A Review of In Vivo and Clinical Studies Applying Scaffolds and Cell Sheet Technology for Periodontal Ligament Regeneration
Abstract
:1. Introduction
1.1. Periodontal Tissues
1.1.1. Periodontal Ligament
1.1.2. Cementum
1.2. Cell-Guided PDL Regeneration
2. Included Studies
2.1. In Vivo Studies
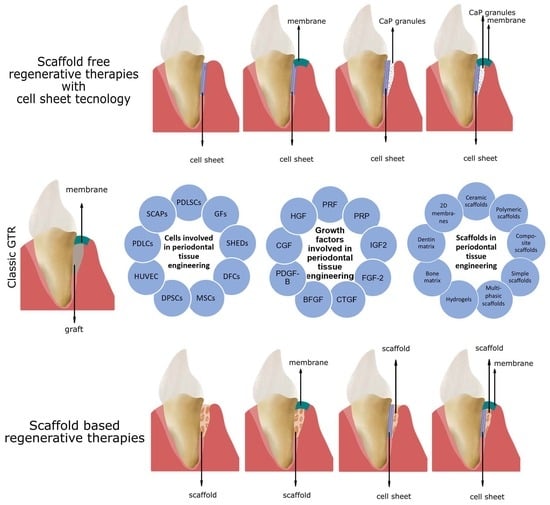
2.1.1. Cell Sheet Engineering
Ectopic Models
Orthotopic Models
2.2. In Vivo Studies with Scaffolds for PDL Regeneration
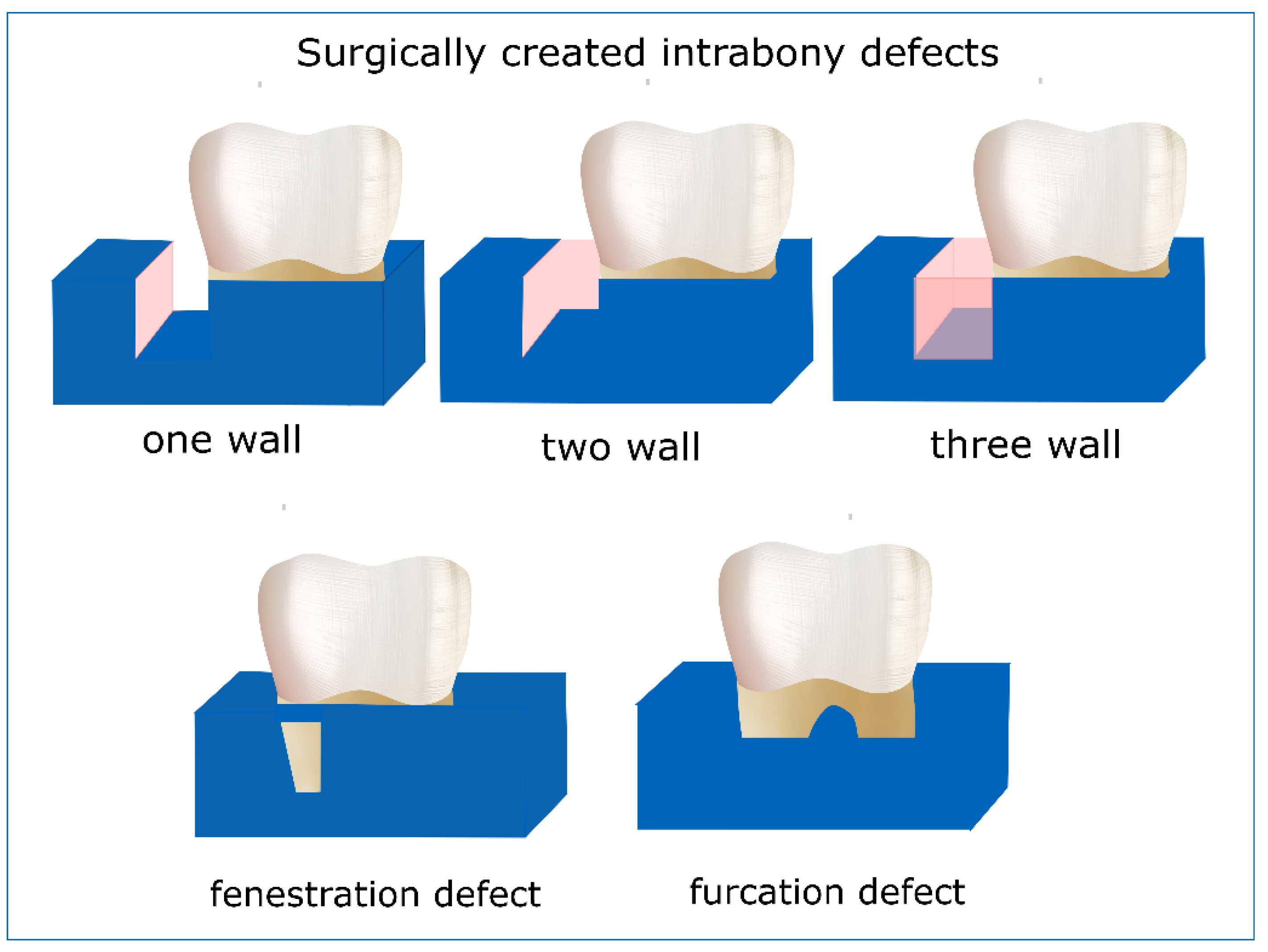
2.2.1. Periodontal Defect Model
2.2.2. Subcutaneous Placement Model
2.2.3. Other Models
2.3. Clinical Studies with Scaffolds for PDL Regeneration
2.3.1. Clinical Studies Involving Scaffolds and Growth Factors
2.3.2. Clinical Studies Involving Caffolds Combined with Cells
3. Discussion and Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABM | Inorganic bovine matrix |
| ACP | Amorphous calcium phosphate |
| Ad-BMP-7, AdBMP7 | Recombinant adenovirus-encoding murine bone morphogenetic protein-7 |
| AdPDGF-B | Adenovirus for platelet-derived growth factor-B |
| AEFC | Acellular extrinsic fiber cementum |
| ALP | Alkaline phosphatase |
| APCs | alveolar periosteal cells |
| BFGF | Basic fibroblast growth factor |
| BGC | Bioactive glass ceramic |
| BMP-2 | Bone morphogenetic protein 2 |
| BMSCs, Bm-MSCs, BMMSCs | Bone marrow mesenchymal stem cells |
| CBB | ceramic bovine bone |
| CCRD | chemical conditioned root dentin |
| CEMP1 | Cementum matrix protein 1 |
| CGF | Concentrated growth factor |
| CHI | Chitosan |
| CIFC | Cellular intrinsic fiber cementum |
| CMSC | Cellular mixed stratified cementum |
| Col | Collagen |
| CTGF | Connective tissue growth factor |
| DBBM | Deproteinized bovine bone mineral |
| DDM | decalcified dentin matrix |
| DePDLSCs | PDLSCs from deciduous teeth |
| DFSCs, DFCs | Dental follicle stem cells |
| dHPDLC sheet | Decellularized human periodontal ligament cell sheet |
| DMOG | Dimethyloxalylglycine |
| dMSCs | Dog mesenchymal stem cells |
| dPDLCs | Dog periodontal ligament cells |
| DPEM | Dental pulp extracellular matrix |
| DPSCs | Dental pulp stem cells |
| DSCs | Epithelial dental stem cells |
| DTT | Dithiothreitol |
| ECM | Extracellular matrix component |
| EMD | Enamel matrix protein derivative |
| ePTFE | Polytetrafluoroethylene |
| FACP | Fluorine containing amorphous calcium phosphate |
| FDM | Fused Deposition Modeling |
| FGF-2, FGF2 | Fibroblast growth factor 2 |
| FN | Fibronectin |
| GC | Gingival cells |
| Gel-MA | Gelatin methacrylate |
| GEN | Genipin |
| G-MSCs | Gingival margin-derived stem/progenitor cells |
| GO | Graphene oxide |
| HA | Hydroxyapatite |
| HA/TCP | Hydroxyapatite/tricalcium phosphate |
| hGFs | Human gingival fibroblasts |
| HGF | Hepatocyte growth factor |
| hJBMMSCs/hBMMSCs | Human (Jaw) bone marrow-derived mesenchymal stem cells |
| hOB | Human osteoblasts |
| HPDLs/HPDLCs | Human periodontal ligament primary cells |
| HPDLSCs | Human periodontal ligament stem cells |
| HPDLSCs | Periodontal ligament stem cells from healthy donors |
| HUVECs | Human umbilical vein endothelial cells |
| IGF2 | Insulin growth factor 2 |
| IMC | Intrafibrillarly mineralized collagen |
| IPCs | Induced pluripotent stem cells |
| JBMMSCs | Jawbone marrow mesenchymal stem cells |
| L-PRF | Leukocyte-PRF |
| LIPUS | low-intensity pulsed ultrasound |
| MBCP | micro/macro-porous biphasic calcium phosphate |
| MCPs | Monolayered cell pellets |
| MCS | Monolayered cell sheet |
| MBCP | Micro/macro-porous biphasic calcium phosphate |
| MUCPs | Multilayered cell pellets |
| MUCS | Multilayered cell sheet |
| OFD | Open flap debridement |
| PCL | Polycaprolactone |
| PCL/GE | Polycaprolactone/gelatin |
| PDGF | Platelet-derived growth factor-B |
| PDL | Periodontal ligament |
| PDLCs | Periodontal ligament cells |
| PDLSCs, PDL-MSCs | Human periodontal ligament stem cells |
| PEG | Poly(ethylene glycol) |
| PEG-DA | Polyethylene glycol diacrylate |
| pFGF-2 | Plasmid DNA encoding fibroblast growth factor-2 |
| PePDLSCs | Periodontal ligament stem cells from permanent teeth |
| PGA | Polyglycolic acid |
| PisPLLA | Polyester poly(isosorbide succin-ate-co-L-lactide) |
| PLAP-1 | Periodontal ligament-associated protein-1 |
| PLGA | Poly-DL-lactic-co-glycolic acid |
| PLLA | Poly(L-lactide) |
| PPD | Pocket probing depth |
| PPDLSCs | Periodontal ligament stem cells from patients with periodontitis |
| PRF | Platelet-rich fibrin |
| PRGF | Platelet-rich in growth factor |
| PRP | Platelet-rich plasma |
| rh-AM, rhAM | Recombinant human amelogenin |
| rh-BMP-2 | Human recombinant bone morphogenetic protein 2 |
| rhCEMP1 | Recombinant human Cementum matrix protein 1 |
| rhFGF-2 | Recombinant human Fibroblast growth factor 2 |
| rh-PDGF-BB | Recombinant human Platelet-Derived Growth Factor-BB |
| SCAPs | Stem cells from apical papilla |
| SDF-1 | Stromal cell-derived factor-1 |
| SDF-1 | Stromal cell-derived factor-1 |
| SHEDs | Stem cells from human exfoliated deciduous teeth |
| TDM | Treated dentin matrix |
| Ti | Titanium |
| USCs | Urine-derived stem cells |
| Vc | Vitamin C |
| XBS | Xenogeneic bone substitute |
| β-TCP | β-tricalcium phosphate |
References
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A.; Genco, R.J. Periodontitis in US Adults: National Health and Nutrition Examination Survey 2009–2014. J. Am. Dent. Assoc. 2018, 149, 576–588.e6. [Google Scholar] [CrossRef]
- Caton, J.; Zander, H.A. Osseous repair of an infrabony pocket without new attachment of connective tissue. J. Clin. Periodontol. 1976, 3, 54–58. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Sculean, A.; Kantarci, A. Regenerative Periodontal Therapy in Intrabony Defects and Long-Term Tooth Prognosis. Dent. Clin. N. Am. 2022, 66, 103–109. [Google Scholar] [CrossRef]
- Tsai, S.J.; Ding, Y.W.; Shih, M.C.; Tu, Y.K. Systematic review and sequential network meta-analysis on the efficacy of periodontal regenerative therapies. J. Clin. Periodontol. 2020, 47, 1108–1120. [Google Scholar] [CrossRef]
- Li, Y.; Jin, F.; Du, Y.; Ma, Z.; Li, F.; Wu, G.; Shi, J.; Zhu, X.; Yu, J.; Jin, Y. Cementum and periodontal ligament-like tissue formation induced using bioengineered dentin. Tissue Eng. Part A 2008, 14, 1731–1742. [Google Scholar] [CrossRef]
- Requicha, J.F.; Viegas, C.A.; Muñoz, F.; Azevedo, J.M.; Leonor, I.B.; Reis, R.L.; Gomes, M.E. A tissue engineering approach for periodontal regeneration based on a biodegradable double-layer scaffold and adipose-derived stem cells. Tissue Eng. Part A 2014, 20, 2483–2492. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Y.; Shi, B.; Cheng, X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials 2007, 28, 1515–1522. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets—A safety and efficacy study in ten patients. Regen. Ther. 2018, 9, 38–44. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, J.; Zhao, L.; Deng, J.; Li, Q. A simvastatin-releasing scaffold with periodontal ligament stem cell sheets for periodontal regeneration. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800019900094. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef]
- Yu, M.; Luo, D.; Qiao, J.; Guo, J.; He, D.; Jin, S.; Tang, L.; Wang, Y.; Shi, X.; Mao, J.; et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact. Mater. 2022, 10, 93–106. [Google Scholar] [CrossRef]
- Daghrery, A.; Ferreira, J.A.; de Souza Araújo, I.J.; Clarkson, B.H.; Eckert, G.J.; Bhaduri, S.B.; Malda, J.; Bottino, M.C. A Highly Ordered, Nanostructured Fluorinated CaP-Coated Melt Electrowritten Scaffold for Periodontal Tissue Regeneration. Adv. Healthc. Mater. 2021, 10, 2101152. [Google Scholar] [CrossRef]
- Huang, R.Y.; Tai, W.C.; Ho, M.H.; Chang, P.C. Combination of a biomolecule-aided biphasic cryogel scaffold with a barrier membrane adhering PDGF-encapsulated nanofibers to promote periodontal regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef]
- Lekic, P.; Mcculloch, C.A.G. Periodontal ligament cell populations: The central role of fibroblasts in creating a unique tissue. Anat. Rec. 1996, 245, 327–341. [Google Scholar] [CrossRef]
- Birn, H. The vascular supply of the periodontal membrane. J. Periodontal Res. 1966, 1, 51–68. [Google Scholar] [CrossRef]
- Trulsson, M. Sensory-motor function of human periodontal mechanoreceptors. J. Oral Rehabil. 2006, 33, 262–273. [Google Scholar] [CrossRef]
- Cho, M.I.; Garant, P.R. Development and general structure of the periodontium. Periodontology 2000 2000, 24, 9–27. [Google Scholar] [CrossRef]
- Xiong, J.; Gronthos, S.; Bartold, P.M. Role of The Epithelial Cell Rests Of Malassez In The Development, Maintenance And Regeneration Of Periodontal Ligament Tissues. Periodontology 2000 2013, 63, 217–233. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontology 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.Q. Signaling Pathways Critical for Tooth Root Formation. J. Dent. Res. 2017, 96, 1221–1228. [Google Scholar] [CrossRef]
- Grant, D.; Bernick, S. Formation of the Periodontal Ligament. J. Periodontol. 1972, 43, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dean, R. The Periodontal Ligament: Development, Anatomy and Function. J. Oral Health Dent. Manag. 2017, 16, 1–7. [Google Scholar]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Strydom, H.; Maltha, J.C.; Kuijpers-Jagtman, A.M.; Von Den Hoff, J.W. The oxytalan fibre network in the periodontium and its possible mechanical function. Arch. Oral Biol. 2012, 57, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Kira-Tatsuoka, M.; Oka, K.; Tsuruga, E.; Ozaki, M.; Sawa, Y. Immunohistochemical expression of fibrillin-1 and fibrillin-2 during tooth development. J. Periodontal Res. 2015, 50, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa-Ohama, R.; Mochizuki, M.; Tamaki, Y.; Suda, N.; Nakahara, T. Heterogeneous Human Periodontal Ligament-Committed Progenitor and Stem Cell Populations Exhibit a Unique Cementogenic Property under in Vitro and in Vivo Conditions. Stem Cells Dev. 2017, 26, 632–645. [Google Scholar] [CrossRef]
- McCulloch, C.A.G.; Bordin, S. Role of fibroblast subpopulations in periodontal physiology and pathology. J. Periodontal Res. 1991, 26, 144–154. [Google Scholar] [CrossRef]
- Gay, I.C.; Chen, S.; MacDougall, M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod. Craniofacial Res. 2007, 10, 149–160. [Google Scholar] [CrossRef]
- Silvério, K.G.; Rodrigues, T.L.; Coletta, R.D.; Benevides, L.; Da Silva, J.S.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H. Mesenchymal Stem Cell Properties of Periodontal Ligament Cells from Deciduous and Permanent Teeth. J. Periodontol. 2010, 81, 1207–1215. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Are cementoblasts a subpopulation of osteoblasts or a unique phenotype? J. Dent. Res. 2005, 84, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Kapila, Y.; Lotz, J.; Kapila, S. Multiple differentiation capacity of STRO-1+/CD146+ PDL Mesenchymal Progenitor Cells. Stem Cells Dev. 2009, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, X.; Huang, Y.; Jia, L.; Li, W. Time series clustering of mRNA and lncRNA expression during osteogenic differentiation of periodontal ligament stem cells. PeerJ 2018, 6, e5214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Z.; Huang, X.; Wang, Z.; Chen, Z.; Wang, R.; Chen, Z.; Liu, W.; Wu, B.; Fang, F.; et al. Dynamic proteomic profiling of human periodontal ligament stem cells during osteogenic differentiation. Stem Cell Res. Ther. 2021, 12, 98. [Google Scholar] [CrossRef]
- Deng, C.; Sun, Y.; Liu, H.; Wang, W.; Wang, J.; Zhang, F. Selective adipogenic differentiation of human periodontal ligament stem cells stimulated with high doses of glucose. PLoS ONE 2018, 13, e0199603. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Cho, T.J.; Kwon, S.K.; Lee, G.; Cho, J. Chondrogenesis of periodontal ligament stem cells by transforming growth factor-β3 and bone morphogenetic protein-6 in a normal healthy impacted third molar. Int. J. Oral Sci. 2013, 5, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, J.; Hilkens, P.; Gervois, P.; Wolfs, E.; Jacobs, R.; Lambrichts, I.; Bronckaers, A. Angiogenic capacity of periodontal ligament stem cells pretreated with deferoxamine and/or fibroblast growth factor-2. PLoS ONE 2016, 11, e0167807. [Google Scholar] [CrossRef]
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Peng, Y.; Umeda, M.; Watabe, T.; Morita, I. Angiogenic effects of secreted factors from periodontal ligament stem cells. Dent. J. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Um, S.; Song, I.-S.; Kim, H.Y.; Seo, B.M. Neurogenic differentiation of human dental stem cells in vitro. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coura, G.S.; Garcez, R.C.; De Aguiar, C.B.N.M.; Alvarez-Silva, M.; Magini, R.S.; Trentin, A.G. Human periodontal ligament: A niche of neural crest stem cells. J. Periodontal Res. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Tanaka, K.; Iwasaki, K.; El Feghali, K.; Komaki, M.; Ishikawa, I.; Izumi, Y. Comparison of characteristics of periodontal ligament cells obtained from outgrowth and enzyme-digested culture methods. Arch. Oral Biol. 2011, 56, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Maeda, H.; Fujii, S.; Wada, N.; Shima, K.; Akamine, A. Development of a multipotent clonal human periodontal ligament cell line. Differentiation 2008, 76, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Sonoyama, W.; Coppe, C.; Stanyon, R.; Shi, S. Recovery of stem cells from cryopreserved periodontal ligament. J. Dent. Res. 2005, 84, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Kim, S.O.; Kim, S.H.; Choi, H.J.; Son, H.K.; Jung, H.S.; Kim, C.S.; Lee, J.H. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng. Part A 2012, 18, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, H.; Zheng, W.; Tang, L.; Yang, Z.; Gao, Y.; Yang, Q.; Wang, C.; Duan, Y.; Jin, Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A 2011, 17, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Menicanin, D.; Shi, S.; Bartold, P.M.; Gronthos, S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009, 219, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, K.; Komaki, M.; Sekiya, I.; Sakaguchi, Y.; Noguchi, K.; Oda, S.; Muneta, T.; Ishikawa, I. Stem cell properties of human periodontal ligament cells. J. Periodontal Res. 2006, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Lackler, K.P.; Cochran, D.L.; Mai Hoang, A.; Takacs, V.; Oates, T.W. Development of an In Vitro Wound Healing Model For Periodontal Cells. J. Periodontol. 2000, 71, 226–237. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, W.; Chen, M.; Zheng, Y.; Zhou, J.; Kim, S.G.; Embree, M.C.; Songhee Song, K.; Marao, H.F.; Mao, J.J. Periodontal Ligament and Alveolar Bone in Health and Adaptation: Tooth Movement. Front. Oral Biol. 2015, 18, 1–8. [Google Scholar]
- Naveh, G.R.S.; Lev-Tov Chattah, N.; Zaslansky, P.; Shahar, R.; Weiner, S. Tooth-PDL-bone complex: Response to compressive loads encountered during mastication—A review. Arch. Oral Biol. 2012, 57, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Beertsen, W.; Mcculloch, C.A.G.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Dangaria, S.J.; Ito, Y.; Luan, X.; Diekwisch, T.G.H. Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem Cells Dev. 2011, 20, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, E. Schroeder The Periodontium. In Handbook of Microscopic Anatomy; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1987; Volume 154. [Google Scholar]
- Schroeder, H.E. Biological Problems of Regenerative Cementogenesis: Synthesis and Attachment of Collagenous Matrices on Growing and Established Root Surfaces. Int. Rev. Cytol. 1992, 142, 1–59. [Google Scholar] [PubMed]
- Bosshardt, D.D.; Selvig, K.A. Dental cementum: The dynamic tissue covering of the root. Periodontology 2000 1997, 13, 41–75. [Google Scholar] [CrossRef]
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1800457. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Q.; Hu, C.H.; Jin, F.; Zhang, L.S.; Xuan, K. Contributions of bioactive molecules in stem cell-based periodontal regeneration. Int. J. Mol. Sci. 2018, 19, 1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, I.; Iwata, T.; Washio, K.; Okano, T.; Nagasawa, T.; Iwasaki, K.; Ando, T. Cell sheet engineering and other novel cell-based approaches to periodontal regeneration. Periodontology 2000 2009, 51, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Abdal-Wahab, M.; Abdel Ghaffar, K.A.; Ezzatt, O.M.; Hassan, A.A.A.; El Ansary, M.M.S.; Gamal, A.Y. Regenerative potential of cultured gingival fibroblasts in treatment of periodontal intrabony defects (randomized clinical and biochemical trial). J. Periodontal Res. 2020, 55, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amghar-Maach, S.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Regeneration of periodontal bone defects with dental pulp stem cells grafting: Systematic Review. J. Clin. Exp. Dent. 2019, 11, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Tsutsumi, Y.; Tsumanuma, Y.; Yano, K.; Srithanyarat, S.S.; Takagi, R.; Ichinose, S.; Meinzer, W.; Yamato, M.; Okano, T.; et al. In Vivo Periodontium Formation Around Titanium Implants Using Periodontal Ligament Cell Sheet. Tissue Eng. Part A 2018, 24, 1273–1282. [Google Scholar] [CrossRef]
- Grounds, M.D. Obstacles and challenges for tissue engineering and regenerative medicine: Australian nuances. Clin. Exp. Pharmacol. Physiol. 2018, 45, 390–400. [Google Scholar] [CrossRef]
- Iwasaki, K.; Washio, K.; Meinzer, W.; Tsumanuma, Y.; Yano, K.; Ishikawa, I. Application of cell-sheet engineering for new formation of cementum around dental implants. Heliyon 2019, 5, e01991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Qi, Y.; Niu, L.; Di, T.; Zhong, J.; Fang, T.; Yan, W. Application of the cell sheet technique in tissue engineering. Biomed. Rep. 2015, 3, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Iwata, T.; Washio, K.; Yoshida, T.; Ishikawa, I.; Ando, T.; Yamato, M.; Okano, T. Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 343–356. [Google Scholar] [CrossRef]
- Okano, T. A novel recoverv svstern for cultured cells using plasrna-trGatid polystyrene dishes grafted with poly(N4sopropylacrylarnide). Langmuir 2008, 24, 7457–7464. [Google Scholar]
- Wei, F.; Song, T.; Ding, G.; Xu, J.; Liu, Y.; Liu, D.; Fan, Z.; Zhang, C.; Shi, S.; Wang, S. Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev. 2013, 22, 1752–1762. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.H.; Hu, L.; Liu, G.L.; Wei, F.L.; Liu, Y.; Liu, Z.H.; Fan, Z.P.; Zhang, C.M.; Wang, J.S.; Wang, S.L. Bio-root and implant-based restoration as a tooth replacement alternative. J. Dent. Res. 2016, 95, 642–649. [Google Scholar] [CrossRef]
- Gao, H.; Li, B.; Zhao, L.; Jin, Y. Influence of nanotopography on periodontal ligament stem cell functions and cell sheet based periodontal regeneration. Int. J. Nanomed. 2015, 10, 4009–4027. [Google Scholar]
- Wei, F.; Qu, C.; Song, T.; Ding, G.; Fan, Z.; Liu, D.; Liu, Y.; Zhang, C.; Shi, S.; Wang, S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J. Cell. Physiol. 2012, 227, 3216–3224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Jin, F.; Zhang, X.; Ma, D.; Han, C.; Huo, N.; Wang, Y.; Zhang, Y.; Lin, Z.; Jin, Y. Tissue engineering of cementum/periodontal-ligament complex using a novel three-dimensional pellet cultivation system for Human periodontal ligament stem cells. Tissue Eng. Part C Methods 2009, 15, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liu, H. A Novel Mixed-Type Stem Cell Pellet for Cementum/Periodontal Ligament–Like Complex. J. Periodontol. 2012, 83, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Feng, Z.H.; Wu, G.F.; Bai, S.Z.; Dong, Y.; Chen, F.M.; Zhao, Y.M. The use of platelet-rich fibrin combined with periodontal ligament and jaw bone mesenchymal stem cell sheets for periodontal tissue engineering. Sci. Rep. 2016, 6, 28126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Song, Z.C.; Xia, Y.R.; Shu, R. Extracellular matrix derived from periodontal ligament cells maintains their stemness and enhances redifferentiation via the wnt pathway. J. Biomed. Mater. Res. Part A 2018, 106, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, C.H.; Yi, T.; Kim, S.N.; Iwata, T.; Yun, J.H. RhBMP-2 pre-treated human periodontal ligament stem cell sheets regenerate a mineralized layer mimicking dental cementum. Int. J. Mol. Sci. 2020, 21, 3767. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhao, B.; Gao, Z.; Xu, J.; Fan, Z.; Zhang, C.; Wang, J.; Wang, S. Regeneration characteristics of different dental derived stem cell sheets. J. Oral Rehabil. 2020, 47, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, W.; Yi, D.; Gong, J.; Zou, Q.; Xie, D.; Chen, Y.; Wu, Y.; Tian, W. Comparative study of human dental follicle cell sheets and periodontal ligament cell sheets for periodontal tissue regeneration. Cell Transplant. 2013, 22, 1061–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, L.; Liu, W.; Li, Q.; Jin, Z.; Jin, Y. Dental follicle cells rescue the regenerative capacity of periodontal ligament stem cells in an inflammatory microenvironment. PLoS ONE 2014, 9, e108752. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, B.; Yuan, L.; Dong, Z.; Zhang, H.; Wang, H.; Sun, J.; Ge, A.; Jin, Y. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J. Tissue Eng. Regen. Med. 2017, 11, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; He, Y.; Tang, X.; Chen, G.; Shi, H.; Gong, K.; Zhou, J.; Wen, L.; Jin, Y. Scaffold-free cell pellet transplantations can be applied to periodontal regeneration. Cell Transplant. 2014, 23, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Zhu, B.; Xu, Q.; Ding, Y.; Jin, Y. Composite cell sheet for periodontal regeneration: Crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res. Ther. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Xiong, X.; Zhou, W.; Feng, G.; Zhang, Y.; Dai, H.; Zhou, J. Effects of human urine-derived stem cells on the cementogenic differentiation of indirectly-cocultured periodontal ligament stem cells. Am. J. Transl. Res. 2020, 12, 361–378. [Google Scholar] [PubMed]
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal Res. 2017, 52, 408–418. [Google Scholar] [CrossRef]
- Yu, Y.; Bi, C.S.; Wu, R.X.; Yin, Y.; Zhang, X.Y.; Lan, P.H.; Chen, F.M. Effects of short-term inflammatory and/or hypoxic pretreatments on periodontal ligament stem cells: In vitro and in vivo studies. Cell Tissue Res. 2016, 366, 311–328. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhu, M.; Ying, S.; Li, L.; Chen, D.; Li, J.; Song, J. Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, G.; Li, J.; Zou, Q.; Xie, D.; Chen, Y.; Wang, H.; Zheng, X.; Long, J.; Tang, W.; et al. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 2012, 33, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wu, Y.; Yu, Y.; Huang, L.; An, S.; Hu, B.; Luo, J.; Song, J. Periodontal ligament-like tissue regeneration with drilled porous decalcified dentin matrix sheet composite. Oral Dis. 2018, 24, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Iwata, T.; Mizutani, M.; Ando, T.; Yamato, M.; Okano, T.; Ishikawa, I. Assessment of cell sheets derived from human periodontal ligament cells: A pre-clinical study. Cell Tissue Res. 2010, 341, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Liu, Y.; Lu, W.; Yang, F.; Yu, J.; Wang, X.; Ma, Q.; Yang, Z.; Wen, L.; Xuan, K. Periodontal tissue engineering with stem cells from the periodontal ligament of human retained deciduous teeth. J. Periodontal Res. 2013, 48, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Hu, L.; Zhou, Y.; Ge, Z.; Wang, H.; Wu, C.T.; Jin, J. A Sandwich Structure of Human Dental Pulp Stem Cell Sheet, Treated Dentin Matrix, and Matrigel for Tooth Root Regeneration. Stem Cells Dev. 2020, 29, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Abe, T.; Tanaka, M.; Hara, Y. Periodontal tissue engineering by transplantation of multilayered sheets of phenotypically modified gingival fibroblasts. J. Periodontal Res. 2008, 43, 681–688. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef] [Green Version]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Zhang, M.; Liu, N.X.; Lv, X.; Zhang, J.; Chen, F.M.; Chen, Y.J. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013, 34, 5506–5520. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Komaki, M.; Yokoyama, N.; Tanaka, Y.; Taki, A.; Honda, I.; Kimura, Y.; Takeda, M.; Akazawa, K.; Oda, S.; et al. Periodontal regeneration using periodontal ligament stem cell-transferred amnion. Tissue Eng. Part A 2014, 20, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Liu, Z.; Xie, Y.; Hu, J.; Wang, H.; Fan, Z.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res. Ther. 2015, 6, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Cao, Y.; Xie, Y.; Wang, H.; Fan, Z.; Wang, J.; Zhang, C.; Wang, J.; Wu, C.T.; Wang, S. Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res. Ther. 2016, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsumanuma, Y.; Iwata, T.; Kinoshita, A.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Yamato, M.; Okano, T.; Izumi, Y. Allogeneic Transplantation of Periodontal Ligament-Derived Multipotent Mesenchymal Stromal Cell Sheets in Canine Critical-Size Supra-Alveolar Periodontal Defect Model. Biores. Open Access 2016, 5, 22–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Kang, J.; Ji, B.; Guo, W.; Ding, Y.; Wu, Y.; Tian, W. Periodontal-Derived Mesenchymal Cell Sheets Promote Periodontal Regeneration in Inflammatory Microenvironment. Tissue Eng. Part A 2017, 23, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Takewaki, M.; Kajiya, M.; Takeda, K.; Sasaki, S.; Motoike, S.; Komatsu, N.; Matsuda, S.; Ouhara, K.; Mizuno, N.; Fujita, T.; et al. MSC/ECM Cellular Complexes Induce Periodontal Tissue Regeneration. J. Dent. Res. 2017, 96, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Hashimi, S.M.; Vaquette, C.; Bartold, P.M.; Hutmacher, D.W.; Ivanovski, S. The effect of decellularized tissue engineered constructs on periodontal regeneration. J. Clin. Periodontol. 2018, 45, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Vaquette, C.; Saifzadeh, S.; Farag, A.; Hutmacher, D.W.; Ivanovski, S. Periodontal Tissue Engineering with a Multiphasic Construct and Cell Sheets. J. Dent. Res. 2019, 98, 673–681. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Hu, Y.; Sun, J.; Guo, W.; Li, H.; Chen, J.; Huo, F.; Tian, W.; Li, S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent. Mater. 2019, 35, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.M.; Huang, J.P.; Lu, K.X.; Sun, W.L.; Tan, J.Y.; Li, B.X.; Chen, L.L.; Wu, Y.M. Regeneration potential of decellularized periodontal ligament cell sheets combined with 15-deoxy-∆12,14-prostaglandin J2 nanoparticles in a rat periodontal defect. Biomed. Mater. 2021, 16, 045008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Abukawa, H.; Troulis, M.J.; Kaban, L.B.; Vacanti, J.P.; Yelick, P.C. Tissue engineered hybrid tooth-bone constructs. Methods 2009, 47, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, L.; Chang, J.; Wei, L.; Chen, D.; Zhang, Y. Porous nagelschmidtite bioceramic scaffolds with improved in vitro and in vivo cementogenesis for periodontal tissue engineering. RSC Adv. 2013, 3, 17843–17850. [Google Scholar] [CrossRef]
- Chantarawaratit, P.; Sangvanich, P.; Banlunara, W.; Soontornvipart, K.; Thunyakitpisal, P. Acemannan sponges stimulate alveolar bone, cementum and periodontal ligament regeneration in a canine class II furcation defect model. J. Periodontal Res. 2014, 49, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Mekhemar, M.K.; Beck-Broichsitter, B.E.; Bähr, T.; Hegab, M.; Receveur, J.; Heneweer, C.; Becker, S.T.; Wiltfang, J.; Dörfer, C.E. Periodontal regeneration employing gingival margin-derived stem/progenitor cells in conjunction with IL-1ra-hydrogel synthetic extracellular matrix. J. Clin. Periodontol. 2015, 42, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Miyaji, H.; Ishizuka, R.; Tokunaga, K.; Inoue, K.; Kosen, Y.; Yokoyama, H.; Sugaya, T.; Tanaka, S.; Sakagami, R.; et al. Combination of Root Surface Modification with BMP-2 and Collagen Hydrogel Scaffold Implantation for Periodontal Healing in Beagle Dogs. Open Dent. J. 2015, 9, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Li, L.; Zhang, D.; Huang, S.; Jing, Z.; Wu, Y.; Zhao, Z.; Zhao, L.; Zhou, S. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015, 25, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miron, R.J.; Li, S.; Shi, B.; Sculean, A.; Cheng, X. Novel MesoPorous BioGlass/silk scaffold containing adPDGF-B and adBMP7 for the repair of periodontal defects in beagle dogs. J. Clin. Periodontol. 2015, 42, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, F.; Yan, X.; Yang, W.; Yu, N.; Oortgiesen, D.A.W.; Wang, Y.; Jansen, J.A.; Walboomers, X.F. Influence of bone marrow-derived mesenchymal stem cells pre-implantation differentiation approach on periodontal regeneration in vivo. J. Clin. Periodontol. 2015, 42, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, X.; Ye, Q.; He, W.; Ge, M.; Zhou, X.; Hu, J.; Zou, S. Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold. J. Biomater. Appl. 2016, 31, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, K.; Miyaji, H.; Kato, A.; Kosen, Y.; Momose, T.; Yoshida, T.; Nishida, E.; Miyata, S.; Murakami, S.; Takita, H.; et al. Periodontal tissue engineering by nano beta-tricalcium phosphate scaffold and fibroblast growth factor-2 in one-wall infrabony defects of dogs. J. Periodontal Res. 2016, 51, 758–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momose, T.; Miyaji, H.; Kato, A.; Ogawa, K.; Yoshida, T.; Nishida, E.; Murakami, S.; Kosen, Y.; Sugaya, T.; Kawanami, M. Collagen Hydrogel Scaffold and Fibroblast Growth Factor-2 Accelerate Periodontal Healing of Class II Furcation Defects in Dog. Open Dent. J. 2016, 10, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; De Moraes, M.S.; Ferreira, L.B.; Carreira, A.C.O.; Kossugue, P.M.; Boaro, L.C.C.; Bentini, R.; Da Silva Garcia, C.R.; Sogayar, M.C.; Arana-Chavez, V.E.; et al. Combination of bioactive polymeric membranes and stem cells for periodontal regeneration: In vitro and in vivo analyses. PLoS ONE 2016, 11, e0152412. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Jin, L.; Kang, S.; Hu, X.; Wang, M.; Wang, J.; Chen, B.; Peng, B.; Wang, Q. Periodontal Wound Healing by Transplantation of Jaw Bone Marrow-Derived Mesenchymal Stem Cells in Chitosan/Anorganic Bovine Bone Carrier into One-Wall Infrabony Defects in Beagles. J. Periodontol. 2016, 87, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Bizenjima, T.; Ishii, Y.; Imamura, K.; Suzuki, E.; Seshima, F.; Saito, A. Enhanced healing of surgical periodontal defects in rats following application of a self-assembling peptide nanofibre hydrogel. J. Clin. Periodontol. 2016, 43, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Jiang, J.; Chen, Y.; Lin, M.; Du, Z.; Xiao, Y.; Luo, K.; Yan, F. Leptin Overexpression in Bone Marrow Stromal Cells Promotes Periodontal Regeneration in a Rat Model of Osteoporosis. J. Periodontol. 2017, 88, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Pilipchuk, S.P.; Fretwurst, T.; Yu, N.; Larsson, L.; Kavanagh, N.M.; Asa’ad, F.; Cheng, K.C.K.; Lahann, J.; Giannobile, W.V. Micropatterned Scaffolds with Immobilized Growth Factor Genes Regenerate Bone and Periodontal Ligament-Like Tissues. Adv. Healthc. Mater. 2018, 7, e1800750. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.H.; Chang, Y.L.; Wang, M.L.; Chuang, J.H.; Yang, Y.C.; Tai, M.C.; Wang, C.Y.; Liu, Y.Y.; Li, H.Y.; Chen, J.T.; et al. Promoting Induced Pluripotent Stem Cell-driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gao, X.; Shen, Z.; Shi, X.; Lin, Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv. 2019, 9, 507–518. [Google Scholar] [CrossRef] [Green Version]
- He, X.T.; Li, X.; Xia, Y.; Yin, Y.; Wu, R.X.; Sun, H.H.; Chen, F.M. Building capacity for macrophage modulation and stem cell recruitment in high-stiffness hydrogels for complex periodontal regeneration: Experimental studies in vitro and in rats. Acta Biomater. 2019, 88, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Yao, Z.; Wang, C. Study of a new nano-hydroxyapatite/basic fibroblast growth factor composite promoting periodontal tissue regeneration. Mater. Express 2020, 10, 1802–1807. [Google Scholar] [CrossRef]
- Ding, T.; Li, J.; Zhang, X.; Du, L.; Li, Y.; Li, D.; Kong, B.; Ge, S. Super-assembled core/shell fibrous frameworks with dual growth factors for: In situ cementum-ligament-bone complex regeneration. Biomater. Sci. 2020, 8, 2459–2471. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontology 2000 2015, 68, 66–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamp, S.E.; Lindhe, J.; Löe, H. Experimental periodontitis in the beagle dog. J. Periodontal Res. 1972, 10, 13–14. [Google Scholar]
- Hugoson, A.; Schmidt, G. Influence of Plaque Control on the Healing of Experimentally-Induced Bone Defects in the Dog. J. Periodontol. 1978, 49, 135–141. [Google Scholar] [CrossRef]
- Kimmel, D.B.; Jee, W.S.S. A quantitative histologic study of bone turnover in young adult beagles. Anat. Rec. 1982, 203, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Hu, K.S.; Song, W.C.; Park, J.T.; Kim, H.J.; Koh, K.S.; Kim, H.J. Innervation patterns of the canine masticatory muscles in comparison to human. Anat. Rec. 2010, 293, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rios, H.F.; Giannobile, W.V. Preclinical Protocols for Periodontal Regeneration. In Osteology Guidelines for Oral & Maxillofacial Regeneration; Giannobile, W.V., Nevins, M., Eds.; Quintessence Publishing Co., Ltd.: New Malden, UK, 2011; pp. 77–102. [Google Scholar]
- Zhang, Y.; Li, S.; Wu, C. The in vitro and in vivo cementogenesis of CaMgSi2O6 bioceramic scaffolds. J. Biomed. Mater. Res. Part A 2014, 102, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.W.; Scapino, R.P. Physiology of feeding in miniature pigs. J. Morphol. 1973, 141, 427–460. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Jones, V.L.; Andry, C.; Kantarci, A. 1-Tetradecanol Complex Reduces Progression of Porphyromonas gingivalis –Induced Experimental Periodontitis in Rabbits. J. Periodontol. 2007, 78, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A.; Ebrahimi, N.; Andry, C.; Holick, M.; Jones, V.L.; Van Dyke, T.E. Topical H2 antagonist prevents periodontitis in a rabbit model. Infect. Immun. 2006, 74, 2402–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slotte, C.; Lundgren, D.; Sennerby, L.; Lundgren, A.K. Influence of preimplant surgical intervention and implant placement on bone wound healing. Clin. Oral Implants Res. 2003, 14, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, S.; Taruscio, D.; Ciaralli, F.; Crateri, P.; Chistolini, P.; Bedini, R.; Colangelo, P.; Pintucci, S. Evaluation of an experimental periodontal ligament for dental implants. Biomaterials 1991, 12, 474–478. [Google Scholar] [CrossRef]
- Khorramirouz, R.; Go, J.L.; Noble, C.; Jana, S.; Maxson, E.; Lerman, A.; Young, M.D. A novel surgical technique for a rat subcutaneous implantation of a tissue engineered scaffold. Acta Histochem. 2018, 120, 282–291. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.J.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; Stupp, S.I.; Sader, R.A.; Kirkpatrick, C.J. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Rios, H.F.; Jin, Q.; Bland, M.E.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010, 31, 5945–5952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.N.; Ma, B.; Shao, J.; Liu, H.; Ge, S. Gingipain-Responsive Thermosensitive Hydrogel Loaded with SDF-1 Facilitates in Situ Periodontal Tissue Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 36880–36893. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, J.; Yang, B.; Li, L.; Luo, X.; Zhang, X.; Feng, L.; Jiang, Z.; Yu, M.; Guo, W.; et al. Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials 2015, 52, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, M.S.; Eltohamy, M.; Kim, T.H.; Kim, H.W. Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering. PLoS ONE 2016, 11, e0149967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, Y.; Miao, L.; Wang, Y.; Ren, S.; Yang, X.; Hu, Y.; Sun, W. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int. J. Nanomed. 2016, 11, 3145–3158. [Google Scholar]
- McGuire, M.K.; Scheyer, E.T. Comparison of recombinant human platelet-derived growth factor-BB plus beta tricalcium phosphate and a collagen membrane to subepithelial connective tissue grafting for the treatment of recession defects: A case series. Int. J. Periodontics Restor. Dent. 2006, 26, 127–133. [Google Scholar]
- Sarment, D.P.; Cooke, J.W.; Miller, S.E.; Jin, Q.; McGuire, M.K.; Kao, R.T.; McClain, P.K.; McAllister, B.S.; Lynch, S.E.; Giannobile, W.V. Effect of rhPDGF-BB on bone turnover during periodontal repair. J. Clin. Periodontol. 2006, 33, 135–140. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Kao, R.T.; Nevins, M.; Lynch, S.E. rhPDGF-BB promotes healing of periodontal defects: 24-month clinical and radiographic observations. Int. J. Periodontics Restor. Dent. 2006, 26, 223–231. [Google Scholar]
- Bhongade, M.L.; Tiwari, I.R. A comparative evaluation of the effectiveness of an anorganic bone matrix/cell binding peptide with an open flap debridement in human infrabony defects: A clinical and radiographic study. J. Contemp. Dent. Pract. 2007, 8, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGuire, M.K.; Scheyer, E.T.; Schupbach, P. Growth Factor–Mediated Treatment of Recession Defects: A Randomized Controlled Trial and Histologic and Microcomputed Tomography Examination. J. Periodontol. 2009, 80, 550–564. [Google Scholar] [CrossRef]
- Jayakumar, A.; Rajababu, P.; Rohini, S.; Butchibabu, K.; Naveen, A.; Reddy, P.K.; Vidyasagar, S.; Satyanarayana, D.; Pavan Kumar, S. Multi-centre, randomized clinical trial on the efficacy and safety of recombinant human platelet-derived growth factor with β-tricalcium phosphate in human intra-osseous periodontal defects. J. Clin. Periodontol. 2011, 38, 163–172. [Google Scholar] [CrossRef]
- Nevins, M.; Kao, R.T.; McGuire, M.K.; McClain, P.K.; Hinrichs, J.E.; McAllister, B.S.; Reddy, M.S.; Nevins, M.L.; Genco, R.J.; Lynch, S.E.; et al. Platelet-Derived Growth Factor Promotes Periodontal Regeneration in Localized Osseous Defects: 36-Month Extension Results From a Randomized, Controlled, Double-Masked Clinical Trial. J. Periodontol. 2013, 84, 456–464. [Google Scholar] [CrossRef]
- Maroo, S.; Murthy, K.R. Treatment of Periodontal Intrabony Defects Using β-TCP Alone or in Combination with rhPDGF-BB: A Randomized Controlled Clinical and Radiographic Study. Int. J. Periodontics Restor. Dent. 2014, 34, 841–847. [Google Scholar] [CrossRef] [Green Version]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef] [Green Version]
- Hamzacebi, B.; Oduncuoglu, B.; Alaaddinoglu, E. Treatment of Peri-implant Bone Defects with Platelet-Rich Fibrin. Int. J. Periodontics Restor. Dent. 2015, 35, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, M.; Akamatsu, M.; Kawanami, M.; Furuichi, Y.; Fujii, T.; Mori, M.; Kunimatsu, K.; Shimauchi, H.; Ogata, Y.; Yamamoto, M.; et al. Randomized Placebo-Controlled and Controlled Non-Inferiority Phase III Trials Comparing Trafermin, a Recombinant Human Fibroblast Growth Factor 2, and Enamel Matrix Derivative in Periodontal Regeneration in Intrabony Defects. J. Bone Miner. Res. 2016, 31, 806–814. [Google Scholar] [CrossRef]
- Cochran, D.L.; Oh, T.J.; Mills, M.P.; Clem, D.S.; McClain, P.K.; Schallhorn, R.A.; McGuire, M.K.; Scheyer, E.T.; Giannobile, W.V.; Reddy, M.S.; et al. A Randomized Clinical Trial Evaluating rh-FGF-2/β-TCP in Periodontal Defects. J. Dent. Res. 2016, 95, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Naineni, R.; Ravi, V.; Subbaraya, D.K.; Prasanna, J.S.; Panthula, V.R.; Koduganti, R.R. Effect of alendronate with β—TCP bone substitute in surgical therapy of periodontal intra-Osseous defects: A randomized controlled clinical trial. J. Clin. Diagn. Res. 2016, 10, ZC113–ZC117. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Kumar, N.; Bansal, M.; Mishra, B. Tinidazole functionalized homogeneous electrospun chitosan/poly (ε-caprolactone) hybrid nanofiber membrane: Development, optimization and its clinical implications. Int. J. Biol. Macromol. 2017, 103, 1311–1326. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Na, H.-J.; Kim, H.-M.; Lee, S.-C.; Lee, J.-Y.; Chung, C.-P.; Seol, Y.-J.; Park, Y. Comparative Study of rhPDGF-BB Plus Equine-Derived Bone Matrix Versus rhPDGF-BB Plus β-TCP in the Treatment of Periodontal Defects. Int. J. Periodontics Restor. Dent. 2017, 37, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshoju, A.K.; Chandra, R.V.; Reddy, A.A.; Reddy, B.H.; Nagarajan, S.; Naveen, A. Efficacy of a Novel Zn-Substituted Monetite-Based Scaffold in the Treatment of Periodontal Osseous Defects. J. Int. Acad. Periodontol. 2017, 19, 2–9. [Google Scholar] [PubMed]
- Kizildağ, A.; Çiçek, Y.; Arabaci, T.; Köse, O. The effect of leukocyte-platelet-rich fibrin on bone morphogenetic protein-2 and insulin-like growth factor-1 levels in patients with chronic periodontitis: A randomized split mouth clinical trail. Growth Factors 2018, 36, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Bizenjima, T.; Takeuchi, T.; Suzuki, E.; Sato, M.; Yoshikawa, K.; Kitamura, Y.; Matsugami, D.; Aoki, H.; Kita, D.; et al. Treatment of intrabony periodontal defects using rhFGF-2 in combination with deproteinized bovine bone mineral or rhFGF-2 alone: A 6-month randomized controlled trial. J. Clin. Periodontol. 2019, 46, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.H.; Jeong, S.N. Adjunctive use of enamel matrix derivatives to porcine-derived xenograft for the treatment of one-wall intrabony defects: Two-year longitudinal results of a randomized controlled clinical trial. J. Periodontol. 2020, 91, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Shoukheba, M.; El Gholy, S.; Gamal, S. Beta-Tricalcium Phosphate and Concentrated Growth Factors in Treatment of Intra-bony Defect Randomized Clinical Trial (RCT). Egypt. Dent. J. 2021, 67, 3103–3112. [Google Scholar] [CrossRef]
- Mangano, C.; Giuliani, A.; De Tullio, I.; Raspanti, M.; Piattelli, A.; Iezzi, G. Case Report: Histological and Histomorphometrical Results of a 3-D Printed Biphasic Calcium Phosphate Ceramic 7 Years After Insertion in a Human Maxillary Alveolar Ridge. Front. Bioeng. Biotechnol. 2021, 9, 614325. [Google Scholar] [CrossRef]
- Deshpande, A.; Baburaj, M.; Tambe, L.; Prasad, U. Extracellular matrix containing nanocomposite bone graft in periodontal regeneration—A randomized controlled clinical and radiographic evaluation. J. Indian Soc. Periodontol. 2021, 25, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Lavu, V.; Balaji, S.K. Clinical efficacy of amniotic membrane with biphasic calcium phosphate in guided tissue regeneration of intrabony defects- a randomized controlled clinical trial. Biomater. Res. 2021, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hsieh, S.C.; Bao, W.; Graves, D.T. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am. J. Physiol.-Cell Physiol. 1997, 272. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, N.; Lin, W.-L.; Kumar, N.M.; Cho, M.I.; Genco, R.J. Mitogenic, Chemotactic, and Synthetic Responses of Rat Periodontal Ligament Fibroblastic Cells to Polypeptide Growth Factors In Vitro. J. Periodontol. 1992, 63, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.W.; Sarment, D.P.; Whitesman, L.A.; Miller, S.E.; Jin, Q.; Lynch, S.E.; Giannobile, W.V. Effect of rhPDGF-BB Delivery on Mediators of Periodontal Wound Repair. Tissue Eng. 2006, 12, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Dhote, R.; Charde, P.; Bhongade, M.; Rao, J. Stem cells cultured on beta tricalcium phosphate (β-TCP) in combination with recombinant human platelet-derived growth factor—BB (RH-PDGF-BB) for the treatment of human infrabony defects. Stem Cells Mediat. Regen. 2016, 10, 17–32. [Google Scholar]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II Trial of Autologous Bone Marrow Stem Cell Transplantation with a Three-Dimensional Woven-Fabric Scaffold for Periodontitis. Stem Cells Int. 2016, 2016, 6205910. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, N.; Fierravanti, L.; Núñez, J.; Vignoletti, F.; González-Zamora, M.; Santamaría, S.; Suárez-Sancho, S.; Fernández-Santos, M.E.; Figuero, E.; Herrera, D.; et al. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J. Clin. Periodontol. 2020, 47, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.A.; Bakopoulou, A.A.; Kouzi-Koliakou, K.; Karagiannis, V.; Konstantinidis, A. A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study. J. Clin. Periodontol. 2021, 48, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, M.; Łysiak-Drwal, K.; Saczko, J.; Kunert-Keil, C.; Gedrange, T. The clinical efficacy of primary culture of human fibroblasts in gingival augmentation procedures-A preliminary report. Ann. Anat. 2012, 194, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Shokrgozar, M.A.; Mofid, R. Culture of Human Gingival Fibroblasts on a Biodegradable Scaffold and Evaluation of Its Effect on Attached Gingiva: A Randomized, Controlled Pilot Study. J. Periodontol. 2007, 78, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Popovic, O.; Bajic, M.; Cakic, S.; Lekovic, V. Clinical application of autologous fibroblast cell culture in gingival recession treatment. J. Periodontal Res. 2015, 50, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Aramoon, M.; Rajabalian, S.; Mohammadi, M.; Khodarahmi, N.; Farzadmoghadam, M. Human gingival fibroblasts culture in an autologous scaffold and assessing its effect on augmentation of attached gingiva in a pilot clinical trial. J. Oral Health Oral Epidemiol. 2017, 6, 211–217. [Google Scholar]
- Jhaveri, H.M.; Chavan, M.S.; Tomar, G.B.; Deshmukh, V.L.; Wani, M.R.; Miller, P.D. Acellular Dermal Matrix Seeded With Autologous Gingival Fibroblasts for the Treatment of Gingival Recession: A Proof-of-Concept Study. J. Periodontol. 2010, 81, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Ledesma-Martínez, E.; Alcauter-Zavala, A.; Mendoza-Núñez, V.M. Retrieval of a periodontally compromised tooth by allogeneic grafting of mesenchymal stem cells from dental pulp: A case report. J. Int. Med. Res. 2018, 46, 2983–2993. [Google Scholar] [CrossRef]
- Kashte, S.; Dwivedi, A.; Gautam, S.; Sharma, R.K.; Kadam, S. Treatment of gingival recession defect using mesenchymal stem cells cultured PCL based bone regenerating scaffold: A randomized controlled clinical study. Int. J. Appl. Pharm. 2020, 12, 31–33. [Google Scholar] [CrossRef]
- Li, X.; Huang, T.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Antimicrobial nanoparticle coatings for medical implants: Design challenges and prospects. Biointerphases 2020, 15, 060801. [Google Scholar] [CrossRef]
- Liu, B.; Lun, D. xing Current application of β-tricalcium phosphate composites in orthopaedics. Orthop. Surg. 2012, 4, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ham, A.; López-Gutierrez, J.; Bermúdez, M.; Aguilar-Medina, M.; Sarmiento-Sánchez, J.I.; López-Camarillo, C.; Sanchez-Schmitz, G.; Ramos-Payan, R. Hydrogel-Based Scaffolds in Oral Tissue Engineering. Front. Mater. 2021, 8, 294. [Google Scholar] [CrossRef]
- Alvarez-Pérez, M.A.; Narayanan, S.; Zeichner-David, M.; Rodríguez Carmona, B.; Arzate, H. Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 2006, 38, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Yacobi, R.; Savion, N.; Narayanan, A.S.; Pitaru, S. A collagenous cementum-derived attachment protein is a marker for progenitors of the mineralized tissue-forming cell lineage of the periodontal ligament. J. Bone Miner. Res. 1997, 12, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M.; Iwasaki, K.; Arzate, H.; Narayanan, A.S.; Izumi, Y.; Morita, I. Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J. Cell. Physiol. 2012, 227, 649–657. [Google Scholar] [CrossRef]
- Iwata, T.; Mizuno, N.; Nagahara, T.; Kaneda-Ikeda, E.; Kajiya, M.; Kitagawa, M.; Takeda, K.; Yoshioka, M.; Yagi, R.; Takata, T.; et al. Identification of regulatory mRNA and microRNA for differentiation into cementoblasts and periodontal ligament cells. J. Periodontal Res. 2021, 56, 69–82. [Google Scholar] [CrossRef]
- Onizuka, S.; Iwata, T. Application of periodontal ligament-derived multipotent mesenchymal Stromal cell sheets for periodontal regeneration. Int. J. Mol. Sci. 2019, 20, 2796. [Google Scholar] [CrossRef] [Green Version]


| Author | Cells | Experimental Groups | Additional Pretreatment | Material | Technique | Experimental Setup | Results |
|---|---|---|---|---|---|---|---|
| Nakajima et al., 2008 [99] | HGFs | FN-ALP sheet group, FN sheet group, control (no treatment in the defect), control (without immunosuppressant FK administration) | None | None | FN matrix-based multilayered cell sheets of hGFs modified to express ALP (FN-ALP) | Orthotopic model of fenestration bone defects in rats | FN- ALP-expressing hGFs supported the regeneration of cementum-like, PDL-like and bone tissue, exhibiting superior regenerative potential. |
| Ding et al., 2010 [100] | minipig PDLSCs | Control group, HA/TCP group, HA/TCP scaffolds +autologous pPDLSCs group, HA/TCP scaffolds + allogeneic Guizhou minipig pPDLSCs group, HA/TCP scaffolds+ autologous heterogenic minipig pPDLCs group | None | HA/TCP | Cell sheet | Orthotopic model of experimental periodontitis in minipigs | Treatment containing either autologous or allogeneic pPDLSCs resulted in PDL-like tissue regeneration. The use of allogeneic cells did not result in immunological rejection. |
| Tsumanuma et al., 2011 [101] | Canine PDLSCs, BMMSCS, and APCs | Control, PDLC group, BMMSC group, APC group | None | Woven PGA, porous β-TCP and 3% type I collagen | Three-layered cell sheets attached with PGA | One-wall defects were surgically created in dog | The PDLC group exhibited enhanced cementum-like and PDL-like tissue regeneration, exhibiting more dense collagen fibers and thicker mineralized tissue. |
| Wei et al., 2012 [75] | PDLSCs | Vc-induced autologous PDLSCs sheet group, UpCell dish PDLSCs sheet group, Gelfoam scaffolds/dissociated autologous PDLSCs group (control) | Vc treatment | Gelfoam scaffold | Cell sheet | Ectopic transplantation in nude mice, and orthotopic transplantation experimental periodontal lesions bone defect in miniature swines | Vc-induced PDLSCs sheet group and UpCell dish PDLSC sheet group application resulted in significantly more bone/cementum-like tissue formation compared to control/Vc-induced PDLSCs sheet group was significantly better. |
| Zhao et al., 2013 [102] | PDLSCs | PDLSCs/PRF construct group, cell sheet fragments group, PRF granules group | None | PRF granules | Combination of fragments from PDLSCs cell sheet and PRF granules | PDLSCs/PRF granules construct in tooth reimplantation in dogs | PDLSCs/PRF construct promoted PDL-like tissue regeneration and exhibited reduction in terms of inflammation and ankylosis. |
| Iwasaki et al., 2014 [103] | PDLSCs | Amnion group, PDLSC-amnion group | None | Decellularized amniotic membrane (amnion) | None | Application of PDLSCs -amnion in a periodontal defect model in rat maxillary molars | Histological and radiographic analysis showed that PDLSC-amnion group promoted PDL-like tissue regeneration. |
| Guo et al., 2014 [86] | Rat PDLSCs | MCPs group, MUCPs group, MCPs/TDM group, MUCPs/TDM group | None | TDM | MCP and MUCPs produced by MCS and MUCS | In vivo transplantation of MCPs or MUCPs into the imental pouch; periodontal defect model and in vivo transplantation of MCPs and MUCPs in rats | All groups promoted cementum-like and PDL-like tissue regeneration, but MUCPs group exhibited superior behavior in terms of mineralization and collagen fiber arrangement compared to MCPs group. |
| Cao et al., 2015 [104] | hDPSCs | Control group, hDPSC injection group, HGF-hDPSC injection group, hDPSC sheet, HGFhDPSC sheets | Adenovirus-mediated transfer of HGF gene to DPSCs | None | Cell sheet of adenovirus-mediated transfer of HGF | 40 periodontitis lesions, three-wall intrabony defects, in the 1st molars of miniature pigs | HGFhDPSC sheet group was able to promote PDL-like tissue formation and alveolar bone regeneration similar to that of native tissue, whereas the other groups provided only limited regeneration. |
| Hu et al., 2016 [105] | hDPSCs | Control group, hDPSC injection group, hDPSC sheep group | None | None | Cell injection or cell sheet transplantation | Three-wall intrabony periodontal defects, in miniature pigs | Both experimental groups effectively promoted periodontal regeneration compared to control. hDPSC sheet application resulted in significantly better bone regeneration compared to the hDPSC injection. |
| Tsumanuma et al., 2016 [106] | Canine PDLSCs | Control group, autologous group, allogeneic group | None | Woven PGA, porous β-TCP and 3% type I collagen | Three-layered cell sheets attached with PGA | Critical size supraalveolar periodontal defect model in dog | Both autologous and allogeneic groups were able to regenerate bone, cementum-like and PDL-like tissue. |
| Yu et al., 2016 [90] | PDLSCs | Inflammation group, hypoxia group, inflammatory plus hypoxic stimuli-dual-stimuli group, no-stimulus group, blank group, CBB group | Inflammatory conditions (inflammation), hypoxic conditions (hypoxia), or a combination of both (dual stimuli) conditions | CBB | Cell sheet | Ectopic trasplantation model (subcutaneously) into the dorsal region, and orthotopic model with surgical creation of periodontal defects (3 mm × 1.5 mm) in nude mice | Hypoxia group exhibited more bone formation compared to other groups, while cementum-like and PDL-like tissue formation was identified in the no-stimulus and hypoxia groups. |
| Guo et al., 2017 [107] | PDLCs and DFCs | Control group, DFC sheet group, PDLC sheet group | P. gingivalis LPS-induced inflammation microenvironment | None | Cell sheet | Canine periodontitis model (two wall intrabony defects), in dogs | DFC sheet application was more effective in terms of bone, cementum-like and PDL-like tissue regeneration compared to the PDLC sheet. |
| Takewaki et al., 2017 [108] | BMMSCs | No graft, C-MSC in growth medium, C-MSC in OIM | Osteoinductive medium (OIM) | None | MSC/ECM complex (C-MSC) | Orthotopic model of class III furcation defect, in beagle dogs | Both C-MSC and C-MSC-OIM exhibited formation of cementum-like, PDL-like and bone formation leading to the regeneration of the periodontal complex. |
| Farag et al., 2018 [109] | PDLCs | Scaffold group, decellularized cell sheet/scaffold group | None | PCL | Decellularized cell sheet | Rat periodontal defect model in the mandible (orthotopic) | PDL-like tissue regeneration was observed in both groups. However, the group with the decellularized cell sheet presented higher detection of PDL fiber attachment with perpendicular orientation. |
| Iwata et al., 2018 [8] | PDLCs | PDL cell sheet group | None | β-TCP granules | cell sheet | Bony defects were filled with three-layered PDL-derived cell sheets and with β-TCP granules (clinical study) | Improvement was observed in terms of bone regeneration and clinical attachment 6 months after application of PDL-cell sheet. |
| Yanget al., 2019 [94] | DFCs and SHEDs | SHEDSs combined with TDM group, DFCSs combined with TDM group, TDM group | None | TDM | Cell sheet | Subcutaneous transplantation into nude mice and orthotopic implantation in Sprague-Dawley rats’ jawbone | Both SHEDs/TDM and DFCs/TDM groups formed PDL-like tissues, enriched in collagen fibers and fibroblasts, with arrangement similar to that of native PDL. |
| Vaquette et al., 2019 [110] | GC, BMMSCs, and PDLCs | Control group (no cells on scaffold), GC group, BMMSC group, and PDLC group | None | PCL | Biphasic PCL scaffold consisting of bone and PDL compartments combined with the cell sheets. | Dehiscence periodontal defects in sheep | Bone, cementum-like and PDL-like tissue regeneration was observed in the BMMSC and PDLC groups compared to the GC and control group. |
| Yang et al., 2019 [111] | hDFCs | Blank group, cDFCSs group, TDMP group, HA/-TCPgroup, TDMP + cDFCSs group, HA/-TCP + cDFCSs group | None | TDM particles or HA/-TCP | Cell sheet | One-wall periodontal intrabony defects in beagle dogs | The use of materials enhanced bone formation. The presence of DFCs promoted the regeneration of bone and PDL-like tissue. |
| Raju et al., 2020 [97] | Rat PDL cells and osteoblastic cells | PDL cell sheet group, MC3T3-E1 cell sheet group, complex cell sheet group (containing both cells) | None | None | Cell sheet | Ectopic and orthotopic transplantation in vivo in mice | Ectopic transplantation of complex cell sheet resulted in PDL-like and bone tissue formation. Only complex cell sheet group was able to regenerate bone and PDL-like tissue similar to the native PDL-bone complex. |
| Jiang et al., 2021 [112] | hPDLCs | dHPDLC group, dHPDLC sheets loaded with PCL/GE group (dHPDLC-PCL/GE), control group | None | PCL/GE nanofibers and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) nanoparticles | Decellularized cell sheet | Periodontal defects (periodontal fenestration defect) in rats | dHPDLC and dHPDLC-PCL/GE groups promoted new bone formation as well as PDL-like and cementum-like tissue regeneration compared to control. dHPDLC-PCL/GE group exhibited irregular and perpendicular fiber orientation in the regenerated PDL-like tissue. |
| Study | Scaffold Type | Cells | In Vivo Animal Model | Animal/ Evaluation Time | Major Findings |
|---|---|---|---|---|---|
| Zhang et al., 2009 [113] | Hybrid tooth constructs from PGA/PLLA and PLGA scaffolds for tooth and bone parts respectively | DSCs | Intrabony defects in the mandible | Yucatan mini pigs 12 and 20 weeks | Cementum-like tissues but absence of periodontal ligament tissues. Scarcely found fibers resembling Sharpey’s fibers penetrated the regenerated cementum-like tissues and surrounding bone |
| Park 2012 et al. [114] | Amorphous and fiber guiding PCL scaffolds | hPDLs | Periodontal defect model with osseous defects on the buccal side of the mandible | Athymic rats 4 weeks | Cementum-like tissue was formed on the dentin surfaces with fiber guiding scaffolds, which displayed similar angulation of fiber orientation to native ligament tissue |
| Inukai 2013 et al. [115] | Absorbable collagen sponges | dMSCs and dPDLCs | One-wall intrabony defects on 2nd, and 4th premolars | Hybrid dogs 4 weeks | New lamellar and woven bone formation and cementum with dense collagen fibers in the MSCs condition medium+ scaffold group |
| Wu 2013 et al. [116] | Porous nagelschmidtite (NAGEL: Ca7P2Si2O16) bioceramic and β-TCP scaffolds | No cells | Periodontal defect model, defects on 2nd and 3rd maxillary premolars and 1st maxillary molar | Beagle dogs 4 and 8 weeks | Both materials presented new bone, cementum and PDL tissue formation, but thicker osteogenic layer was observed for the NAGEL group compared to β-TCP |
| Chantarawaratit 2014 et al. [117] | Acemannan sponges | No cells | Class II furcation defects of maxillary and mandibular 2nd and 3rd premolars | Mondrel dogs 30 and 60 days | New bone, cementum and PDL formation at 30 d. Accelerated regeneration for the acemannan treated groups |
| Fawzy El-Sayed 2015 et al. [118] | IL-1-receptor-antagonist (IL-1ra) releasing hyaluronic acid synthetic extracellular matrix (HA-sECM). | G-MSCs | Periodontal defect model with defects on premolars/molars | Miniature pigs 16 weeks | Cementum-like substance, bone and PDL were regenerated in the IL-1ra/G-MSCs/HA-sECM, and G-MSCs/HA-sECM groups and Sharpey’s fibers similar to normal periodontal tissues |
| Kato 2015 et al. [119] | Collagen hydrogel scaffold (Col) | No cells | One wall intrabony defects on mandibular 2nd and 4th premolars | Beagle dogs 4 weeks | Scaffolds with rh-BMP-2: considerable new trabecular alveolar bone, thick, cellular cementum like tissue with Sharpey’s fiber insertion. Fiber-rich PDL |
| Jiang 2015 et al. [120] | Three-dimensional multilayered scaffold (3D): aligned (AL) and random (RD) biodegradable PCL-PEG (PCE) copolymer electrospun nanofibrous mats into porous chitosan (CHI) | No cells | Periodontal defect model with fenestration defects on maxillary 1st molars | Sprague–Dawley rats 8 weeks | strong topographical guidance of scaffolds to the PDL regeneration. 3D-RD and 3D-ALscaffolds lead to the regeneration of tissues with mostly defined orientation, while 3D-AL scaffolds resulted in cementum-like tissue formation on dentin surfaces. |
| Zhang 2015 et al. [121] | MesoPorous BioGlass/silk scaffold containing adPDGF-B and adBMP7 | No cells | Periodontal defect model with defects on 2nd and 3rd maxillary premolars | Beagle dogs 8 weeks | Best results with the adPDGF-B+ BMP scaffolds” PDL regeneration at 90% of its original height along with both alveolar bone and cementum formation with multiple new Sharpey’s fibers |
| Cai 2015 et al. [122] | PLGA-PCL scaffold by electrospinning | BMSCs cultured in multilineage differentiation (FGF-2), osteogenic (O+) and chondrogenic (C+) medium | Periodontal defect model with intrabony three-wall defects on maxillary 1st molars | Fischer rats 6 weeks | Newbone and ligament, cementum formation limited to the apical root surface. Collagen fibers with an oblique orientation in the FGF-2 and C+ groups, while cartilage-like tissue formation in the C+ group. Bone formation was more profound but limited collagen fibres were observed in the O+ group |
| Liu 2016 et al. [123] | Collagen-hydroxyapatite scaffold (CH) | BMSCs | Labial alveolar intrabony defects in 2nd premolars | Beagle dogs 12 and 24 weeks | Newly formed alveolar bone, PDL and cementum after 12 weeks and after 24 weeks mineralized bone and well-organized and defined tissues |
| Ogawa 2016 et al. [124] | Nano β-TCP and FGF-2-loaded nano-β-TCP scaffold, collagen scaffold as control | No cells | One wall intrabony defects on mandibular 2nd and 4th premolars | Beagle dogs 10 days and 4 weeks | FGF2-treated scaffold: acellular cementum-like tissue in continuity with pre-existing root cementum and PDL-like tissue |
| Momose 2016 et al. [125] | Collagen Hydrogel Scaffold and FGF2 | No cells | Artificial buccal class II furcation defects on the mandibular 2nd, 3rd and 4th premolars | Beagle dogs 10 days and 4 weeks | New bone and vessel-like structures in the FGF2-loaded scaffolds. Formation of woven bone. Only fibrous tissue on the root surface but not PDL attachment. Inhibition of epithelial tissue infiltration |
| Gonçalves 2016 et al. [126] | PisPLLA and PLLA, PLLA-30% HA, PLLA-COL-30% HA, PLLA-COL-30% HA-BMP7 membranes | SHEDs | Periodontal defect model with fenestration defects on 1st mandibular molars | Wistar rats 4 weeks | Both PLLA/COL/HA and PisPLLA/COL/HA membranes presented high bone and PDL regeneration, but the PLLA/COL/HA presented thicker cellular cementum and remained intact for the testing period. The presence of cells inhibited bone regeneration. |
| Zang 2016 et al. [127] | Chitosan/anorganic bovine bone (C/ABB) scaffolds | hJBMMSCs | One-wall intrabony defects on 3rd premolars and 1st molars | Beagle dogs 8 weeks | Bone and cementum formation were greater in groups C/ABB and C/ABB+cell, with the later presenting more lamellar bone and dense PDL with oblique or perpendicular embedding in the new formed tissues |
| Takeuchi 2016 et al. [128] | Self-assembling peptide hydrogel (RADA16)) | No cells | Periodontal defect model with bilateral defects on 2nd maxillary molars | Wistar rats 4 weeks | Ambudant new bone formation was observed. PDL-like collagen bundles with oblique orientation to root surface |
| Zheng 2017 et al. [129] | β-TCP scaffolds | Ad-hLEP-EGFP and Ad-EGFP transfected BMSCs | Periodontal defect model with defects on 1st and 2nd molars | Nude BALB/c mice 10 days and 4 weeks | New well-organized PDL fibersincerting cementum-like tissue for the cell seeded scaffolds. Cementum generation was more pronounced at the β-TCP scaffolds +Ad-hLEP-EGFP-transfected BMSCs group and completely absent in the β-TCP group |
| Sowmya 2017 et al. [10] | Tri-layered scaffold: a. Cementum: CHI- PLGA)/nBGC/ CEMP1, b. PDL: CHI–PLGA/FGF 2, and c. Bone: CHI–PLGA/nBGC/PRP | No cells | Periodontal defect model with maxillary defects | New Zealand white rabbits 4 and 12 weeks | More formation of new cementum, fibrous PDL, and alveolar bone with well-defined bony trabeculae for scaffolds with growth factors |
| Pilipchuk 2018 et al. [130] | Biphasic scaffods. PDL structure: Micropatterned, PLGA/PCL (AdPDGF-BB) Bone: amorphous PCL (AdBMP-7). | Scaffolds’ bone region was seeded with hGFs and the PDL region with hPDLs | Periodontal defect mode with fenestration defects on 1st mandibular molars | Athymic rats 3 and 6 weeks | Soft tissue for all groups by 3 weeks obliquely aligned in the patterned scaffolds. New soft tissue was more mature and PDL-like tissue for the groups with combined patterning and gene delivery. |
| Chien 2018 et al. [131] | Injectable and thermosensitive chitosan/gelatin/glycerol phosphate hydrogel | IPCs, loading with BMP-6 | Periodontal defect model with defects on maxillary 1st molars | Sprague Dawley rats 4 weeks | Only the iPSCs-BMP-6-hydrogel group showed new bone, cementum and PDL formation |
| Farag 2018 et al. [109] | PCL melt electrospun scaffolds and electrospun PCL sheet as barrier to cover the periodontal defect | Primary hPDLCs decellularized cell sheet that enveloped the scaffold | Periodontal defect model with intrabony defects in the mandible | Athymic rats 2 and 4 weeks | PCL scaffolds: fiber orientation parallel to the root surface with few isolated areas of inserted fibers into the cementum surface. Decellularized scaffold constructs: organized fibers mostly inserted perpendicularly to the tooth surface |
| Vaquette 2019 et al. [110] | Biphasic electrospun PCL scaffold + β-TCP 20% wt | Scaffolds seeded or not with PDLCs, GCs, and BMMSCs | Periodontal defect model with defects adjacent to the 2nd pre-molar and 1st molar of the mandible | Sheeps 5 and 10 weeks | Newly formed cementum and bone, oblique PDL fiber insertion and periodontal regeneration with vascularized PDL significantly higher in the PDLCs and BMMSCs |
| Yang 2019 et al. [132] | 2D PCL nanofibers and 3D PCL nanofibrous (aligned or random) scaffolds | PDLSCs | Periodontal defect model with fenestration defects in mandibular buccal sides | Sprague-Dawley rats 3 and 6 weeks | PDL-like thick collagenous tissue, well aligned and inserted into the newly formed bone for the aligned 3D scaffolds |
| He 2019 et al. [133] | Transglutaminase crosslinked gelatin hydrogel (TG-gel) | No cells Interleukin IL-4 stromal cell-derived factor SDF-1a | Periodontal defect model with defects on 2nd molars | Sprague-Dawley rats 1, 4, and 8 weeks | Newly formed and oriented PDL, new bone and new cementum in all hydrogel groups. The presence of two cytokines provided the best outcome |
| Wang 2020 et al. [134] | nHA/BFGF composite scaffold | No cells Geistlich bio-Gide (GBG) membrane | Periodontal defect model with defects in root bifurcation area of premolars | Dogs 6 weeks | More new bone, cementum and PDL formation for the nHAC/BFGF/GBG implantation group |
| Huang 2020 et al. [13] | Biphasic scaffold: gelatin and β-TCP/HA particles (BH) and biphasic cryogel scaffold (BCS) | No cells. BMP-2 infusion in scaffolds and EMD on root surface prior to implantation | Periodontal defect model with two-walled intrabony defects on mandibular 2nd and 4th premolars | Beagle dogs 12 weeks | Cementum with interposing Obliquely inserted ligament-like fibers to the newly formed bone. The functionally graded membrane provided additional limited benefit |
| Ding 2020 et al. [135] | Composite PLLA-PLGA fibrous scaffolds through coaxial electrospinning of core and shell solutions | No cells | Intrabony bone defects distal to the front of the mandible, 1 mm apical to the alveolar bone crest | Wistar rats 1, 2, 4, and 8 weeks | New PDL formation with similar angulation with the natural PDL and in general in situ cementum–ligament–bone complex regeneration with the growth factors (bFGF and BMP-2) loaded scaffolds |
| Shang 2021 et al. [136] | PLGA fibrous membranes incorporating DMOG and nanosilicate (nSi) | No cells | Intrabony defects in the mandible | Wistar rats 1, 2, 4, 8 weeks | Comparative angulation of fiber orientation of the developed PDL to native PDL and thicker cementum formation |
| Daghrery 2021 et al. [12] | PCL scaffolds were fabricated via Melt ElectroWriting (MEW) and were subsequently applied to F/CaP coating process | No cells | Periodontal defect model with fenestration defects bilaterally in the mandible. | Fischer 344 rats 3 and 6 weeks | Bone formation after 3 and 6 weeks significantly enhanced in F/CaP-coated scaffolds. Regeneration of new alveolar bone, cementum, and PDL even after 3 weeks and connective tissue fibers orientation similar to normal PDL |
| Yu 2022 et al. [11] | Bilayer construct: self-assembly and microstamping strategies IMC scaffold with CGF | No cells | Periodontal defect model with fenestration defects in mandibular 1st molars | Sprague-Dawley rats 8 weeks | Regeneration of both mineralized (cementum and bone) and non-mineralized soft connective tissues (PDL) with structure and fibrous orientation similar to normal PD. |
| Author, Year | Type of Scaffold | Research Type | Experimental Groups | Number of Subjects, Term | Outcome Measurements | Results |
|---|---|---|---|---|---|---|
| McGuire & Scheyer 2006 [161] | β-TCP in combination with rh-PDGF-BB and collagen membrane | Clinical case series | Patients with recession defects > 3 mm, in contralateral quadrants of maxilla, excluding molars: (1) rhPDGF-BB and collagen membrane and β-TCP (2) subepithelial connective tissue graft (CTG) | 7 patients, up to 24 weeks | Clinical measurements of recession depth | The use of new graft material has comparable results to CTG method in treatment of gum recession. |
| Sarment et al., 2006 [162] | β-TCP in combination with rh-PDGF-BB | Clinical study | Patients with vertical bone defects: (1) β-TCP (active control n = 15), (2) β-TCP + 0.3 mg/mL of rhPDGF-BB (n = 14), or (3) β-TCP + 1.0 mg/mL of rhPDGF-BB (n = 18). | 47 patients, 24 weeks | Wound fluid analysis by radioimmunoassay for pyridinoline crosslinked carboxyterminal telopeptide of type I collagen (ICTP) | Increase in the amount of ICTP up to 6 weeks was detected in the 0.3 and 1.0 mg/mL PDGF-BB treatment groups, indicating bone turnover |
| McGuire et al., 2006 [163] | rhPDGF-BB with synthetic β-TCP | Clinical case series | Group 1: sites treated with 0.3 mg/mL rhPDGF-BB + β-TCP. Group 2: beta-TCP with buffer solution (control) | 4 patients, 24 months | Clinical and radiographic parameters | Significant improvements of clinical and radiographic parameters in sites treated with rhPDGF-BB + β-TCP. |
| Bhongade & Tivari, 2007 [164] | Type-I collagen and cell binding peptide (P-15) with anorganic bovine matrixABM | Clinical study | Test group: OFD with a bovine-derived xenograft enriched with a cell binding peptide P-15, Control group: only OFD | 20 interproximal intraosseous defects in 16 patients, 6 months | Clinical and radiographic assessments | Experimental group demonstrated significantly increased mean defect fill. |
| McGuire et al., 2009 [165] | β-TCP in combination with rh-PDGF-BB and collagen dressing | Randomized control trial | Patients with Miller Class II buccal gingival recession, >3 mm: (1) rhPDGF-BB and collagen dressing and β-TCP, (2) subepithelial connective tissue graft (CTG) | 30 patients, 6 months | Histologic/micro-CT | Evidence of regeneration of bone, cementum and PDL with connective tissue fibers insertion, whereas neither CTG-treated site exhibited any signs of periodontal regeneration |
| Jayakumar et al., 2011 [166] | rhPDGFBB and β-TCP | Multi-centre, randomized clinical trial | Two groups with moderate and advanced periodontitis: (1) β-TCP graft with rhPDGF-BB (n = 27) (2) β-TCP (control, n = 27). | 54 patients, 3 and 6 months | Clinical and radiographic parameters (linear bone growth (LBG) and percent bone fill (%BF) | Significantly higher linear bone growth and percent bone fill in the experimental group |
| Nevins et al., 2013 [167] | β-TCP scaffold matrix and PDGF-BB | Multicenter, randomized, controlled clinical trial | Three groups: (1) (β-TCP) (scaffold) with sodium acetate buffer alone; (2) β-TCP with 0.3 mg/mL rhPDGF-BB; and (3) β-TCP with 1.0 mg/mL rhPDGF-BB in patients with advanced periodontal defects. | 135 patients, 36 months | Clinical and radiographic evaluation | rhPDGF-BB at 0.3 mg/mL resulted in significantly greater clinical and radiographic finfings, in moderate to severe 2- and 3-wall periodontal intrabony defects |
| Maroo & Murthy, 2014 [168] | β-TCP in combination with rh-PDGF-BB | Randomized clinical trial | 30 sites were randomly divided into test group (β-TCP) in with rh-PDGF-BB and control group-only β-TCP | 15 patients, 9 months | Clinical and radiographic examination | Sites with rhPDGF + β-TCP demonstrated significantly greater reduction of pocket depth and gain in clinical attachment level. Significantly higher amount and percentage of defect fill in test sites. |
| Rasperini et al., 2015 [169] | 3D printed PCL and 4% of HA scaffold. Internal part: pegs for PDL guidance and compartment for rhPDGF-BB delivery | Clinical case report | Scaffold implantation in region of #43 tooth | 1 patient, 14 months | Clinical examination | The scaffold remained covered for 12 months but was removed after exposure after 13 months. Clinical partial root coverage and 3 mm attachment gain were observed. No signs of chronic inflammation or dehiscence. |
| Hamzacebi et al., 2015 [170] | PRFmembrane and plug | Clinical study | Two groups: (1) patients who recieved PRF scaffold and (2) (control) patients who received only the access flap. | 19 patients with peri-implant bone loss, 6 months | Clinical assessment | Significantly higher mean reduction of probing depth and clinical attachment gain compared to the control. |
| Kitamura et al., 2016 [171] | Hydroxypropyl cellulose with rhFGF-2 | Multicenter, randomized clinical trial | Study A. Patients with advanced periodontitis received 0.3% rhFGF-2 or Placebo after flap surgery Study B. Patients received rhFGF-2, enamel matrix derivative (EMD) therapy, or flap surgery. | Study A: 328 patients Study B: 274 patients, 36 weeks | Serum antibodies measurement, clinical and radiographic data | Study A: significantly higher percentage of bone fill in the rhFGF-group, no significant differences in clinical attachment level between groups. Study B: significantly higher linear alveolar bone growth in rhFGF-2 group as compared to EMD group in the EMD group, efficacy of rhFGF-2 treatment in smokers. |
| Cochran et al., 2016 [172] | β-TCP loaded with rhFGF-2 | Double-blinded, dose-verification, externally monitored clinical study | Patients with vertical bone defects: 1 Group: β-TCP alone (control) 2 Group: β-TCP + 0.1% rh-FGF-2, 3 Group: β-TCP + 0.3% rh-FGF-20.3% 4 Group: β-TCP + 0.4% rh-FGF-2 | 88 patients, 6 months | Clinical and radiographic evaluation | Groups 3 and 4 showed significant clinical improvements as compared to others. |
| Naineni et al., 2016 [173] | β-TCP loaded with Alendronate (ALN), | Randomized prospective clinical study | Patients with vertical periodontal defects (>4 mm): (1) 400 μg ALN + β-TCP + Saline (test) and (2) β-TCP + Saline (active-control). | 32 patients, 6 months | Clinical and radiographic evaluation | Experimental scaffold improved soft tissue parameters, inhibited alveolar crestal resorption and enhanced bone formation, compared to β-TCP |
| Khan et al., 2017 [174] | Tinidazole (TNZ) functionalized biodegradable chitosan/PCL mucoadhesive hybrid nanofiber membrane | Preliminary clinical trial | 3 periodontal sites in patients with chronical periodontitsis: (1) scaling and root planning; (2) placebo and fiber; (3) medicated nanofiber | 10 patients, 8 weeks | Clinical examination | Significant decrease in clinical markers of periodontitis in the experimental group |
| Lee et al., 2017 [175] | Equine-derived bone matrix vs. β-TCP with rh-PDGF-BB | A single blinded comparative study | Patients with advanced periodontitis: (1) rhPDGF-BB + equine-derived bone matrix, (2) rhPDGF-BB + β-TCP (control) | 32 patients, 6 months | Clinical examination and X ray | Group 1 showed significant CAL gain. No statistically significant change in radiographic bone level betweengroups. |
| Deshoju et al., 2017 [176] | Zn-substituted monetite-based scaffold | Randomized controlled clinical trial (split-mouth, double-blind) | Patients with chronical periodontitis and vertical bone loss: (1) open flap debridement (OFD) + Sil-Oss®, (2) OFD + HA bone graft (control). | 30 patients, 9 months | Clinical and radiographic analysis. Histological evaluation after 7 months (bone biopsy) | No significant differences in clinical parameters between groups after 6 months and significant increase in bone fill of the experimental material as compared to HA. |
| Kizildag et al., 2018 [177] | Leukocyte-PRF membrane | Randomized controlled trial | Patients with chronic periodontitis with horizontal bone loss, were treated by OFD alone (control) or L-PRF + OFD. | 16 patients, 6 months | Clinical examination and biochemical detection of levels of growth factors in gingival crevicular fluid | Significantly higher PD reduction and CAL gain were observed in the L-PRF treated sites and increased BMP-2 and IGF-1 at 2 weeks. |
| Saito et al., 2019 [178] | (rhFGF)-2 REGROTH® with deproteinized bovine bone mineral (DBBM) | Randomized clinical trial | Patients with moderate to severe chronic periodontitis: (1) 0.3% rhFGF-2 + DBBM (n = 22) and (2) rhFGF-2 alone (n = 22, control). | Number of patients not stated 6 months | Clinical and radiographic parameters | No significant difference in clinical attachment gain was observed, improved radiographic outcome in Group 1. |
| Lee et al., 2020 [179] | EMD in combination with demineralized porcine bone matrix (DPBM) | Randomized controlled clinical trial. | Patients with one-wall intrabony defects in the molar regions: (1) DPBM + EMD (n = 20), (2) DPBM (control, n = 22). | 42 patients, 24 months | Clinical and radiographic parameters | No severe adverse effects, no statistically significant differences between groups, better wound healing in Group 1. |
| Shoukheba et al., 2021 [180] | β-TCP gelatin sponge soaked in CGF | Randomized clinical trial | Patients with moderate and severe periodontitis: (1) surgery plus biodegradable gelatin/β-TCP sponges, (control group, n = 10), (2) gelatin/β-TCP sponges socked in CGF(n = 10). | 20 patients, 6 months | Clinical examination, CBCT (bone defect area and density) | Investigated scaffolds enhanced the outcome of periodontal regeneration, as evidenced by improved bone density and reduction in the defect area. |
| Mangano et al., 2021 [181] | 30% HA-70% β-TCP BCP 3D-printed scaffold, | Case report | Implantation of 3D-printed biphasic-HA block and implant placement in the area of #15 (unloaded) in 2013 and bridge placement in the area 14–17 | 1 patient, 7 years | Micro CT and histomorphometrical analyses. | Regeneration of lamellar bone, scaffold integration and signs of scaffold degragation. 57% of newly formed bone detected by micro-CT. |
| Deshpante et al., 2021 [182] | ECM component—natural collagen to nHA bone graft | Randomized controlled clinical study | Group 1: nHA + natural collagen (20 sites) Group 2: nHA with natural collagen and Group B: nHA (20 sites) | 40 patients, 3 and 6 months | Clinical and radiographic evaluation. | Statistically significant improvement in clinical attachment indexes was noted in Group A after 3 months, while the results were similar in both groups after 6 months. |
| Venkatesan et al., 2021 [183] | Amniotic membrane or porcine collagen membrane with Biphasic calcium phosphate BCP(60% HA 40% β TCP). | Randomized clinical study | Patients with localized moderate to severe periodontitis: (1) Collagen membrane + BCP and (2) Amniotic membrane + BCP. | 50 patients, 6 months |
Radiographic bone fill and clinical measurements | No statistically significant difference between the groups |
| Author, Year | Type of Scaffold | Type of Cells | Research Type | Experimental Groups | Number of Subjects, Term | Outcome Measurements | Results |
|---|---|---|---|---|---|---|---|
| Mohammadi et al., 2007 [195] | Collagen gel | hGF | Climical study | hGF from attached gingiva was added to collagen gel. Each patient: 1 tooth treated with a periosteal fenestration technique (control group) or a tissue engineered mucosal graft (test group). | 9 patients (18 sites), 3 months | Clinical parameters: width of keratinized tissue, probing depth, and width of attached gingiva | The mean amount of attached gingiva was significantly higher at test sites than at control sites. |
| Jhaveri et al., 2010 [198] | Acellular dermal matrix allograft (ADMA) seeded with autologous GFs | autologous hGF | Split-mouth, controlled, double-masked clinical case series | Patients with Class I or II recessions of maxillary canines or premolars: subepithelial connective tissue graft (control group) or an ADMA seeded with autologous GFs (test group). | 10 patients, 3 and 6 months | Clinical parameters, healing time and inflammation were assessed. | No significant differences between test and control sites. The test sites showed less inflammation in the early postoperative period |
| Dhote et al., 2016 [188] | (β-TCP) in combination with rh-PDGF-BB | Umbical cord MSCs | Randomized Clinical trial | The control group (n = 12 sites) was treated by an open flap debridement (OFD) only, while the test group (n = 12 sites) was treated by a MSCs cultured on β-TCP in combination with rh-PDGF-BB. | 14 patients with moderate to advanced periodontitis, 6 months | Clinical measurements and radiographic analysis | Significant improvrmrnts in clinical parameters & greater radiographic defect depth reduction and defect fill in test group. |
| Chen et al., 2016 [191] | Bio-Oss | Autologous PDLSCs | Single center, randomized clinical trial | Group 1: GTR and PDLSC + Bio-oss® and Group 2: GTR + Bio-oss® (control group). | 30 patients, 12 months | Clinical and radiographic evaluation | No statistically significant differences were detected between groups. |
| Baba et al., 2016 [190] | biodegradable three-dimensional (3D) woven fabric PLLA resin scaffold and PRP | Iliac bone marrow autologous MSC | phase I/II clinical study | Implantation of scaffolds with MSC and PRP, no control group | 10 patients, 36 months | Clinical parameters, laboratory tests of blood and urine samples | Improvement of all clinical parameters during the entire follow-up period. |
| Aramoon et al., 2017 [197] | PRGF | Autologous hGF | Pilot clinical study | Patients with gingival recession: (1) Periosteal fenestration on one side (control) and (2) tissue-engineered mucosal graft (test) | 4 patients (8 sites), 3 months | Probing depth (PD), width of keratinized and attached gingiva | Significantly incerased width of keratinized gingiva in test group. |
| Iwata et al., 2018 [8] | β-TCP | Autologous PDL-erived cell sheets | A single-arm and single-institute clinical study; | 3-layered PDL-derived cell sheets were fabricated and applied to bone defects with β-TCP granules. | 10 patients, 6 months and 55 ± 19 months follow up | Clinical examination and CBCT | Improvement of clinical parameters and increase of bone height. |
| Hernandez-Monjaraz et al., 2018 [199] | lyophilized collagen-polyvinylpyrrolidone sponge | MSCs from dental pulp of a deciduous tooth | Case report | Tooth #35 with pocket depth 6.5 mm and II stage of mobility, underwent flap surgery and scaffold placement | 1 patient, 6 months | Clinical and radiographic evaluation | Decrease in tooth mobility, periodontal pocket depth and bone defect area. |
| Ferrarotti et al., 2018 [189] | Collagen sponge | Autologous DPSCs | Randomized controlled trial | Patients with severe periodontitis: (1) DPSC micrografts seeded onto collagen sponge (n = 15), (2) collagen sponge alone (n = 14, control). | 29 patients, 12 months | Clinical and radiographic evaluation | Significantly greater clinical attachment level gain and bone defect fill in test groups. |
| Abdal-Wahab et al., 2020 [62] | β TCP | Autogenous hGF and associated mesenchymal stem cells (GMSC) | Randomized controlled clinical trial and biochemical study | Patients with advanced periodontitis: (1) β-TCP +collagen membrane (n = 10), (2) β-TCP scaffold with seeded GF and collagen membrane (n = 10) | 20 patients, 6 months | Clinical and CBCT examination, quantitative measurement of PDGF-BB and BMP-2 in gingival crevicular fluid. | Significant improvements in clinical measurements in the test group. Statistically higher radiographic bone gain in the test group and higher concentration of PDGF-BB on days 1, 3, and 7. |
| Kashte et al., 2020 [200] | PCL-GO-Cissus quadrangularis (CQ)(PCL-GO-CQ) scaffold | Human umbilical cord Wharton’s jelly derived MSCs | Case report | Multiple gingival recessions (Miller’s class II) | 1 patient, 2 months | Clinical examination | Significant reduction of gingival recession with over 70% of root coverage |
| Sanchez et al., 2020 [192] | Xenogeneic bone substitute (XBS) | PDL-MSCs | quasi-randomized controlled pilot clinical trial | Patients with moderate and severe chronic periodontitis with one or two wall defects: (1) XBS + PDLMSCs and (2) XBS (control) | 20 patients, 12 months | Clinical and radiographic evaluation | No significant differences between groups, low morbidity, and safety of cell-based therapyll-based therapy |
| Apatzidou et al., 2021 [193] | Collagen scaffolds with autologous fibrin/platelet lysate (aFPL). | Autologous alveolar bone marrow MSCs | A proof-of-principle randomized clinical study | Group-1 (n = 9) BMMSCs seeded into collagen scaffolds, and aFPL. Group-2 (n = 10), the collagen scaffold/aFPL seeded with a BMMSCs Group-3 (n = 8) no scaffold, minimal access flap surgery | 27 subjects with advanced periodontitis, 12 months | Radiographic bone fill and clinical measurements | Significant clinical improvements with no inter-group differences Better clinical outcomes in Groups 1 and 3, over 2nd. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousnaki, M.; Beketova, A.; Kontonasaki, E. A Review of In Vivo and Clinical Studies Applying Scaffolds and Cell Sheet Technology for Periodontal Ligament Regeneration. Biomolecules 2022, 12, 435. https://doi.org/10.3390/biom12030435
Bousnaki M, Beketova A, Kontonasaki E. A Review of In Vivo and Clinical Studies Applying Scaffolds and Cell Sheet Technology for Periodontal Ligament Regeneration. Biomolecules. 2022; 12(3):435. https://doi.org/10.3390/biom12030435
Chicago/Turabian StyleBousnaki, Maria, Anastasia Beketova, and Eleana Kontonasaki. 2022. "A Review of In Vivo and Clinical Studies Applying Scaffolds and Cell Sheet Technology for Periodontal Ligament Regeneration" Biomolecules 12, no. 3: 435. https://doi.org/10.3390/biom12030435
APA StyleBousnaki, M., Beketova, A., & Kontonasaki, E. (2022). A Review of In Vivo and Clinical Studies Applying Scaffolds and Cell Sheet Technology for Periodontal Ligament Regeneration. Biomolecules, 12(3), 435. https://doi.org/10.3390/biom12030435