Harnessing Exosomes: A Brief Overview of Nature’s Nanocarriers and a Glimpse into Their Implications in Pituitary Neuroendocrine Tumors (PitNETs)
Abstract
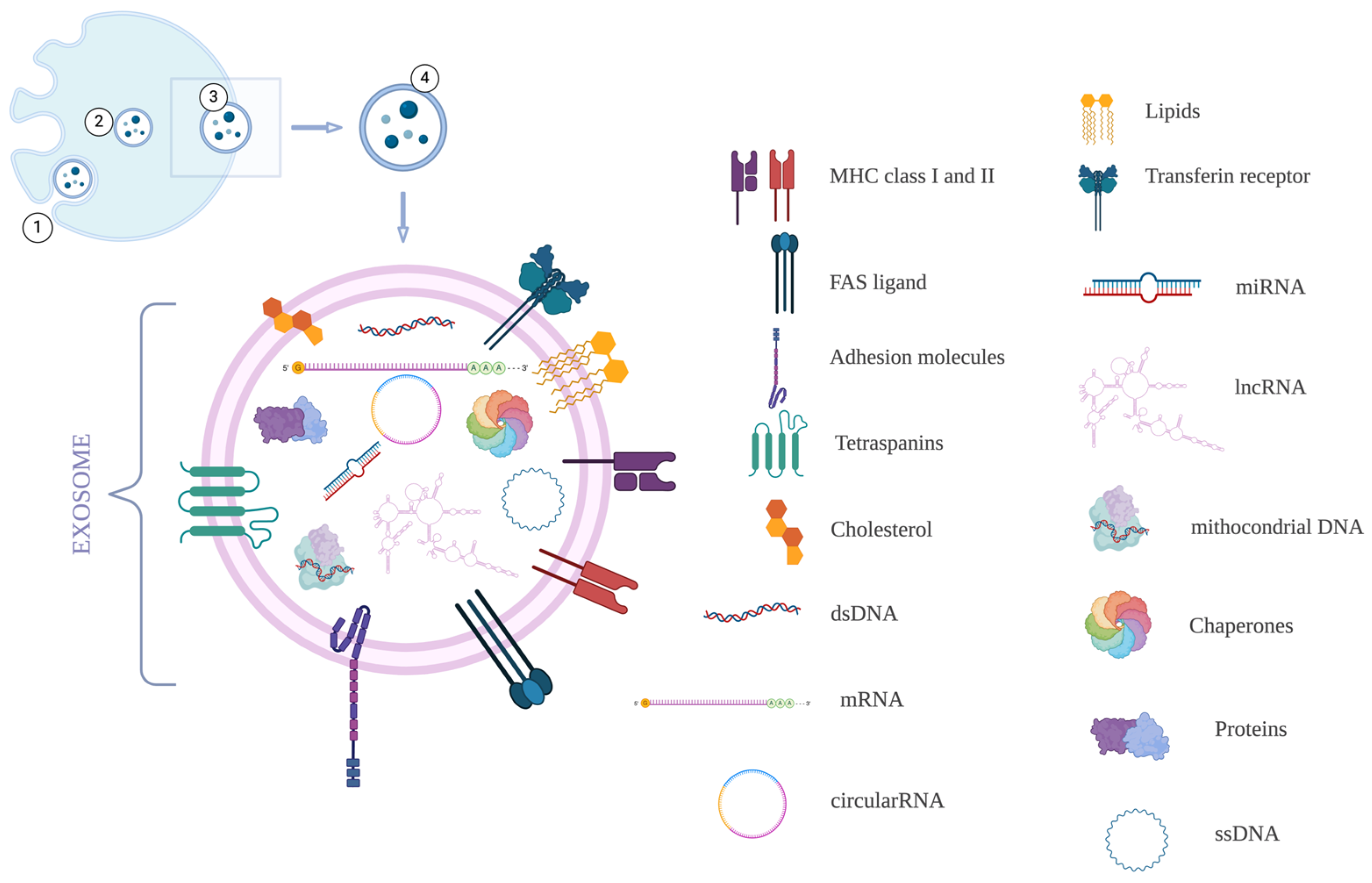
1. Introduction
2. Roles of Exosomes
- Roles in infection and pregnancy
- Transfer of oncogenic signals between cells
- Communication between neoplastic cells and the tumor microenvironment
- Reshaping cellular metabolism
- Promoting angiogenesis
- Mediating immune evasion
- Biomarkers for diagnosis and prognosis
- Drug delivery vehicles
- Therapeutic target
2.1. Roles in Infection and Pregnancy
2.2. Transfer of Oncogenic Signals Between Cells
2.3. Communication Between Neoplastic Cells and the Tumor Microenvironment
2.4. Reshaping Cellular Metabolism
2.5. Promoting Angiogenesis
2.6. Mediating Immune Evasion
2.7. Biomarkers for Diagnosis and Prognosis
2.8. Drug Delivery Vehicles
2.9. Therapeutic Target
3. Researched Implications of Exosomes in PitNETs
3.1. Exosomes as Biomarkers in PitNETs
3.2. Exosomes as a Potential Therapeutic Target in PitNETs
3.3. Mechanistic Insights on the Influence of Exosomes in PitNETs
4. Conclusions
Funding
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Yokoi, A.; Ochiya, T. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J.A. Ectosomes as modulators of inflammation and immunity. Clin. Exp. Immunol. 2011, 163, 26–32. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Liu, C.; Zhao, Y. Extracellular vesicles in cancers: Mechanisms, biomarkers, and therapeutic strategies. MedComm 2024, 5, e70009. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Zöller, M. Exosomes in Cancer Disease. Methods Mol. Biol. 2016, 1381, 111–149. [Google Scholar] [CrossRef]
- Liu, M.; Wen, Z.; Zhang, T.; Zhang, L.; Liu, X.; Wang, M. The role of exosomal molecular cargo in exosome biogenesis and disease diagnosis. Front. Immunol. 2024, 15, 1417758. [Google Scholar] [CrossRef]
- Lisiewicz, P.; Szelachowska, M.; Krętowski, A.J.; Siewko, K. The prospective roles of exosomes in pituitary tumors. Front. Endocrinol. 2024, 15, 1482756. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, M.; Comandatore, A.; Franczak, M.A.; Smolenski, R.T.; Peters, G.J.; Morelli, L.; Giovannetti, E. Role of exosomes in transferring chemoresistance through modulation of cancer glycolytic cell metabolism. Cytokine Growth Factor. Rev. 2023, 73, 163–172. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Donker, R.B.; Mouillet, J.F.; Chu, T.; Bayer, A.; Ouyang, Y.; Wang, T.; Stolz, D.B.; Sarkar, S.N.; Morelli, A.E.; et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. USA 2013, 110, 12048–12053. [Google Scholar] [CrossRef]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C. Circulating Exosomal miRNA Profile During Term and Preterm Birth Pregnancies: A Longitudinal Study. Endocrinology 2019, 160, 249–275. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Trivedi, J.; Yellon, S.M.; Menon, R. Exosomes Cause Preterm Birth in Mice: Evidence for Paracrine Signaling in Pregnancy. Sci. Rep. 2019, 9, 608. [Google Scholar] [CrossRef]
- Salomon, C.; Torres, M.J.; Kobayashi, M.; Scholz-Romero, K.; Sobrevia, L.; Dobierzewska, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS ONE 2014, 9, e98667. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Tai, Y.L.; Chen, K.C.; Hsieh, J.T.; Shen, T.L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, T.; Liu, R.; Bai, M.; Zhou, L.; Wang, X.; Li, S.; Wang, X.; Yang, H.; Li, J.; et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat. Commun. 2017, 8, 15016. [Google Scholar] [CrossRef]
- Wu, S.; Luo, M.; To, K.K.W.; Zhang, J.; Su, C.; Zhang, H.; An, S.; Wang, F.; Chen, D.; Fu, L. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol. Cancer 2021, 20, 17. [Google Scholar] [CrossRef]
- Jin, H.; Liu, P.; Wu, Y.; Meng, X.; Wu, M.; Han, J.; Tan, X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018, 109, 2946–2956. [Google Scholar] [CrossRef]
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010, 70, 9621–9630. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef]
- Vu, L.T.; Peng, B.; Zhang, D.X.; Ma, V.; Mathey-Andrews, C.A.; Lam, C.K.; Kiomourtzis, T.; Jin, J.; McReynolds, L.; Huang, L.; et al. Tumor-secreted extracellular vesicles promote the activation of cancer-associated fibroblasts via the transfer of microRNA-125b. J. Extracell. Vesicles 2019, 8, 1599680. [Google Scholar] [CrossRef]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef]
- Yan, W.; Wu, X.; Zhou, W.; Fong, M.Y.; Cao, M.; Liu, J.; Liu, X.; Chen, C.H.; Fadare, O.; Pizzo, D.P.; et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat. Cell Biol. 2018, 20, 597–609. [Google Scholar] [CrossRef]
- Rai, A.; Greening, D.W.; Chen, M.; Xu, R.; Ji, H.; Simpson, R.J. Exosomes Derived from Human Primary and Metastatic Colorectal Cancer Cells Contribute to Functional Heterogeneity of Activated Fibroblasts by Reprogramming Their Proteome. Proteomics 2019, 19, e1800148. [Google Scholar] [CrossRef]
- Sagar, G.; Sah, R.P.; Javeed, N.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Giorgadze, N.; Tchkonia, T.; Kirkland, J.L.; Chari, S.T.; et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 2016, 65, 1165–1174. [Google Scholar] [CrossRef]
- Hu, W.; Ru, Z.; Xiao, W.; Xiong, Z.; Wang, C.; Yuan, C.; Zhang, X.; Yang, H. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem. Biophys. Res. Commun. 2018, 506, 122–129. [Google Scholar] [CrossRef]
- Wong, S.K.; Mohamad, N.V.; Giaze, T.R.; Chin, K.Y.; Mohamed, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef]
- Taverna, S.; Flugy, A.; Saieva, L.; Kohn, E.C.; Santoro, A.; Meraviglia, S.; De Leo, G.; Alessandro, R. Role of exosomes released by chronic myelogenous leukemia cells in angiogenesis. Int. J. Cancer 2012, 130, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013, 32, 2747–2755. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; De Veirman, K.; Faict, S.; Frassanito, M.A.; Ribatti, D.; Vacca, A.; Menu, E. Multiple myeloma exosomes establish a favourable bone marrow microenvironment with enhanced angiogenesis and immunosuppression. J. Pathol. 2016, 239, 162–173. [Google Scholar] [CrossRef]
- Wilson, C.M.; Naves, T.; Vincent, F.; Melloni, B.; Bonnaud, F.; Lalloué, F.; Jauberteau, M.O. Sortilin mediates the release and transfer of exosomes in concert with two tyrosine kinase receptors. J. Cell Sci. 2014, 127, 3983–3997. [Google Scholar] [CrossRef]
- Kucharzewska, P.; Christianson, H.C.; Welch, J.E.; Svensson, K.J.; Fredlund, E.; Ringnér, M.; Mörgelin, M.; Bourseau-Guilmain, E.; Bengzon, J.; Belting, M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc. Natl. Acad. Sci. USA 2013, 110, 7312–7317. [Google Scholar] [CrossRef]
- Aga, M.; Bentz, G.L.; Raffa, S.; Torrisi, M.R.; Kondo, S.; Wakisaka, N.; Yoshizaki, T.; Pagano, J.S.; Shackelford, J. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene 2014, 33, 4613–4622. [Google Scholar] [CrossRef]
- de Jong, O.G.; van Balkom, B.W.; Gremmels, H.; Verhaar, M.C. Exosomes from hypoxic endothelial cells have increased collagen crosslinking activity through up-regulation of lysyl oxidase-like 2. J. Cell Mol. Med. 2016, 20, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Gao, L.; Tang, X.; He, Q.; Sun, G.; Wang, C.; Qu, H. Exosome-transmitted circCOG2 promotes colorectal cancer progression via miR-1305/TGF-β2/SMAD3 pathway. Cell Death Discov. 2021, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.E.; Nowicki, M.O.; Ricklefs, F.L.; Chiocca, E.A. Immune Escape Mediated by Exosomal PD-L1 in Cancer. Adv. Biosyst. 2020, 4, e2000017. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zeng, W.; Wu, B.; Wang, L.; Wang, Z.; Tian, H.; Wang, L.; Jiang, Y.; Clay, R.; Wei, X.; et al. PPARα Inhibition Overcomes Tumor-Derived Exosomal Lipid-Induced Dendritic Cell Dysfunction. Cell Rep. 2020, 33, 108278. [Google Scholar] [CrossRef]
- Chen, L.; Huan, X.; Gao, X.D.; Yu, W.H.; Xiao, G.H.; Li, T.F.; Wang, Z.Y.; Zhang, Y.C. Biological Functions of the DNA Glycosylase NEIL3 and Its Role in Disease Progression Including Cancer. Cancers 2022, 14, 5722. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, R.; Li, B.; Qi, Y.; Qiu, W.; Guo, Q.; Zhang, S.; Zhao, S.; Xu, H.; Li, M.; et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol. Cancer 2022, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Kugeratski, F.G.; Kalluri, R. Exosomes as mediators of immune regulation and immunotherapy in cancer. FEBS J. 2021, 288, 10–35. [Google Scholar] [CrossRef]
- Essola, J.M.; Zhang, M.; Yang, H.; Li, F.; Xia, B.; Mavoungou, J.F.; Hussain, A.; Huang, Y. Exosome regulation of immune response mechanism: Pros and cons in immunotherapy. Bioact. Mater. 2024, 32, 124–146. [Google Scholar] [CrossRef]
- Suvasini, R.; Shruti, B.; Thota, B.; Shinde, S.V.; Friedmann-Morvinski, D.; Nawaz, Z.; Prasanna, K.V.; Thennarasu, K.; Hegde, A.S.; Arivazhagan, A.; et al. Insulin growth factor-2 binding protein 3 (IGF2BP3) is a glioblastoma-specific marker that activates phosphatidylinositol 3-kinase/mitogen-activated protein kinase (PI3K/MAPK) pathways by modulating IGF-2. J. Biol. Chem. 2011, 286, 25882–25890. [Google Scholar] [CrossRef]
- Taylor, D.D.; Bender, D.P.; Gerçel-Taylor, C.; Stanson, J.; Whiteside, T.L. Modulation of TcR/CD3-zeta chain expression by a circulating factor derived from ovarian cancer patients. Br. J. Cancer 2001, 84, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xing, J.; Xu, K.; Liu, D.; Zhuo, Y. Exosomes in the tumor microenvironment: Promoting cancer progression. Front. Immunol. 2022, 13, 1025218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kamohara, H.; Kinoshita, K.; Kurashige, J.; Ishimoto, T.; Iwatsuki, M.; Watanabe, M.; Baba, H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 2013, 119, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hou, L.; Li, A.; Duan, Y.; Gao, H.; Song, X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed. Res. Int. 2014, 2014, 864894. [Google Scholar] [CrossRef]
- Machida, T.; Tomofuji, T.; Maruyama, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Miyai, H.; Mizuno, H.; Kato, H.; Tsutsumi, K.; et al. miR-1246 and miR-4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol. Rep. 2016, 36, 2375–2381. [Google Scholar] [CrossRef]
- Zhou, H.; Cheruvanky, A.; Hu, X.; Matsumoto, T.; Hiramatsu, N.; Cho, M.E.; Berger, A.; Leelahavanichkul, A.; Doi, K.; Chawla, L.S.; et al. Urinary exosomal transcription factors, a new class of biomarkers for renal disease. Kidney Int. 2008, 74, 613–621. [Google Scholar] [CrossRef]
- Dijkstra, S.; Birker, I.L.; Smit, F.P.; Leyten, G.H.; de Reijke, T.M.; van Oort, I.M.; Mulders, P.F.; Jannink, S.A.; Schalken, J.A. Prostate cancer biomarker profiles in urinary sediments and exosomes. J. Urol. 2014, 191, 1132–1138. [Google Scholar] [CrossRef]
- Oshikawa, S.; Sonoda, H.; Ikeda, M. Aquaporins in Urinary Extracellular Vesicles (Exosomes). Int. J. Mol. Sci. 2016, 17, 957. [Google Scholar] [CrossRef]
- Zhou, H.; Pisitkun, T.; Aponte, A.; Yuen, P.S.; Hoffert, J.D.; Yasuda, H.; Hu, X.; Chawla, L.; Shen, R.F.; Knepper, M.A.; et al. Exosomal Fetuin-A identified by proteomics: A novel urinary biomarker for detecting acute kidney injury. Kidney Int. 2006, 70, 1847–1857. [Google Scholar] [CrossRef]
- Zhou, H.; Kajiyama, H.; Tsuji, T.; Hu, X.; Leelahavanichkul, A.; Vento, S.; Frank, R.; Kopp, J.B.; Trachtman, H.; Star, R.A.; et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Renal Physiol. 2013, 305, F553–F559. [Google Scholar] [CrossRef]
- Graner, M.W.; Alzate, O.; Dechkovskaia, A.M.; Keene, J.D.; Sampson, J.H.; Mitchell, D.A.; Bigner, D.D. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009, 23, 1541–1557. [Google Scholar] [CrossRef]
- San Lucas, F.A.; Allenson, K.; Bernard, V.; Castillo, J.; Kim, D.U.; Ellis, K.; Ehli, E.A.; Davies, G.E.; Petersen, J.L.; Li, D.; et al. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Ann. Oncol. 2016, 27, 635–641. [Google Scholar] [CrossRef]
- Natasha, G.; Gundogan, B.; Tan, A.; Farhatnia, Y.; Wu, W.; Rajadas, J.; Seifalian, A.M. Exosomes as immunotheranostic nanoparticles. Clin. Ther. 2014, 36, 820–829. [Google Scholar] [CrossRef]
- Yong, T.; Wang, D.; Li, X.; Yan, Y.; Hu, J.; Gan, L.; Yang, X. Extracellular vesicles for tumor targeting delivery based on five features principle. J. Control. Release 2020, 322, 555–565. [Google Scholar] [CrossRef]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef]
- Kalani, A.; Kamat, P.K.; Chaturvedi, P.; Tyagi, S.C.; Tyagi, N. Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci. 2014, 107, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Du, Y.; Zhang, C.; Pan, F.; Yao, Y.; Zhang, T.; Peng, Q. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019, 86, 1–14. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles 2018, 7, 1440131. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef]
- Li, X.; Corbett, A.L.; Taatizadeh, E.; Tasnim, N.; Little, J.P.; Garnis, C.; Daugaard, M.; Guns, E.; Hoorfar, M.; Li, I.T.S. Challenges and opportunities in exosome research-Perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019, 3, 011503. [Google Scholar] [CrossRef]
- Arafah, B.M.; Nasrallah, M.P. Pituitary tumors: Pathophysiology, clinical manifestations and management. Endocr. Relat. Cancer 2001, 8, 287–305. [Google Scholar] [CrossRef]
- Daly, A.F.; Beckers, A. The Epidemiology of Pituitary Adenomas. Endocrinol. Metab. Clin. N. Am. 2020, 49, 347–355. [Google Scholar] [CrossRef]
- Esposito, D.; Olsson, D.S.; Ragnarsson, O.; Buchfelder, M.; Skoglund, T.; Johannsson, G. Non-functioning pituitary adenomas: Indications for pituitary surgery and post-surgical management. Pituitary 2019, 22, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.U.; Lonser, R.R. Management of hormone-secreting pituitary adenomas. Neuro Oncol. 2017, 19, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S. Pituitary-Tumor Endocrinopathies. N. Engl. J. Med. 2020, 382, 937–950. [Google Scholar] [CrossRef]
- Németh, K.; Darvasi, O.; Likó, I.; Szücs, N.; Czirják, S.; Reiniger, L.; Szabó, B.; Krokker, L.; Pállinger, É.; Igaz, P.; et al. Comprehensive Analysis of Circulating miRNAs in the Plasma of Patients With Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 4151–4168. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Li, H.; Chen, C.; Yu, Y.; Wang, L.; Yin, S.; Hu, Y.; Jiang, S.; Ye, F.; Zhou, P. Exosomal miRNA Profiling is a Potential Screening Route for Non-Functional Pituitary Adenoma. Front. Cell Dev. Biol. 2021, 9, 771354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, J.; Feng, J.; Li, Z.; Liu, Q.; Lv, P.; Wang, F.; Gao, H.; Zhang, Y. Identification of Serum miRNA-423-5p Expression Signature in Somatotroph Adenomas. Int. J. Endocrinol. 2019, 2019, 8516858. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.T.; Tang, H.; Xie, W.Q.; Yao, H.; Gu, W.T.; Zheng, Y.Z.; Shang, H.B.; Wang, Y.; Wei, Y.X.; et al. Exosome-Transmitted lncRNA H19 Inhibits the Growth of Pituitary Adenoma. J. Clin. Endocrinol. Metab. 2019, 104, 6345–6356. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.; Kang, X.; Liu, P.; Qian, L.; Shi, Y.; Osman, R.A.; Yang, Z.; Zhang, G. EMT-Related Markers in Serum Exosomes are Potential Diagnostic Biomarkers for Invasive Pituitary Adenomas. Neuropsychiatr. Dis. Treat. 2021, 17, 3769–3780. [Google Scholar] [CrossRef]
- Chang, M.; Jiang, S.; Guo, X.; Gao, J.; Liu, P.; Bao, X.; Feng, M.; Wang, R. Exosomal RNAs in the development and treatment of pituitary adenomas. Front. Endocrinol. 2023, 14, 1142494. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Du, Q.; Bian, P.; Chen, Y.; Liu, Z.; Zheng, J.; Sai, K.; Mou, Y.; Chen, Z.; et al. CircVPS13C promotes pituitary adenoma growth by decreasing the stability of IFITM1 mRNA via interacting with RRBP1. Oncogene 2022, 41, 1550–1562. [Google Scholar] [CrossRef]
- Wan, H.; Gao, X.; Yang, Z.; Wei, L.; Qu, Y.; Liu, Q. Exosomal CircMFN2 Enhances the Progression of Pituitary Adenoma via the MiR-146a-3p/TRAF6/NF-κB Pathway. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2025, 86, 135–147. [Google Scholar] [CrossRef]
- Xia, Y.; Pei, T.; Zhao, J.; Wang, Z.; Shen, Y.; Yang, Y.; Liang, J. Long noncoding RNA H19: Functions and mechanisms in regulating programmed cell death in cancer. Cell Death Discov. 2024, 10, 76. [Google Scholar] [CrossRef]
- Wu, Z.R.; Yan, L.; Liu, Y.T.; Cao, L.; Guo, Y.H.; Zhang, Y.; Yao, H.; Cai, L.; Shang, H.B.; Rui, W.W.; et al. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat. Commun. 2018, 9, 4624. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, D.; Li, W.; Zhang, G.; Zhang, H.; Peng, Q. Exosomal AFAP1-AS1 Promotes the Growth, Metastasis, and Glycolysis of Pituitary Adenoma by Inhibiting HuR Degradation. Mol. Neurobiol. 2025, 62, 2212–2229. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, N.; Sheida, A.; Rajabi, M.; Heidari, M.M.; Tobeiha, M.; Esfahani, P.V.; Ahmadi Asouri, S.; Hamblin, M.R.; Mohamadzadeh, O.; Motamedzadeh, A.; et al. Non-coding RNAs and exosomal non-coding RNAs in pituitary adenoma. Pathol. Res. Pract. 2023, 248, 154649. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tang, Y.; Fan, F.; Zeng, Y.; Li, C.; Zhou, G.; Hu, Z.; Zhang, L.; Liu, Z. Exosomal hsa-miR-21-5p derived from growth hormone-secreting pituitary adenoma promotes abnormal bone formation in acromegaly. Transl. Res. 2020, 215, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, Y.; Bao, X.; Feng, M.; Xing, B.; Lian, W.; Yao, Y.; Wang, R. Diagnosis of invasive non-functional pituitary adenomas using exosomal biomarkers. Clin. Chim. Acta 2022, 529, 25–33. [Google Scholar] [CrossRef]
- Wang, H.; Chen, K.; Yang, Z.; Li, W.; Wang, C.; Zhang, G.; Zhu, L.; Liu, P.; Yang, Y. Diagnosis of Invasive Nonfunctional Pituitary Adenomas by Serum Extracellular Vesicles. Anal. Chem. 2019, 91, 9580–9589. [Google Scholar] [CrossRef]
- Fagotto, F. EpCAM as Modulator of Tissue Plasticity. Cells 2020, 9, 2128. [Google Scholar] [CrossRef]
- Nawaz, F.Z.; Kipreos, E.T. Emerging roles for folate receptor FOLR1 in signaling and cancer. Trends Endocrinol. Metab. 2022, 33, 159–174. [Google Scholar] [CrossRef]
- Chen, X.; Pang, B.; Liang, Y.; Xu, S.C.; Xin, T.; Fan, H.T.; Yu, Y.B.; Pang, Q. Overexpression of EpCAM and Trop2 in pituitary adenomas. Int. J. Clin. Exp. Pathol. 2014, 7, 7907–7914. [Google Scholar]
- Liu, X.; Ma, S.; Yao, Y.; Li, G.; Feng, M.; Deng, K.; Dai, C.; Cai, F.; Li, Y.; Zhang, B.; et al. Differential expression of folate receptor alpha in pituitary adenomas and its relationship to tumor behavior. Neurosurgery 2012, 70, 1274–1280; discussion 1280. [Google Scholar] [CrossRef]
- Sundström, K.; Cedervall, T.; Ohlsson, C.; Camacho-Hübner, C.; Sävendahl, L. Combined treatment with GH and IGF-I: Additive effect on cortical bone mass but not on linear bone growth in female rats. Endocrinology 2014, 155, 4798–4807. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Caliskan, A.; Arga, K.Y. Overview of omics biomarkers in pituitary neuroendocrine tumors to design future diagnosis and treatment strategies. EPMA J. 2021, 12, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Sav, A. Editorial: Diagnosis and treatment of non-functioning pituitary tumors. Front. Endocrinol. 2025, 16, 1558988. [Google Scholar] [CrossRef] [PubMed]
| Biomarker/Therapeutic Target | Molecular Role | Clinical Potential |
|---|---|---|
| miR-149-5p | Tumor suppressor; inhibits proliferation, migration, and invasion | Therapeutic delivery via exosomes |
| miR-99a-3p | Tumor suppressor involved in regulating tumor dynamics | Diagnostic and therapeutic use |
| miR-21-5p | Promotes osteoblast activity and contributes to acromegaly development | Biomarker for acromegaly severity |
| miR-1180-3p | Potential early diagnostic biomarker for non-functional PitNETs | Liquid biopsy candidate |
| miR-143-3p | Downregulated in FSH/LH adenomas | Subtype-specific diagnostic marker |
| miR-486-5p | Significantly dysregulated in non-functional PitNETs | High sensitivity and specificity as a diagnostic biomarker |
| miR-423-5p | Decreased in somatotrophinomas; inhibits growth hormone secretion | Therapeutic target in somatotropinomas |
| lncRNA H19 | Inhibits tumoral proliferation; involved in the regulation of cellular apoptosis | Predicts medical response; therapeutic candidate |
| E-cad, N-cad, Epcam | EMT-related markers upregulated in invasive PitNETs | Serum biomarker panel for invasiveness |
| circMFN2 | Promotes tumoral growth via miR-146a/TRAF6/NF-κB signaling | Potential target for aggressive PitNETs |
| AFAP1-AS1 | Enhances tumoral growth, migration, and glycolysis via HuR stabilization | Targetable axis for metabolic intervention |
| FOLR1 | Downregulated in invasive non-functioning PitNETs | Biomarker for tumor invasiveness |
| Smad7/Runx2 Pathway | Stimulated by GH3-derived exosomes; activates osteoblasts | Mechanistic link to bone changes in acromegaly |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tataranu, L.G. Harnessing Exosomes: A Brief Overview of Nature’s Nanocarriers and a Glimpse into Their Implications in Pituitary Neuroendocrine Tumors (PitNETs). Curr. Issues Mol. Biol. 2025, 47, 310. https://doi.org/10.3390/cimb47050310
Tataranu LG. Harnessing Exosomes: A Brief Overview of Nature’s Nanocarriers and a Glimpse into Their Implications in Pituitary Neuroendocrine Tumors (PitNETs). Current Issues in Molecular Biology. 2025; 47(5):310. https://doi.org/10.3390/cimb47050310
Chicago/Turabian StyleTataranu, Ligia Gabriela. 2025. "Harnessing Exosomes: A Brief Overview of Nature’s Nanocarriers and a Glimpse into Their Implications in Pituitary Neuroendocrine Tumors (PitNETs)" Current Issues in Molecular Biology 47, no. 5: 310. https://doi.org/10.3390/cimb47050310
APA StyleTataranu, L. G. (2025). Harnessing Exosomes: A Brief Overview of Nature’s Nanocarriers and a Glimpse into Their Implications in Pituitary Neuroendocrine Tumors (PitNETs). Current Issues in Molecular Biology, 47(5), 310. https://doi.org/10.3390/cimb47050310






