Evaluation of Sapindus mukorossi Gaertn Flower Water Extract on In Vitro Anti-Acne Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.1.1. Plant Materials
2.1.2. Cell Culture
2.1.3. Chemicals
2.2. Phytochemical Measurements
2.2.1. Determination of Saponin, Total Flavonoid, and Total Phenol Contents
2.2.2. Untargeted Metabolomics Analysis
2.2.3. HPLC Conditions
2.3. In Vitro Antioxidant Assays
2.3.1. DPPH Radical Scavenging Assay
2.3.2. Superoxide Anion Radical Scavenging Assay
2.3.3. ABTS Radical Scavenging Activity
2.3.4. FRAP Assay
2.4. Antibacterial Assay
2.5. In Vitro Anti-Inflammatory Activity
2.5.1. Cell Viability Assay
2.5.2. Cytokine Measurement
2.6. RNA-Seq Analysis
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of SMFW
3.1.1. Total Saponin, Phenolic, and Flavonoid Contents
3.1.2. Untargeted Metabolomics Analysis of SMFW
3.1.3. HPLC Analysis
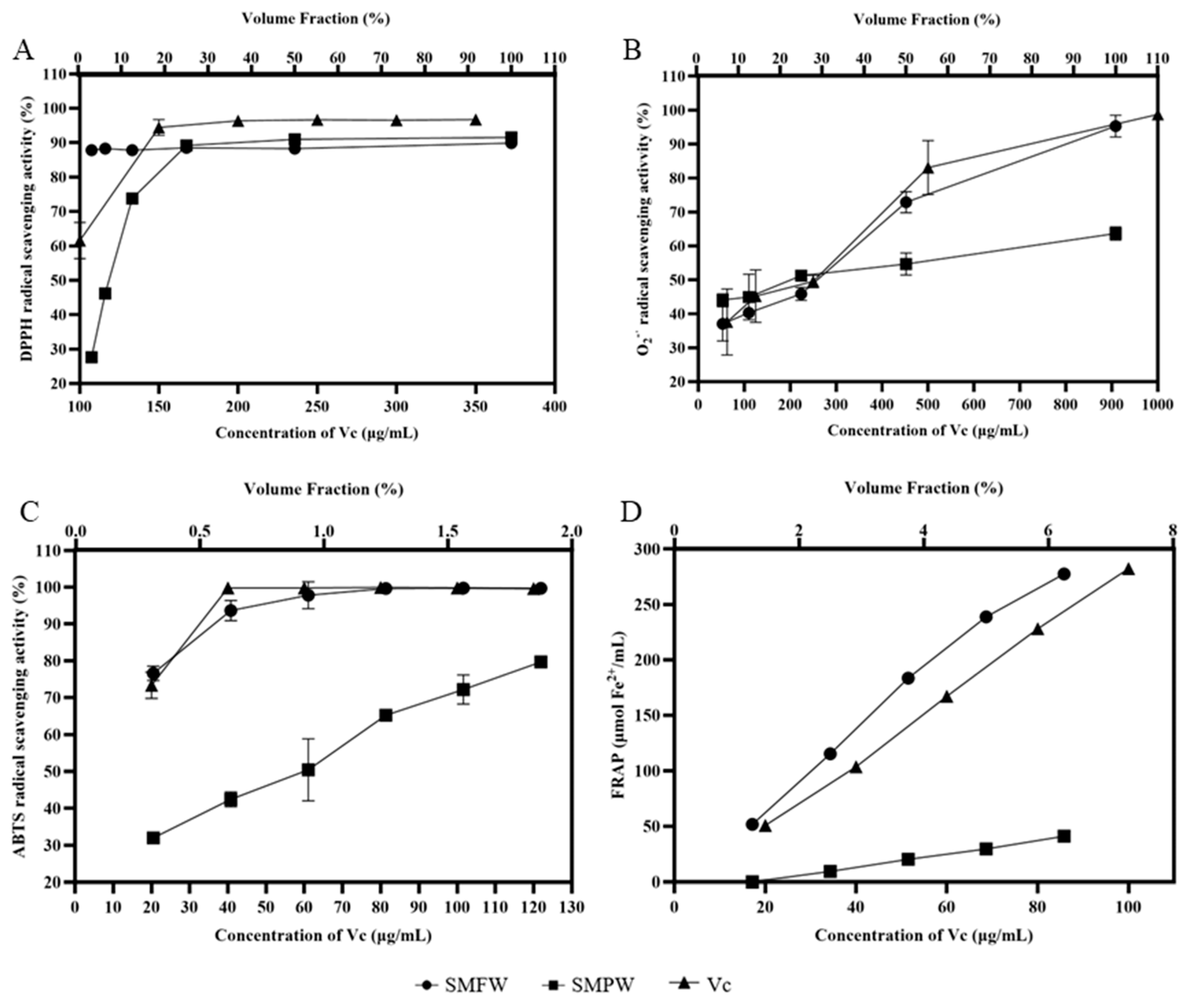
3.2. Antioxidant Activity
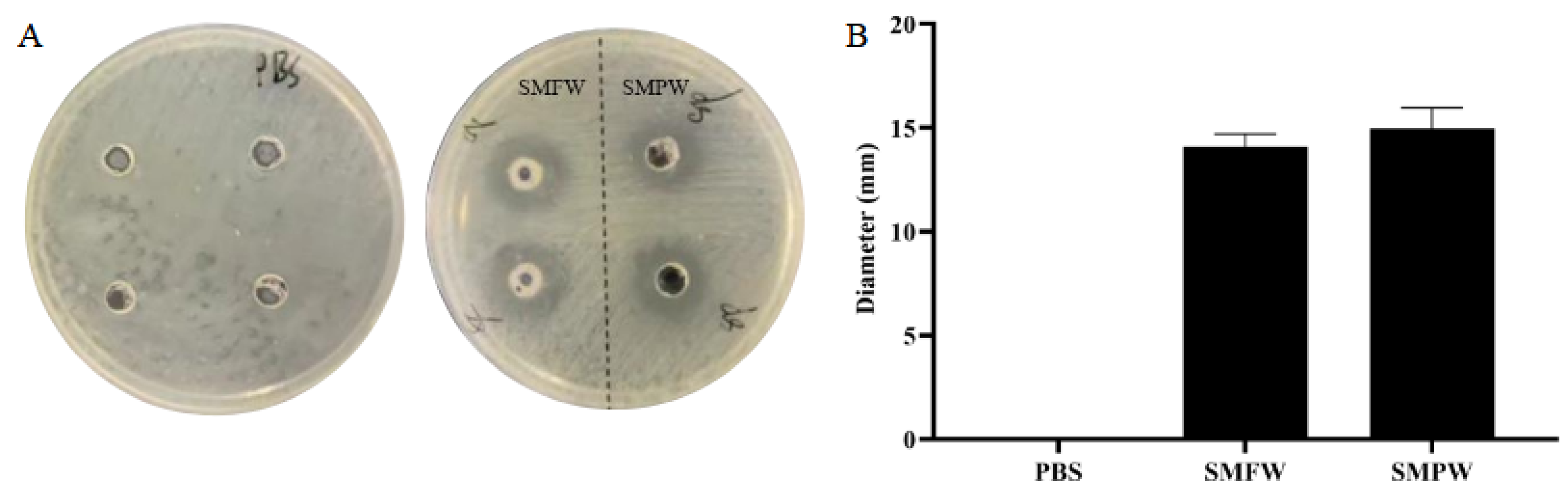
3.3. Antibacterial Activity Against C. acnes
3.4. Anti-Inflammatory Activity
3.5. Transcriptome Analysis
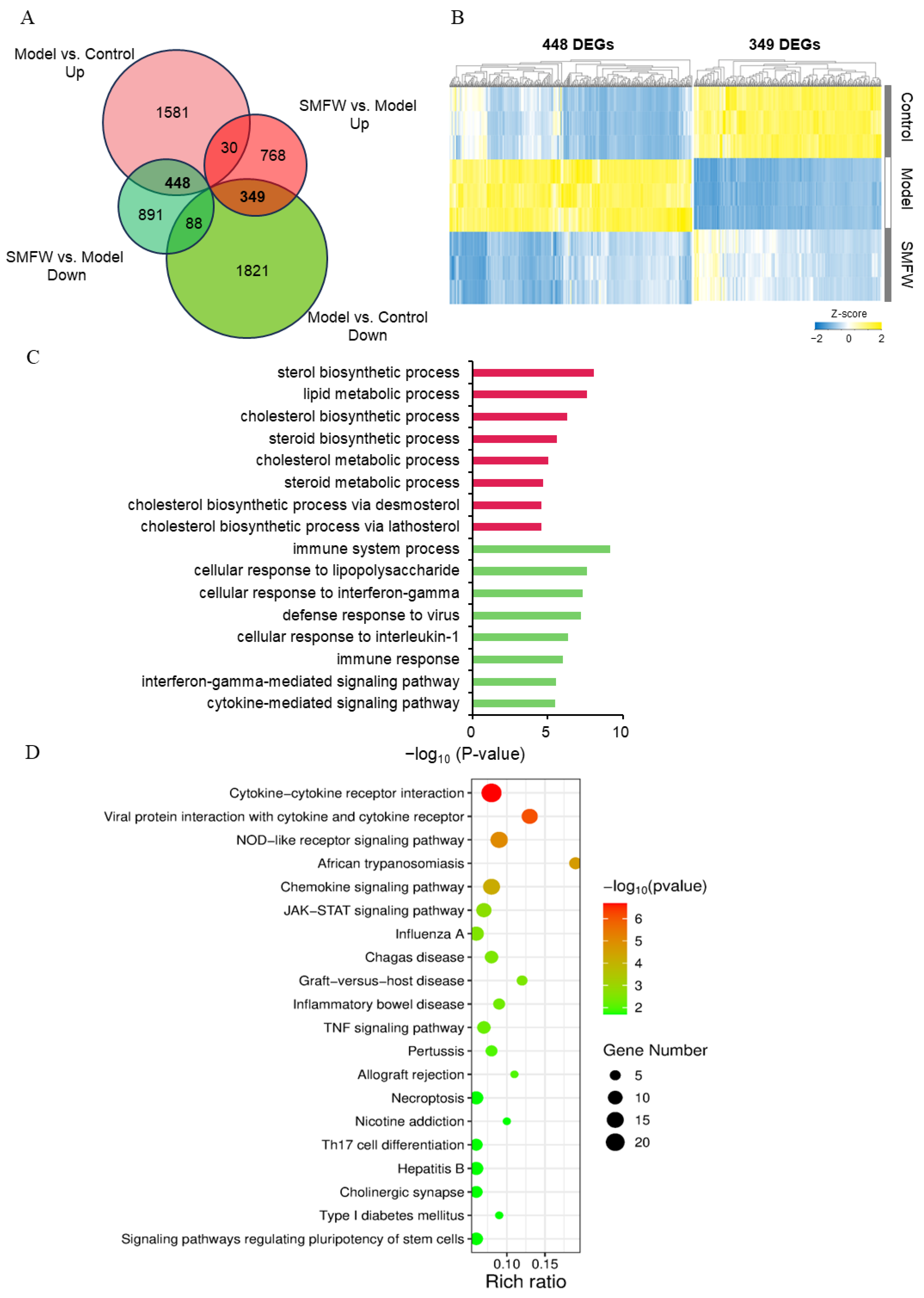
3.5.1. DEGs Between Model and Control
3.5.2. DEGs Between SMFW and Model
3.5.3. DEGs with Inverse Trends in Model vs. Control and SMFW vs. Model
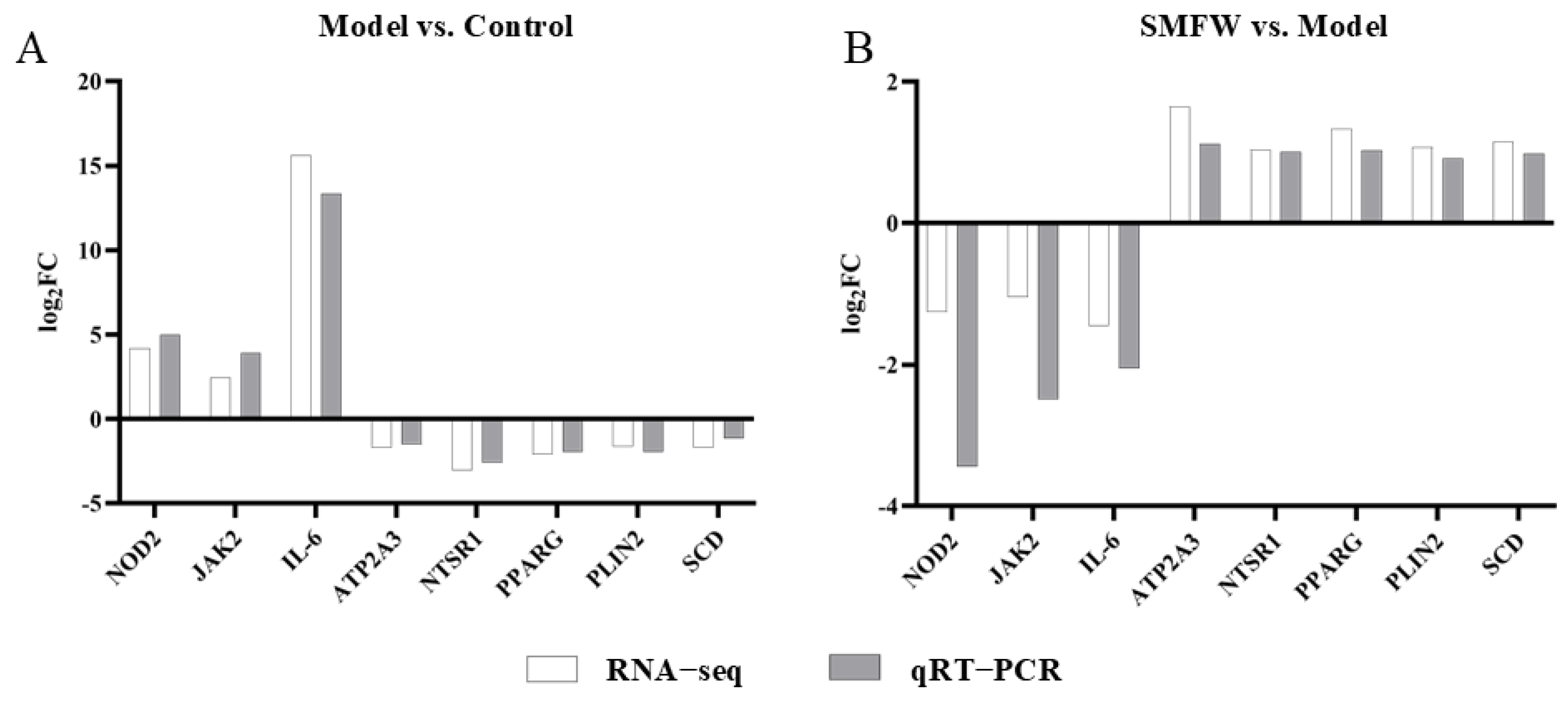
3.5.4. Validation of Gene Expression by qRT-PCR
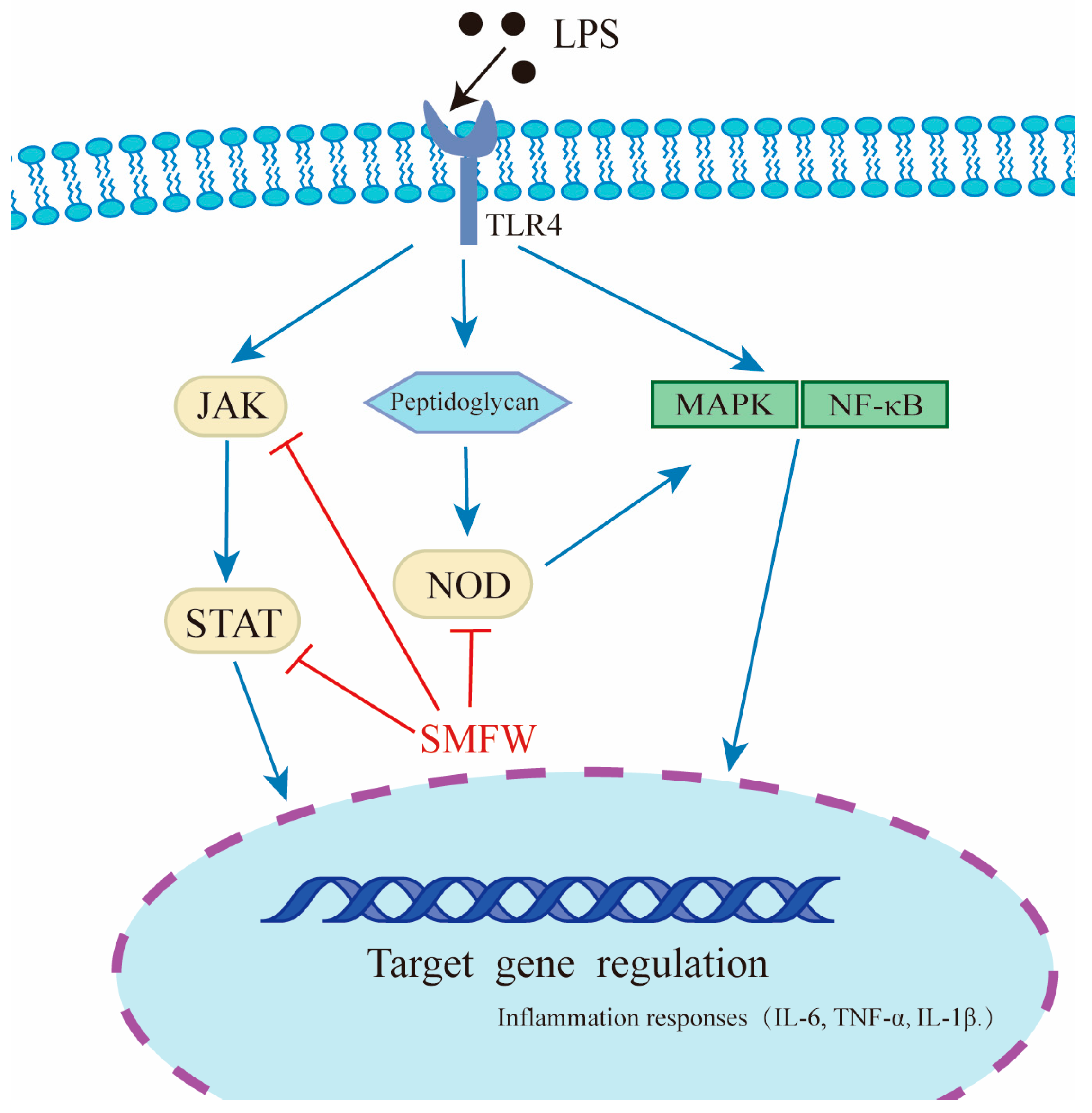
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMFW | S. mukorossi flower water extract |
| SMPW | S. mukorossi pericarp water extract |
| LPS | Lipopolysaccharide |
| DMX | Dexamethasone |
| DEG | Differential expressed genes |
| S. mukorossi | Sapindus mukorossi Gaertn |
References
- Singh, R.; Sharma, B. Biotechnological Advances, Phytochemical Analysis and Ethnomedical Implications of Sapindus Species; Springer: Singapore, 2019. [Google Scholar]
- Sochacki, M.; Vogt, O. Triterpenoid Saponins from Washnut (Sapindus mukorossi Gaertn.)—A Source of Natural Surfactants and Other Active Components. Plants 2022, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, L.; Wang, N.; Guo, Y.; Weng, Z.; Sun, Z.; Xu, D.; Xie, Y.; Yao, W. Extraction and Fermentation-Based Purification of Saponins from Sapindus mukorossi Gaertn. J. Surfactants Deterg. 2015, 18, 429–438. [Google Scholar]
- Xu, Y.; Zhou, S.; Chen, Z.; Zhao, G.; Liu, J.; Wang, L.; Wang, X.; Jia, L.; Zhang, D. Contents of the total saponins and total flavonoids in different organs of Sapindus mukorossi. J. Nanjing For. Univ. 2021, 45, 83. [Google Scholar]
- Liu, M.; Chen, Y.-L.; Kuo, Y.-H.; Lu, M.-K.; Liao, C.-C. Aqueous extract of Sapindus mukorossi induced cell death of A549 cells and exhibited antitumor property in vivo. Sci. Rep. 2018, 8, 4831. [Google Scholar] [CrossRef]
- Wei, M.; Qiu, J.; Li, L.; Xie, Y.; Yu, H.; Guo, Y.; Yao, W. Saponin fraction from Sapindus mukorossi Gaertn as a novel cosmetic additive: Extraction, biological evaluation, analysis of anti-acne mechanism and toxicity prediction. J. Ethnopharmacol. 2021, 268, 113552. [Google Scholar] [CrossRef]
- Shah, M.; Parveen, Z.; Khan, M.R. Evaluation of antioxidant, anti-inflammatory, analgesic and antipyretic activities of the stem bark of Sapindus mukorossi. BMC Complement. Altern. Med. 2017, 17, 526. [Google Scholar] [CrossRef]
- Chen, C.C.; Nien, C.J.; Chen, L.G.; Huang, K.Y.; Chang, W.J.; Huang, H.M. Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing. Int. J. Mol. Sci. 2019, 20, 2579. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Qin, B.; Wang, Y. Four new tirucallane-type triterpenoids from Sapindus mukorossi Gaertn. flowers induced neurite outgrowth in PC12 cells related to insulin-like growth factor 1 receptor/phosphoinositide 3-kinase/extracellular regulated protein kinase signaling pathway. Phytochem. Lett. 2019, 34, 5–12. [Google Scholar] [CrossRef]
- Reynolds, R.V.; Yeung, H.; Cheng, C.E.; Cook-Bolden, F.; Desai, S.R.; Druby, K.M.; Freeman, E.E.; Keri, J.E.; Gold, L.F.S.; Tan, J.K.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2024, 90, 1006.e1–1006.e30. [Google Scholar] [CrossRef]
- Feng, Y.; Li, J.; Mo, X.; Ju, Q. Macrophages in acne vulgaris: Mediating phagocytosis, inflammation, scar formation, and therapeutic implications. Front. Immunol. 2024, 15, 1355455. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Lee, W.-R.; Wang, P.-H.; Cheng, K.-T.; Chen, Y.-C.; Shen, S.-C. Propionibacterium acnes-induced iNOS and COX-2 protein expression via ROS-dependent NF-κB and AP-1 activation in macrophages. J. Dermatol. Sci. 2013, 69, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Tschismarov, R.; Pilz, A.; Straubinger, S.; Carotta, S.; McDowell, A.; Decker, T. Cutibacterium acnes Infection Induces Type I Interferon Synthesis Through the cGAS-STING Pathway. Front. Immunol. 2020, 11, 571334. [Google Scholar] [CrossRef] [PubMed]
- Sarici, G.; Cinar, S.; Armutcu, F.; Altinyazar, C.; Koca, R.; Tekin, N.S. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Srinivasarao, M.; Lakshminarasu, M.; Anjum, A.; Ibrahim, M. Comparative study on phytochemical, antimicrobial and antioxidant activity of Sapindus mukorossi Gaertn. and Rheum emodi Wall. ex Meissn.: In vitro studies. Ann. Phytomed. 2015, 4, 93–97. [Google Scholar]
- Wan, Z.; Zhu, J.; Tian, R.; Yang, W.; Chen, Z.; Hu, Q.; Zeng, Z. Quality evaluation for Dipacus asperoides from Enshi areas and optimization extraction of saponins and organic acids and its application. Arab. J. Chem. 2021, 14, 103107. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Olive (Olea europaea) Leaves, and Evaluation of Their Antioxidant and Anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Xu, S.; Fang, D.; Tian, X.; Xu, Y.; Zhu, X.; Wang, Y.; Lei, B.; Hu, P.; Ma, L. Subcritical water extraction of bioactive compounds from waste cotton (Gossypium hirsutum L.) flowers. Ind. Crop. Prod. 2021, 164, 113369. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Sharma, S.; Pradhan, R.; Manickavasagan, A.; Thimmanagari, M.; Saha, D.; Singh, S.S.; Dutta, A. Production of antioxidative protein hydrolysates from corn distillers solubles: Process optimization, antioxidant activity evaluation, and peptide analysis. Ind. Crop. Prod. 2022, 184, 115107. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Wu, W.; Zhang, G.; Zheng, Y.; Ma, C.; Li, W.; Yan, Y.; Xu, Z. Green extraction of active ingredients from finger citron using subcritical water and assessment of antioxidant activity. Ind. Crop. Prod. 2023, 200, 116821. [Google Scholar] [CrossRef]
- Jurić, T.; Pavlović, R.Ž.; Uka, D.; Beara, I.; Majkić, T.; Savić, S.; Žekić, M.; Popović, B.M. Natural deep eutectic solvents-mediated extraction of rosmarinic acid from Lamiaceae plants: Enhanced extractability and anti-inflammatory potential. Ind. Crop. Prod. 2024, 214, 118559. [Google Scholar] [CrossRef]
- Mustarichie, R.; Sulistyaningsih, S.; Runadi, D. Antibacterial Activity Test of Extracts and Fractions of Cassava Leaves (Manihot esculenta Crantz) against Clinical Isolates of Staphylococcus epidermidis and Propionibacterium acnes Causing Acne. Int. J. Microbiol. 2020, 2020, 1975904. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Hernández, D.; Demuner, A.J.; Barbosa, L.C.; Csuk, R.; Heller, L. Hederagenin as a triterpene template for the development of new antitumor compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Park, E.; Kato, H. Anti-inflammatory Activities of Hederagenin and Crude Saponin isolated from Sapindus mukorossi GAERTN. Chem. Pharm. Bull. 1980, 28, 1183–1188. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kumar, J.; Parmar, B.S. Structure-biological activity relationships in triterpenic saponins: The relative activity of protobassic acid and its derivatives against plant pathogenic fungi. Pest Manag. Sci. 2010, 66, 825–831. [Google Scholar] [CrossRef]
- Upadhyay, A.; Singh, D. Molluscicidal activity of Sapindus mukorossi and Terminalia chebula against the freshwater snail Lymnaea acuminata. Chemosphere 2011, 83, 468–474. [Google Scholar] [CrossRef]
- Jie, P. Ultrasonic-assisted Extraction Process of Ferulic Acid from Sapindus. Chin. J. Trop. Crop. 2012, 87, 562–567. [Google Scholar]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of phenolic acid, tannins, stilbenes, lignans and flavonoids in human health—A review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Ma, E.Z.; Khachemoune, A. Flavonoids and their therapeutic applications in skin diseases. Arch. Dermatol. Res. 2023, 315, 321–331. [Google Scholar] [CrossRef]
- Jaeger, R.; Cuny, E. Terpenoids with Special Pharmacological Significance: A Review. Nat. Prod. Commun. 2016, 11, 1373–1390. [Google Scholar] [CrossRef]
- Lu, S.-H.; Guan, J.-H.; Huang, Y.-L.; Pan, Y.-W.; Yang, W.; Lan, H.; Huang, S.; Hu, J.; Zhao, G.-P. Experimental Study of Antiatherosclerosis Effects with Hederagenin in Rats. Evid. Based Complement. Altern. Med. 2015, 2015, 456354. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-J.; Song, D.H.; Yoo, H.S.; Chung, K.-H.; Lee, K.J.; An, J.H. Hederagenin Supplementation Alleviates the Pro-Inflammatory and Apoptotic Response to Alcohol in Rats. Nutrients 2017, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Guo, Y.; Liu, C.; Yang, Y.; Fan, X.; Yang, H.; Liu, Y.; Ma, T. Function and inhibition of P38 MAP kinase signaling: Targeting multiple inflammation diseases. Biochem. Pharmacol. 2024, 220, 115973. [Google Scholar] [CrossRef]
- Xiong, H.; Li, X.; Mou, X.; Huang, C.; Yi, S.; Xiong, X.; Zhou, Y.; Chen, Y. Syringic acid suppresses Cutibacterium acnes-induced inflammation in human keratinocytes via regulating the NLRP3/caspase-1/IL-1β signaling axis by activating PPARγ/Nrf2-antioxidant pathway. Int. Immunopharmacol. 2024, 139, 112708. [Google Scholar] [CrossRef]
- Bungau, A.F.; Radu, A.F.; Bungau, S.G.; Vesa, C.M.; Tit, D.M.; Endres, L.M. Oxidative stress and metabolic syndrome in acne vulgaris: Pathogenetic connections and potential role of dietary supplements and phytochemicals. Biomed. Pharmacother. 2023, 164, 115003. [Google Scholar] [CrossRef]
- Zhou, X.; Su, Y.; Wang, H.; Kang, X.; Zhang, L.; He, G.; Jiang, X. Hydroxy-α-sanshool has anti-inflammatory and antioxidant effects and affects the NF-κB/STAT3 pathway via TRPV1 in acne. J. Funct. Foods 2023, 110, 105823. [Google Scholar] [CrossRef]
- Amorati, R.; Valgimigli, L. Methods To Measure the Antioxidant Activity of Phytochemicals and Plant Extracts. J. Agric. Food Chem. 2018, 66, 3324–3329. [Google Scholar] [CrossRef]
- Zhang, Y.; He, H.; Wang, D.; Song, L.; He, C. Evaluation of in vitro anti-acne activities of Ocimum basilicum L. water extract. Ind. Crop. Prod. 2022, 186, 115205. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Singh, R.; Kumari, N. Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn.—A valuable medicinal tree. Ind. Crop. Prod. 2015, 73, 1–8. [Google Scholar] [CrossRef]
- Huang, L.; Yang, S.; Yu, X.; Fang, F.; Zhu, L.; Wang, L.; Zhang, X.; Yang, C.; Qian, Q.; Zhu, T. Association of different cell types and inflammation in early acne vulgaris. Front. Immunol. 2024, 15, 1275269. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.G.; Martins, G.R.; Nucci, L.M.B.; Granero, F.O.; Figueiredo, C.C.M.; Santiago, P.S.; Silva, L.P. Antiglycation, antioxidant, antiacne, and photoprotective activities of crude extracts and triterpene saponin fraction of Sapindus saponaria L. fruits: An in vitro study. Asian Pac. J. Trop. Biomed. 2022, 12, 391–399. [Google Scholar] [CrossRef]
- Wei, M.; Yu, H.; Guo, Y.; Cheng, Y.; Xie, Y.; Yao, W. Synergistic antibacterial combination of Sapindoside A and B changes the fatty acid compositions and membrane properties of Cutibacterium acnes. Microbiol. Res. 2022, 255, 126924. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.; Wang, Y.; Yan, Y.; Wang, Y.; Su, M.; Lv, H.; Li, K.; Hao, X.; Xing, X.; et al. Application of lipopolysaccharide in establishing inflammatory models. Int. J. Biol. Macromol. 2024, 279, 135371. [Google Scholar] [CrossRef]
- Mytych, J.; Romerowicz-Misielak, M.; Koziorowski, M. Long-term culture with lipopolysaccharide induces dose-dependent cytostatic and cytotoxic effects in THP-1 monocytes. Toxicol. Vitr. 2017, 42, 1–9. [Google Scholar] [CrossRef]
- Shu, J.; He, X.; Zhang, L.; Li, H.; Wang, P.; Huang, X. Human amnion mesenchymal cells inhibit lipopolysaccharide-induced TNF-α and IL-1β production in THP-1 cells. Biol. Res. 2015, 48, 69. [Google Scholar] [CrossRef]
- Spangler, J.B.; Moraga, I.; Mendoza, J.L.; Garcia, K.C. Insights into cytokine-receptor interactions from cytokine engineering. Annu. Rev. Immunol. 2014, 33, 139–167. [Google Scholar] [CrossRef]
- Qin, M.; Pirouz, A.; Kim, M.-H.; Krutzik, S.R.; Garbán, H.J.; Kim, J. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J. Investig. Dermatol. 2013, 134, 381–388. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.; Zhang, W. NOD-like Receptor Signaling Pathway in Gastrointestinal Inflammatory Diseases and Cancers. Int. J. Mol. Sci. 2023, 24, 14511. [Google Scholar] [CrossRef]
- Liu, R.; Guo, Y.; Yu, J.; Wei, X.; Zhou, F.; Yuan, X.; Cai, L.; Yu, C. Protective effect of N-(E)-p-coumaroyltyrosine on LPS-induced acute inflammatory injury and signaling pathway analysis. Fish Shellfish Immunol. 2024, 144, 109242. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.M.; Tawfik, Y.M.; El-Mokhtar, M.A.; El-Gazzar, A.F.; Motaleb, A.A.A. Activation of Janus kinase signaling pathway in acne lesions. Dermatol. Ther. 2020, 34, e14563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-C.; Liao, L.-X.; Zhao, M.-B.; Dong, X.; Zeng, K.-W.; Tu, P.-F. Protosappanin A exerts anti-neuroinflammatory effect by inhibiting JAK2-STAT3 pathway in lipopolysaccharide-induced BV2 microglia. Chin. J. Nat. Med. 2017, 15, 674–679. [Google Scholar]
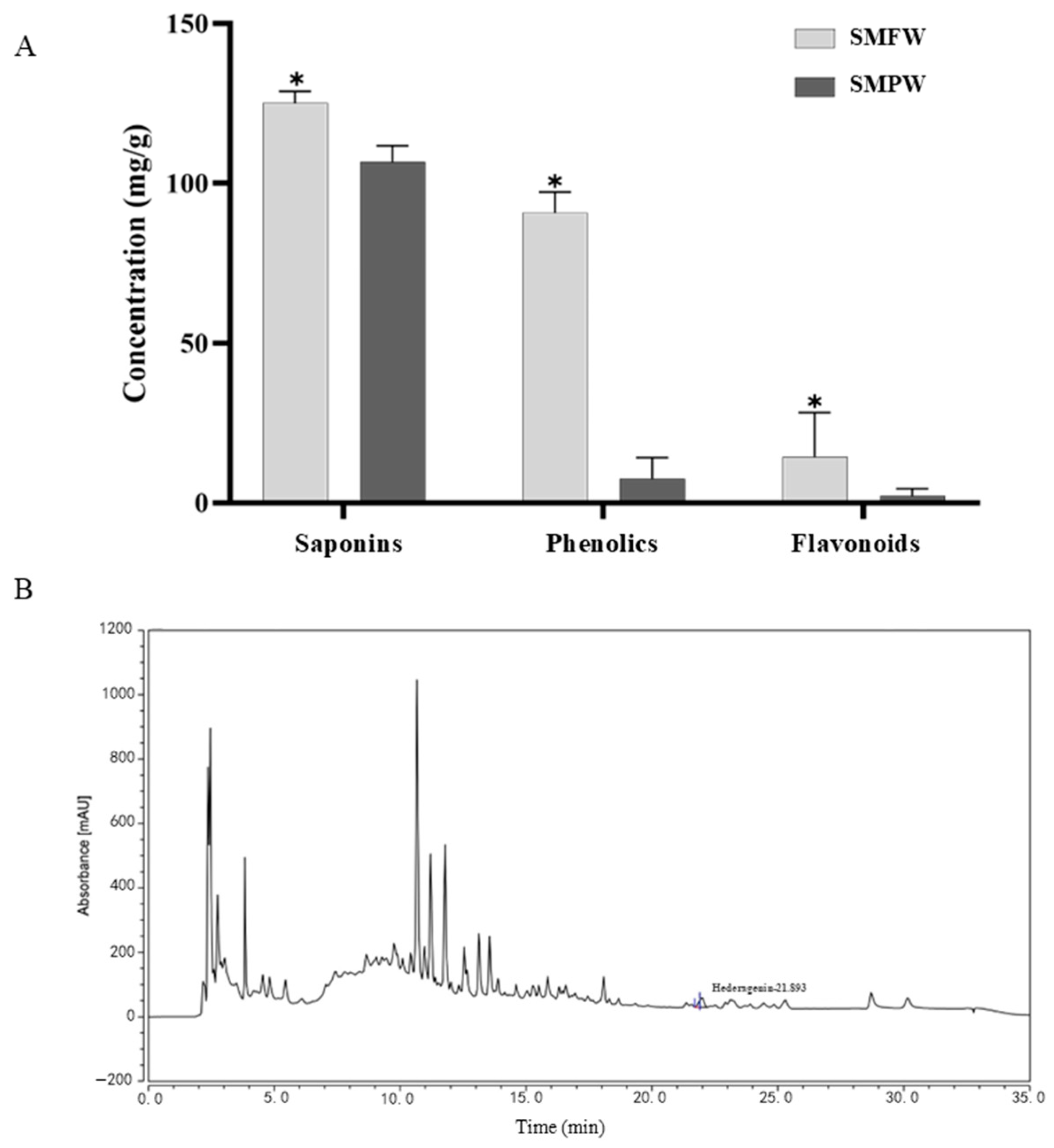


| Compound | Calibration Equation a | Retention Time (tr) | Correlation Coefficient (r2) |
|---|---|---|---|
| Hederagenin | Y = 0.0644x + 0.0306 | 21.893 min | 0.9999 |
| In Vitro Activity | Volume Fraction | SMFW | SMPW | |
|---|---|---|---|---|
| Antioxidant activity | DPPH scavenging rates (%) | 6.25% | 88.72 ± 0.66 | 46.53 ± 0.96 |
| O2− scavenging rate (%) | 6.25% | 36.94 ± 3.73 | 44.01 ± 3.15 | |
| ABTS scavenging rate (%) | 1.25% | 99.52 ± 0.23 | 65.05 ± 1.4 | |
| FRAP value | 1.25% | 52.11 ± 0.96 | 0.43 ± 0.35 | |
| Antibacterial assay | Inhibition diameter sizes (mm) | 100% | 14.08 ± 0.63 | 14.98 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, A.; Song, L.; He, C.; He, H. Evaluation of Sapindus mukorossi Gaertn Flower Water Extract on In Vitro Anti-Acne Activity. Curr. Issues Mol. Biol. 2025, 47, 316. https://doi.org/10.3390/cimb47050316
Zhao Z, Zhang A, Song L, He C, He H. Evaluation of Sapindus mukorossi Gaertn Flower Water Extract on In Vitro Anti-Acne Activity. Current Issues in Molecular Biology. 2025; 47(5):316. https://doi.org/10.3390/cimb47050316
Chicago/Turabian StyleZhao, Zibing, Aohuan Zhang, Liya Song, Congfen He, and Huaming He. 2025. "Evaluation of Sapindus mukorossi Gaertn Flower Water Extract on In Vitro Anti-Acne Activity" Current Issues in Molecular Biology 47, no. 5: 316. https://doi.org/10.3390/cimb47050316
APA StyleZhao, Z., Zhang, A., Song, L., He, C., & He, H. (2025). Evaluation of Sapindus mukorossi Gaertn Flower Water Extract on In Vitro Anti-Acne Activity. Current Issues in Molecular Biology, 47(5), 316. https://doi.org/10.3390/cimb47050316






