Impact of Two Strains of Rhizobium leguminosarum on the Adaptation to Terminal Water Deficit of Two Cultivars Vicia faba
Abstract
:1. Introduction
2. Results
2.1. Growth Parameters Analyses on Vicia faba with Different Rhizobium leguminosarum Strains under Drought Stress
2.2. Stomatal Conductance and Photosynthetic Pigments Responses to Water-Limited Conditions
2.3. Accumulation of Osmoprotectants in Response to Water Deficit
2.4. Measurement of Oxidative Stress Markers
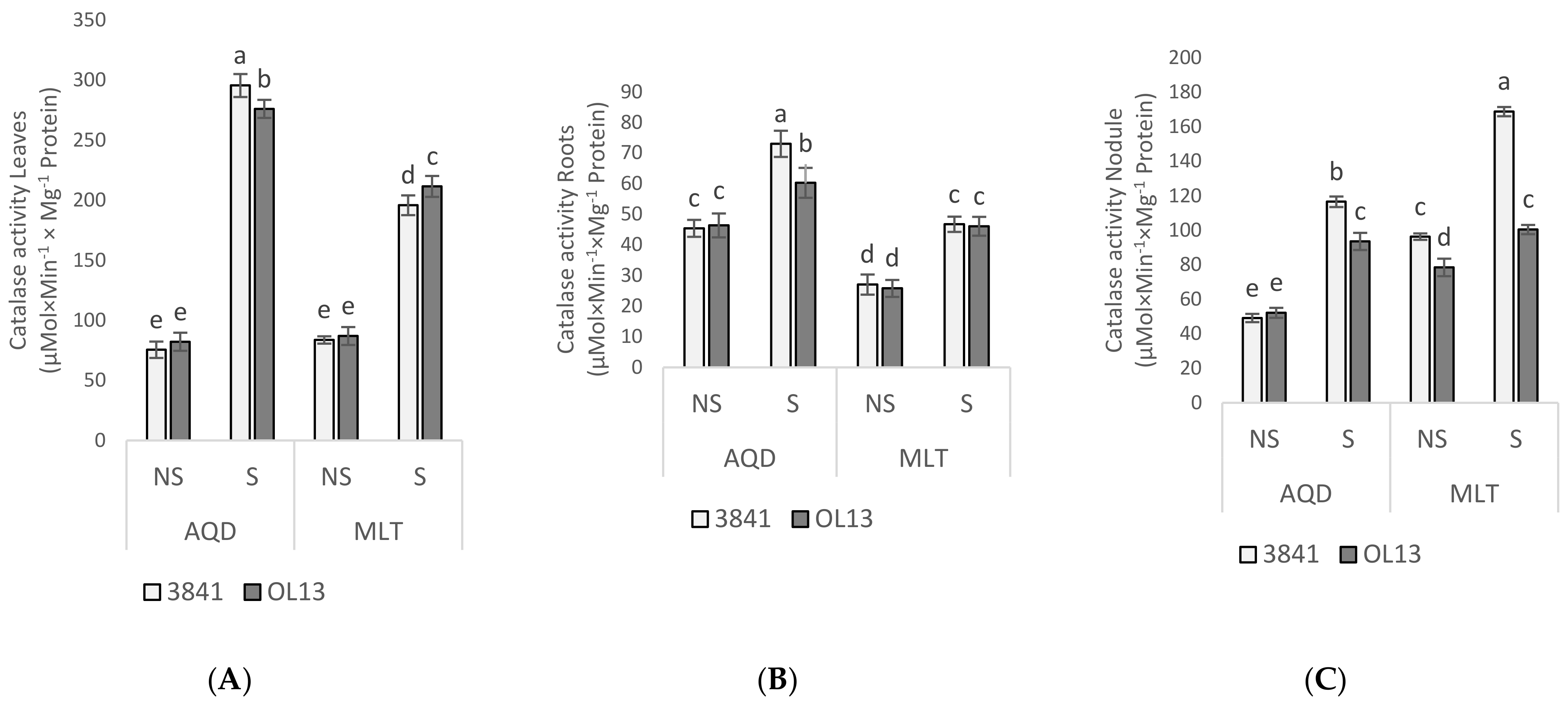
2.5. Enzymatic Activities Responses to Water Deficit
3. Discussion
4. Materials and Methods
4.1. Biological Material and Growth Conditions
4.2. Assessment of Physiological and Biochemical Parameters
4.3. Proline and Soluble Sugar Contents
4.4. Lipid Peroxidation and Hydrogen Peroxide Content
4.5. Determination of Antioxidant Enzymes Activities
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Furlan, A.L.; Bianucci, E.; Castro, S.; Dietz, K.J. Metabolic features involved in drought stress tolerance mechanisms in peanut nodules and their contribution to biological nitrogen fixation. Plant Sci. 2017, 263, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernández, J.A.; Vågen, I.M.; Rewald, B.; Alsiņa, I.; Kronberga, A.; Balliu, A.; Olle, M.; et al. Faba bean cultivation–revealing novel managing practices for more sustainable and competitive European cropping systems. Front. Plant Sci. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Mattoo, A.K.; Schmidt, M.A. Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 2021, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, F.; Sacco, D.; Casiello, G.; Ventrella, A.; Sacco, A. Chemical profile of the Carpino broad bean by conventional and innovative physicochemical analyses. J. Food Qual. 2015, 38, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Amede, T.; Schubert, S. Mechanisms of drought resistance in grain II: Stomatal regulation and root growth. SINET Ethiop. J. Sci. 2003, 26, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.R.; Paull, J.G.; Siddique, K.H.M.; Stoddard, F.L. Faba bean breeding for drought-affected environments: A physiologcal and agronomic perspective. Field Crop. Res. 2010, 115, 279–286. [Google Scholar] [CrossRef]
- Khazaei, H.; Street, K.; Santanen, A.; Bari, A.; Stoddard, F.L. Do faba bean (Vicia faba L.) accessions from environments with contrasting seasonal moisture availabilities differ in stomatal characteristics and related traits? Genet. Resour. Crop. Evol. 2013, 60, 2343–2357. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.B.; Mahmood, S.A.J.I.D.; Ahmed, N.I.A.Z.; Nawaz, H. Rhizobial inoculation for improving growth physiology, nutrition and yield of maize under drought stress conditions. Pak. J. Bot. 2018, 50, 1681–1689. [Google Scholar]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in le-gumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [Green Version]
- Maalouf, F.M.; Mohammed, N.; Aladdin, H.; Ahmed, A.; Xuxiao, Z.; Shiying, B.; Tao, Y. Faba bean. In Genetic and Genomic Re-Sources of Grain Legume Improvement; Elsevier: Oxford, UK, 2013; Chapter 5; pp. 113–136. [Google Scholar]
- Muktadir, M.A.; Adhikari, K.N.; Merchant, A.; Belachew, K.Y.; Vandenberg, A.; Stoddard, F.L.; Khazaei, H. Physiological and biochemical basis of faba bean breeding for drought adaptation—A review. Agronomy 2020, 10, 1345. [Google Scholar] [CrossRef]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 1999, 11, 1–11. [Google Scholar] [CrossRef]
- Katerji, N.; Mastrorilli, M.; Lahmer, F.Z.; Maalouf, F.; Oweis, T. Faba bean productivity in saline–drought conditions. Eur. J. Agron. 2011, 35, 2–12. [Google Scholar] [CrossRef]
- Khan, H.R.; Link, W.; Hocking, T.J.; Stoddard, F.L. Evaluation of physiological traits for improving drought tolerance in faba bean (Vicia faba L.). Plant Soil 2007, 292, 205–217. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Al-Shameri, A.M.; Migdadi, H.M.; Ammar, M.H.; El-Harty, E.H.; Khan, M.A.; Farooq, M. Physiological and molecular characterization of faba bean (Vicia faba L.) genotypes for adaptation to drought stress. J. Agron. Crop. Sci. 2015, 201, 401–409. [Google Scholar] [CrossRef]
- Girma, F.; Haile, D. Effects of supplemental irrigation on physiological parameters and yield of faba bean (Vicia faba L.) varie-ties in the highlands of Bale, Ethiopia. J. Agron. 2014, 13, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Kabbadj, A.; Makoudi, B.; Mouradi, M.; Pauly, N.; Frendo, P.; Ghoulam, C. Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PLoS ONE 2017, 12, e0190284. [Google Scholar] [CrossRef] [Green Version]
- Mansour, E.; Desoky, E.S.M.; Ali, M.M.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying drought-tolerant genotypes of faba bean and their agro-physiological responses to different water regimes in an arid Mediterranean environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Hayatu, M.; Muhammad, S.Y.; Abdu, H.U. Effect of water stress on the leaf relative water content and yield of some cow-pea (Vigna unguiculata (L) Walp.) genotype. Int. J. Sci. Technol. Res. 2014, 3, 148–152. [Google Scholar]
- Benjamin, J.G.; Nielsen, D.C.; Vigil, M.F.; Mikha, M.M.; Calderon, F. Water deficit stress effects on corn (Zea mays, L.) root: Shoot ratio. Open J. Soil Sci. 2014, 4, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.B.; Peng, L.X.; Zhao, J.L.; Zou, L.; Zhao, G.; Song, C. Effect of drought stress on yield, chlorophyll contents and photosynthesis in tartary buckwheat (Fagopyrum tataricum). J. Food Agric. Environ. 2013, 11, 1358–1363. [Google Scholar]
- Pandey, V.; Shukla, A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci. 2015, 22, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Nasr Esfahani, M.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Mechanisms of physiological adjustment of N2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: Single or multi-factor controls. Plant J. 2014, 79, 964–980. [Google Scholar] [CrossRef]
- Dhanushkodi, R.; Matthew, C.; McManus, M.T.; Dijkwel, P.P. Drought-induced senescence of Medicago truncatula nodules involves serpin and ferritin to control proteolytic activity and iron levels. New Phytol. 2018, 220, 196–208. [Google Scholar] [CrossRef] [Green Version]
- Mohammadkhani, N.; Heidari, R. Effects of drought stress on soluble proteins in two maize varieties. Turk. J. Biol. 2008, 32, 23–30. [Google Scholar]
- Siddiqui, M.H.; Khan, M.N.; Mohammad, F.; Khan, M.M.A. Role of nitrogen and gibberellin (GA3) in the regulation of en-zyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008, 194, 214–224. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Stress-triggered redox signalling: What’s in pROSpect? Plant Cell Environ. 2016, 39, 951–964. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Riah, N. Diversité et Structure Génétique des Populations de Rhizobium leguminosarum Symbiovar Viciae Isolées du Pois (Pisum sativum) et de la Lentille (Lens culinaris) Cultivés dans Deux Zones Éco-Climatiques Subhumide et Semi-Aride de l’est Algérien. Ph.D. Thesis, University of Constantine 1, Constantine, Algerie, 2015. [Google Scholar]
- El-Tayeb, M.; Hassanein, A.M. Germination, seedling growth, some organic solutes and peroxidase expression of different Vicia faba lines as influenced by water sterss. Acta Agron. Hung. 2000, 48, 11–20. [Google Scholar] [CrossRef]
- Le Thiec, D.; Manninen, S. Ozone and water deficit reduced growth of Aleppo pine seedlings. Plant Physiol. Biochem. 2003, 41, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Devi, S.P.S.; Sujatha, B. Drought-induced accumulation of soluble sugars and proline in two pigeon pea (Cajanus Cajan L. Millsp.) cultivars. Int. J. Innov. Res. Dev. 2014, 3, 302–306. [Google Scholar]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.L.M.; Maffei, M.; Navari-Izzo, F. Antioxidative enzymes in wheat subjected to increasing water deficit and rewa-tering. J. Plant Physiol. 2000, 157, 273–279. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Chiboub, M.; Jebara, S.H.; Abid, G.; Jebara, M. Co-inoculation effects of Rhizobium sullae and Pseudomonas sp. on growth, antioxidant status, and expression pattern of genes associated with heavy metal tolerance and accumulation of cadmium in Sulla co-ronaria. J. Plant Growth Regul. 2020, 39, 216–228. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhang, P.; Cao, Y.; Hu, T.; Yang, P. Rhizobium symbiosis contribution to short-term salt stress tolerance in alfalfa (Medicago sativa L.). Plant Soil 2016, 402, 247–261. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.H.; Lin, Y.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Sahrawat, K.L.; Upadhyaya, H.D.; Mengoni, A.; Galardini, M.; Bazzicalupo, M.; Biondi, E.G.; Hungria, M.; Kaschuk, G.; Blair, M.W.; et al. Advances in host plant and rhizobium genomics to enhance symbiotic nitrogen fixation in grain legumes. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2015; Volume 129, pp. 1–116. [Google Scholar]
- Abid, G.; M’hamdi, M.; Mingeot, D.; Aouida, M.; Aroua, I.; Muhovski, Y.; Sassi, K.; Souissi, F.; Mannai, K.; Jebara, M. Effect of drought stress on chlorophyll fluorescence, antioxidant enzyme activities and gene expression patterns in faba bean (Vicia faba L.). Arch. Agron. Soil Sci. 2017, 63, 536–552. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, C.L.; Chen, Y.; Chen, Z.; Jiang, Q.B.; Wu, C.; Pinyopusarerk, K. Improving drought tolerance of Casuarina equisetifolia seedlings by arbuscular mycorrhizas under glasshouse conditions. New For. 2010, 40, 261–271. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Ait Barka, E. Advances in wheat physiology in response to drought and the role of plant growth promoting rhizobacteria to trigger drought tolerance. Microorganisms 2021, 9, 687. [Google Scholar] [CrossRef]
- Kocsy, G.; Laurie, R.; Szalai, G.; Szilágyi, V.; Simon-Sarkadi, L.; Galiba, G.; De Ronde, J.A. Genetic manipulation of proline le-vels affects antioxidants in soybean subjected to simultaneous drought and heat stresses. Physiol. Plant. 2005, 124, 227–235. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of rhizobium-soybean symbiosis and nitrogen fixation under drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef] [Green Version]
- Irshad, A.; Rehman, R.N.U.; Abrar, M.M.; Saeed, Q.; Sharif, R.; Hu, T. Contribution of rhizobium–legume symbiosis in salt stress tolerance in medicago truncatula evaluated through photosynthesis, antioxidant enzymes, and compatible solutes accumulation. Sustainability 2021, 13, 3369. [Google Scholar] [CrossRef]
- Verslues, P.E.; Kim, Y.S.; Zhu, J.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate: Glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007, 64, 205–217. [Google Scholar] [CrossRef]
- Gurrieri, L.; Merico, M.; Trost, P.; Forlani, G.; Sparla, F. Impact of drought on soluble sugars and free proline content in selected Arabidopsis mutants. Biology 2020, 9, 367. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Jebara, M.; Limam, F.; Aouani, M.E. Rhizobial strain involvement in plant growth, nodule protein composition and antioxidant enzyme activities of chickpea-rhizobia symbioses: Modulation by salt stress. Plant Physiol. Biochem. 2004, 42, 717–722. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Jebara, M.; Zitoun, A.; Limam, F.; Aouani, M.E. Symbiotic effectiveness and response to mannitol-mediated osmotic stress of various chickpea–rhizobia associations. World J. Microbiol. Biotechnol. 2008, 24, 1027–1035. [Google Scholar] [CrossRef]
- Jebara, S.; Jebara, M.; Limam, F.; Aouani, M.E. Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J. Plant Physiol. 2005, 162, 929–936. [Google Scholar] [CrossRef]
- Nandwal, A.S.; Kukreja, S.; Kumar, N.; Sharma, P.K.; Jain, M.; Mann, A.; Singh, S. Plant water status, ethylene evolution, N2-fixing efficiency, antioxidant activity and lipid peroxidation in Cicer arietinum L. nodules as affected by short-term salinization and desalinization. J. Plant Physiol. 2007, 164, 1161–1169. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Fotopoulos, V.; Djebali, N.; Polidoros, A.N.; Aouani, M.E. Behaviours of Medicago truncatula–Sinorhizobium meliloti symbioses under osmotic stress in relation with the symbiotic partner input: Effects on nodule functioning and protection. J. Agron. Crop Sci. 2009, 195, 225–231. [Google Scholar] [CrossRef]
- Türkan, I.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Nan, Z.B. Growth and anti-oxidative systems changes in Elymus dahuricus is affected by Neotyphodium endophyte under contrasting water availability. J. Agron. Crop Sci. 2007, 193, 377–386. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Dalton, D.A.; Ramos, J.; Clemente, M.R.; Rubio, M.C.; Becana, M. Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol. 2003, 133, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Kumari, V.; Germida, J.; Vujanovic, V. Legume endosymbionts: Drought stress tolerance in second-generation chickpea (Cicer arietinum) seeds. J. Agron. Crop Sci. 2018, 204, 529–540. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; University of California, College of Agriculture, California Agricultural Experiment Station: Davis, CA, USA, 1938; p. 347. [Google Scholar]
- Takele, A. Differential responses of electrolyte leakage and pigment compositions in maize and sorghum after exposure to and recovery from pre-and post-flowering dehydration. Agric. Sci. China 2010, 9, 813–824. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Troll, W.; Lindsley, J. A photometric method for the determination of proline. J. Biol. Chem. 1955, 215, 655–660. [Google Scholar] [CrossRef]
- Monneveux, P.; Nemmar, M. Contribution à l’étude de la résistance à la sécheresse chez le blé tendre (Triticum aestivum L.) et chez le blé dur (Triticum durum Desf.): Étude de l’accumulation de la proline au cours du cycle de développement. Agronomie 1986, 6, 583–590. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. The assay of catalases and peroxidases. Methods Biochem. Anal. 1955, 136, 764–775. [Google Scholar]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.X.; Asada, K. Inactivation of ascorbate peroxidase by thiols requires hydrogen peroxide. Plant Cell Physiol. 1992, 33, 117–123. [Google Scholar]
- Fielding, J.L.; Hall, J.L. A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum: II. distribution of enzymes in relation to root development. J. Exp. Bot. 1978, 29, 983–991. [Google Scholar] [CrossRef]
- Klapheck, S.; Zimmer, I.; Cosse, H. Scavenging of hydrogen peroxide in the endosperm of Ricinus communis by ascorbate peroxidase. Plant Cell Physiol. 1990, 31, 1005–1013. [Google Scholar]








| MDA Content (nmol × g−1 FW) | |||||
|---|---|---|---|---|---|
| Organs | Leaves | Roots | |||
| Genotype | Rhiz-treatmnt | 80% FC (NS) | 40% FC (S) | 80% FC (NS) | 40% FC (S) |
| AQD | 3841 | 2.75 ± 0.21 d | 4.26 ± 0.28 b | 1.68 ± 0.19 b | 2.32 ± 0.20 a |
| OL13 | 2.69 ± 0.21 d | 3.76 ± 0.23 c | 1.65 ± 0.17 b | 2.35 ± 0.10 a | |
| MLT | 3841 | 3.08 ± 0.10 d | 4.86 ± 0.23 a | 1.66 ± 0.16 b | 2.69 ± 0.16 a |
| OL13 | 2.97 ± 0.13 d | 4.56 ± 0.32 ab | 1.61 ± 0.17 b | 2.52 ± 0.23 a | |
| H2O2 Content (nmol × g−1 FW) | |||||
|---|---|---|---|---|---|
| Organs | Leaves | Roots | |||
| Genotype | Rhiz.treatmnt | 80% FC (NS) | 40% FC (S) | 80% FC (NS) | 40% FC (S) |
| AQD | 3841 | 32.62 ± 3.22 c | 51.19 ± 2.30 b | 15.95 ± 1.09 c | 20.24 ± 1.49 b |
| OL13 | 29.05 ± 3.38 cd | 50.24 ± 0.82 b | 14.29 ± 0.71 cd | 24.29 ± 3.11 b | |
| MLT | 3841 | 26.43 ± 1.89 d | 60.71 ± 3.27 a | 15.71 ± 0.71 d | 31.90 ± 3.93 a |
| OL13 | 25.48 ± 2.30 d | 57.14 ± 1.89 a | 15.71 ± 2.47 d | 26.43 ± 2.58 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amine-Khodja, I.R.; Boscari, A.; Riah, N.; Kechid, M.; Maougal, R.T.; Belbekri, N.; Djekoun, A. Impact of Two Strains of Rhizobium leguminosarum on the Adaptation to Terminal Water Deficit of Two Cultivars Vicia faba. Plants 2022, 11, 515. https://doi.org/10.3390/plants11040515
Amine-Khodja IR, Boscari A, Riah N, Kechid M, Maougal RT, Belbekri N, Djekoun A. Impact of Two Strains of Rhizobium leguminosarum on the Adaptation to Terminal Water Deficit of Two Cultivars Vicia faba. Plants. 2022; 11(4):515. https://doi.org/10.3390/plants11040515
Chicago/Turabian StyleAmine-Khodja, Ihsein Rokia, Alexandre Boscari, Nassira Riah, Maya Kechid, Rim Tinhinen Maougal, Nadir Belbekri, and Abdelhamid Djekoun. 2022. "Impact of Two Strains of Rhizobium leguminosarum on the Adaptation to Terminal Water Deficit of Two Cultivars Vicia faba" Plants 11, no. 4: 515. https://doi.org/10.3390/plants11040515





