Applications of Growth Factors in Implant Dentistry
Abstract
:1. Introduction
2. Aims
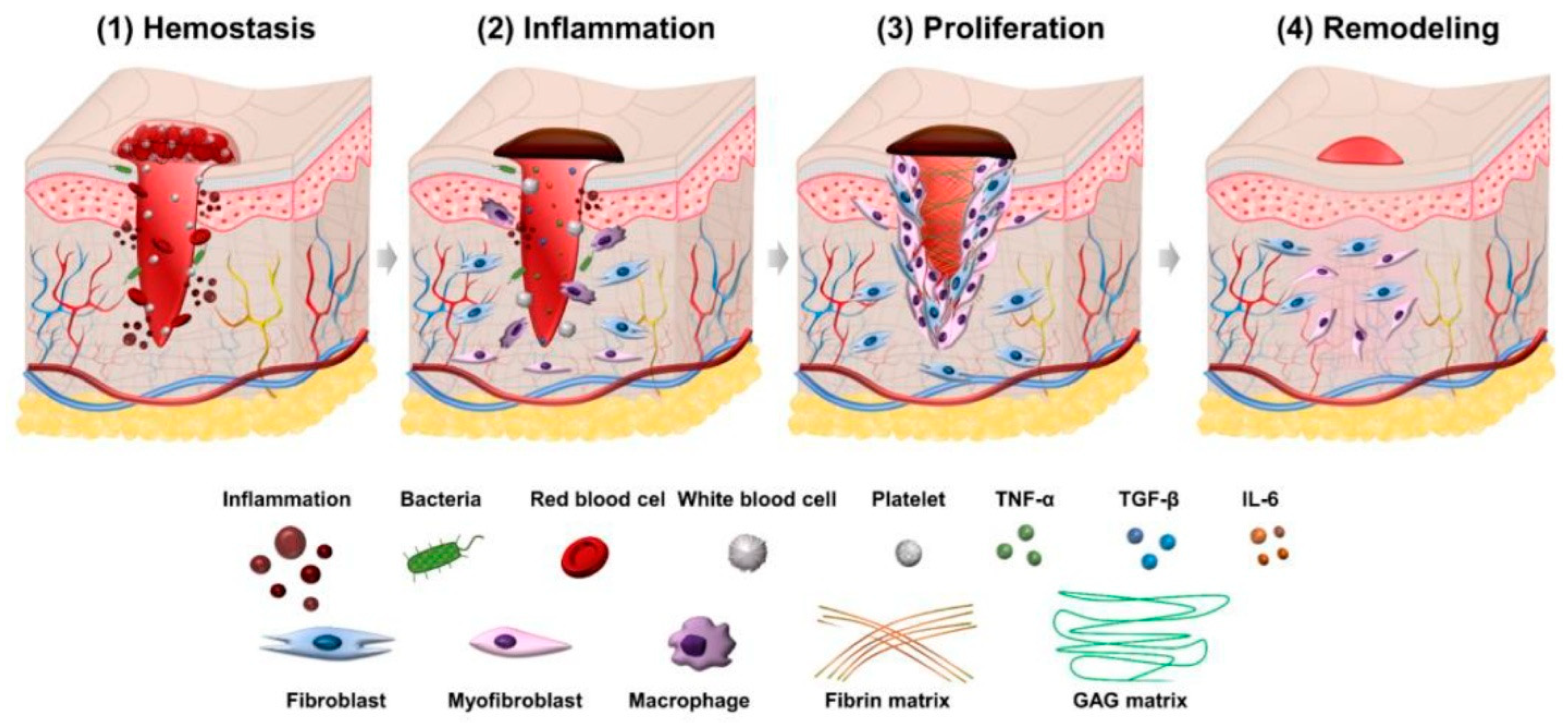
3. Physiology of Wound Healing
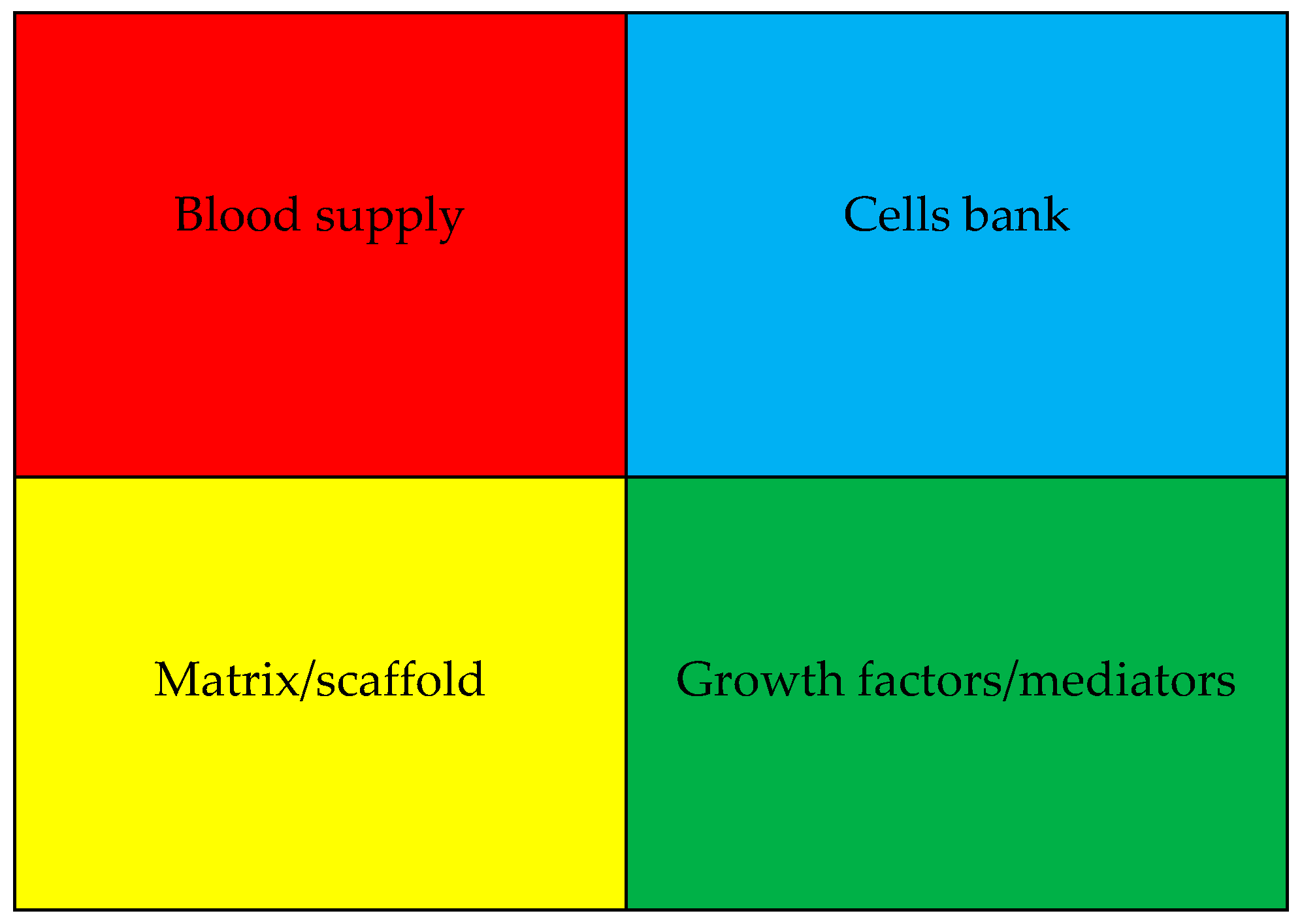
4. Role of Growth Factors in Tissue Regeneration, Healing, and Repair
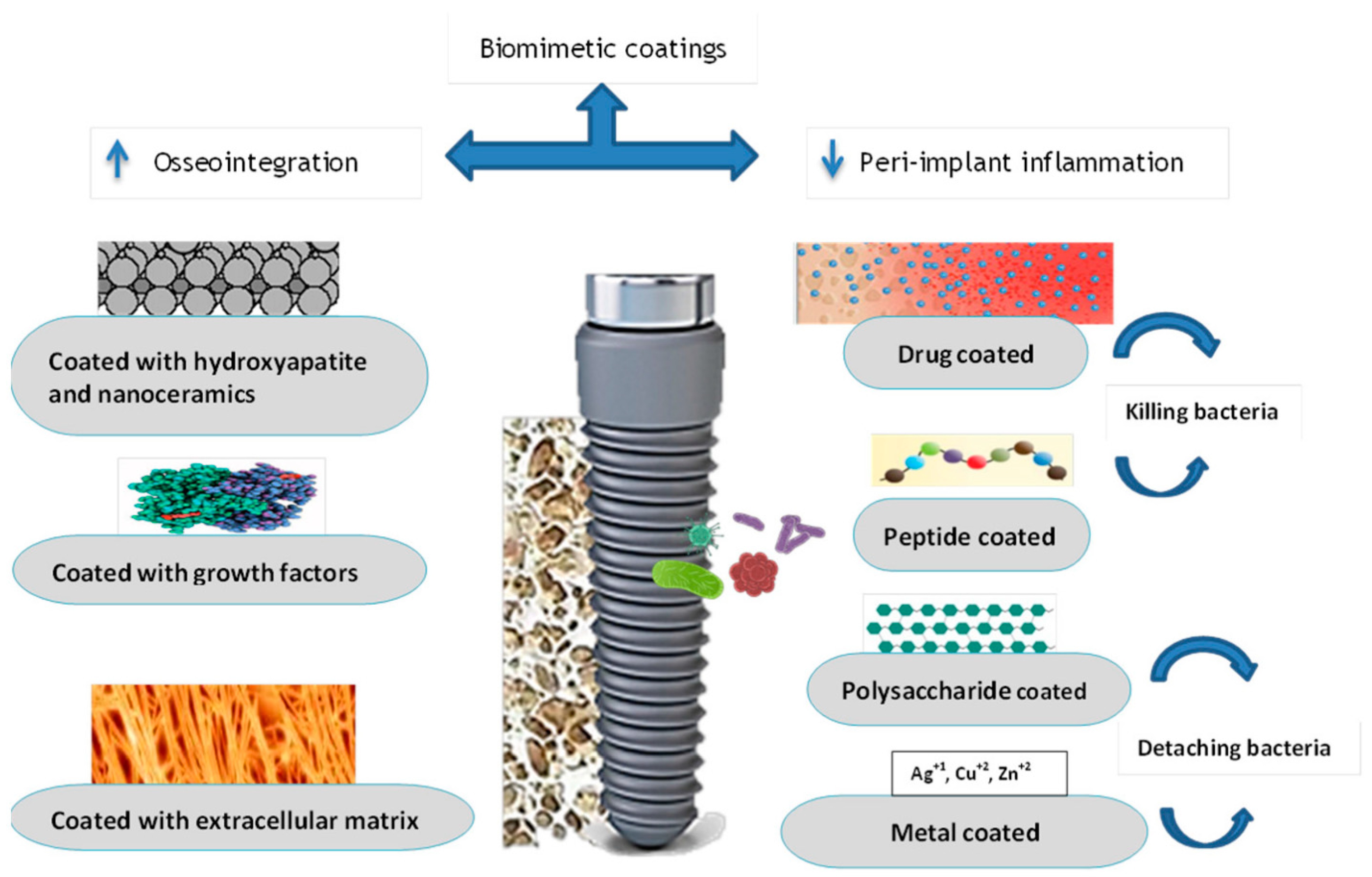
5. Application of Growth Factors in Implant Dentistry
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hedin, U.; Roy, J. Growth Factors. In Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2006; pp. 730–734. [Google Scholar] [CrossRef]
- Mohammed, A.; Zardawi, F.; Gul, S. A Retrospective CBCT Survey on Severity and Pattern of Alveolar Bone Loss Among a Selected Sample in the City of Sulaimani, Kurdistan Region of Iraq. Sulaimani Dent. J. 2021, 8, 10–17. [Google Scholar] [CrossRef]
- Shimono, K.; Oshima, M.; Arakawa, H.; Kimura, A.; Nawachi, K.; Kuboki, T. The effect of growth factors for bone augmentation to enable dental implant placement: A systematic review. Jpn. Dent. Sci. Rev. 2010, 46, 43–53. [Google Scholar] [CrossRef]
- Kaigler, D.; Avila, G.; Wisner-Lynch, L.; Nevins, M.L.; Nevins, M.; Rasperini, G.; Lynch, S.E.; Giannobile, W.V. Platelet-derived growth factor applications in periodontal and peri-implant bone regeneration. Expert Opin. Biol. Ther. 2011, 11, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Egierska, D.; Perszke, M.; Mazur, M.; Duś-Ilnicka, I. Platelet-rich plasma and platelet-rich fibrin in oral surgery: A narrative review. Dent. Med. Probl. 2023, 60, 177–186. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Galindo-Moreno, P.; Herford, A.S.; Spagnuolo, G.; Cicciù, M. Growth factors in oral tissue engineering: New perspectives and current therapeutic options. BioMed. Res. Int. 2021, 2021, 8840598. [Google Scholar] [CrossRef] [PubMed]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic coatings in implant dentistry: A quick update. J. Funct. Biomater. 2023, 15, 15. [Google Scholar] [CrossRef]
- Abdulghafor, M.A.; Amin, Z.M. The impact of hyaluronic acid coating on polyether ether ketone dental implant surface: An in vitro analysis. Saudi Dent. J. 2024, 36, 1326–1332. [Google Scholar] [CrossRef]
- Abdulghfor, M.A.; Mahmood, M.K.; Noori, Z.F.; Kurda, H.A.; Qadir, B.H.; Othman, K.S.; Fatih, M.T.; Amin, S.; Mahmood, N.K.; Abdulghfor, M. Coating Polyetheretherketone Implant Surface with Titanium Oxide Nanoparticles and Hyaluronic Acid: An In Vitro Study. Cureus 2025, 17, e77383. [Google Scholar] [CrossRef]
- Mohammad, C.A.; Mirza, B.A.; Mahmood, Z.S.; Zardawi, F.M. The effect of hyaluronic acid gel on periodontal parameters, pro-inflammatory cytokines and biochemical markers in periodontitis patients. Gels 2023, 9, 325. [Google Scholar] [CrossRef]
- Taher, B.B.; Rasheed, T.A. The impact of adding chitosan nanoparticles on biofilm formation, cytotoxicity, and certain physical and mechanical aspects of directly printed orthodontic clear aligners. Nanomaterials 2023, 13, 2649. [Google Scholar] [CrossRef]
- Lokwani, B.V.; Gupta, D.; Agrawal, R.S.; Mehta, S.; Nirmal, N.J. The use of concentrated growth factor in dental implantology: A systematic review. J. Indian Prosthodont. Soc. 2020, 20, 3–10. [Google Scholar] [PubMed]
- Greenhalgh, D.G. Wound Healing. In Burn Care for General Surgeons and General Practitioners; Greenhalgh, D.G., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 95–116. [Google Scholar] [CrossRef]
- Stone, W.L.; Leavitt, L.; Varacallo, M. Physiology, Growth Factor; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Bjelić, D.; Finšgar, M. The role of growth factors in bioactive coatings. Pharmaceutics 2021, 13, 1083. [Google Scholar] [CrossRef] [PubMed]
- Bartold, M.; Ivanovski, S. Biological processes and factors involved in soft and hard tissue healing. Periodontology 2000 2025, 97, 16–42. [Google Scholar] [CrossRef]
- Schultz, G.S.; Chin, G.A.; Moldawer, L.; Diegelmann, R.F. Principles of wound healing. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Vasalou, V.; Kotidis, E.; Tatsis, D.; Boulogeorgou, K.; Grivas, I.; Koliakos, G.; Cheva, A.; Ioannidis, O.; Tsingotjidou, A.; Angelopoulos, S. The effects of tissue healing factors in wound repair involving absorbable meshes: A narrative review. J. Clin. Med. 2023, 12, 5683. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, N.B.; Valerio, M.S.; Goldman, S.M.; Dearth, C.L. The role of the inflammatory response in mediating functional recovery following composite tissue injuries. Int. J. Mol. Sci. 2021, 22, 13552. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating inflammatory markers in wound healing: Understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical role of transforming growth factor beta in different phases of wound healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef]
- Fernández-Guarino, M.; Hernández-Bule, M.L.; Bacci, S. Cellular and molecular processes in wound healing. Biomedicines 2023, 11, 2526. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A comprehensive review of natural compounds for wound healing: Targeting bioactivity perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, É.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in growth factor delivery for bone tissue engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.-H.; Choi, I. Implications of insulin-like growth factor-1 in skeletal muscle and various diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Gadina, M.; Siegel, R.M. Cytokines and cytokine receptors. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–155. [Google Scholar]
- Kaigler, D.; Cirelli, J.A.; Giannobile, W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin. Drug Deliv. 2006, 3, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Wound healing essentials: Let there be oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Braunagel, S.; Rosenblum, B.I. Growth factors in wound healing: The present and the future? Clin. Podiatr. Med. Surg. 2015, 32, 109–119. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Nurden, A.T.; Nurden, P.; Orive, G.; Andía, I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006, 24, 227–234. [Google Scholar] [CrossRef]
- Carlson, N.E.; Roach, R.B., Jr. Platelet-rich plasma: Clinical applications in dentistry. J. Am. Dent. Assoc. 2002, 133, 1383–1386. [Google Scholar] [CrossRef]
- Schliephake, H. Bone growth factors in maxillofacial skeletal reconstruction. Int. J. Oral Maxillofac. Surg. 2002, 31, 469–484. [Google Scholar] [CrossRef]
- Plachokova, A.S.; Nikolidakis, D.; Mulder, J.; Jansen, J.A.; Creugers, N.H. Effect of platelet-rich plasma on bone regeneration in dentistry: A systematic review. Clin. Oral Implant. Res. 2008, 19, 539–545. [Google Scholar] [CrossRef]
- Glavina, A.; Vucicevic Boras, V.; Gabric, D.; Susic, M.; Granic, M.; Pelivan, I. Plasma rich in growth factors in dentistry. Australas. Med. J. 2017, 10, 497–501. [Google Scholar] [CrossRef]
- Adamska, P.; Kaczoruk-Wieremczuk, M.; Pylińska-Dąbrowska, D.; Stasiak, M.; Bartmański, M.; Zedler, A.; Studniarek, M. Treatment of Oroantral Communication and Fistulas with the Use of Blood-Derived Platelet-Rich Preparations Rich in Growth Factors: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 11507. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, S.; Mahendra, A. Gene therapy with growth factors for periodontal tissue engineering—A review. Med. Oral Patol. Oral Cir. Bucal 2011, 17, e301. [Google Scholar] [CrossRef]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef]
- Anitua, E.; Orive, G.; Aguirre, J.J.; Andía, I. Clinical outcome of immediately loaded dental implants bioactivated with plasma rich in growth factors: A 5-year retrospective study. J. Periodontol. 2008, 79, 1168–1176. [Google Scholar] [CrossRef]
- Mozzati, M.; Arata, V.; Giacomello, M.; Del Fabbro, M.; Gallesio, G.; Mortellaro, C.; Bergamasco, L. Failure risk estimates after dental implants placement associated with plasma rich in growth factor-Endoret in osteoporotic women under bisphosphonate therapy. J. Craniofac. Surg. 2015, 26, 749–755. [Google Scholar] [CrossRef]
- Triplett, R.G.; Nevins, M.; Marx, R.E.; Spagnoli, D.B.; Oates, T.W.; Moy, P.K.; Boyne, P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2009, 67, 1947–1960. [Google Scholar] [CrossRef]
- Simonpieri, A.; Gasparro, R.; Pantaleo, G.; Mignogna, J.; Riccitiello, F.; Sammartino, G. Four-year post-loading results of full-arch rehabilitation with immediate placement and immediate loading implants: A retrospective controlled study. Quintessence Int. 2017, 48, 315–324. [Google Scholar] [PubMed]
- Shah, S.A.; Singh, B.P.; Rao, J.; Kumar, L.; Singh, M.; Singh, P.K. Biological and esthetic outcome of immediate dental implant with the adjunct pretreatment of immediate implants with platelet-rich plasma or photofunctionalization: A randomized controlled trial. J. Indian Prosthodont. Soc. 2021, 21, 348–355. [Google Scholar] [PubMed]
- Boyne, P.J.; Lilly, L.C.; Marx, R.E.; Moy, P.K.; Nevins, M.; Spagnoli, D.B.; Triplett, R.G. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2005, 63, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.-S.; Heo, J.-U.; Kwak, D.-H.; Kim, D.-E.; Kim, J.-M.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef]
- Isler, S.C.; Soysal, F.; Ceyhanlı, T.; Bakırarar, B.; Unsal, B. Regenerative surgical treatment of peri-implantitis using either a collagen membrane or concentrated growth factor: A 12-month randomized clinical trial. Clin. Implant Dent. Relat. Res. 2018, 20, 703–712. [Google Scholar] [CrossRef]
- Amorfini, L.; Migliorati, M.; Signori, A.; Silvestrini-Biavati, A.; Benedicenti, S. Block allograft technique versus standard guided bone regeneration: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2014, 16, 655–667. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Boggian, C.; Taschieri, S. Immediate implant placement into fresh extraction sites with chronic periapical pathologic features combined with plasma rich in growth factors: Preliminary results of single-cohort study. J. Oral Maxillofac. Surg. 2009, 67, 2476–2484. [Google Scholar] [CrossRef]
- Torkzaban, P.; Khoshhal, M.; Ghamari, A.; Tapak, L.; Houshyar, E. Efficacy of application of platelet-rich fibrin for improvement of implant stability: A clinical trial. J. Long. Term Eff. Med. Implant. 2018, 28, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ntounis, A.; Geurs, N.; Vassilopoulos, P.; Reddy, M. Clinical assessment of bone quality of human extraction sockets after conversion with growth factors. Int. J. Oral Maxillofac. Implant. 2015, 30, 196–201. [Google Scholar] [CrossRef]
- Anis, M.; Abdelrahman, A.R.; Attia, R.; Zahran, A. Tomographic assessment of bone changes in atrophic maxilla treated by split-crest technique and dental implants with platelet-rich fibrin and NanoBone® versus platelet-rich fibrin alone: Randomized controlled trial. BMC Oral Health 2024, 24, 691. [Google Scholar] [CrossRef]
- Diana, C.; Mohanty, S.; Chaudhary, Z.; Kumari, S.; Dabas, J.; Bodh, R. Does platelet-rich fibrin have a role in osseointegration of immediate implants? A randomized, single-blind, controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2018, 47, 1178–1188. [Google Scholar] [CrossRef]
- Cheruvu, R.N.S.; Katuri, K.K.; Dhulipalla, R.; Kolaparthy, L.; Adurty, C.; Thota, K.M. Evaluation of soft tissue and crestal bone changes around non-submerged implants with and without a platelet-rich fibrin membrane: A randomized controlled clinical trial. Dent. Med. Probl. 2023, 60, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Pirpir, C.; Yilmaz, O.; Candirli, C.; Balaban, E. Evaluation of effectiveness of concentrated growth factor on osseointegration. Int. J. Implant Dent. 2017, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, T.; Horii, K.; Senga, Y.; Shibuya, Y. Crestal approach to sinus floor elevation for atrophic maxilla using platelet-rich fibrin as the only grafting material: A 1-year prospective study. Implant Dent. 2016, 25, 32–38. [Google Scholar] [CrossRef]
- Jung, R.E.; Glauser, R.; Schärer, P.; Hämmerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on guided bone regeneration in humans: A randomized, controlled clinical and histomorphometric study. Clin. Oral Implant. Res. 2003, 14, 556–568. [Google Scholar] [CrossRef]
- ArRejaie, A.; Al-Harbi, F.; Alagl, A.S.; Hassan, K.S. Platelet-rich plasma gel combined with bovine-derived xenograft for the treatment of dehiscence around immediately placed conventionally loaded dental implants in humans: Cone beam computed tomography and three-dimensional image evaluation. Int. J. Oral Maxillofac. Implant. 2016, 31, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.B.; Santana, C.M. A clinical comparison of guided bone regeneration with platelet-derived growth factor-enhanced bone ceramic versus autogenous bone block grafting. Int. J. Oral Maxillofac. Implant. 2015, 30, 700–706. [Google Scholar] [CrossRef]
- Öncü, E.; Erbeyoğlu, A.A. Enhancement of Immediate Implant Stability and Recovery Using Platelet-Rich Fibrin. Int. J. Periodontics Restor. Dent. 2019, 39, e58–e63. [Google Scholar] [CrossRef]
- Singhal, L.; Belludi, S.A.; Pradhan, N.; Manvi, S. A comparative evaluation of the effect of platelet rich fibrin matrix with and without peripheral blood mesenchymal stem cells on dental implant stability: A randomized controlled clinical trial. J. Tissue Eng. Regen. Med. 2022, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Naeimi Darestani, M.; Asl Roosta, H.; Mosaddad, S.A.; Yaghoubee, S. The effect of leukocyte-and platelet-rich fibrin on the bone loss and primary stability of implants placed in posterior maxilla: A randomized clinical trial. Int. J. Implant Dent. 2023, 9, 23. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Z.; Zheng, D.; Lin, P.; Cai, Y.; Hong, S.; Lai, Y.; Wu, D. Inlay osteotome sinus floor elevation with concentrated growth factor application and simultaneous short implant placement in severely atrophic maxilla. Sci. Rep. 2016, 6, 27348. [Google Scholar] [CrossRef]
- Hartlev, J.; Schou, S.; Isidor, F.; Nørholt, S.E. A clinical and radiographic study of implants placed in autogenous bone grafts covered by either a platelet-rich fibrin membrane or deproteinised bovine bone mineral and a collagen membrane: A pilot randomised controlled clinical trial with a 2-year follow-up. Int. J. Implant Dent. 2021, 7, 8. [Google Scholar]
- Amer, O.; Shemais, N.; Fawzy El-Sayed, K.; Saleh, H.A.; Darhous, M. Does Injectable Platelet-Rich Fibrin Combined with Autogenous Demineralized Dentine Enhance Alveolar Ridge Preservation? A Randomized Controlled Trial. Clin. Oral Implants Res. 2025, 36, 166–177. [Google Scholar] [CrossRef]
- Koyuncu, B.Ö.; Çelik, K.İ.; Yüce, M.Ö.; Günbay, T.; Çömlekoğlu, M. The role of concentrated growth factor on implant stability: A preliminary study. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Sohn, D.-S.; Bae, M.-S.; Moon, J.-W.; Lee, J.-H.; Park, I.-S. Flapless transcrestal sinus augmentation using hydrodynamic piezoelectric internal sinus elevation with autologous concentrated growth factors alone. Implant Dent. 2014, 23, 168–174. [Google Scholar] [CrossRef]
- Palermo, A.; Giannotti, L.; Di Chiara Stanca, B.; Ferrante, F.; Gnoni, A.; Nitti, P.; Calabriso, N.; Demitri, C.; Damiano, F.; Batani, T. Use of CGF in oral and implant surgery: From laboratory evidence to clinical evaluation. Int. J. Mol. Sci. 2022, 23, 15164. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ position paper on medication-related osteonecrosis of the jaws—2022 update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
| Wound Healing Phase | Growth Factor Type | Cell of Origin | Functions |
|---|---|---|---|
| INFLAMMATORY | PDGF | Platelets | Enhances neutrophil and monocyte chemotaxis. |
| TGF-β | Platelets, leukocytes, and fibroblasts | Promotes neutrophil and monocyte chemotaxis. Additional cytokines (TNF-α, IL-1β, PDGF, and chemokines) are produced by autocrine expression. | |
| VEGF | Platelets, leukocytes, and fibroblasts | Increase vascular permeability. | |
| PROLIFERATIVE | EGF | Macrophages, mesenchymal cells, and platelets | Promotes the migration and proliferation of epithelial cells. |
| FGF-2 | Macrophages and endothelial cells | Promotes the growth of fibroblasts and the production of extracellular matrix. Boost endothelial cell chemotaxis, proliferation, and differentiation. | |
| KGF(FGF-7) | Keratinocytes and fibroblasts | Promotes the migration and proliferation of epithelial cells. | |
| PDGF | Macrophages and endothelial cells | Promotes the growth of fibroblasts and the production of extracellular matrix. Boost endothelial cell chemotaxis, proliferation, and differentiation. | |
| TGF-β | Macrophages, fibroblasts, and leukocytes | Promotes the migration and proliferation of epithelial cells. Promotes the growth of fibroblasts and the production of extracellular matrix. Increases the synthesis of inhibitors and inhibits proteases. | |
| VEGF | Macrophages | Enhances endothelial progenitor cell chemotaxis. Promotes the growth of endothelial cells. | |
| BONE REMODELLING AND MATRIX SYNTHESIS | BMPs 2–4 | Osteoblasts | Promotes the migration of progenitor cells from mesenchyme. |
| BMP-7 | Osteoblasts | Enhances the differentiation of osteoblasts and chondroblasts. | |
| FGF-2 | Macrophages and endothelial cells | Activates progenitor cell migration from the mesenchyme. | |
| IGF-2 | Macrophages and fibroblasts | Promotes proliferation of osteoblasts and the synthesis of bone matrix. | |
| PDGF | Macrophages | Enhance differentiation of fibroblasts into myofibroblasts. Promotes the proliferation of progenitor cells from mesenchyme. | |
| TGF-β | Fibroblasts and osteoblasts | Prompts the apoptosis of endothelial cells and fibroblasts. Prompts the differentiation of fibroblasts to become myofibroblasts. Activates survival of osteoblasts and chemotaxis. | |
| VEGF | Macrophages | Chemotaxis of stem cells from the mesenchyme, antiapoptotic effect on bone-forming cells, and promotes angiogenesis. |
| Sections | Description |
|---|---|
| Problem/ Population | Does the application of growth factors positively affect dental implant outcomes clinically? Complete and partially edentulous human patients receiving dental implants. |
| Intervention | Use of growth factors alone or with other bone graft materials. |
| Comparison | Intragroup comparison between baseline and after treatment or intergroup group comparison with the controls. |
| Outcome | All the reported clinical, radiological, and statistical outcomes. |
| Study design/ Time | All the primary research study designs except single case reports and series with few cases (<10 cases). All the published literature in the mentioned databases between 2000 and 2025. |
| Author (Year) [Reference] | Sample Size | Type of GF Used | Follow-Up Duration | Measured Outcomes | Findings |
|---|---|---|---|---|---|
| Anitua (2008) [41] | 241 patients (1139 implants) | Implants with platelet-rich growth facor (PRGF) | 5 years | Implant survival | The corresponding survival rates for the implant-, surgery-, and patient-based analyses were 99.3%, 96.8%, and 96.9%. |
| Mozzati (2015) [42] | 235 female patients on bisphosphonates (BPs) (1267 implants) | Plasma rich in growth factor (PRGF)–Endoret | 10 years | BP-related osteonecrosis of the jaws (BRONJ) and failure rate | Survival rate was 98.7% and 93.2% on implant basis and patient basis, respectively. No cases of BRONJ were reported. |
| Triplett (2009) [43] | 160 patients (490 implants) | Human morphogenetic protein-2 (rhBMP-2) and collagen sponge (ACS) compared to autogenous bone graft | 6 months | New bone formation, placement integration, and functional loading | Implants placed in rhBMP-2/ACS and bone graft groups performed similarly after functional loading. |
| Simonpieri (2017) [44] | 42 patients (334 implants) | PRF in buccal bone augmentation | 4 months | Implant survival and radiographic bone loss | Implants in the maxilla had 97.8% survival; the mandible, 98.1%; immediate implants, 98.3%; and delayed implants, 96.9%. There were no significant differences (p > 0.05) in mean radiographic bone loss between immediate and delayed implants or anterior and posterior implants. |
| Shah (2021) [45] | 90 patients (90 implants) | Photofunctionalization (PF group) or platelet-rich plasma (PRP group) | 1, 2, 4, 6, and 12 months | Biological outcomes (mean marginal bone loss and implant stability), esthetic outcomes (pink esthetic score and white esthetic score), and survival rate | PF and PRP groups had similar mean marginal loss compared to control group. The PF and PRP groups had considerably higher implant stability than the control group. Pink and white estheticscores were similar across groups. |
| Boyne (2005) [46] | 48 patients (219 implants); 18 patients received 0.75 mg/mL of rhBMP-2/ACS 17 patients received 1.50 mg/mL of rhBMP-2/ACS; 13 patients (bone graft) | Group 1: 0.75 mg/mL of rhBMP-2/ACS; group 2: 1.50 mg/mL of rhBMP-2/ACS; group 3: standard bone graft material | 6 months (bone density) and 36 months (implant survival and functionality) | Bone density and implant survival | For the bone graft, 0.75 mg/mL, and 1.50 mg/mL rhBMP-2/ACS therapy groups, the new bone density at 4 months post-operatively was 350 mg/cc, 84 mg/cc, and 134 mg/cc, respectively. These differences were statistically significant. Post functional survival at 36 months was 67%, 76%, and 62% in group 1, group 2, and group 3, respectively. |
| Sohn (2011) [47] | 53 patients (113 implants) | Fibrin-rich blocks with concentrated growth factors (CGFs) | 10 months | Bone formation and implant survival | On both conventional and cone-beam computed tomograms, new bone consolidation was seen along the implants in every instance. After loading, the implant’s success rate was 98.2%. |
| Isler (2018) [48] | 52 patients with at least one peri-implantitis case | A bone graft combined with either collagen membrane (CM) or concentrated growth factor (CGF) | 6–12 months | Bleeding on probing (BOP), gingival index (GI), clinical attachment level (CAL), probing depth (PD), and mucosal recession (MR) | Significant reductions were obtained in the studied parameters at both 6 and 12 months post-operatively for both treatments. The mean PD, CAL, and vertical defect depth (VDD) values were statistically significant in favor of the CM group at 12 months (p < 0.05), but at 6 months, no statistically significant difference was seen for any of the clinical parameters between the groups. |
| Amorfini (2013) [49] | 16 patients (50 implants) | Autologous bone supplemented with bovine bone, either alone or in combination with recombinant human platelet-derived growth factor-BB (rhPDGF-BB) | 12 months | Quantity of bone variation | The two groups’ changes in bone volume did not differ substantially (p-value = 0.25). |
| Fabbro (2009) [50] | 30 patients (61 implants) | Implants coated with plasma rich in growth factors | 1 year | Implant survival | Implant survival was 98.4% at 1 year of function. |
| Torkzaban (2018) [51] | 10 patients (50 implants) | PRF group vs. no PRF group | Surgery day (T1), at 1 week (T2), and at 1 month (T3) | Implant stability measured by resonance frequency | After 1 week (T2), the PRF group had a mean implant stability quotient (ISQ) of 59.85 ± 5.32, while the non-PRF group was 55.99 ± 3.39. The ISQ rose to 0.12 ± 0.47 (p = 1.000) in the PRF group and fell to 2.42 ± 0.36 (p < 0.001) in the non-PRF group compared to the baseline. At 1 month post-op, ISQ significantly rose by 6.89 ± 0.96 in PRF and by 4.82 ± 0.92 in non-PRF compared to the baseline (p < 0.001). |
| Ntounis (2015) [52] | 41 patients (40 implants) | Group 1, collagen plug (control); Group 2, FDBA/β-tricalcium phosphate (β-TCP)/collagen plug; Group 3, FDBA/β-TCP/platelet-rich plasma (PRP)/collagen plug; Group 4, FDBA/β-TCP/recombinant human platelet-derived growth factor BB (rhPDGF-BB)/collagen plug | After 8 weeks of healing, implants were placed | Subjective assessment of bone quality | Bone grafting changed D4 bone to D3 bone. PRP in bone grafting changed D4 bone, establishing D3 and D2 bones (56% vs. 42%). Combining rhPDGF-BB with β-TCP with bone grafting yields similar effects, but D2 quality is less common. Sockets with growth factors showed less remaining bone graft particles compared to those with FDBA/β-TCP/collagen plug alone. |
| Anis (2024) [53] | 40 patients with atrophic maxilla treated by split-crest technique (40 implants) | Control group (PRF membrane) and test group (PRF membrane + Nanobone®) | 5 months | Bone resorption and gain measured by CBCT | Horizontal bone width gain was 1.46 ± 0.44 mm for the control group and 1.29 ± 0.73 mm for the test group, with no statistical significance. |
| Diana (2018) [54] | 29 patients (39 implants) | PRF group vs. non PRF group | 3 months and 1 year | Implant stability | Both study and control groups showed significant increases in implant stability over 3 months (implant stability quotient: from 56.58 ± 18.81 to 71.32 ± 7.82; control group: from 60.61 ± 11.49 to 70.06 ± 8.96; p = 0.01). Implant stability was similar between groups. |
| Cheruvu (2023) [55] | 40 patients with edentulous posterior mandibular sites | Group I received implants with a PRF membrane; group II was treated with implants alone | Baseline, and at 3-month and 6-month follow-ups. | Modified plaque index (mPI), gingival index (GI), width of keratinized tissue (WKT), thickness of keratinized tissue (TKT), and crestal bone level (CBL), assessed using digital intraoral periapical radiography (IOPA) | Significant increases in WKT and TKT were seen in both groups at 3 and 6 months post-op compared to the baseline (p < 0.05). Group I showed significant increases compared to group II (p < 0.05). Both groups showed significant increases in CBL at 3 and 6 months post-op (p < 0.05), with no distinguishing differences. CBL reduced in group I compared to group II at 3- and 6-month intervals (p < 0.05). |
| Pirpir (2017) [56] | 12 patients (40 implants) | In contrast to the control group’s conventional implants, the study group’s implant cavities were coated with CGF prior to implant insertion | 1 and 4 weeks | Implant stability quotient (ISQ) | By the first week, the study group’s mean ISQ value was 79.40, and the control group’s was 73.50; by the fourth week, they were 78.60 and 73.45, respectively. There were statistically significant differences between the groups (p < 0.05). |
| Kanayama (2016) [57] | 27 patients (39 implants) | PRF as the only graft in sinus floor elevation. Two implant types were used: hydroxyapatite (HA) and sandblasted acid-etched (SA) | 1 year | Bone gain | SA and HA groups had mean residual bone measures of 2.85 and 2.68 mm before surgery. The SA and HA groups had 4.38 and 4.00 mm mean annual bone increases. |
| Jung (2003) [58] | 11 patients (34 implants) | Xenogenic bone graft and collagen membrane coated with rhBMP-2 (test); xenogenic bone graft and collagen membrane (control) | 6 months | Bone volume, density, and maturation | The mean peri-implant bone defect was 5.8 mm in the control baseline and 7 mm in the test. The mean dropped to 0.02 for the test and 0.04 for the control at re-entry. There was statistical significance in this outcome (p < 0.01). According to a histological analysis, test sites had an average area density of 37% newly produced bone, while control sites had an area density of 30%. |
| ArRejaie (2016) [59] | 16 patients (32 implants) | PRP gel plus bovine-derived xenograft vs. withour PRP | 6 and 12 months | Dehiscence around immediate implants | Both treatments significantly improved the bone fill and marginal bone level. Statistically significant variations in bone density were seen between the control and the combined therapy (p ≤ 0.01). |
| Santana (2015) [60] | 30 patients (30 implants) | rhPDGF with beta-tricalcium phosphate (β-TCP)/hydroxyapatite compared to autogenous bone block | 6 months | Bone crest width (BCW) and implant torque | The experimental group’s mean baseline BCW reading was 3:03 mm, while the control group’s was 3:13 mm. In 87% of the experimental sites and 93% of the control sites, the implant was positioned with torque values greater than 35 N/cm. These two findings were not statistically significant. |
| Öncü (2019) [61] | 26 patients (60 implants: 30 tests and 30 controls) | Test sockets were coated with leukocyte platelet-rich fibrin (L-PRF), and control sockets were not | 1 week and 1 month | Implant stability and marginal bone loss | After 1 week and 1 month, the test group’s stability was higher (p < 0.002), and the group that received the PRF had a significantly smaller mean marginal bone resorption difference (p ≤ 0.05). |
| Singhal (2022) [62] | 15 patients (30 implants) | Platelet-rich fibrin matrix (PRFM) with and without peripheral blood mesenchymal stem cells (PBMSCs) | 1 week, 1 month, and 3 months | Implant stability | G1 and G2 insertion torque values were not significantly different (p = 0.81). PBMSCs and a platelet-rich fibrin matrix improved implant stability, with highly significant ISQ values at 1 week (p = 0.18), 1 month (p ≤ 0.001), and 3 months (p ≤ 0.001) in the G2 group. |
| Darestani (2023) [63] | 14 patients (28 implants) | Eukocyte- and platelet-rich fibrin (L-PRF) vs. control | 1, 2, 4, 6, 8, and 12 weeks | Implant stability | Both groups had significant stability results (p < 0.001; Eta2 = 0.322), although there was no significant difference in ISQ scores between the two groups (p > 0.05). |
| Chen (2016) [64] | 16 patients (25 implants) | Immediate implants placed after sinus floor elevation with CGF application | 19.8 months | Implant survival and vertical bone gain (VBG) | Implants had a 100% survival rate. Immediately following surgery, the mean VBG was 9.21 mm. The alveolar bone height (2.90 ± 0.22 mm) was significantly reduced 6 months later (p < 0.05). Additional alveolar bone resorption (0.14 ± 0.11 mm) was observed during the second 6-month period; however, it was not significant (p > 0.05). |
| Hartlev (2021) [65] | 27 patients (27 implants) | Bone grafts covered by either a (PRF) membrane (PRF group) or coverage of the bone graft with deproteinised bovine bone mineral and a resorbable collagen membrane (control group) | 2 years | Survival and marginal bone loss | The control group lost two implants (85% survival rate), while the PRF group lost none (100% survival rate). At follow-up, the PRF group had a mean marginal bone level of 0.26 mm (95% CI: 0.01–0.50 mm) and the control group, 0.68 mm (95% CI: 0.41–0.96 mm). A statistically significant difference between groups was −0.43 mm (95% CI: −0.80 to −0.05 mm; p = 0.03). At the final follow-up, both groups had healthy peri-implant soft tissue. |
| Amer (2024) [66] | 24 patients (24 implants) | Autogenous demineralized dentin graft with injectable platelet-rich fibrin (ADDG + i-PRF) versus ADDG alone | 6 months | Alveolar ridge preservation | ADDG alone or in combination with i-PRF produces similar clinical effects for ARP, osseous tissue quality, and patient satisfaction. However, adding i-PRF to ADDG preserves keratinized tissue and reduces post-operative discomfort. |
| Ozveri (2020) [67] | 12 patients (24 implants) | CGF placed in implant sockets compared to conventional implants | 1, 2, and 4 weeks | Implant stability | The study group’s mean ISQ was 67.00 ± 4.573, while the control group’s was 64.75 ± 5.065. There was no statistically significant difference. |
| Kim (2014) [68] | 11 patients (16 implants) | Immediate implants placed with CGF | 23.8 weeks | Bone gain measured by CBCT and implant survival | Survival was 100%. Average bone gain above the sinus floor was 8.23 ± 2.88 mm in the axial aspect of CBCT. |
| Palermo (2022) [69] | 10 patients (20 implants: 10 tests and 10 controls) | 10 implants coated with CGF compared to non-coated controls | 6 months | Crestal bone level, probing depth, and bleeding | CGF-coated group showed better crestal levels and probing and less bleeding when compared with the controls that showed bone resorption problems. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qadir, B.H.; Abdulghafor, M.A.; Mahmood, M.K.; Zardawi, F.M.; Fatih, M.T.; Kurda, H.A.; Noori, Z.F.; Tassery, H.; Tardivo, D.; Falguiere, A.; et al. Applications of Growth Factors in Implant Dentistry. Curr. Issues Mol. Biol. 2025, 47, 317. https://doi.org/10.3390/cimb47050317
Qadir BH, Abdulghafor MA, Mahmood MK, Zardawi FM, Fatih MT, Kurda HA, Noori ZF, Tassery H, Tardivo D, Falguiere A, et al. Applications of Growth Factors in Implant Dentistry. Current Issues in Molecular Biology. 2025; 47(5):317. https://doi.org/10.3390/cimb47050317
Chicago/Turabian StyleQadir, Balen Hamid, Mohammed Aso Abdulghafor, Mohammed Khalid Mahmood, Faraedon Mostafa Zardawi, Mohammed Taib Fatih, Handren Ameer Kurda, Zana Fuad Noori, Herve Tassery, Delphine Tardivo, Arthur Falguiere, and et al. 2025. "Applications of Growth Factors in Implant Dentistry" Current Issues in Molecular Biology 47, no. 5: 317. https://doi.org/10.3390/cimb47050317
APA StyleQadir, B. H., Abdulghafor, M. A., Mahmood, M. K., Zardawi, F. M., Fatih, M. T., Kurda, H. A., Noori, Z. F., Tassery, H., Tardivo, D., Falguiere, A., Romao, V., & Lan, R. (2025). Applications of Growth Factors in Implant Dentistry. Current Issues in Molecular Biology, 47(5), 317. https://doi.org/10.3390/cimb47050317








