17 Inhibitors for Cardiovascular Disease, Venous Thromboembolism and Cancer in Psoriatic Arthritis and Axial Spondyloarthritis. Ann. Rheum. Dis. 2024, 83, 352. [Google Scholar] [CrossRef]
- Santos-Sierra, S. Targeting Toll-like Receptor (TLR) Pathways in Inflammatory Arthritis: Two Better Than One? Biomolecules 2021, 11, 1291. [Google Scholar] [CrossRef]
- Bakinowska, E.; Bratborska, A.W.; Kiełbowski, K.; Ćmil, M.; Biniek, W.J.; Pawlik, A. The Role of Mesenchymal Stromal Cells in the Treatment of Rheumatoid Arthritis. Cells 2024, 13, 915. [Google Scholar] [CrossRef]
- Jonny; Sitepu, E.C.; Nidom, C.A.; Wirjopranoto, S.; Sudiana, I.K.; Ansori, A.N.M.; Putranto, T.A. Ex Vivo-Generated Tolerogenic Dendritic Cells: Hope for a Definitive Therapy of Autoimmune Diseases. Curr. Issues Mol. Biol. 2024, 46, 4035–4048. [Google Scholar] [CrossRef]
- Moe, R.H.; Vliet Vlieland, T.P.M. Current and Future Challenges for Rehabilitation for Inflammatory Arthritis. J. Clin. Med. 2024, 13, 1808. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Li, X.; Li, G.; Yang, B.; Lu, X.; Gao, Y.; Sun, F. Nano-Based Co-Delivery System for Treatment of Rheumatoid Arthritis. Molecules 2022, 27, 5973. [Google Scholar] [CrossRef]
- Hosseinikhah, S.M.; Barani, M.; Rahdar, A.; Madry, H.; Arshad, R.; Mohammadzadeh, V.; Cucchiarini, M. Nanomaterials for the Diagnosis and Treatment of Inflammatory Arthritis. Int. J. Mol. Sci. 2021, 22, 3092. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L.; Duarte, N. Triterpenes as Potential Drug Candidates for Rheumatoid Arthritis Treatment. Life 2023, 13, 1514. [Google Scholar] [CrossRef]
- Bakinowska, E.; Stańska, W.; Kiełbowski, K.; Szwedkowicz, A.; Boboryko, D.; Pawlik, A. Gut Dysbiosis and Dietary Interventions in Rheumatoid Arthritis—A Narrative Review. Nutrients 2024, 16, 3215. [Google Scholar] [CrossRef]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- Navarini, L.; Afeltra, A.; Gallo Afflitto, G.; Margiotta, D.P.E. Polyunsaturated Fatty Acids: Any Role in Rheumatoid Arthritis? Lipids Health Dis. 2017, 16, 197. [Google Scholar] [CrossRef]
- Sigaux, J.; Mathieu, S.; Nguyen, Y.; Sanchez, P.; Letarouilly, J.-G.; Soubrier, M.; Czernichow, S.; Flipo, R.-M.; Sellam, J.; Daïen, C. Impact of Type and Dose of Oral Polyunsaturated Fatty Acid Supplementation on Disease Activity in Inflammatory Rheumatic Diseases: A Systematic Literature Review and Meta-Analysis. Arthritis Res. Ther. 2022, 24, 100. [Google Scholar] [CrossRef]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and Cardiovascular Disease: Are Marine Phospholipids the Answer? Food Funct 2020, 11, 2861–2885. [Google Scholar] [CrossRef]
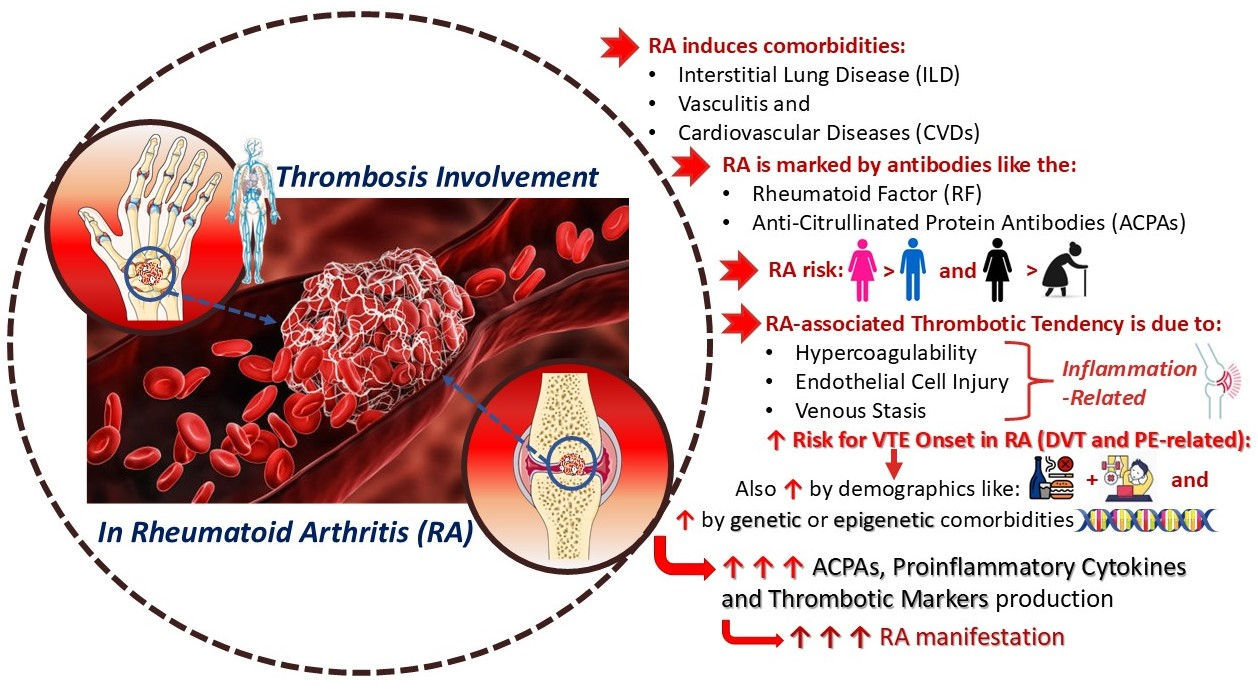
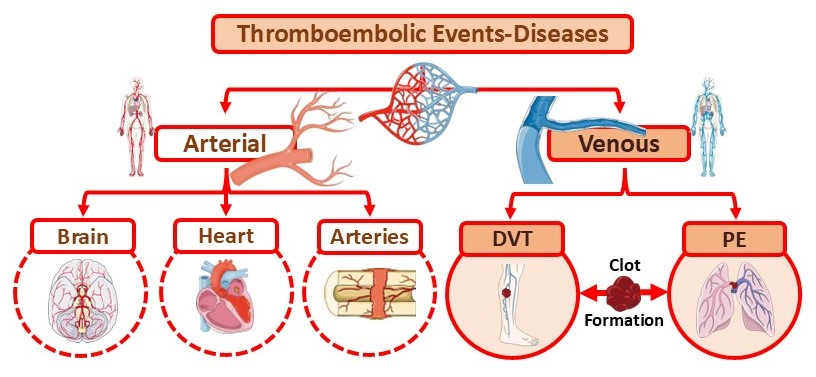
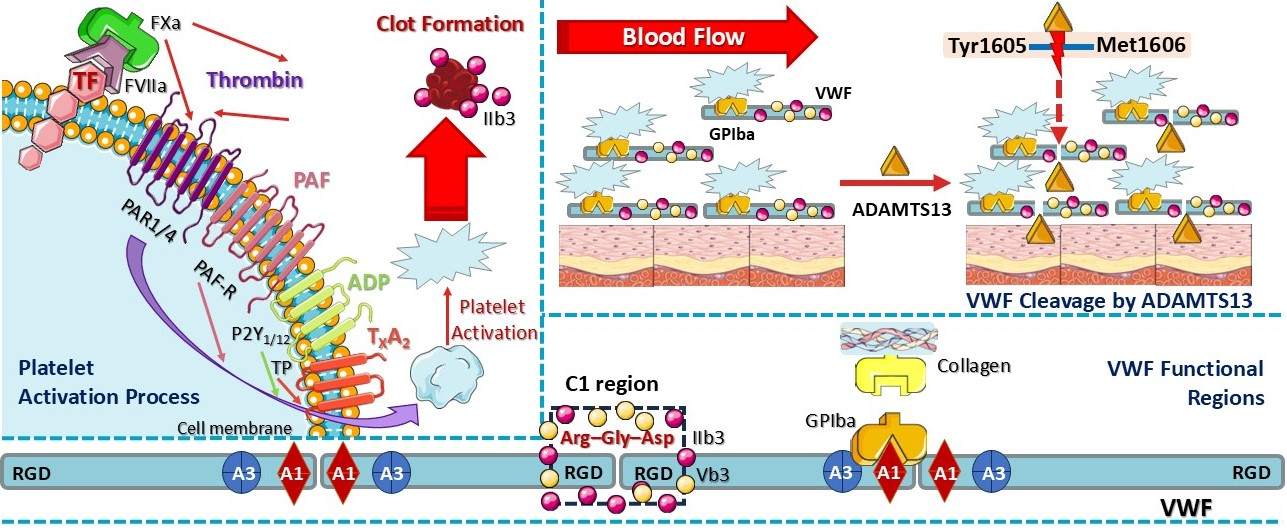
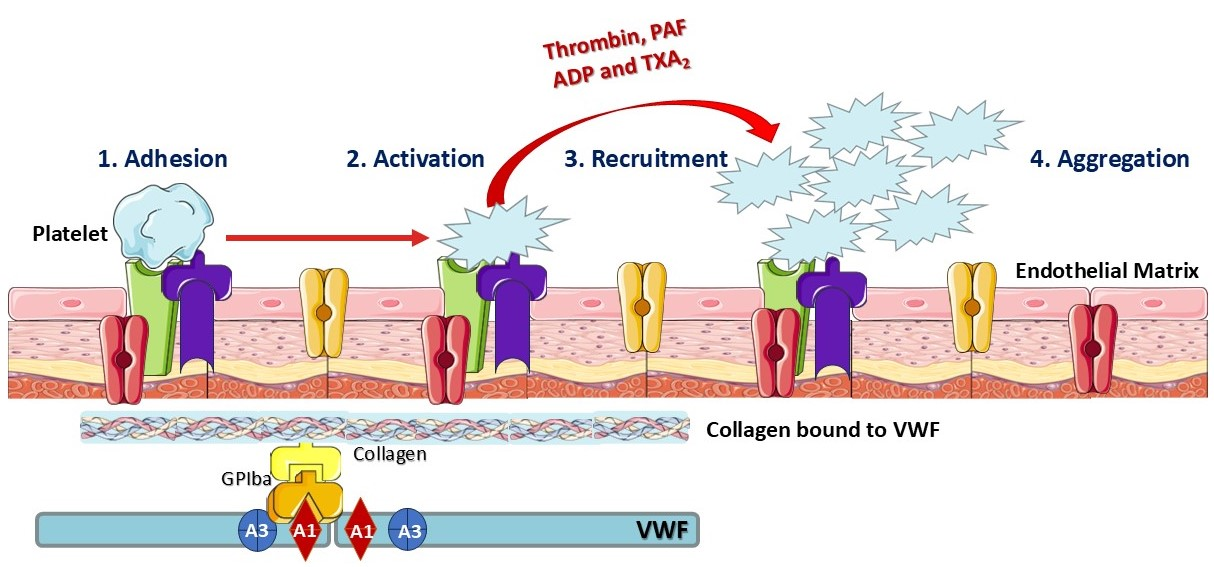



| Mechanisms of RA-Associated Thrombosis 1 | Description | Therapeutic Approaches | Adverse Effects and Risks | References |
|---|---|---|---|---|
| Endothelial Injury |
|
|
| [8,38,80,94,160,162,163,164,173,229,276,277] |
| Hypercoagulability |
|
|
| [38,61,165,166,167,173,256,278,279] |
| Venous Stasis |
|
|
| [8,35,38,86,168,169,170,172,173,176,177] |
| Medication-induced Risk |
|
|
| [38,173,177,178,179,227,245,261] |
| Platelet Activation |
|
|
| [8,38,62,91,99,167,173,203,205,205,206,233,254,278] |
| PAF Activation |
|
|
| [27,62,109,173,185,195,196,197,199] |
| Inflammatory Cytokines |
|
|
| [38,143,144,158,159,160,177,201,232,233,238,244,245,246,249,250,251,256,259,261,262,280,281,282] |
| Oxidative Stress |
|
|
| [38,105,107,108,147,157,174,175,176,283] |
| Surgery-related Risks |
|
|
| [17,133,208,263,266,271,274,275,284,285] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamantidi, T.; Pisioti, M.S.; Pitsouni, S.; Maria, C.; Georgios, K.; Dania, V.; Vordos, N.; Krokidis, X.; Tsoupras, A. The Inflammatory Link of Rheumatoid Arthritis and Thrombosis: Pathogenic Molecular Circuits and Treatment Approaches. Curr. Issues Mol. Biol. 2025, 47, 291. https://doi.org/10.3390/cimb47040291
Adamantidi T, Pisioti MS, Pitsouni S, Maria C, Georgios K, Dania V, Vordos N, Krokidis X, Tsoupras A. The Inflammatory Link of Rheumatoid Arthritis and Thrombosis: Pathogenic Molecular Circuits and Treatment Approaches. Current Issues in Molecular Biology. 2025; 47(4):291. https://doi.org/10.3390/cimb47040291
Chicago/Turabian StyleAdamantidi, Theodora, Maria Stavroula Pisioti, Sofia Pitsouni, Chatzikamari Maria, Karamanis Georgios, Vasiliki Dania, Nikolaos Vordos, Xenophon Krokidis, and Alexandros Tsoupras. 2025. "The Inflammatory Link of Rheumatoid Arthritis and Thrombosis: Pathogenic Molecular Circuits and Treatment Approaches" Current Issues in Molecular Biology 47, no. 4: 291. https://doi.org/10.3390/cimb47040291
APA StyleAdamantidi, T., Pisioti, M. S., Pitsouni, S., Maria, C., Georgios, K., Dania, V., Vordos, N., Krokidis, X., & Tsoupras, A. (2025). The Inflammatory Link of Rheumatoid Arthritis and Thrombosis: Pathogenic Molecular Circuits and Treatment Approaches. Current Issues in Molecular Biology, 47(4), 291. https://doi.org/10.3390/cimb47040291









