Natural Products and Health Care Functions of Inonotus obliquus
Abstract
:1. Introduction
2. Materials and Methods
3. Main Active Components of I. obliquus
3.1. Polysaccharides
3.2. Triterpenes
3.3. Polyphenols
3.4. Others
4. Health Care Functions of I. obliquus
4.1. Antitumor Activity
4.2. Anti-Inflammatory Activity
4.3. Hypoglycemic Effects
4.4. Hypolipidemic Effects
4.5. Antiviral Activity
4.6. Antioxidant Activity
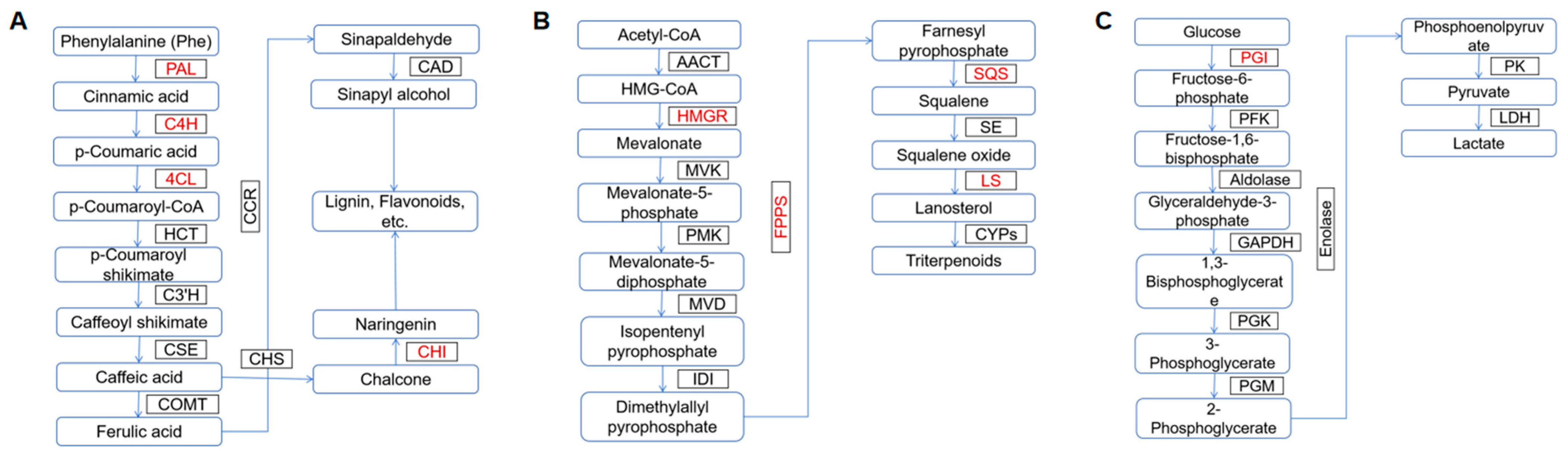
5. Mechanism of Metabolite Synthesis
5.1. Polyphenols
5.2. Triterpenes
5.3. Polysaccharides
6. Discussion
6.1. Pharmacological Effects and Diversity of Active Ingredients
6.2. Production Technology Innovation and Scale Challenges
6.3. Limitations in Clinical Application and Product Development
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.W.; Hur, H.; Chang, K.C.; Lee, T.S.; Ka, K.H.; Jankovsky, L. Introduction to Distribution and Ecology of Sterile Conks of Inonotus obliquus. Mycobiology 2008, 36, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Song, D.; Mu, H.; Zhang, W.; Sun, F.; Duan, J. Investigation of three lignin complexes with antioxidant and immunological capacities from Inonotus obliquus. Int. J. Biol. Macromol. 2016, 86, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, M.; Zhao, Y.; Wang, Y.; Miao, K.; Wei, Z. Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Bioresour. Technol. 2009, 100, 1327–1335. [Google Scholar] [CrossRef]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpinski, T.M.; Aruci, E.; Atrooz, O.M. A brief overview of the medicinal and nutraceutical importance of Inonotus obliquus (chaga) mushrooms. Heliyon 2024, 10, e35638. [Google Scholar] [CrossRef]
- Beltrame, G.; Trygg, J.; Hemming, J.; Han, Z.; Yang, B. Comparison of Polysaccharides Extracted from Cultivated Mycelium of Inonotus obliquus with Polysaccharide Fractions Obtained from Sterile Conk (Chaga) and Birch Heart Rot. J. Fungi 2021, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.S.; Kim, S.H.; Moon, S.Y.; Chung, M.J.; Cui, C.B.; Han, E.K.; Chung, C.K.; Choe, M. Antimutagenic effects of subfractions of Chaga mushroom (Inonotus obliquus) extract. Mutat. Res. 2009, 672, 55–59. [Google Scholar] [CrossRef]
- Maza, P.; Lee, J.H.; Kim, Y.S.; Sun, G.M.; Sung, Y.J.; Ponomarenko, L.P.; Stonik, V.A.; Ryu, M.; Kwak, J.Y. Inotodiol From Inonotus obliquus Chaga Mushroom Induces Atypical Maturation in Dendritic Cells. Front. Immunol. 2021, 12, 650841. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skora, B.; Pomianek, T.; Gminski, J. Inonotus obliquus—From folk medicine to clinical use. J. Tradit. Complement. Med. 2021, 11, 293–302. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P. The pharmacological potential and possible molecular mechanisms of action of Inonotus obliquus from preclinical studies. Phytother. Res. 2019, 33, 1966–1980. [Google Scholar] [CrossRef]
- Yan, K.; Zhou, H.; Wang, M.; Li, H.; Sang, R.; Ge, B.; Zhao, X.; Li, C.; Wang, W.; Zhang, X. Inhibitory Effects of Inonotus obliquus Polysaccharide on Inflammatory Response in Toxoplasma gondii-Infected RAW264.7 Macrophages. Evid. Based Complement. Altern. Med. 2021, 2021, 2245496. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, X.; Liu, D.; Wu, H.; Qu, L. Identification of Inonotus obliquus polysaccharide with broad-spectrum antiviral activity against multi-feline viruses. Int. J. Biol. Macromol. 2017, 95, 160–167. [Google Scholar] [CrossRef]
- Song, F.Q.; Liu, Y.; Kong, X.S.; Chang, W.; Song, G. Progress on understanding the anticancer mechanisms of medicinal mushroom: Inonotus obliquus. Asian Pac. J. Cancer Prev. 2013, 14, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Sato, Y.; Konishi, T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem. Pharm. Bull. 2007, 55, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.Y.; Park, Y.; Ko, E.J.; Ban, T.H.; Chung, B.H.; Lee, H.S.; Yang, C.W. Development of End Stage Renal Disease after Long-Term Ingestion of Chaga Mushroom: Case Report and Review of Literature. J. Korean Med. Sci. 2020, 35, e122. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, E.J.; Kim, S.H. Ethanol extract of Innotus obliquus (Chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr. Res. Pract. 2015, 9, 111–116. [Google Scholar] [CrossRef]
- Diao, B.Z.; Jin, W.R.; Yu, X.J. Protective Effect of Polysaccharides from Inonotus obliquus on Streptozotocin-Induced Diabetic Symptoms and Their Potential Mechanisms in Rats. Evid. Based Complement. Altern. Med. 2014, 2014, 841496. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, Y.; Zhang, Z.; Gu, Z.; Zhong, H.; Zha, Q.; Yang, L.; Zhu, C.; Chen, E. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann. Transl. Med. 2020, 8, 816. [Google Scholar] [CrossRef]
- Bolwell, P.P.; Page, A.; Pislewska, M.; Wojtaszek, P. Pathogenic infection and the oxidative defences in plant apoplast. Protoplasma 2001, 217, 20–32. [Google Scholar] [CrossRef]
- Rao, H.; Tan, J.B.L. Polysaccharide-based hydrogels for atopic dermatitis management: A review. Carbohydr. Polym. 2025, 349, 122966. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Yang, Y.; Tian, X.; Jin, Y.; Jiang, W.; He, H.; Xu, Y.; Liu, Y. Polysaccharide-based biomaterials for regenerative therapy in intervertebral disc degeneration. Mater. Today Bio 2025, 30, 101395. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhou, J.; Shen, X.; Chalamaiah, M.; Lv, S.; Luo, H.; Chen, L. Bioactive Properties of Peptides and Polysaccharides Derived from Peanut Worms: A Review. Mar. Drugs 2021, 20, 10. [Google Scholar] [CrossRef]
- Seweryn, E.; Ziala, A.; Gamian, A. Health-Promoting of Polysaccharides Extracted from Ganoderma lucidum. Nutrients 2021, 13, 2725. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Cong, H.; Li, C.; Wu, J.; Li, L.; Jiang, J.; Cao, X. Cultivable Endophyte Resources in Medicinal Plants and Effects on Hosts. Life 2023, 13, 1695. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, W.; Deng, X.; Sun, M.; Sun, R.; Li, Q.; Ren, J.; Jiang, W.; Wang, Y.; Liu, S.; et al. Structural analysis of polysaccharide from Inonotus obliquus and investigate combined impact on the sex hormones, intestinal microbiota and metabolism in SPF male mice. Int. J. Biol. Macromol. 2024, 262, 129686. [Google Scholar] [CrossRef]
- Su, B.; Yan, X.; Li, Y.; Zhang, J.; Xia, X. Effects of Inonotus obliquus Polysaccharides on Proliferation, Invasion, Migration, and Apoptosis of Osteosarcoma Cells. Anal. Cell Pathol. 2020, 2020, 4282036. [Google Scholar] [CrossRef]
- Ding, M.; Yang, Y.; Zhang, Z.; Liu, H.; Dai, Y.; Wang, Z.; Ma, S.; Liu, Y.; Wang, Q. Structural characterization of the polysaccharide from the black crystal region of Inonotus obliquus and its effect on AsPC-1 and SW1990 pancreatic cancer cell apoptosis. Int. J. Biol. Macromol. 2024, 268, 131891. [Google Scholar]
- Miranda, R.S.; de Jesus, B.; da Silva Luiz, S.R.; Viana, C.B.; Adao Malafaia, C.R.; Figueiredo, F.S.; Carvalho, T.; Silva, M.L.; Londero, V.S.; da Costa-Silva, T.A.; et al. Antiinflammatory activity of natural triterpenes-An overview from 2006 to 2021. Phytother. Res. 2022, 36, 1459–1506. [Google Scholar] [CrossRef]
- Kim, J.; Yang, S.C.; Hwang, A.Y.; Cho, H.; Hwang, K.T. Composition of Triterpenoids in Inonotus obliquus and Their Anti-Proliferative Activity on Cancer Cell Lines. Molecules 2020, 25, 4066. [Google Scholar] [CrossRef]
- Sagayama, K.; Tanaka, N.; Fukumoto, T.; Kashiwada, Y. Lanostane-type triterpenes from the sclerotium of Inonotus obliquus (Chaga mushrooms) as proproliferative agents on human follicle dermal papilla cells. J. Nat. Med. 2019, 73, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Lee, I.K.; Kim, Y.S.; Jang, Y.W.; Jung, J.Y.; Yun, B.S. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg Med. Chem. Lett. 2007, 17, 6678–6681. [Google Scholar] [CrossRef]
- Wang, Y.; Ouyang, F.; Teng, C.; Qu, J. Optimization for the extraction of polyphenols from Inonotus obliquus and its antioxidation activity. Prep. Biochem. Biotechnol. 2021, 51, 852–859. [Google Scholar] [CrossRef]
- Burmasova, M.A.; Utebaeva, A.A.; Sysoeva, E.V.; Sysoeva, M.A. Melanins of Inonotus Obliquus: Bifidogenic and Antioxidant Properties. Biomolecules 2019, 9, 248. [Google Scholar] [CrossRef]
- Zou, C.X.; Hou, Z.L.; Bai, M.; Guo, R.; Lin, B.; Wang, X.B.; Huang, X.X.; Song, S.J. Highly modified steroids from Inonotus obliquus. Org. Biomol. Chem. 2020, 18, 3908–3916. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Sun, Y.; Jiang, J. Inonotus obliquus sclerotia epidermis were different from internal tissues in compound composition, antioxidant activity, and associated fungi. FEMS Microbiol. Lett. 2023, 370, fnad126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, W. Deciphering the antitumoral potential of the bioactive metabolites from medicinal mushroom Inonotus obliquus. J. Ethnopharmacol. 2021, 265, 113321. [Google Scholar] [CrossRef]
- Zhang, S.D.; Yu, L.; Wang, P.; Kou, P.; Li, J.; Wang, L.T.; Wang, W.; Yao, L.P.; Zhao, X.H.; Fu, Y.J. Inotodiol inhibits cells migration and invasion and induces apoptosis via p53-dependent pathway in HeLa cells. Phytomedicine 2019, 60, 152957. [Google Scholar] [CrossRef]
- Li, J.; Qu, C.; Li, F.; Chen, Y.; Zheng, J.; Xiao, Y.; Jin, Q.; Jin, G.; Huang, X.; Jin, D. Inonotus obliquus Polysaccharide Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Cancer in Mice via Activation of the NLRP3 Inflammasome. Front. Pharmacol. 2020, 11, 621835. [Google Scholar] [CrossRef] [PubMed]
- Sang, R.; Sun, F.; Zhou, H.; Wang, M.; Li, H.; Li, C.; Sun, X.; Zhao, X.; Zhang, X. Immunomodulatory effects of Inonotus obliquus polysaccharide on splenic lymphocytes infected with Toxoplasma gondii via NF-kappaB and MAPKs pathways. Immunopharmacol. Immunotoxicol. 2022, 44, 129–138. [Google Scholar] [CrossRef]
- Kou, R.W.; Han, R.; Gao, Y.Q.; Li, D.; Yin, X.; Gao, J.M. Anti-neuroinflammatory polyoxygenated lanostanoids from Chaga mushroom Inonotus obliquus. Phytochemistry 2021, 184, 112647. [Google Scholar] [CrossRef]
- Park, J.; Nguyen, T.M.N.; Park, H.A.; Nguyen, M.T.T.; Lee, N.Y.; Ban, S.Y.; Park, K.B.; Lee, C.K.; Kim, J.; Park, J.T. Protective Effects of Lanostane Triterpenoids from Chaga Mushroom in Human Keratinocytes, HaCaT Cells, against Inflammatory and Oxidative Stresses. Int. J. Mol. Sci. 2023, 24, 12803. [Google Scholar] [CrossRef] [PubMed]
- Delgersaikhan, N.; Odkhuu, E.; Khaltar, P.; Samdan, E.; Dugarsuren, U.; Dalkhsuren, S.O.; Dorjkhuu, A.; Aldartsogt, D.; Amgalanbaatar, A. Antidiabetic activity of Inonotus obliquus water extract in alloxan-induced diabetic mice. J. Complement. Integr. Med. 2024, 21, 472–480. [Google Scholar]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed. Pharmacother. 2017, 95, 1669–1677. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Gong, L.; Han, Z.; Zhang, Y.; Li, R.; Liao, H. Inonotus obliquus (Chaga) against HFD/STZ-induced glucolipid metabolism disorders and abnormal renal functions by regulating NOS-cGMP-PDE5 signaling pathway. Chin. J. Nat. Med. 2024, 22, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, G.; Wang, X.; Lv, C.; Tian, Y. Inonotus obliquus polysaccharide ameliorates serum profiling in STZ-induced diabetic mice model. BMC Chem. 2021, 15, 64. [Google Scholar] [CrossRef]
- Ding, G.; Guo, X.; Li, X.; An, L.; Shi, H. Study of active components and mechanisms mediating the hypolipidemic effect of Inonotus obliquus polysaccharides. Food Sci. Nutr. 2024, 12, 2833–2845. [Google Scholar] [CrossRef]
- Yang, M.; Hu, D.; Cui, Z.; Li, H.; Man, C.; Jiang, Y. Lipid-Lowering Effects of Inonotus obliquus Polysaccharide In Vivo and In Vitro. Foods 2021, 10, 3085. [Google Scholar] [CrossRef]
- Teplyakova, T.V.; Pyankov, O.V.; Safatov, A.S.; Ovchinnikova, A.S.; Kosogova, T.A.; Skarnovich, M.O.; Filippova, E.I.; Poteshkina, A.L. Water Extract of the Chaga Medicinal Mushroom, Inonotus obliquus (Agaricomycetes), Inhibits SARS-CoV-2 Replication in Vero E6 and Vero Cell Culture Experiments. Int. J. Med. Mushrooms 2022, 24, 23–30. [Google Scholar] [CrossRef]
- Eid, J.I.; Das, B.; Al-Tuwaijri, M.M.; Basal, W.T. Targeting SARS-CoV-2 with Chaga mushroom: An in silico study toward developing a natural antiviral compound. Food Sci. Nutr. 2021, 9, 6513–6523. [Google Scholar] [CrossRef] [PubMed]
- Kou, R.W.; Xia, B.; Han, R.; Li, Z.Q.; Yang, J.R.; Yin, X.; Gao, Y.Q.; Gao, J.M. Neuroprotective effects of a new triterpenoid from edible mushroom on oxidative stress and apoptosis through the BDNF/TrkB/ERK/CREB and Nrf2 signaling pathway in vitro and in vivo. Food Funct. 2022, 13, 12121–12134. [Google Scholar] [CrossRef]
- Zhao, Y.; Xi, Q.; Xu, Q.; He, M.; Ding, J.; Dai, Y.; Keller, N.P.; Zheng, W. Correlation of nitric oxide produced by an inducible nitric oxide synthase-like protein with enhanced expression of the phenylpropanoid pathway in Inonotus obliquus cocultured with Phellinus morii. Appl. Microbiol. Biotechnol. 2015, 99, 4361–4372. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, H.; Lu, T.; Zhao, Y.; Zheng, W. Ultraviolet radiation promotes the production of hispidin polyphenols by medicinal mushroom Inonotus obliquus. Fungal Biol. 2022, 126, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, M.; Wu, Z.; Liang, X.; Zheng, Q.; Li, D.; Wang, G.; An, T. The microbial biosynthesis of noncanonical terpenoids. Appl. Microbiol. Biotechnol. 2024, 108, 226. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.X.; Yu, X.D.; Fan, J.; Pickett, J.A.; Jones, H.D.; Zhou, J.J.; Birkett, M.A.; Caulfield, J.; Napier, J.A.; et al. Molecular characterization of two isoforms of a farnesyl pyrophosphate synthase gene in wheat and their roles in sesquiterpene synthesis and inducible defence against aphid infestation. New Phytol. 2015, 206, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, Z.-F.; Li, C.-T. Effects of Exogenous Elicitors on Triterpenoids Accumulation and Expression of Farnesyl Diphosphate Synthase Gene in Inonotus obliquus. Biotechnol. Bioprocess. Eng. 2020, 25, 580–588. [Google Scholar] [CrossRef]
- Hua, L.; Shi, H.; Lin, Q.; Wang, H.; Gao, Y.; Zeng, J.; Lou, K.; Huo, X. Selection and Genetic Analysis of High Polysaccharide-Producing Mutants in Inonotus obliquus. Microorganisms 2024, 12, 1335. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Han, J.; Yang, Z.; Zhu, J.; Ren, A.; Shi, L.; Yu, H.; Zhao, M. Cloning and characterization of phosphoglucose isomerase in Lentinula edodes. J. Basic. Microbiol. 2022, 62, 740–749. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Fang, M.; Li, T.; Liang, Y.; Mei, Y. Carbon Source Affects Synthesis, Structures, and Activities of Mycelial Polysaccharides from Medicinal Fungus Inonotus obliquus. J. Microbiol. Biotechnol. 2021, 31, 855–866. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Wang, C.; Zhang, H.; Liu, P. Necessity of Different Lignocellulose on Exopolysaccharide Synthesis and Its Hypoglycemic Activity In Vitro of Inonotus obliquus. Appl. Biochem. Biotechnol. 2024, 196, 3420–3440. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, Z.; Zhou, X.; Hu, J.; Xue, J.; Liu, X.; Zhang, J.; Liu, P.; Tong, S. Simultaneous Use of Stimulatory Agents to Enhance the Production and Hypoglycaemic Activity of Polysaccharides from Inonotus obliquus by Submerged Fermentation. Molecules 2019, 24, 4400. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Shi, Y.; Li, L.; Chu, J.; Li, J.; Lin, W.; Yu, T.; Hou, D. Integrated omic profiling of the medicinal mushroom Inonotus obliquus under submerged conditions. BMC Genom. 2023, 24, 554. [Google Scholar] [CrossRef] [PubMed]
- Ern, P.T.Y.; Quan, T.Y.; Yee, F.S.; Yin, A.C.Y. Therapeutic properties of Inonotus obliquus (Chaga mushroom): A review. Mycology 2024, 15, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Krejsa, J.; Sima, J.; Kobera, M.; Seda, M.; Svoboda, L. Detrimental and essential elements in fruiting bodies of mushrooms with ecological relationship to birch (Betula sp.) collected in the Bohemian Forest, the Czech Republic. Environ. Sci. Pollut. Res. Int. 2021, 28, 67852–67862. [Google Scholar] [CrossRef]
- Azeem, U.; Shri, R.; Dhingra, G.S. Mineral Elements and Vitamins from Wild Wood Inhabiting Basidiocarps of Some Medicinal Mushrooms (Agaricomycetes) from India. Int. J. Med. Mushrooms 2022, 24, 53–62. [Google Scholar] [CrossRef]
- Tee, P.Y.E.; Krishnan, T.; Cheong, X.T.; Maniam, S.A.P.; Looi, C.Y.; Ooi, Y.Y.; Chua, C.L.L.; Fung, S.Y.; Chia, A.Y.Y. A review on the cultivation, bioactive compounds, health-promoting factors and clinical trials of medicinal mushrooms Taiwanofungus camphoratus, Inonotus obliquus and Tropicoporus linteus. Fungal Biol. Biotechnol. 2024, 11, 7. [Google Scholar] [CrossRef]
- Drenkhan, R.; Kaldmae, H.; Silm, M.; Adamson, K.; Bleive, U.; Aluvee, A.; Erik, M.; Raal, A. Comparative Analyses of Bioactive Compounds in Inonotus obliquus Conks Growing on Alnus and Betula. Biomolecules 2022, 12, 1178. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Zeng, L.; Peng, J.; Yao, X.; Zhou, T.; Tan, Z.; Wu, W.; Sun, Y.; Jiang, J.; et al. Revitalization of the Endophytic Fungus Acremonium sp. MEP2000 and Its Impact on the Growth and Accumulation of Bioactive Compounds in Inonotus obliquus. J. Microbiol. Biotechnol. 2025, 35, e2410037. [Google Scholar] [CrossRef]
- Huynh, N.; Beltrame, G.; Tarvainen, M.; Suomela, J.P.; Yang, B. Supercritical CO2 Extraction of Triterpenoids from Chaga Sterile Conk of Inonotus obliquus. Molecules 2022, 27, 1880. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Mock, M.B.; Summers, R.M. Microbial metabolism of caffeine and potential applications in bioremediation. J. Appl. Microbiol. 2024, 135, lxae080. [Google Scholar] [CrossRef]
- Antonio, J.; Antonio, B.; Arent, S.M.; Candow, D.G.; Escalante, G.; Evans, C.; Forbes, S.; Fukuda, D.; Gibbons, M.; Harty, P.; et al. Common Questions and Misconceptions About Energy Drinks: What Does the Scientific Evidence Really Show? Nutrients 2024, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Di Caprio, F.; Pedram, N.; Brugnoli, B.; Francolini, I.; Altimari, P.; Pagnanelli, F. Exploring different processes for starch extraction from microalgae and synthesis of starch-chitosan plastic films. Bioresour. Technol. 2024, 413, 131516. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, N.; Waqar, M.A.; Khan, A.M.; Asif, Z.; Alvi, A.S.; Virk, A.A.; Amir, S. A comprehensive insight of innovations and recent advancements in nanocarriers for nose-to-brain drug targeting. Des. Monomers Polym. 2025, 28, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Mazloomi, N.; Safari, B.; Can Karaca, A.; Karimzadeh, L.; Moghadasi, S.; Ghanbari, M.; Assadpour, E.; Sarabandi, K.; Jafari, S.M. Loading bioactive peptides within different nanocarriers to enhance their functionality and bioavailability; in vitro and in vivo studies. Adv. Colloid. Interface Sci. 2024, 334, 103318. [Google Scholar] [CrossRef]
- Sun, R.; Jin, D.; Fei, F.; Xu, Z.; Cao, B.; Li, J. Mushroom polysaccharides from Grifola frondosa (Dicks.) Gray and Inonotus obliquus (Fr.) Pilat ameliorated dextran sulfate sodium-induced colitis in mice by global modulation of systemic metabolism and the gut microbiota. Front. Pharmacol. 2023, 14, 1172963. [Google Scholar] [CrossRef]
- Wang, T.; Yue, S.; Jin, Y.; Wei, H.; Lu, L. Advances allowing feasible pyrG gene editing by a CRISPR-Cas9 system for the edible mushroom Pleurotus eryngii. Fungal Genet. Biol. 2021, 147, 103509. [Google Scholar] [CrossRef]
- Jain, D.; Kalia, A.; Sharma, S.; Manchanda, P. Genome editing tools based improved applications in macrofungi. Mol. Biol. Rep. 2024, 51, 873. [Google Scholar] [CrossRef]
- Chan, I.C.W.; Chen, N.; Hernandez, J.; Meltzer, H.; Park, A.; Stahl, A. Future avenues in Drosophila mushroom body research. Learn. Mem. 2024, 31, a053863. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’Hara, I.; Harrison, M.D. Filamentous fungi for future functional food and feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef] [PubMed]
- Lysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production-A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Pecchia, J.A. Bacterial Community Patterns in the Agaricus bisporus Cultivation System, from Compost Raw Materials to Mushroom Caps. Microb. Ecol. 2022, 84, 20–32. [Google Scholar] [CrossRef] [PubMed]
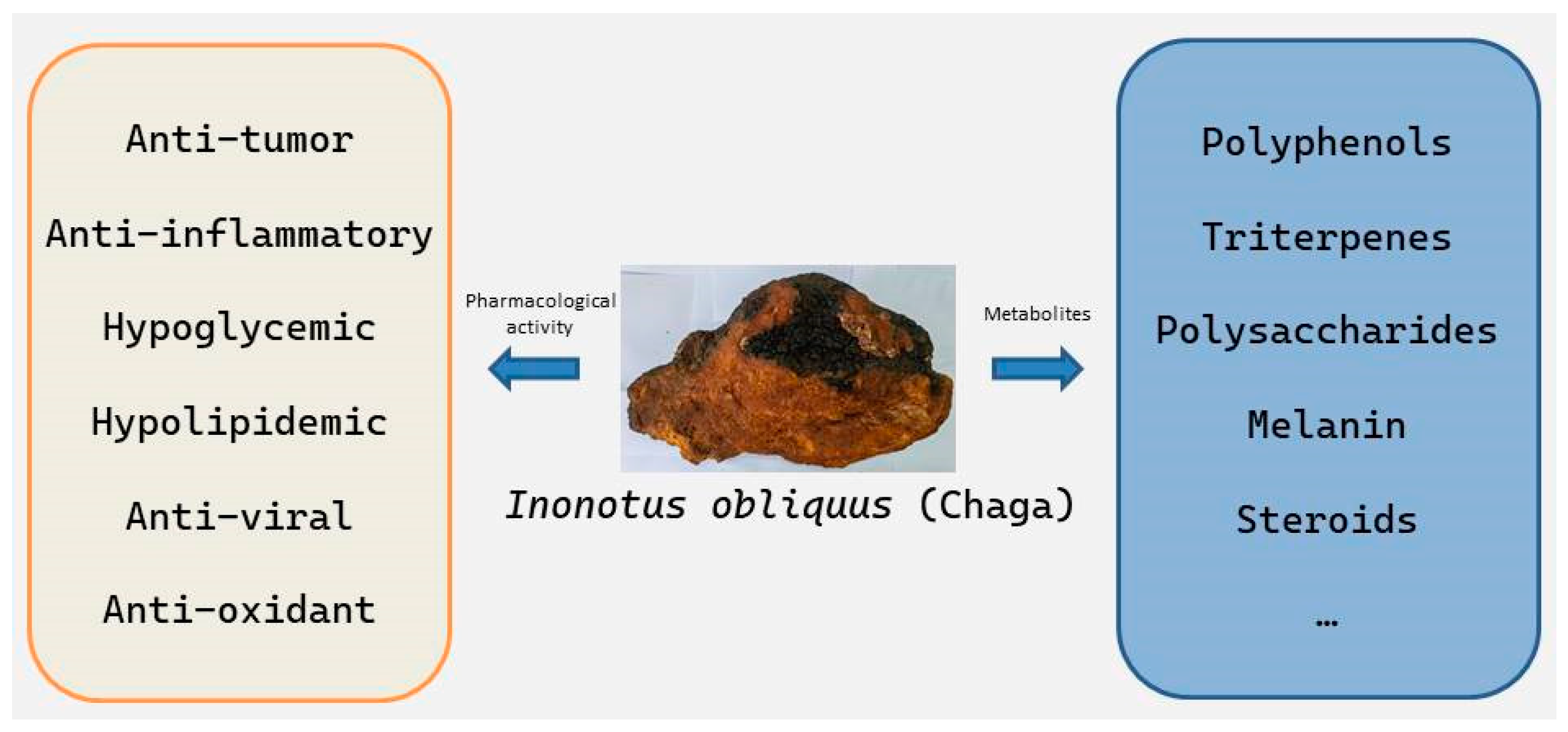

| Main Active Components of Inonotus obliquus | Specific Ingredients | Functions | References |
|---|---|---|---|
| Polysaccharide | A six-carbon pyarose Glc unit with an alpha configuration with a major glycosidyl linkage at the (1 → 4) position | Glycerophospholipid metabolism and other lipid metabolic pathways | [26] |
| Mannose, rhamnose, glucose, galactose, xylose, and arabinose | Antitumor activity against osteosarcoma | [27] | |
| Polysaccharide contained mainly →4)-α-Glcp-(1→, α-Glcp-(1→, →6)-β-Glcp-(1→, →4, 6)-β-Glcp-(1→, →3)-α-Glcp-(1 → and →3, 6)-β-Glcp-(1→ | Inhibit pancreatic cancer cell proliferation and induce cell cycle arrest in AsPC-1 and SW1990 cells | [28] | |
| Triterpenes | Betulinic acid, betulin, trametenolic acid, inopodiol, and an unknown triterpenoid | - | [30] |
| Lanostane-type triterpenes | Stimulate the growth of hair | [31] | |
| Polyphenols | Inonoblins A (1), B (2), and C (3) as well as phelligridins D (4), E (5), and G (6) | - | [34] |
| Procyanidin, caffeic acid, p-coumaric acid, isorhamnetin-3-o-glucoside, astilbin, tangeretin, gallic acid, kaempferol, quercetin, and catechin 7-xyloside | Antioxidant activity | [35] | |
| Others | Precipitated melanin | - | [36] |
| Six undescribed steroids | - | [37] | |
| Three water-soluble macromolecules belong to lignin–carbohydrate complexes | - | [2] |
| Health Care Functions of Inonotus obliquus | Related Components and Mechanisms | Specific Biological Activities | References |
|---|---|---|---|
| Antitumor activity | A six-carbon pyarose Glc unit with an alpha configuration with a major glycosidyl linkage at the (1 → 4) position; glycerophospholipid metabolism and other lipid metabolic pathways | To reduce the incidence of cancer in a healthy population | [39] |
| Inhibit the activation of Akt/mTOR signaling pathway | Regulates the proliferation, migration, invasion and apoptosis of osteosarcoma cells | [27] | |
| Contains oleanolic acid, betulin, betulin, inopodiol (a triterpenoid compound), and an unknown triterpenoid compound | Triterpenoid components exhibit dose-dependent anti-proliferative activities against AGS, MCF-7, and PC3 cells | [30] | |
| Downregulate the level of matrix metalloproteinases (MMPs) | Inhibit the migration and invasion of human cervical cancer cell line HeLa cells and induce apoptosis by regulating the relationship between pro-apoptotic proteins and anti-apoptotic proteins | [40] | |
| Promote the activation of the NLRP3 inflammasome induced by AOM/DSS; upregulate the protein levels of ASC, caspase-1 and NLRP3; enhance the secretion of cytokines IL-1β and IL-18 in tumor cells | The potential therapeutic effect on inflammation-associated cancers | [41] | |
| Anti-inflammatory Activity | Downregulate the expression of TLR2 and TLR4, inhibit the excessive phosphorylation of NF-κB p65, as well as IκBα (an inhibitor of the NF-κB signaling pathway) and the components of the mitogen-activated protein kinase (MAPK) signaling pathway, p38 and c-Jun N-terminal kinase (JNK) | Inhibit excessive inflammatory response | [42] |
| Inhibit LPS-induced iNOS expression, and inonotusols I and L have strong interactions with the iNOS protein | Beneficial to the treatment of neurodegenerative diseases | [43] | |
| Reduce the expression of pro-inflammatory cytokines induced by ultraviolet (UV) and tumor necrosis factor (TNF-α), which may be due to its inhibition of the activation of the NF-κB signaling pathway | The anti-inflammatory effects of inotodiol and its concentrates are demonstrated | [44] | |
| Regulate the TLR2/TLR4-NF-κB/MAPKs pathway | Inhibit the inflammatory response caused by Toxoplasma gondii (T. gondii) infection and exert its anti-Toxoplasma effect in vitro | [11] | |
| Hypoglycemic Effects | Restore body weight, reduce fasting blood glucose levels, improve glucose tolerance, and increase insulin levels | Powerful hypoglycemic ability and the function of enhancing islet cell function | [32] |
| PI3K/Akt signaling pathway | Restore the body weight and fat mass of diabetic mice, reduce fasting blood glucose levels, improve glucose tolerance, increase hepatic glycogen levels, and ameliorate insulin resistance | [46] | |
| Increase the levels of endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) isoenzymes; reduce the level of inducible nitric oxide synthase (iNOS) to alleviate pro-inflammatory responses, and inhibit phosphodiesterase 5 (PDE5) to reduce the hydrolysis of cyclic guanosine monophosphate (cGMP); regulate the NOS-cGMP-PDE5 signaling pathway | Counteracting glucose and lipid metabolism as well as renal function disorders induced by high-fat diet (HFD)/streptozotocin (STZ) | [47] | |
| Ameliorating serum profiling | Reduce blood glucose | [48] | |
| Hypolipidemic Effects | Increase the expression of CYP 7A1 and SR-B1 proteins | Lower cholesterol levels | [49] |
| Stimulate the gene and protein expression of AMPK, SREBP-1C, FAS, and ACC | Reduce blood lipids | [50] | |
| Antiviral Activity | Exhibit high antiviral activity against SARS-CoV-2 | Therapeutic and preventive agents | [51] |
| Chaga mushroom components, beta glycan, galactomannan, and betulinic acid exhibited strong binding interaction with the S1-carboxy-terminal domain of the receptor-binding domain of SARS-CoV-2, mainly at TRP-436, ASN-437, and ASN-440 sites | An effective natural antiviral | [52] | |
| A variety of virus activities were inhibited using the IOPSs | IOPSs effectively inhibited at least five different families, including RNA viruses (Caliciviridae, Coronaviridae, and orthomyxoviridae) and DNA viruses (alphaherpesviridae and parvoviruses) | [12] | |
| Antioxidant Activity | Alleviate H2O2-induced oxidative stress injury, reactive oxygen species (ROS) accumulation, and mitochondrial damage in SH-SY5Y cells; Nrf2 and BDNF/TrkB/ERK/CREB signaling pathways | Neuroprotective effect | [53] |
| Reduce the levels of reactive oxygen species (ROS) and malondialdehyde (MDA), while enhancing the activities of superoxide dismutase (SOD) and catalase (CAT) | Alleviate oxidative stress | [53] |
| Relevant Metabolism | Biosynthesis | Genes | References |
|---|---|---|---|
| Polyphenols | Phenylpropanoid pathway | Phenylalanine ammonia lyase (PAL), cinnamic acid 4-hydroxylase (C4H), 4-coumarate-CoA ligase (4CL), and chalcone isomerase (CHI) catalyze the formation of hispidin precursors | [54] |
| Phenylpropanoid pathway | UV radiation upregulated the expression of metabolism-related genes and enhanced the activity of PAL and CHI in the phenylpropanoid pathway | [55] | |
| Triterpenes | Mevalonate (MVA) pathway | 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), farnesyl pyrophosphate synthase (FPS), squalene synthase (SQS), and lanosterol synthase (LS) are key enzymes in this metabolic pathway | [56] |
| The effects of elicitors extracted from host and microbial sources, specifically birch bark (BB) and birch rhizosphere soil (BS), on the accumulation of triterpenes and FPS expression levels in I. obliquus | BB can increase triterpene accumulation to some extent, while BS has an inhibitory effect on triterpene accumulation, with significant differences in its inhibitory effects on BB | [58] | |
| Polysaccharides | The mutant genes associated with polysaccharide biosynthesis include those encoding glucose-1-phosphate uridylyltransferase, phosphoglucose isomerase, glycosidases, and glycosyltransferases | Phosphoglucose isomerase (PGI) is involved in the pentose phosphate pathway, glycolysis/gluconeogenesis, and amino sugar and nucleotide sugar metabolism | [59] |
| Different carbon sources significantly affected the expression levels of key genes in the polysaccharide biosynthesis pathway, PGI and UDP-Glc4-epimase (UGE) | Carbon sources differentially regulate the expression levels of polysaccharide biosynthesis-related genes, which in turn affect the synthesis, structure, and function of I. obliquus polysaccharides | [61] | |
| Lignocellulosic materials | Increased polysaccharide content and α-glucosidase inhibition rate and advanced the onset of α-glucosidase inhibitory activity, thereby promoting polysaccharide synthesis | [62] | |
| Used elicitors such as VB6, VB1, betulin, and birch extract to regulate Inonotus obliquus | Promoted EPS production and enhanced the inhibitory activity of polysaccharides against α-glucosidase | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Gu, J.; Wu, J.; Xu, Y.; Liu, Y.; Li, F.; Liu, Q.; Lu, K.; Liang, T.; Hao, J.; et al. Natural Products and Health Care Functions of Inonotus obliquus. Curr. Issues Mol. Biol. 2025, 47, 269. https://doi.org/10.3390/cimb47040269
Wang Y, Gu J, Wu J, Xu Y, Liu Y, Li F, Liu Q, Lu K, Liang T, Hao J, et al. Natural Products and Health Care Functions of Inonotus obliquus. Current Issues in Molecular Biology. 2025; 47(4):269. https://doi.org/10.3390/cimb47040269
Chicago/Turabian StyleWang, Yiming, Jingsheng Gu, Jiaying Wu, Yuxuan Xu, Yiting Liu, Fengxiu Li, Qiao Liu, Kailun Lu, Ting Liang, Jingwen Hao, and et al. 2025. "Natural Products and Health Care Functions of Inonotus obliquus" Current Issues in Molecular Biology 47, no. 4: 269. https://doi.org/10.3390/cimb47040269
APA StyleWang, Y., Gu, J., Wu, J., Xu, Y., Liu, Y., Li, F., Liu, Q., Lu, K., Liang, T., Hao, J., Li, L., Cao, X., & Jiang, J. (2025). Natural Products and Health Care Functions of Inonotus obliquus. Current Issues in Molecular Biology, 47(4), 269. https://doi.org/10.3390/cimb47040269









