Fraxin Alleviates Atherosclerosis by Inhibiting Oxidative Stress and Inflammatory Responses via the TLR4/PI3K/Akt Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Potential Targets of Fraxin
2.2. Collection of Potential Targets of AS
2.3. Construction of Protein–Protein Interaction Network
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analyses
2.5. Molecular Docking
2.6. Cell Viability Assay
2.7. Oil-Red O Staining
2.8. Quantification of Total Cholesterol (TC) Content
2.9. Determination of Glutathione (GSH) and Malondialdehyde (MDA) Content
2.10. RNA Extraction and qRT–PCR Analysis
2.11. Western Blot Assay
2.12. ROS Detection Assay
2.13. Data Processing and Statistical Analyses
3. Results
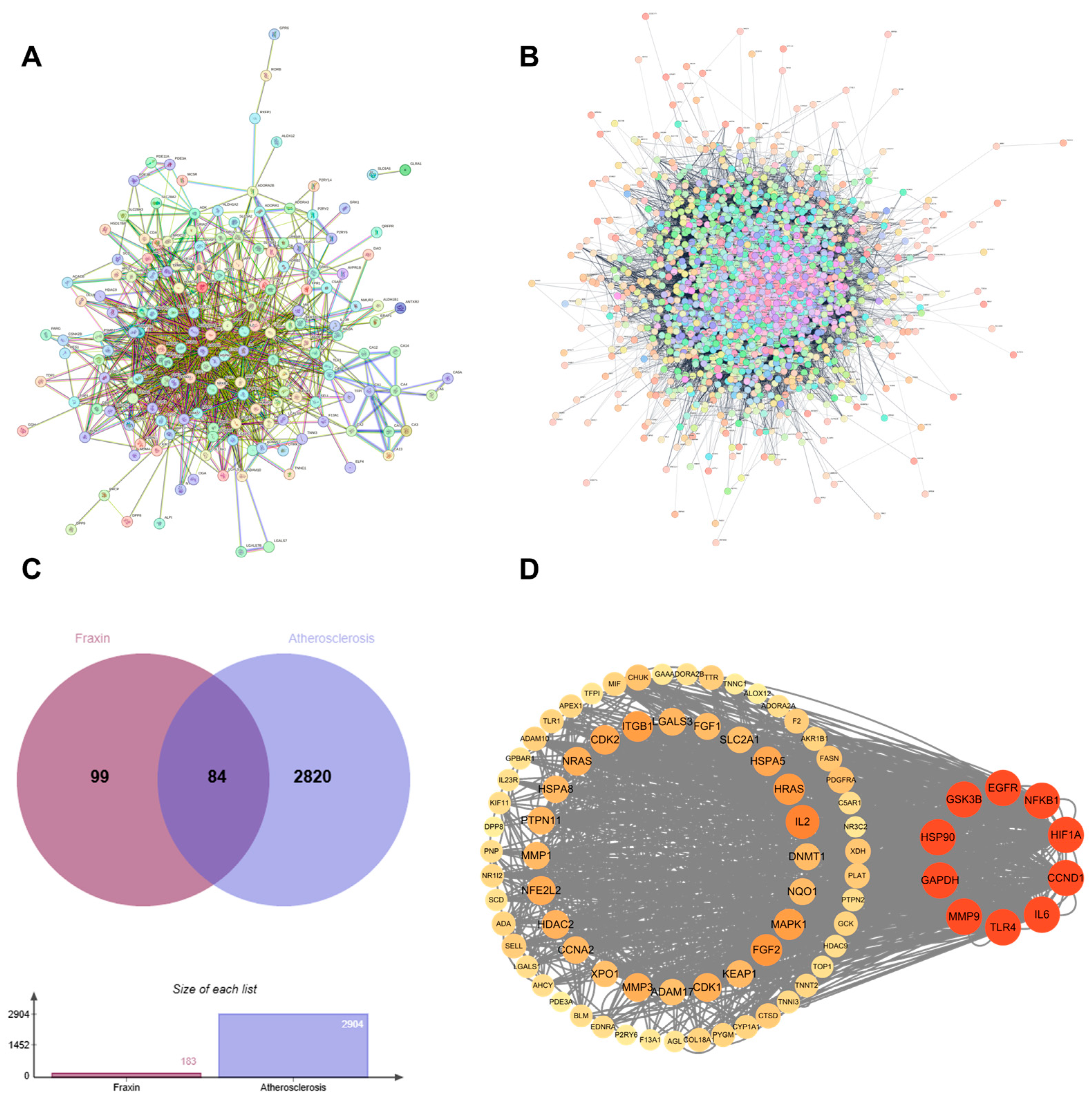
3.1. Network Pharmacology Prediction
3.1.1. Identification of Targets of Fraxin Against AS
3.1.2. Protein–Protein Interaction Network
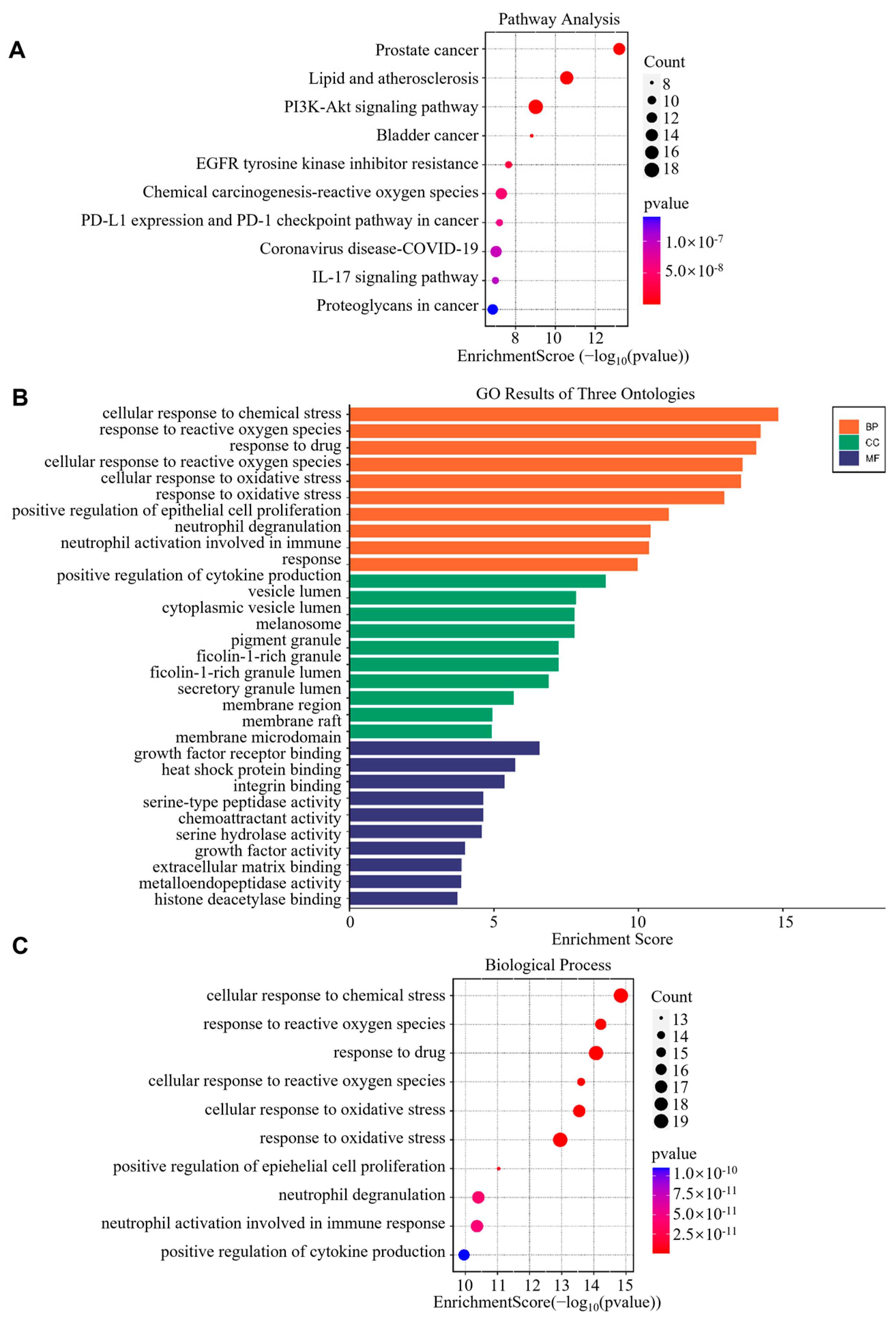
3.1.3. GO and KEGG Pathway Enrichment Analysis
3.1.4. Verification of Results by Molecular Docking
3.2. In Vitro Experimental Verification
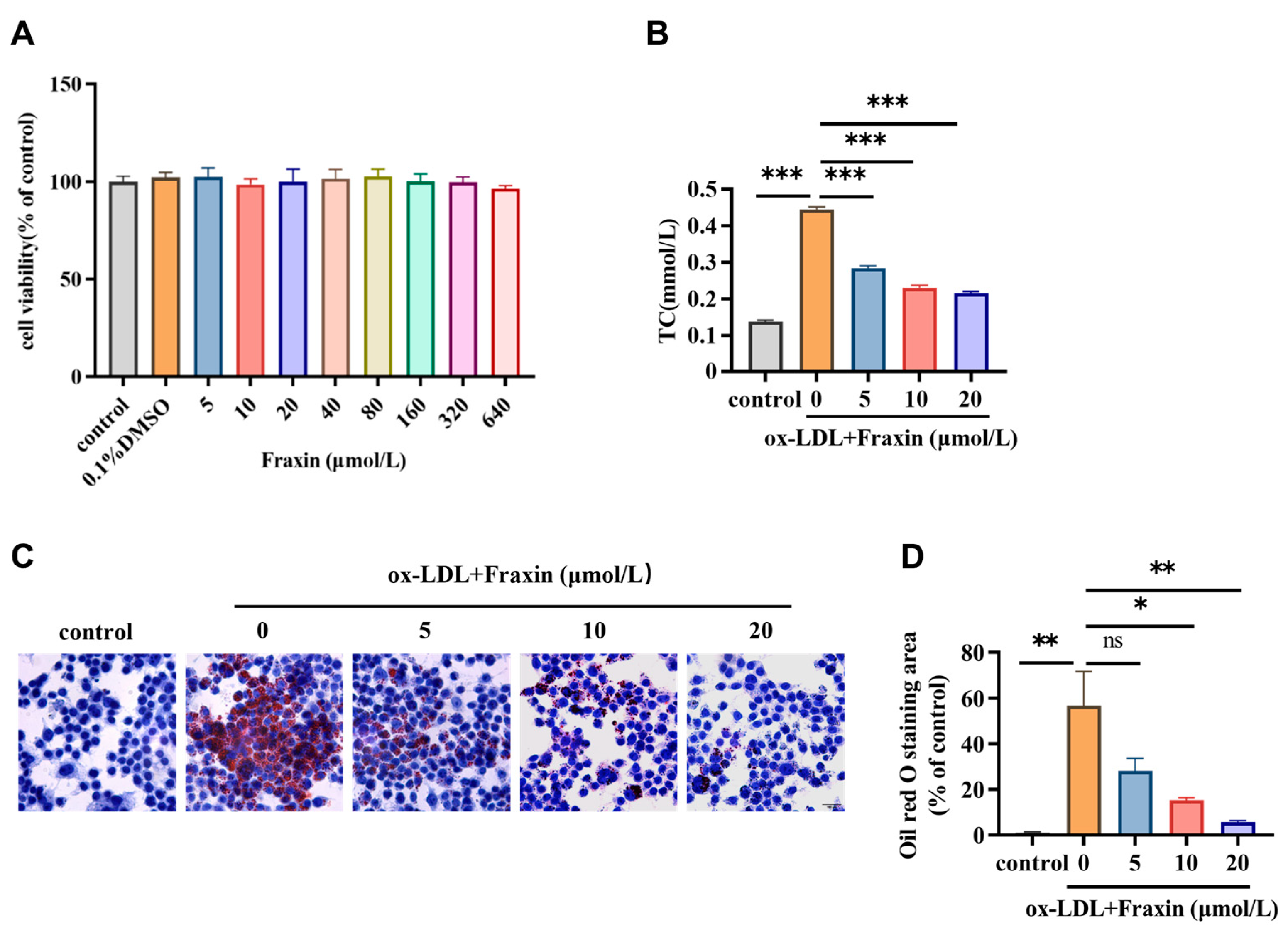
3.2.1. Fraxin Inhibits Lipid Accumulation and Reduces Cholesterol Content in Macrophages
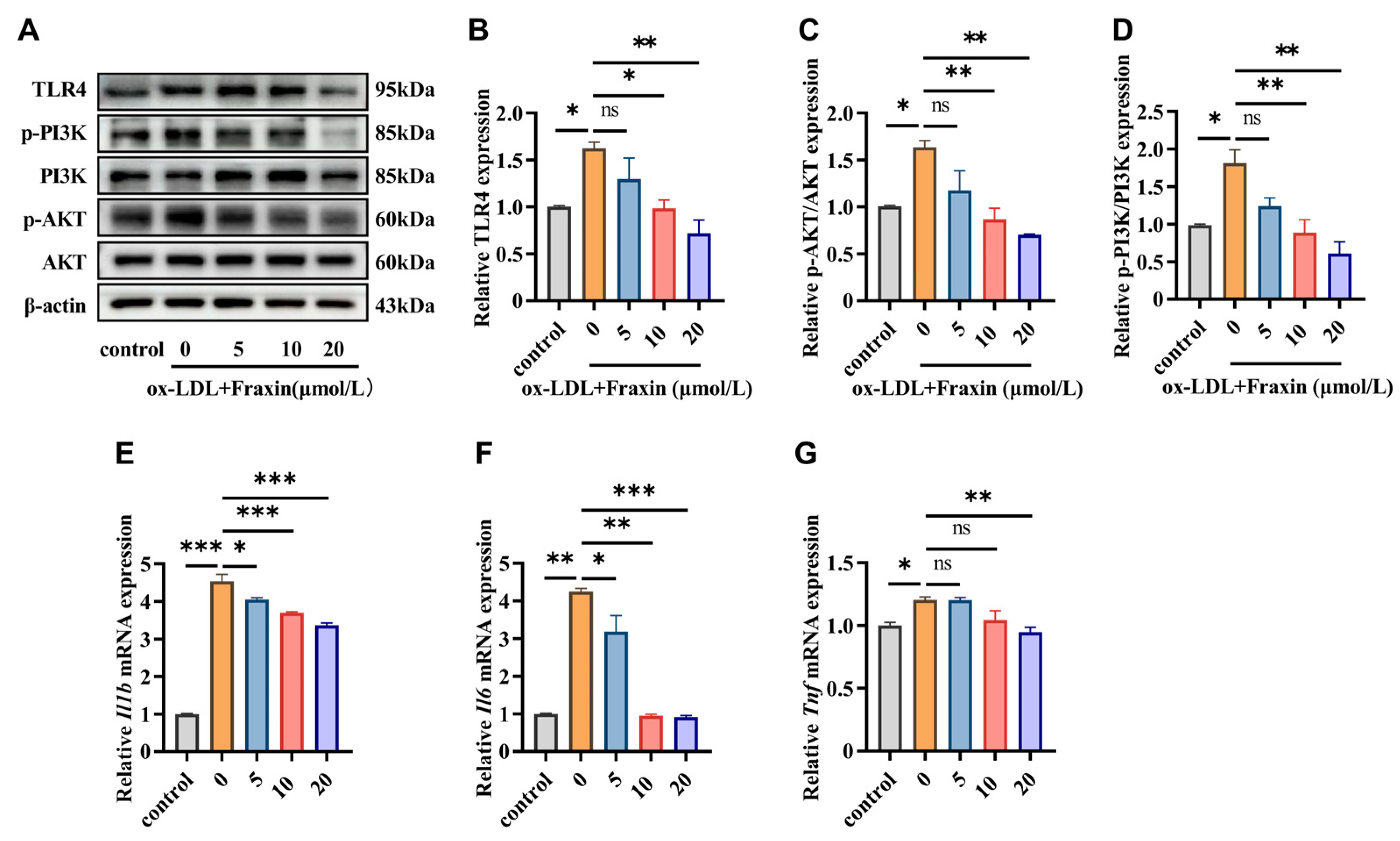
3.2.2. Fraxin Inhibits TLR4/PI3K/Akt Pathway and Suppresses Macrophage Inflammatory
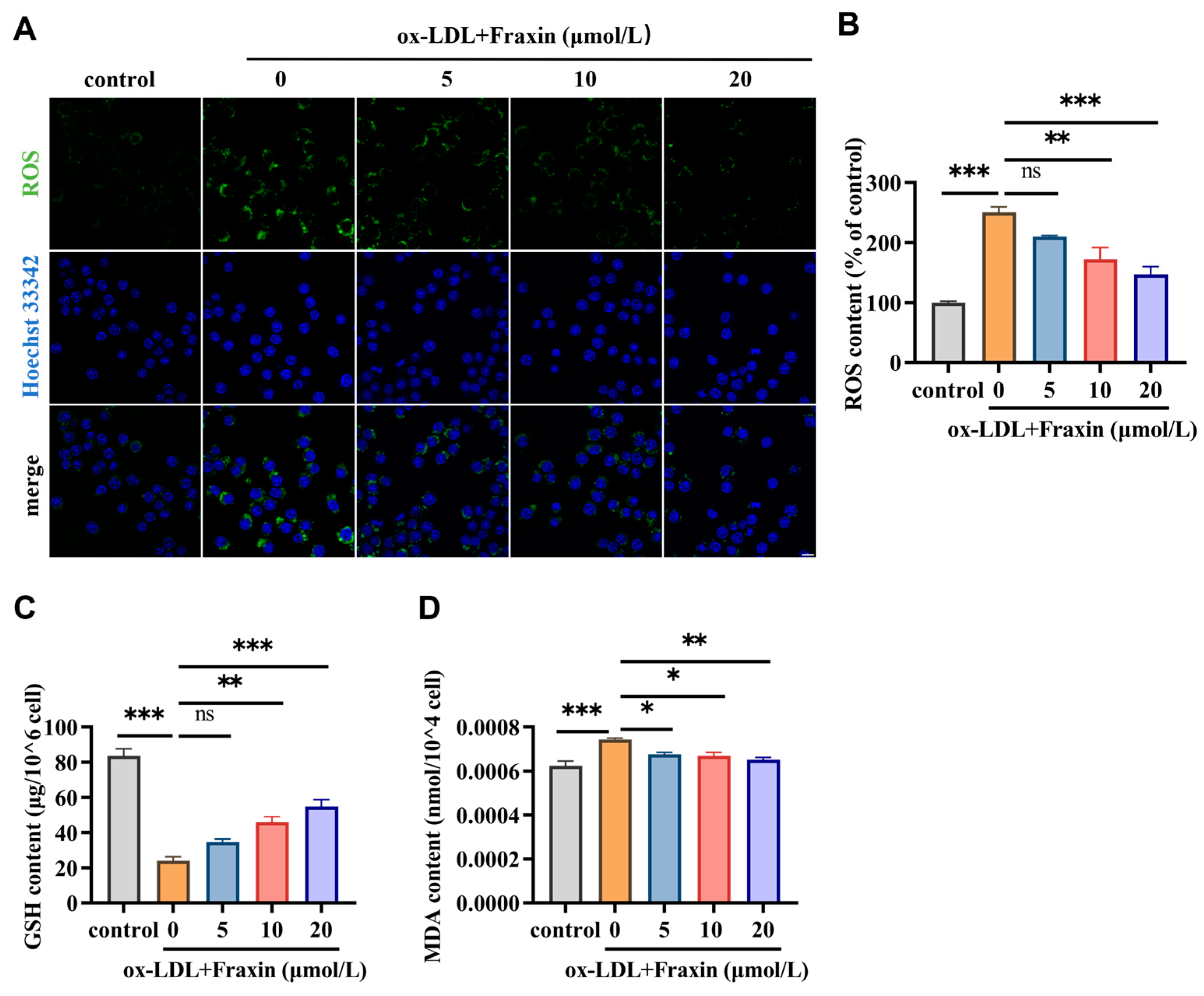
3.2.3. Fraxin Significantly Reverses the Redox Steady State Disrupted by ox-LDL
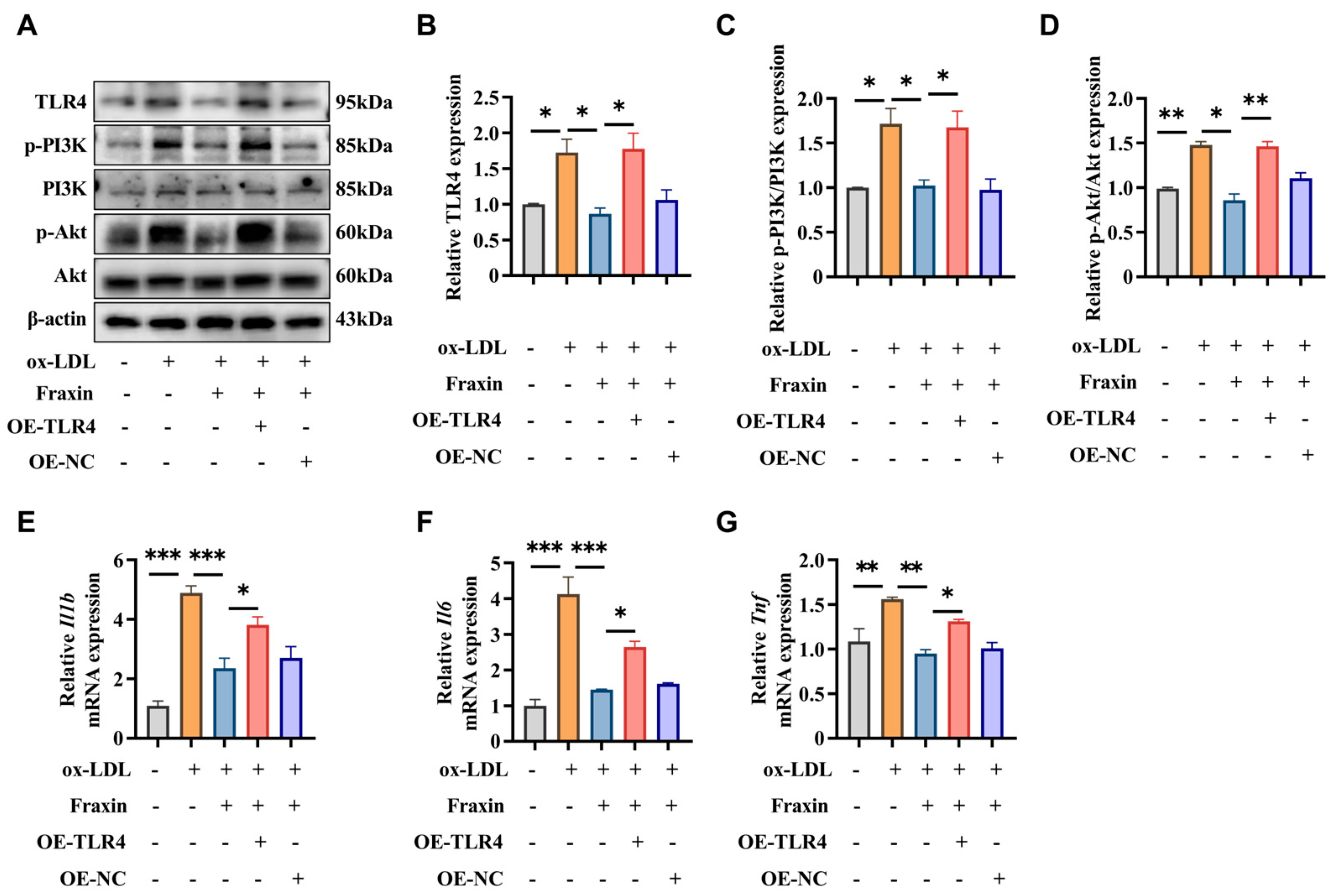
3.2.4. Overexpression of TLR4 Abrogated the Effects of Fraxin
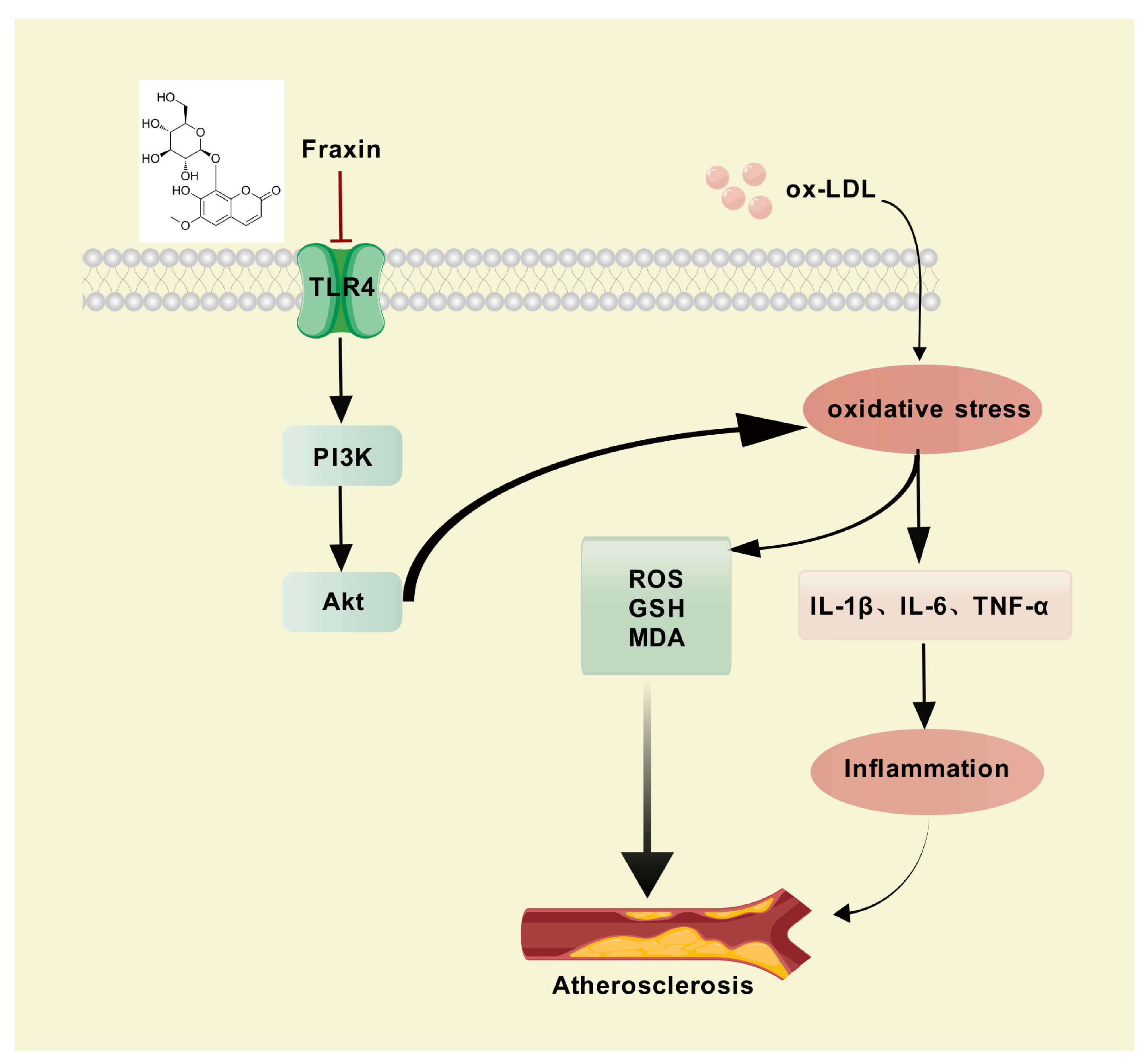
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Bornfeldt, K.E.; Tall, A.R. Atherosclerosis: Successes, Surprises, and Future Challenges. Circ. Res. 2016, 118, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar]
- Bułdak, Ł. Cardiovascular Diseases-A Focus on Atherosclerosis, Its Prophylaxis, Complications and Recent Advancements in Therapies. Int. J. Mol. Sci. 2022, 23, 4695. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Corr, E.M.; Erbay, E.; Moore, K.J. Regulation of macrophage immunometabolism in atherosclerosis. Nat. Immunol. 2018, 19, 526–537. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, J.; Li, X.; Xue, F.; Cao, L.; Meng, L.; Sui, W.; Zhang, M.; Zhao, Y.; Xi, B.; et al. NPRC deletion mitigated atherosclerosis by inhibiting oxidative stress, inflammation and apoptosis in ApoE knockout mice. Signal Transduct. Target. Ther. 2023, 8, 290. [Google Scholar] [CrossRef]
- Hu, R.; Dai, C.; Dong, C.; Ding, L.; Huang, H.; Chen, Y.; Zhang, B. Living Macrophage-Delivered Tetrapod PdH Nanoenzyme for Targeted Atherosclerosis Management by ROS Scavenging, Hydrogen Anti-inflammation, and Autophagy Activation. ACS Nano 2022, 16, 15959–15976. [Google Scholar] [CrossRef]
- Cao, H.; Jia, Q.; Yan, L.; Chen, C.; Xing, S.; Shen, D. Quercetin Suppresses the Progression of Atherosclerosis by Regulating MST1-Mediated Autophagy in ox-LDL-Induced RAW264.7 Macrophage Foam Cells. Int. J. Mol. Sci. 2019, 20, 6093. [Google Scholar] [CrossRef]
- Yan, Q.; Liu, S.; Sun, Y.; Chen, C.; Yang, S.; Lin, M.; Long, J.; Yao, J.; Lin, Y.; Yi, F.; et al. Targeting oxidative stress as a preventive and therapeutic approach for cardiovascular disease. J. Transl. Med. 2023, 21, 519. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Sun, T.; Ding, X.; Gu, X.; Wang, Y.; Ma, X.; Li, Z.; Gao, H.; Ge, S.; Feng, Q. The exoprotein Gbp of Fusobacterium nucleatum promotes THP-1 cell lipid deposition by binding to CypA and activating PI3K-AKT/MAPK/NF-κB pathways. J. Adv. Res. 2024, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Du, M.; Li, S.; Zhang, Y.; Ding, J.; Wang, J.; Wang, Y.; Liu, P. Hydroxysafflor yellow A regulates lymphangiogenesis and inflammation via the inhibition of PI3K on regulating AKT/mTOR and NF-κB pathway in macrophages to reduce atherosclerosis in ApoE-/- mice. Phytomedicine 2023, 112, 154684. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef]
- Zhao, C.; Li, S.; Zhang, J.; Huang, Y.; Zhang, L.; Zhao, F.; Du, X.; Hou, J.; Zhang, T.; Shi, C.; et al. Current state and future perspective of cardiovascular medicines derived from natural products. Pharmacol. Ther. 2020, 216, 107698. [Google Scholar] [CrossRef]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An Overview of Coumarin as a Versatile and Readily Accessible Scaffold with Broad-Ranging Biological Activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef]
- Tang, F.L.; Xie, L.W.; Tang, L.F.; Lu, H.Y.; Zhu, R.Q.; Wang, D.F.; Tian, Y.; Cai, S.; Li, M. Fraxin (7-hydroxy-6-methoxycoumarin 8-glucoside) confers protection against ionizing radiation-induced intestinal epithelial injury in vitro and in vivo. Int. Immunopharmacol. 2024, 129, 111637. [Google Scholar] [CrossRef]
- Li, W.; Li, W.; Zang, L.; Liu, F.; Yao, Q.; Zhao, J.; Zhi, W.; Niu, X. Fraxin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting the NF-κB and NLRP3 signalling pathways. Int. Immunopharmacol. 2019, 67, 1–12. [Google Scholar] [CrossRef]
- Zeng, J.; Liang, L.; Chen, R.; Li, C.; Pan, L.; Wen, M.; Lv, D.; Liu, M.; Xu, Z.; Huang, H. Fraxin represses NF-κB pathway via inhibiting the activation of epidermal growth factor receptor to ameliorate diabetic renal tubulointerstitial fibrosis. Eur. J. Pharmacol. 2023, 955, 175915. [Google Scholar] [CrossRef]
- Chang, B.Y.; Jung, Y.S.; Yoon, C.S.; Oh, J.S.; Hong, J.H.; Kim, Y.C.; Kim, S.Y. Fraxin Prevents Chemically Induced Hepatotoxicity by Reducing Oxidative Stress. Molecules 2017, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yu, X.; Zeng, H.; Hu, Y.; Jiang, L.; Huang, J.; Fu, C.; Chen, J.; Zeng, Q. Fraxin inhibits melanogenesis by suppressing the ERK/MAPK pathway and antagonizes oxidative stress by activating the NRF2 pathway. Heliyon 2023, 9, e18929. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ye, F.C.; Sun, T.; Liu, F.J.; Wu, M.J.; Zheng, W.H.; Wu, L.F. Delay the progression of glucocorticoid-induced osteoporosis: Fraxin targets ferroptosis via the Nrf2/GPX4 pathway. Phytother. Res. 2024, 38, 5203–5224. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Song, Q.; Fan, F.; Hu, Z.; Cheng, G.; Zhang, Y. miR-223 Inhibits Lipid Deposition and Inflammation by Suppressing Toll-Like Receptor 4 Signaling in Macrophages. Int. J. Mol. Sci. 2015, 16, 24965–24982. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Lin, S.; Wu, Y.; He, D.; Qu, P. PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp. Anim. 2021, 70, 488–497. [Google Scholar] [CrossRef]
- Ha, S.J.; Lee, J.; Song, K.M.; Kim, Y.H.; Lee, N.H.; Kim, Y.E.; Jung, S.K. Ultrasonicated Lespedeza cuneata extract prevents TNF-α-induced early atherosclerosis in vitro and in vivo. Food Funct. 2018, 9, 2090–2101. [Google Scholar] [CrossRef]
- Gomez, D.; Baylis, R.A.; Durgin, B.G.; Newman, A.A.C.; Alencar, G.F.; Mahan, S.; St Hilaire, C.; Müller, W.; Waisman, A.; Francis, S.E.; et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 2018, 24, 1418–1429. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Wang, Y.; Huai, L.; Shi, B.; Zhang, D.; Xu, W.; Cui, D. Interleukin-6 related signaling pathways as the intersection between chronic diseases and sepsis. Mol. Med. 2025, 31, 34. [Google Scholar] [CrossRef]
- Xu, L.; Ren, H.; Xie, D.; Zhang, F.; Hu, X.; Fang, S.; Wang, H.; He, D. Rac2 mediate foam cell formation and associated immune responses in THP-1 to promote the process of atherosclerotic plaques. Mol. Immunol. 2023, 163, 196–206. [Google Scholar] [CrossRef]
- Bai, T.; Li, M.; Liu, Y.; Qiao, Z.; Wang, Z. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Ma, S.; Ma, F.; Ding, N.; Xie, L.; Yang, A.; Shen, J.; Jiao, Y.; Wu, K.; Chai, Y.; Bai, Z.; et al. Homocysteine Promotes the Pathogenesis of Atherosclerosis through the Circ-PIAS1-5/miR-219a-2-3p/TEAD1 Axis. Adv. Sci. 2025, e2415563. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Sviridov, D.; Miller, Y.I. Oxidized cholesteryl esters and inflammation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 393–397. [Google Scholar] [CrossRef]
- Li, S.; Navia-Pelaez, J.M.; Choi, S.H.; Miller, Y.I. Macrophage inflammarafts in atherosclerosis. Curr. Opin. Lipidol. 2023, 34, 189–195. [Google Scholar] [CrossRef]
- Zeng, F.; Zheng, J.; Shen, L.; Herrera-Balandrano, D.D.; Huang, W.; Sui, Z. Physiological mechanisms of TLR4 in glucolipid metabolism regulation: Potential use in metabolic syndrome prevention. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 38–46. [Google Scholar] [CrossRef]
- Ferdous, J.; Bhuia, M.S.; Chowdhury, R.; Rakib, A.I.; Aktar, M.A.; Al Hasan, M.S.; Melo Coutinho, H.D.; Islam, M.T. Pharmacological Activities of Plant-Derived Fraxin with Molecular Mechanisms: A Comprehensive Review. Chem. Biodivers. 2024, 21, e202301615. [Google Scholar] [CrossRef]
- Totoń-Żurańska, J.; Mikolajczyk, T.P.; Saju, B.; Guzik, T.J. Vascular remodelling in cardiovascular diseases: Hypertension, oxidation, and inflammation. Clin. Sci. 2024, 138, 817–850. [Google Scholar] [CrossRef]
- Kao, T.W.; Huang, C.C. Inflammatory Burden and Immunomodulative Therapeutics of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 804. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gong, W.; Zhang, S.; Shen, J.; Liu, X.; Wang, Y.; Chen, Y.; Meng, G. Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation. Biochem. Pharmacol. 2021, 192, 114665. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Feng, X.; Li, S.; Wang, J.; Liu, P.; Wang, Y. Taoren Honghua Decoction alleviates atherosclerosis by inducing autophagy and inhibiting the PI3K-AKT signaling pathway to regulate cholesterol efflux and inflammatory responses. Int. Immunopharmacol. 2025, 144, 113629. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Piao, X.; Niu, W.; Zhang, Q.; Ma, C.; Wu, T.; Gu, Q.; Cui, T.; Li, S. Kuijieyuan Decoction Improved Intestinal Barrier Injury of Ulcerative Colitis by Affecting TLR4-Dependent PI3K/AKT/NF-κB Oxidative and Inflammatory Signaling and Gut Microbiota. Front. Pharmacol. 2020, 11, 1036. [Google Scholar] [CrossRef]

| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Il1b | gaaatgccaccttttgacagtg | tggatgctctcatcaggacag |
| Il6 | ctgcaagagacttccatccag | agtggtatagacaggtctgttgg |
| Tnf | caggcggtgcctatgtctc | cgatcaccccgaagttcagtag |
| Gapdh | aggtcggtgtgaacggatttg | tgtagaccatgtagttgaggtca |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, B.; Leng, M.; He, J.; Zhao, Y.; Xia, H.; Luo, H.; Bai, X. Fraxin Alleviates Atherosclerosis by Inhibiting Oxidative Stress and Inflammatory Responses via the TLR4/PI3K/Akt Pathway. Curr. Issues Mol. Biol. 2025, 47, 308. https://doi.org/10.3390/cimb47050308
Wang Y, Wei B, Leng M, He J, Zhao Y, Xia H, Luo H, Bai X. Fraxin Alleviates Atherosclerosis by Inhibiting Oxidative Stress and Inflammatory Responses via the TLR4/PI3K/Akt Pathway. Current Issues in Molecular Biology. 2025; 47(5):308. https://doi.org/10.3390/cimb47050308
Chicago/Turabian StyleWang, Yaru, Bailing Wei, Mingyang Leng, Jiali He, Yicheng Zhao, Haohao Xia, Haibin Luo, and Xue Bai. 2025. "Fraxin Alleviates Atherosclerosis by Inhibiting Oxidative Stress and Inflammatory Responses via the TLR4/PI3K/Akt Pathway" Current Issues in Molecular Biology 47, no. 5: 308. https://doi.org/10.3390/cimb47050308
APA StyleWang, Y., Wei, B., Leng, M., He, J., Zhao, Y., Xia, H., Luo, H., & Bai, X. (2025). Fraxin Alleviates Atherosclerosis by Inhibiting Oxidative Stress and Inflammatory Responses via the TLR4/PI3K/Akt Pathway. Current Issues in Molecular Biology, 47(5), 308. https://doi.org/10.3390/cimb47050308







