Male Infertility in the XXI Century: Are Obesogens to Blame?
Abstract
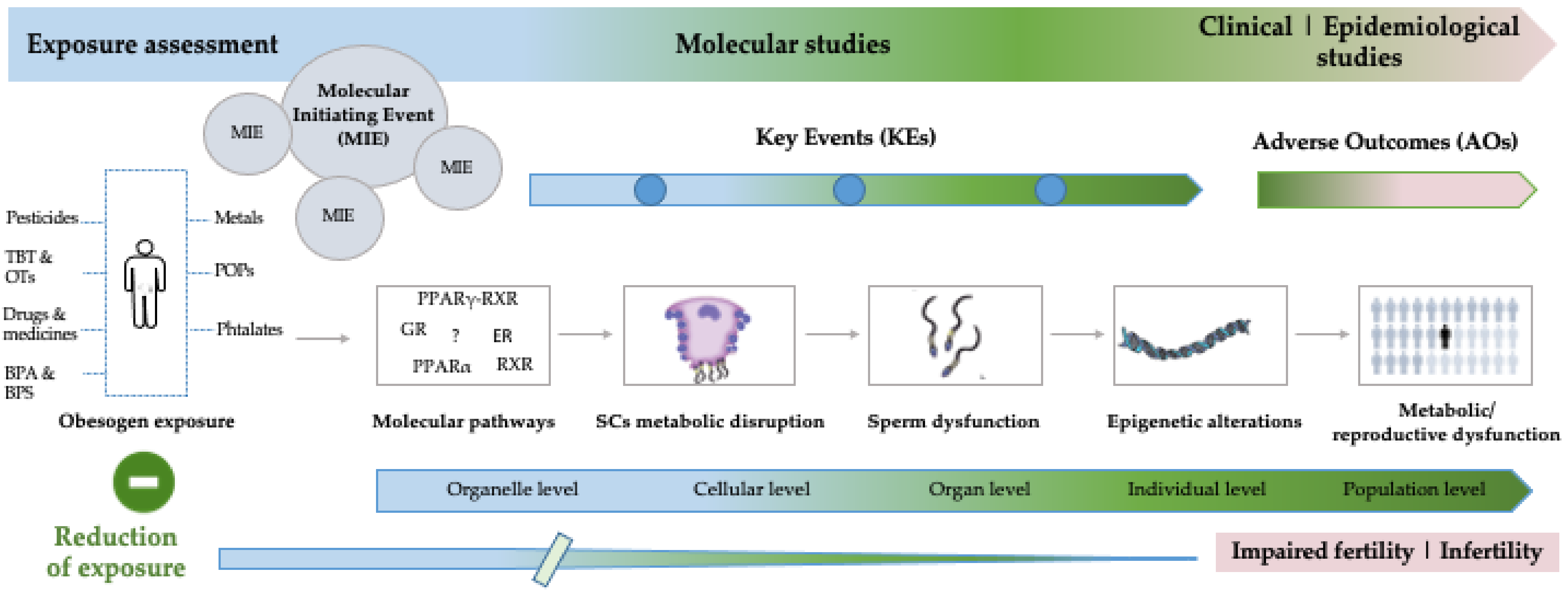
:1. Introduction
2. A Brief Overview of Testicular Metabolism
3. Molecular Connections between Obesogen Toxicity and Testicular Metabolic Pathways: From Fundamental to Clinical Evidence
4. Evaluation of Obesogen Toxicity in the XXI Century
4.1. In Silico Models
4.2. In Vitro and Ex Vivo Models
5. Gaps and Future Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Grün, F.; Blumberg, B. Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 2006, 147, s50–s55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrempft, S.; van Jaarsveld, C.H.M.; Fisher, A.; Herle, M.; Smith, A.D.; Fildes, A.; Llewellyn, C.H. Variation in the Heritability of Child Body Mass Index by Obesogenic Home Environment. JAMA Pediatr. 2018, 172, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Needleman, H.L.; Gunnoe, C.; Leviton, A.; Reed, R.; Peresie, H.; Maher, C.; Barrett, P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 1979, 300, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Bergman, Å; Heindel, J.J.; Jobling, S.; Kidd, K.; Zoeller, T.R.; World Health Organization, United Nations Environment Programme, Inter-Organization Programme for the Sound Management of Chemicals. State of the Science of Endocrine Disrupting Chemicals 2012: Summary for Decision-Makers; World Health Organization: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/handle/10665/78102 (accessed on 8 March 2022).
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- OCDE. Guidance Document on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; OCDE: Paris, France, 2014.
- OCDE. Revised Guidance Document 150 on Standardised Test Guidelines for Evaluating Chemicals for Endocrine Disruption; OCDE series on Testing and Assessment, No. 150; OECD Publishing: Paris, France, 2018. [CrossRef]
- Oliveira, P.F.; Cheng, C.Y.; Alves, M.G. Emerging Role for Mammalian Target of Rapamycin in Male Fertility. Trends Endocrinol. Metab. 2017, 28, 165–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roulet, V.; Denis, H.; Staub, C.; Le Tortorec, A.; Delaleu, B.; Satie, A.P.; Patard, J.J.; Jegou, B.; Dejucq-Rainsford, N. Human testis in organotypic culture: Application for basic or clinical research. Hum. Reprod. 2006, 21, 1564–1575. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, A.M.; Alves, M.G.; Sousa, A.C.; Jarak, I.; Carvalho, R.A.; Oliveira, P.F.; Cavaco, J.E.; Rato, L. The effects of the obesogen tributyltin on the metabolism of Sertoli cells cultured ex vivo. Arch. Toxicol. 2018, 92, 601–610. [Google Scholar] [CrossRef]
- Mitra, S.; Srivastava, A.; Khandelwal, S. Tributyltin chloride induced testicular toxicity by JNK and p38 activation, redox imbalance and cell death in sertoli-germ cell co-culture. Toxicology 2013, 314, 39–50. [Google Scholar] [CrossRef]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg Effect Revisited—Lesson from the Sertoli Cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, M.; Gupta, G.; Setty, B. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtens, J.L.; Ploen, L. Improvement of spermatogenesis in adult cryptorchid rat testis by intratesticular infusion of lactate. Biol. Reprod. 1999, 61, 154–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erkkila, K.; Aito, H.; Aalto, K.; Pentikainen, V.; Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Hum. Reprod. 2002, 8, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, M.G.; Socorro, S.; Silva, J.; Barros, A.; Sousa, M.; Cavaco, J.E.; Oliveira, P.F. In vitro cultured human Sertoli cells secrete high amounts of acetate that is stimulated by 17beta-estradiol and suppressed by insulin deprivation. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1389–1394. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.P.; Wang, H.K.; Wu, H.; Chen, Y.M.; Han, D.S. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 2009, 137, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, G.R.; Monteiro, S.C.; Gelain, D.P.; Souza, L.F.; Perry, M.L.; Bernard, E.A. Metabolism of amino acids by cultured rat Sertoli cells. Clin. Exp. 2005, 54, 515–521. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Duarte, A.I.; Santos, M.S.; Moreira, P.I.; Cavaco, J.E.; Oliveira, P.F. Testosterone deficiency induced by progressive stages of diabetes mellitus impairs glucose metabolism and favors glycogenesis in mature rat Sertoli cells. Int. J. Biochem. Cell Biol. 2015, 66, 1–10. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Pu, Y.; Gingrich, J.; Padmanabhan, V. Obesogenic Endocrine Disrupting Chemicals: Identifying Knowledge Gaps. Trends Endocrinol. Metab. 2018, 29, 607–625. [Google Scholar] [CrossRef]
- Bardot, O.; Aldridge, T.C.; Latruffe, N.; Green, S. PPAR-RXR heterodimer activates a peroxisome proliferator response element upstream of the bifunctional enzyme gene. Biochem. Biophys. Res. Commun. 1993, 192, 37–45. [Google Scholar] [CrossRef]
- Comninos, A.N.; Jayasena, C.N.; Dhillo, W.S. The relationship between gut and adipose hormones, and reproduction. Hum. Reprod. Update 2014, 20, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Neuhaus-Oliveira, A.; Moreira, P.; Socorro, S.; Oliveira, P. Exposure to 2, 4-dichlorophenoxyacetic acid alters glucose metabolism in immature rat Sertoli cells. Reprod. Toxicol. 2013, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Cavaco, J.E.; Oliveira, P.F. High-energy diets: A threat for male fertility? Obes. Rev. 2014, 15, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Sousa, M.; Silva, B.M.; Monteiro, M.P.; Alves, M.G. Obesity, energy balance and spermatogenesis. Reproduction 2017, 153, R173–R185. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Sharma, P.M.; Mistry, D.S.; Chang, R.J.; Olefsky, J.M.; Mellon, P.L.; Webster, N.J. PPARG regulates gonadotropin-releasing hormone signaling in LbetaT2 cells in vitro and pituitary gonadotroph function in vivo in mice. Biol. Reprod. 2011, 84, 466–475. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Alves, M.G.; Mathur, P.P.; Oliveira, P.F.; Cavaco, J.E.; Rato, L. Obesogens and male fertility. Obes Rev. 2017, 18, 109–125. [Google Scholar] [CrossRef] [Green Version]
- Aquila, S.; Bonofiglio, D.; Gentile, M.; Middea, E.; Gabriele, S.; Belmonte, M.; Catalano, S.; Pellegrino, M.; Ando, S. Peroxisome proliferator-activated receptor (PPAR)gamma is expressed by human spermatozoa: Its potential role on the sperm physiology. J. Cell. Physiol. 2006, 209, 977–986. [Google Scholar] [CrossRef]
- Wang, C.; Yang, L.; Wang, S.; Zhang, Z.; Yu, Y.; Wang, M.; Cromie, M.; Gao, W.; Wang, S.-L. The classic EDCs, phthalate esters and organochlorines, in relation to abnormal sperm quality: A systematic review with meta-analysis. Sci. Rep. 2016, 6, 19982. [Google Scholar] [CrossRef]
- Shi, M.; Sekulovski, N.; MacLean, I.I.J.A.; Hayashi, K. Prenatal Exposure to Bisphenol A Analogues on Male Reproductive Functions in Mice. Toxicol. Sci. 2018, 163, 620–631. [Google Scholar] [CrossRef] [Green Version]
- National Research Council. Toxicity Testing in the 21st Century: A Vision and a Strategy; National Academies Press: Washington, DC, USA, 2007. [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef]
- Małgorzata, N.; Jenny, O.; Sharon, M. Adverse Outcome Pathway: Peroxisome Proliferator-Activated Receptor α Activation and Reproductive Toxicity—Development and Application in Assessment of Endocrine Disruptors/Reproductive Toxicants. Appl. Vitr. Toxicol. 2017, 3, 234–249. [Google Scholar] [CrossRef]
- Platts, A.E.; Dix, D.J.; Krawetz, S.A. Considerations When Using Array Technologies for Male Factor Assessment. In The Genetics of Male Infertility; Carrell, D.T., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 37–54. [Google Scholar]
- Harada, S.; Hiromori, Y.; Nakamura, S.; Kawahara, K.; Fukakusa, S.; Maruno, T.; Noda, M.; Uchiyama, S.; Fukui, K.; Nishikawa, J.-I.; et al. Structural basis for PPARγ transactivation by endocrine-disrupting organotin compounds. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- le Maire, A.; Grimaldi, M.; Roecklin, D.; Dagnino, S.; Vivat-Hannah, V.; Balaguer, P.; Bourguet, W. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep. 2009, 10, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.A.; Tanabe, S.; Pastorinho, M.R. Organotins: Sources and Impacts on Health and Environment. In Encyclopedia of the Anthropocene; Dellasala, D.A., Goldstein, M.I., Eds.; Elsevier: Oxford, UK, 2018; pp. 133–139. [Google Scholar]
- Frontera, A.; Bauzá, A. S Sn Tetrel Bonds in the Activation of Peroxisome Proliferator-Activated Receptors (PPARs) by Organotin Molecules. Chem. Eur. J. 2018, 24, 16582–16587. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshida, K.; Nozawa, S.; Yoshiike, M.; Arai, M.; Otoi, T.; Iwamoto, T.; Sato, Y.; Yoshida, K.; Nozawa, S.; et al. Establishment of adult mouse Sertoli cell lines by using the starvation method. Reproduction 2013, 145, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, R.; McKinnell, C.; Kivlin, C.; Fisher, J.; Sharpe, R.; McKinnell, C.; Kivlin, C.; Fisher, J. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef]
- Reis, M.M.; Moreira, A.C.; Sousa, M.; Mathur, P.P.; Oliveira, P.F.; Alves, M.G. Sertoli cell as a model in male reproductive toxicology: Advantages and disadvantages. J. Appl. Toxicol. 2015, 35, 870–883. [Google Scholar] [CrossRef]
- Gao, Y.; Mruk, D.D.; Cheng, C.Y. Sertoli cells are the target of environmental toxicants in the testis—A mechanistic and therapeutic insight. Expert Opin. Ther. Targets 2015, 19, 1073–1090. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Carvalho, R.A.; Cavaco, J.E.; Oliveira, P.F. Metabolic modulation induced by oestradiol and DHT in immature rat Sertoli cells cultured in vitro. Biosci. Rep. 2012, 32, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Rato, L.; Meneses, M.J.; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016, 31, 499–513. [Google Scholar]
- Yu, X.; Hong, S.; Moreira, E.G.; Faustman, E.M.J. Improving in vitro Sertoli cell/gonocyte co-culture model for assessing male reproductive toxicity: Lessons learned from comparisons of cytotoxicity versus genomic responses to phthalates. Toxicol. Appl. Pharmacol. 2009, 239, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saldutti, L.P.; Beyer, B.K.; Breslin, W.; Brown, T.R.; Chapin, R.E.; Campion, S.; Enright, B.; Faustman, E.; Foster, P.; Hartung, T. In vitro testicular toxicity models: Opportunities for advancement via biomedical engineering techniques. ALTEX 2013, 30, 353–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, K.M.; Seyler, D.E.; Durand, P.; Perrard, M.-H.; Baker, T.K.J. Use of a rat ex-vivo testis culture method to assess toxicity of select known male reproductive toxicants. Reprod. Toxicol. 2016, 60, 92–103. [Google Scholar] [CrossRef]
- Rothbauer, M.; Rosser, J.M.; Zirath, H.; Ertl, P. Tomorrow today: Organ-on-a-chip advances towards clinically relevant pharmaceutical and medical in vitro models. Curr. Opin. Biotechnol. 2019, 55, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Saglam-Metiner, P.; Gulce-Iz, S.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 686, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Komeya, M.; Kimura, H.; Nakamura, H.; Yokonishi, T.; Sato, T.; Kojima, K.; Hayashi, K.; Katagiri, K.; Yamanaka, H.; Sanjo, H.; et al. Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device. Sci. Rep. 2016, 6, 21472. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, S.C.; Jubendradass, R.; Jayakanthan, M.; Rani, S.J.A.; Mathur, P.P. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: An in vivo and in silico study. Food Chem. Toxicol. 2012, 50, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, S.C.; Jubendradass, R.; Mathur, P.P. Bisphenol A induces oxidative stress and decreases levels of insulin receptor substrate 2 and glucose transporter 8 in rat testis. Reprod. Sci. 2012, 19, 163–172. [Google Scholar] [CrossRef]
- Sai, L.; Li, X.; Liu, Y.; Guo, Q.; Xie, L.; Yu, G.; Bo, C.; Zhang, Z.; Li, L. Effects of chlorpyrifos on reproductive toxicology of male rats. Environ. Toxicol. 2014, 29, 1083–1088. [Google Scholar] [CrossRef]
- Batarseh, L.I.; Welsh, M.J.; Brabec, M.J. Effect of lead acetate on Sertoli cell lactate production and protein synthesis in vitro. Cell Biol. Toxicol. 1986, 2, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Raychoudhury, S.S.; Flowers, A.F.; Millette, C.F.; Finlay, M.F. Toxic effects of polychlorinated biphenyls on cultured rat Sertoli cells. J. Androl. 2000, 21, 964–973. [Google Scholar] [PubMed]
- Meneses, M.J.; Bernardino, R.L.; Sa, R.; Silva, J.; Barros, A.; Sousa, M.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Pioglitazone increases the glycolytic efficiency of human Sertoli cells with possible implications for spermatogenesis. Int. J. Biochem. Cell Biol. 2016, 79, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Itsuki-Yoneda, A.; Kimoto, M.; Tsuji, H.; Hiemori, M.; Yamashita, H. Effect of a hypolipidemic drug, di (2-ethylhexyl) phthalate, on mRNA-expression associated fatty acid and acetate metabolism in rat tissues. Biosci. Biotechnol. Biochem. 2007, 71, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Moss, E.J.; Cook, M.W.; Thomas, L.V.; Gray, T.J. The effect of mono-(2-ethylhexyl) phthalate and other phthalate esters on lactate production by Sertoli cells in vitro. Toxicol. Lett. 1988, 40, 77–84. [Google Scholar] [CrossRef]
- Hauser, R.; Skakkebaek, N.E.; Hass, U.; Toppari, J.; Juul, A.; Andersson, A.M.; Kortenkamp, A.; Heindel, J.J.; Trasande, L. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab. 2015, 100, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, P.; Volpe, M.G.; Lorenzetti, S.; Mantovani, A.; Notari, T.; Cocca, E.; Cerullo, S.; Di Stasio, M.; Cerino, P.; Montano, L. Human semen as an early, sensitive biomarker of highly polluted living environment in healthy men: A pilot biomonitoring study on trace elements in blood and semen and their relationship with sperm quality and RedOx status. Reprod. Toxicol. 2016, 66, 1–9. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Sundaram, R.; Sweeney, A.M.; Schisterman, E.F.; Maisog, J.; Kannan, K. Urinary bisphenol A, phthalates, and couple fecundity: The Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil. Steril. 2014, 101, 1359–1366. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.H.; Afeiche, M.C.; Gaskins, A.J.; Williams, P.L.; Petrozza, J.C.; Tanrikut, C.; Hauser, R.; Chavarro, J.E. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum. Reprod. 2015. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Kałużny, P.; Klimowska, A.; Radwan, P.; Hanke, W. Environmental levels of triclosan and male fertility. Environ. Sci. Poll. Res. 2018, 25, 5484–5490. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-J.; Yeh, C.-Y.; Chen, R.-Y.; Tzeng, C.-R.; Han, B.-C.; Chien, L.-C. Biomonitoring of blood heavy metals and reproductive hormone level related to low semen quality. J. Hazard. Mater. 2015, 300, 815–822. [Google Scholar] [CrossRef]
- Santi, D.; Vezzani, S.; Granata, A.R.M.; Roli, L.; De Santis, M.C.; Ongaro, C.; Donati, F.; Baraldi, E.; Trenti, T.; Setti, M.; et al. Sperm quality and environment: A retrospective, cohort study in a Northern province of Italy. Environ. Res. 2016, 150, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Xu, W.; Huang, Q.; Liu, L.; Tian, M.; Xia, Y.; Zhang, W.; Shen, H. Low-level environmental arsenic exposure correlates with unexplained male infertility risk. Sci. Total Environ. 2016, 571, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, X.-M.; He, D.-L.; Zou, X.-M.; Wu, C.-Q.; Guo, W.-Z.; Feng, W. Evaluation of urinary metal concentrations and sperm DNA damage in infertile men from an infertility clinic. Environ. Toxicol. Pharmacol. 2016, 45, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Smarr, M.M.; Sun, L.; Chen, Z.; Honda, M.; Wang, W.; Karthikraj, R.; Weck, J.; Kannan, K. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environ. Res. 2018, 163, 64–70. [Google Scholar] [CrossRef]
- Gabrielsen, J.S.; Tanrikut, C. Chronic exposures and male fertility: The impacts of environment, diet, and drug use on spermatogenesis. Andrology 2016, 4, 648–661. [Google Scholar] [CrossRef]
- Buck Louis, G.; Sundaram, R.; Schisterman, E.; Sweeney, A.; Lynch, C.; Gore-Langton, R.; Maisog, J.; Kim, S.; Chen, Z.; Barr, D. Persistent Environmental Pollutants and Couple Fecundity: The LIFE Study. Environ. Health Perspect. 2013, 121, 231–236. [Google Scholar] [CrossRef]
- Le Magueresse-Battistoni, B.; Vidal, H.; Naville, D. Environmental Pollutants and Metabolic Disorders: The Multi-Exposure Scenario of Life. Front. Endocrinol. 2018, 9, 582. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something from “Nothing”—Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef]
- Nelms, M.D.; Simmons, J.E.; Edwards, S.W. Adverse Outcome Pathways to Support the Assessment of Chemical Mixtures. In Chemical Mixtures and Combined Chemical and Nonchemical Stressors: Exposure, Toxicity, Analysis, and Risk; Rider, C.V., Simmons, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 177–201. [Google Scholar]
- Chamorro-García, R.; Blumberg, B. Transgenerational effects of obesogens and the obesity epidemic. Curr. Opin. Pharmacol. 2014, 19, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Chamorro-Garcia, R.; Diaz-Castillo, C.; Shoucri, B.M.; Käch, H.; Leavitt, R.; Shioda, T.; Blumberg, B. Ancestral perinatal obesogen exposure results in a transgenerational thrifty phenotype in mice. Nat. Commun. 2017, 8, 2012. [Google Scholar] [CrossRef] [Green Version]
- Chamorro-García, R.; Sahu, M.; Abbey, R.J.; Laude, J.; Pham, N.; Blumberg, B. Transgenerational Inheritance of Increased Fat Depot Size, Stem Cell Reprogramming, and Hepatic Steatosis Elicited by Prenatal Obesogen Tributyltin in Mice. Environ. Health Perspect. 2013, 121, 359–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anway, M.D.; Cupp, A.S.; Uzumcu, M.; Skinner, M.K. Epigenetic Transgenerational Actions of Endocrine Disruptors and Male Fertility. Science 2005, 308, 1466–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2018, 4, dvy016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Obesogens | Specie(s)/ Tissue(s)/Cells | Doses | Effects on Metabolism | |
|---|---|---|---|---|
| 2,4-D | Rat SCs | 100 nM, 10 µM, 1 mM | ↓GLUT3, PFK1 LDH mRNA, ↓Lactate production [25] | |
| BPA | Rat testis | 0.005, 0.5, 50, 500 µg/kg body wg/day | ↓IRS-1, ↓GLUT2 [53] ↓HEX, ↓PFK [54] | |
| CPYF | Rat testis | 0, 2.7, 5.4, 12.8 mg/kg body wg | ↑LDH [55] | |
| Lead | Rat SCs | 0.01, 0.05, 0.1 mM | ↑Lactate production [56] | |
| PCBs | Rat SCs | 10−7 M (PCB22) 10−8 M (PCB77) | ↑Lactate production [57] | |
| PIO | Rat SCs | 1, 10, 100 µM | ↑Glucose uptake ↓GLUT3 ↑Lactate production ↑LDH ↑MCT4 [58] | |
| PTLs | Rat | Testis | CE-2 diet with 2%(mass) of DEHP | ↓ACC ↑LCAD ↑3KACT [59] |
| SCs | 0.1–200 µM | ↑Pyruvate production ↑Lactate production [60] | ||
| TBT | Rat SCs | 0.1 nM, 10 nM | ↓Glucose uptake ↓Pyruvate uptake ↓GLUT1 ↓Lactate production [11] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, A.C.A.; Alves, M.G.; Oliveira, P.F.; Silva, B.M.; Rato, L. Male Infertility in the XXI Century: Are Obesogens to Blame? Int. J. Mol. Sci. 2022, 23, 3046. https://doi.org/10.3390/ijms23063046
Sousa ACA, Alves MG, Oliveira PF, Silva BM, Rato L. Male Infertility in the XXI Century: Are Obesogens to Blame? International Journal of Molecular Sciences. 2022; 23(6):3046. https://doi.org/10.3390/ijms23063046
Chicago/Turabian StyleSousa, Ana C. A., Marco G. Alves, Pedro F. Oliveira, Branca M. Silva, and Luís Rato. 2022. "Male Infertility in the XXI Century: Are Obesogens to Blame?" International Journal of Molecular Sciences 23, no. 6: 3046. https://doi.org/10.3390/ijms23063046
APA StyleSousa, A. C. A., Alves, M. G., Oliveira, P. F., Silva, B. M., & Rato, L. (2022). Male Infertility in the XXI Century: Are Obesogens to Blame? International Journal of Molecular Sciences, 23(6), 3046. https://doi.org/10.3390/ijms23063046









