Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic
Abstract
:1. Introduction
2. Results
2.1. Biochemical Analysis
Phytochemical Screening
2.2. Antioxidant and Free Radical Scavenging Activities of Garlic Extract
2.2.1. Assay for Ferric Reducing Antioxidant Power (FRAP)
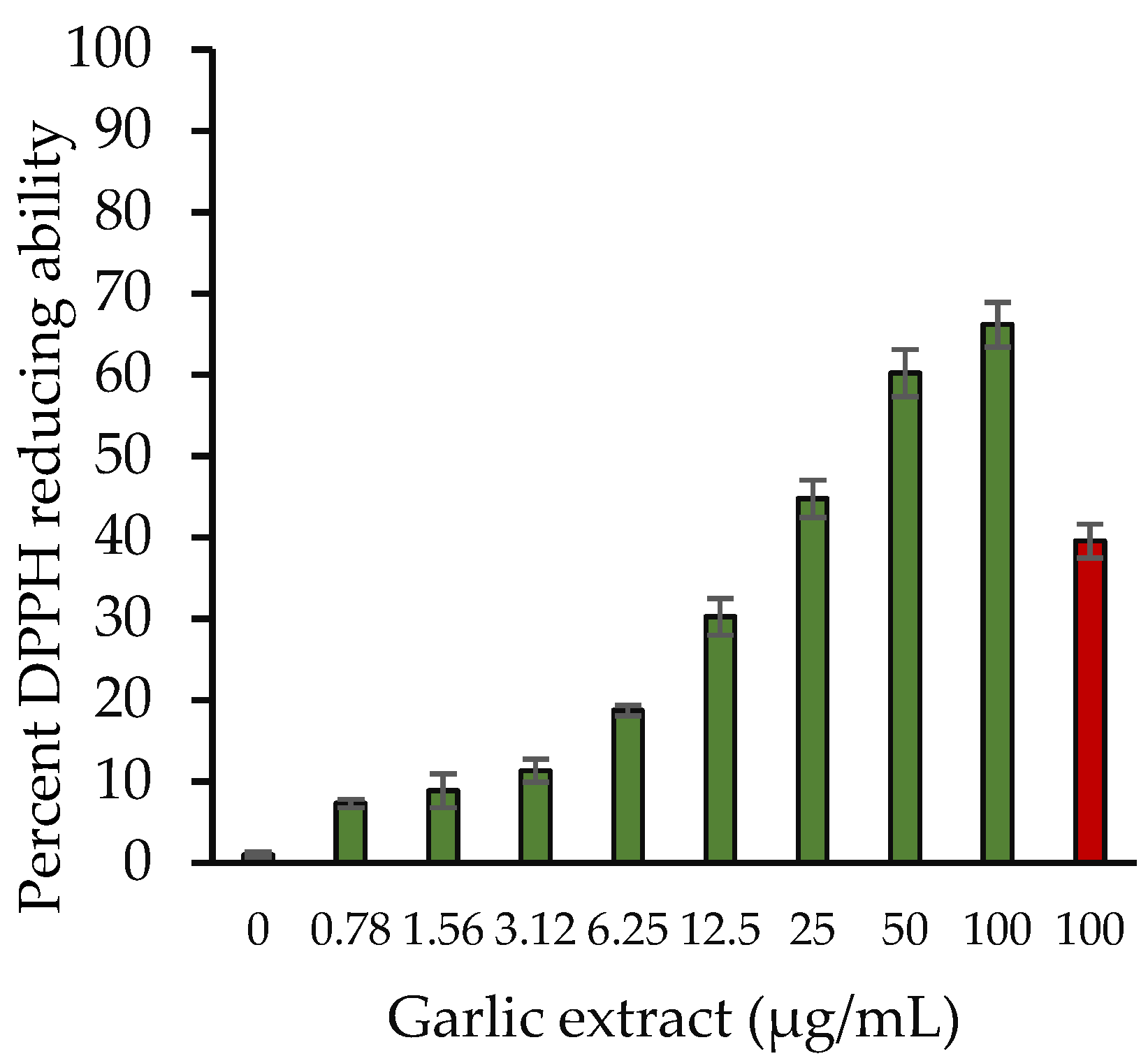
2.2.2. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
2.3. Inhibition of Structural Changes by Garlic Extract
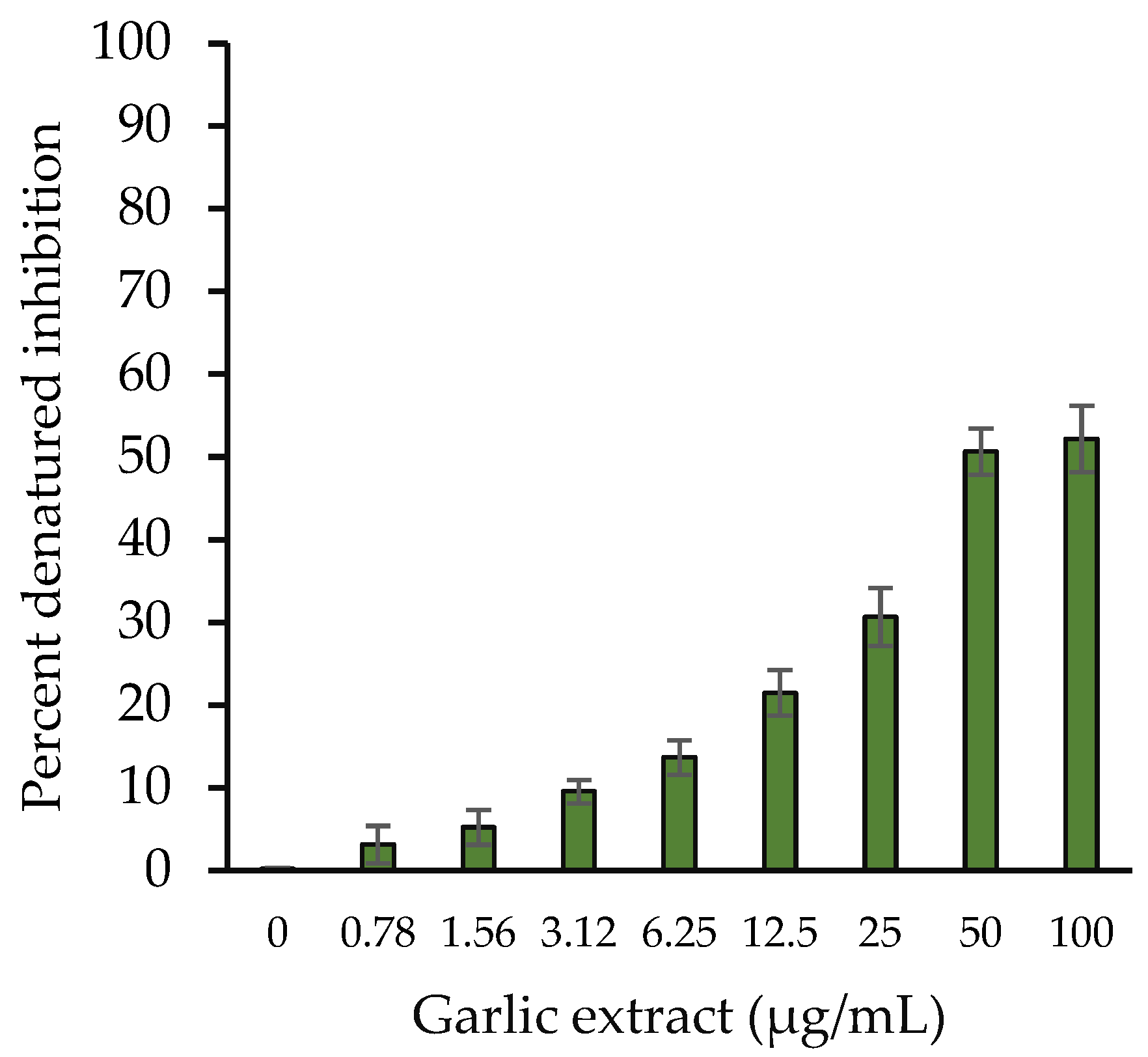
2.3.1. Protein Denaturation Inhibition
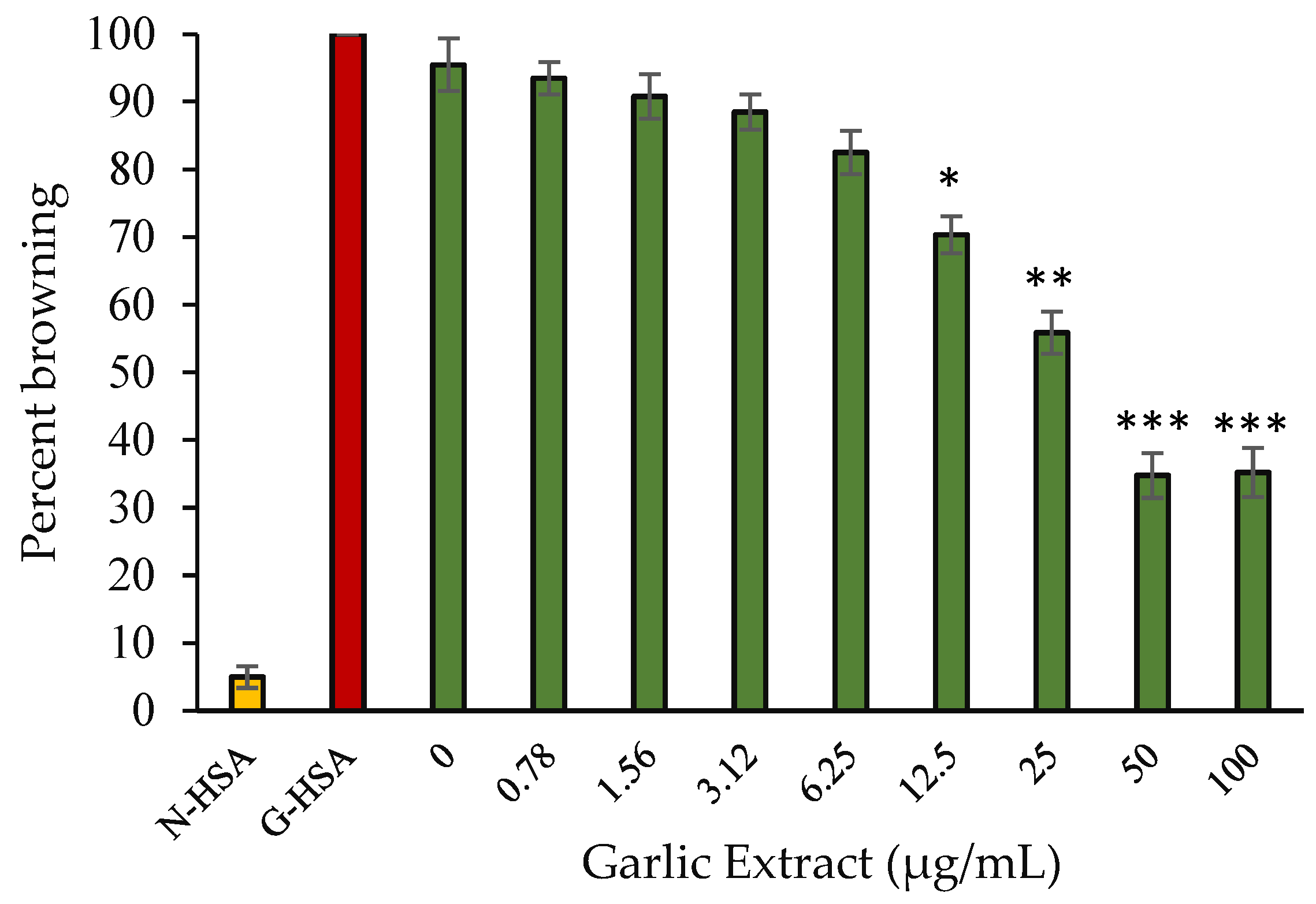
2.3.2. Protein Browning Inhibition
2.3.3. Inhibition in Protein Aggregate Formation
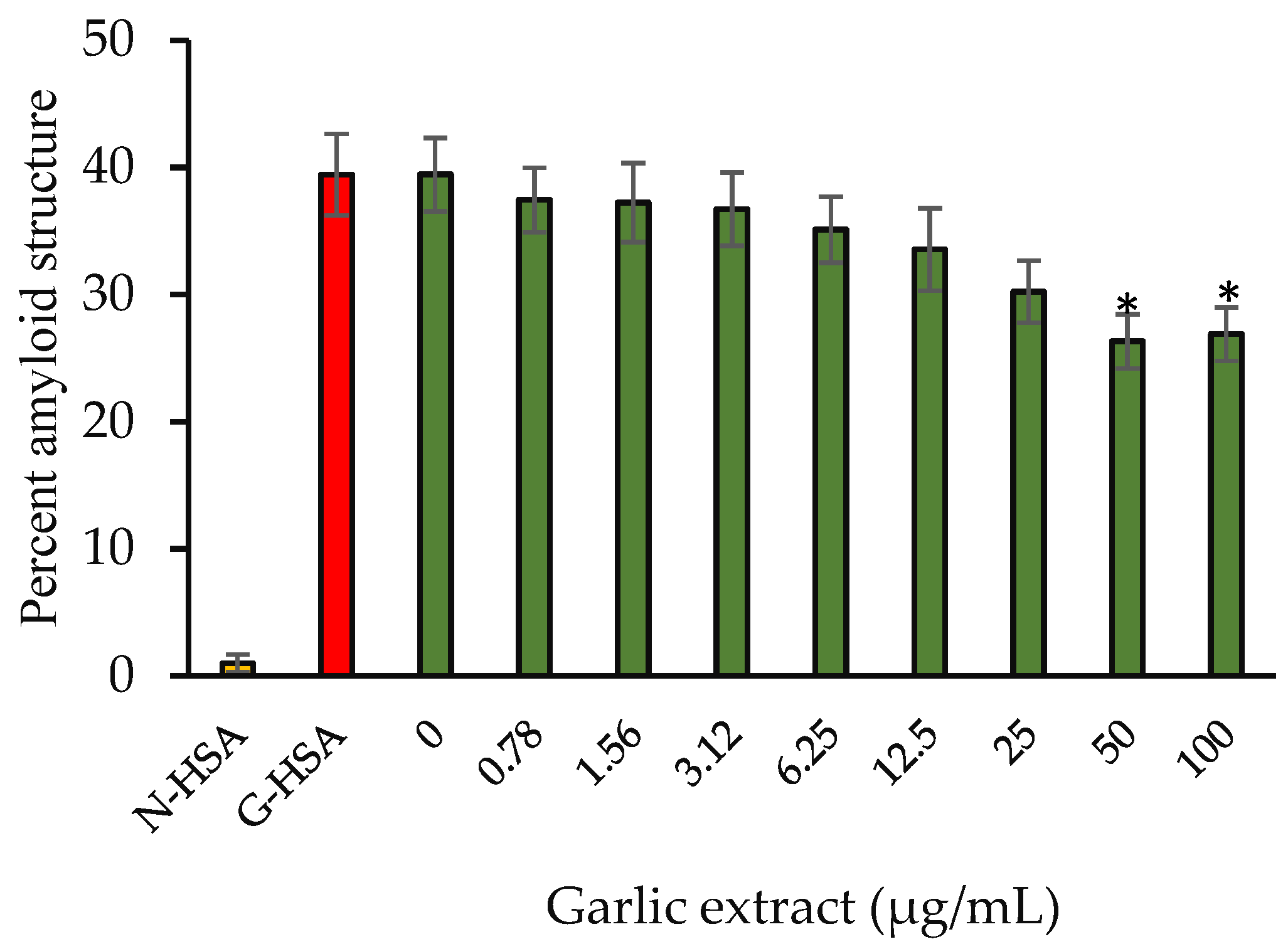
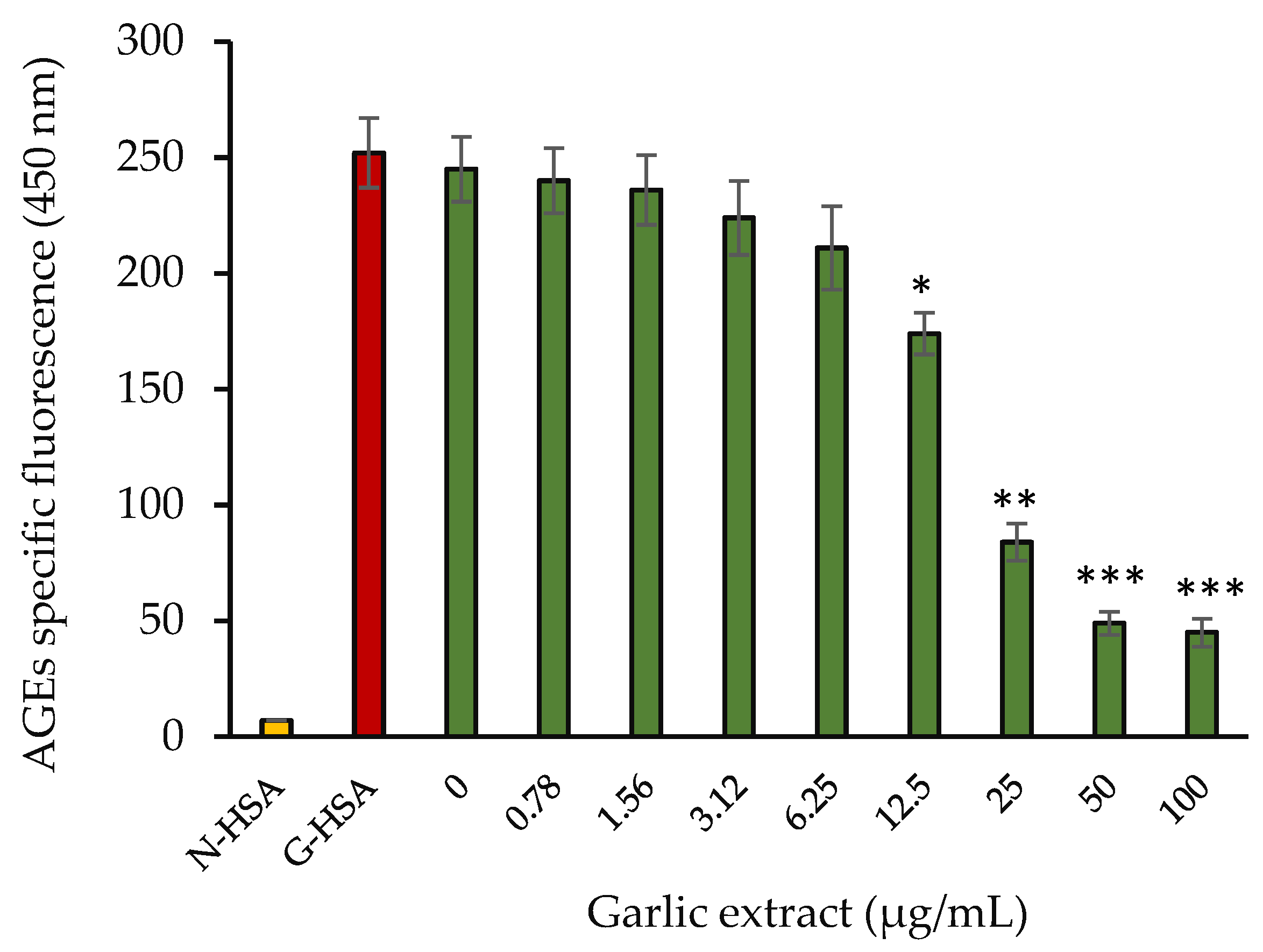
2.3.4. Amyloid Structure Inhibition
2.3.5. Spectral Studies
2.3.6. Circular Dichroism
2.4. Molecular Docking Studies for Potential Natural Product Metabolites as Inhibitors of Glycation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemical and Biological Studies
4.2.1. Preparation of Aqueous Solution of Garlic Extract
4.2.2. Qualitative and Quantitative Analysis for Flavonoids and Phenolics
4.2.3. Antioxidant and Free Radical Scavenging Activities of Extract
4.3. Modification of HSA by Glucose
4.4. Protein Denaturation Inhibition by Garlic Extract
4.5. Measurement of Browning in Glycated Samples
4.6. Effect of Garlic Extract on Protein Aggregation Index
4.7. Spectral Studies
4.8. Circular Dichroism
4.9. Structural Details of Potential Natural Product Ligands/Inhibitors of HSA Glycation
4.10. Molecular Docking and Scoring of Ligand Poses
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khan, M.W.A.; Rasheed, Z.; Khan, W.A.; Ali, R. Biochemical, biophysical, and thermodynamic analysis of in vitro glycated human serum albumin. Biochemistry 2007, 72, 146–152. [Google Scholar]
- Khan, M.W.; Al Otaibi, A.; Al-Zahrani, S.A.; Alshammari, E.M.; Haque, A.; Alouffi, S.; Khan, W.A.; Khan, S.N. Experimental and theoretical insight into resistance to glycation of bovine serum albumin. J. Mol. Struct. 2021, 1230, 129645. [Google Scholar] [CrossRef]
- Sirangelo, I.; Iannuzzi, C. Understanding the role of protein glycation in the amyloid aggregation process. Int. J. Mol. Sci. 2021, 22, 6609. [Google Scholar] [CrossRef]
- Khanam, A.; Ahmad, S.; Husain, A.; Rehman, S.; Farooqui, A.; Yusuf, M.A. Glycation and Antioxidants: Hand in the glove of antiglycation and natural antioxidants. Curr. Protein Pept. Sci. 2020, 21, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diab. Rep. 2014, 14, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Groot, L.; Hinkema, H.; Westra, J.; Smit, A.J.; Kallenberg, C.G.; Bijl, M.; Posthumus, M.D. Advanced glycation endproducts are increased in rheumatoid arthritis patients with controlled disease. Arthritis Res. Ther. 2011, 13, R205. [Google Scholar] [CrossRef] [Green Version]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef]
- Indyk, D.; Bronowicka-Szydełko, A.; Gamian, A.; Kuzan, A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021, 11, 13264. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective effects of ginger extract against glycation and oxidative stress-induced health complications: An in vitro study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.; Almatoudi, A.; AlSahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A. Natural products: Implication in cancer prevention and treatment through modulating various biological activities. Anticancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef]
- Koch, H.P.; Lawson, L.D. Garlic: The Science and Therapeutic Application of Allium Sativum L. and Related Species, 2nd ed.; Williams & Wilkins: Baltimore, MD, USA, 1996. [Google Scholar]
- Murray, M.T. The Healing Power of Herbs: The Enlightened Person’s Guide to the Wonders of Medicinal Plants, 2nd ed.; Prima: Rocklin, CA, USA, 1995. [Google Scholar]
- Colín-González, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chánez-Cárdenas, M.E.; Santamaría, A.; Maldonado, P.D. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxid. Med. Cell. Longev. 2012, 2012, 907162. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2018, 24, 119. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Yang, J.T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem. Biophys. Res. Commun. 1971, 44, 1285–1291. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Shi, X.; Luo, Z.; Lin, D.; Huang, M. Structural mechanism of ring-opening reaction of glucose by human serum albumin. J. Biol. Chem. 2013, 288, 15980–15987. [Google Scholar] [CrossRef] [Green Version]
- Safari, M.R.; Azizi, O.; Heidary, S.S.; Kheiripour, N.; Ravan, A.P. Antiglycation and antioxidant activity of four Iranian medical plant extracts. J. Pharmacopunct. 2018, 21, 82–89. [Google Scholar] [CrossRef]
- Khan, M.W.A.; Banga, K.; Mashal, S.N.; Khan, W.A. Detection of autoantibodies against reactive oxygen species modified glutamic acid decarboxylase-65 in type 1 diabetes associated complications. BMC Immunol. 2011, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.W.A.; Qadrie, Z.L.; Khan, W.A. Antibodies against gluco-oxidative modified HSA-detected in diabetes associated complications. Int. Arch. Allergy Immunol. 2010, 153, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.A.; Sherwani, S.; Khan, W.A.; Moinuddin; Ali, R. Characterization of hydroxyl radical modified GAD65: A potential autoantigen in type 1 diabetes. Autoimmunity 2009, 42, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tuso, P.J.; Ismail, M.H.; Ha, B.P.; Bartolotto, C. Nutritional update for physicians: Plant-based diets. Perm. J. 2013, 17, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.W.A.; Otaibi, A.A.; Alhumaid, A.F.M.; Alsukaibi, A.K.D.; Alshamari, A.K.; Alshammari, E.M.; Al-Zahrani, S.A.; Almudyani, A.Y.M.; Sherwani, S. Garlic Extract: Inhibition of Biochemical and Biophysical Changes in Glycated HSA. Appl. Sci. 2021, 11, 11028. [Google Scholar] [CrossRef]
- Nagella, P.; Thiruvengadam, M.; Ahmad, A.; Yoon, J.Y.; Chung, I.M. Comosition of polyphenols and antioxidant activity of garlic bulbs collected from different locations of Korea. Asian J. Chem. 2014, 26, 897–902. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Angel, G.R.; Vimala, B.; Nambisan, B. Antioxidant and anti-inflammatory activities of proteins isolated from eight Curcuma species. J. Phytopharm. 2013, 4, 96–105. [Google Scholar]
- Ruiz-Ruiz, J.C.; Moguel-Ordoñez, Y.B.; Segura-Campos, M.R. Biological activity of Stevia rebaudiana Bertoni and their relationship to health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2680–2690. [Google Scholar] [CrossRef]
- Raimundo, A.F.; Ferreira, S.; Martins, I.C.; Menezes, R. Islet Amyloid Polypeptide: A partner in crime with Aβ in the pathology of Alzheimer’s disease. Front. Mol. Neurosci. 2020, 13, 35. [Google Scholar] [CrossRef]
- Bouma, B.; Kroon-Batenburg, L.M.; Wu, Y.P.; Brünjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef] [Green Version]
- Monacelli, F.; Storace, D.; D’Arrigo, C.; Sanguineti, R.; Borghi, R.; Pacini, D.; Furfaro, A.L.; Pronzato, M.A.; Odetti, P.; Traverso, N. Structural alterations of human serum albumin caused by glycative and oxidative stressors revealed by circular dichroism analysis. Int. J. Mol. Sci. 2013, 14, 10694–10709. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, A.; Moosavi-Movahedi, A.A.; Sattarahmady, N.; Hosseinpour-Faizi, M.A.; Aminbakhsh, M.; Ahmad, F.; Saboury, A.A.; Ganjali, M.R.; Norouzi, P. Spectroscopic studies of the effects of glycation of human serum albumin on l-trp binding. Protein Pept. Lett. 2007, 14, 13–18. [Google Scholar] [CrossRef] [PubMed]
- GhoshMoulick, R.; Bhattacharya, J.; Roy, S.; Basak, S.; Dasgupta, A.K. Compensatory secondary structure alterations in protein glycation. Biochim. Biophys. Acta. 2007, 1774, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Miwa, I.; Maeda, K.; Okuda, J. Anomeric compositions of D-glucose in tissues and blood of rat. Experientia 1978, 34, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.A.; Anwar, S.; Alzahrani, F.M.; Almatroudi, A.; Alfheeaid, H.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Health promoting effect of Phyllanthus emblica and Azadiractha indica against advanced glycation end products formation. Appl. Sci. 2021, 11, 8819. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Lsla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Sakat, S.S.; Juvekar, A.R.; Gambhire, M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Pandey, A.K.; Kashyap, P.P.; Kaur, C.D. Anti-inflammatory activity of novel Schiff bases by in vitro models. Bangladesh J. Pharmacol. 2017, 12, 41–43. [Google Scholar] [CrossRef] [Green Version]
- Anwar, S.; Younus, H. Inhibitory effect of alliin from Allium sativum on the glycation of superoxidedismutase. Int. J. Biol. Macromol. 2017, 103, 182–193. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vangone, A.; Schaarschmidt, J.; Koukos, P.; Geng, C.; Citro, N.; Trellet, M.E.; Xue, L.C.; Bonvin, A.M. Large-scale prediction of binding affinity in protein–small ligand complexes: The PRODIGY-LIG web server. Bioinformatics 2019, 35, 1585–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurkcuoglu, Z.; Koukos, P.I.; Citro, N.; Trellet, M.E.; Rodrigues, J.P.G.L.M.; Moreira, I.S.; Roel-Touris, J.; Melquiond, A.S.J.; Geng, C.; Schaarschmidt, J.; et al. Performance of HADDOCK and a simple contact-based protein-ligand binding affinity predictor in the D3R Grand Challenge 2. J. Comp. Aid. Mol. Des. 2018, 32, 175–185. [Google Scholar] [CrossRef] [PubMed]





| Preliminary Screening | Garlic Extract |
|---|---|
| Weight of dry powder | 50 g |
| Yield | 5.19% |
| Extract | Aqueous |
| Flavonoids | + |
| Polyphenolic compounds | + |
| Total phenolic compounds | 21.45 ± 0.02 mg gallic acid equivalent/g dry weight of extract |
| Total flavonoid content | 16.58 ± 0.03 mg quercetin equivalent/g dry weight of the extract |
| Conformation | N-HSA | G-HSA | G-HSA with Garlic Extracts (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | 0.78 | 1.56 | 3.12 | 6.25 | 12.5 | 25 | 50 | 100 | AG | ||
| α-helix | 42.7 ± 0.6 | 38.2 ± 0.3 (−10.5%) | 38.2 ± 0.3 (−10.5%) | 38.3 ± 0.3 (−10.3%) | 38.4 ± 0.3 (−10.0%) | 38.8 ± 0.3 (−9.1%) | 39.8 ± 0.3 * (−6.8%) | 40.5 ± 0.3 * (−5.2%) | 40.9 ± 0.3 ** (−4.2%) | 41.5 ± 0.3 *** (−2.8%) | 40.8 ± 0.3 ** (−4.4%) |
| β-sheet | 26.2 ± 0.5 | 30.1 ± 0.2 (+14.9%) | 30.1 ± 0.2 (+14.9%) | 30.0 ± 0.2 (+14.5%) | 29.9 ± 0.2 (+14.1%) | 29.6 ± 0.2 (+13.0%) | 28.8 ± 0.2 * (+9.9%) | 28.1 ± 0.2 ** (+7.3%) | 27.9 ± 0.2 ** (+6.5%) | 27.4 ± 0.2 *** (+4.6%) | 27.8 ± 0.2 ** (+6.1%) |
| β-turn | 18.5 ± 0.2 | 19.4 ± 0.3 (+4.9%) | 19.4 ± 0.3 (+4.9%) | 19.4 ± 0.3 (+4.9%) | 19.4 ± 0.3 (+4.9%) | 19.2 ± 0.3 (+4.3%) | 19.1 ± 0.3 * (+3.2%) | 19.0 ± 0.3 ** (+2.7%) | 18.8 ± 0.3 ** (+1.6%) | 18.6 ± 0.3 ** (+0.5%) | 19.0 ± 0.3 ** (+2.7%) |
| Random coil | 12.6 ± 0.5 | 12.3 ± 0.4 (−2.4%) | 12.3 ± 0.4 (−2.4%) | 12.3 ± 0.4 (−2.4%) | 12.3 ± 0.4 (−2.4%) | 12.3 ± 0.4 (−2.4%) | 12.3 ± 0.4 (−2.4%) | 12.4 ± 0.4 * (−1.6%) | 12.4 ± 0.4 * (−1.6%) | 12.5 ± 0.4 *** (−0.8%) | 12.4 ± 0.4 * (−1.6%) |
| Ligand/Inhibitor Name (Common Name) | IUPAC Name, Mol Formula, and PubChem ID | Binding Affinity Kcal/Mol | Number of Hydrogen Bonds | Other Interactions * |
|---|---|---|---|---|
| Glucose (Cyclic form) | (3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Mol formula: C6H12O6 PubChem ID: 5793 | −6.2 | 6 (3 × Arg257, 1 × Arg221, 1 × Tyr150, 1 × His242) | 1 (1 × Lys199) Leu238 and Ala291 (Hydrophobic interactions) |
| Catechin | (2S,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol Mol formula: C15H14O6 PubChem ID: 73160 | −6.7 | 1 (1 × His 288) | 13 (1 × Arg 257, 1 × His 288, 1 × Glc 602, 1 × Ala 291, 1 × Tyr 150, 1 × Glu 153, 1 × Glu 292, 1 × Lys 195, 1 × Ala 191, 1 × Phe 157, 1 × Ser 192, 1 × Lys 436, 1 × Glu 188 Carbon H Bond) |
| Caffeic Acid | (E)-3-(3,4-dihydroxyphenyl) prop-2-enoic acid Mol formula: C9H8O4 PubChem ID:689043 | −6.6 | 3 (1 × Arg 222, 2 × Ser 192) | 12 (1 × Arg 218, 1 × Po 4603, 1 × Glc 602, 1 × Leu 238, 1 × Tyr 150, 1 × Lys 195, 1 × Glu 153, 1 × Ala 291, 1 × His 288, 1 × Phe 157,1 × Gln 196, 1 × Lys 199) |
| Gallic Acid | 3,4,5-trihydroxybenzoic acid Mol formula: C7H6O5 PubChem ID: 370 | −6.4 | 4 (2 × Lys 199, 1 × Gln 196, 1 × Glu 153) | 10 (1 × His 242, 1 × Gln 196, 1 × Ser 192, 1 × Lys 195, 1 × Tyr 150, 1 × Ala 291, 1 × Phe 157, 1 × His 288, 1 × Glu 292, 1 × Arg 257) |
| m-Coumaric Acid | E)-3-(3-hydroxyphenyl)prop-2-enoic acid Mol formula: C9H8O3 PubChem ID: 637541 | −6.3 | 4 (1 × Arg 222, 1 × PO 4603, 1 × Glc 602, 1 × Ser 192) | 6 (1 × Arg 222, 1 × PO 4603, 1 × Glc 602, 1 × Ser 192, 1 × Gln 196 Unfavorable donor–donor, 1 × Gln 196 Unfavorable donor–donor) |
| Quercetin | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one Mol formula: C15H10O7 PubChem ID: 5280343 | −8.1 | 4 (1 × Ser 192, 1 × Lys 199, 1 × Arg 257, 1 × Arg 222) | 17 (1 × Lys 195, 1 × Lys 199, 1 × Gln 196, 1 × His 242, 1 × Tyr 150, 3 × Ala 291, 2 × Leu 238, 1 × Leu 260, 1 × Ala 261, 1 × Ser 287, 1 × Ile 290, 1 × Leu 219,1 × Arg 222, 1 × Glu 153) |
| Pyrogallol | benzene-1,2,3-triol Mol formula: C6H6O3 PubChem ID: 1057 | −5.4 | 3 (2 × Ser 192, 1 × Gln 196) | 8 (1 × Tyr 150, 1 × Glu 153, 1 × Phe 157, 1 × Lys 199, 1 × Ala 291, 1 × Lys 195, 1 × His 242, 1 × Arg 257) |
| Dihydroxybenzoic acid | 2,3-dihydroxybenzoic acid Mol formula: C7H6O4 PubChem ID: 19 | −6.2 | 3 (1 × Arg 222, 1 × Ser 287, 1 × Arg 257) | 9 (1 × Leu 260, 1 × Ile 290, 1 × Leu 238, 1 × Ala 291, 1 × Arg 257, 1 × Leu 219, 1 × Ile 264, 1 × Ala 261, 1 × Tyr 150) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.W.A.; Otaibi, A.A.; Alsukaibi, A.K.D.; Alshammari, E.M.; Al-Zahrani, S.A.; Sherwani, S.; Khan, W.A.; Saha, R.; Verma, S.R.; Ahmed, N. Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic. Molecules 2022, 27, 1868. https://doi.org/10.3390/molecules27061868
Khan MWA, Otaibi AA, Alsukaibi AKD, Alshammari EM, Al-Zahrani SA, Sherwani S, Khan WA, Saha R, Verma SR, Ahmed N. Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic. Molecules. 2022; 27(6):1868. https://doi.org/10.3390/molecules27061868
Chicago/Turabian StyleKhan, Mohd W. A., Ahmed A. Otaibi, Abdulmohsen K. D. Alsukaibi, Eida M. Alshammari, Salma A. Al-Zahrani, Subuhi Sherwani, Wahid A. Khan, Ritika Saha, Smita R. Verma, and Nessar Ahmed. 2022. "Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic" Molecules 27, no. 6: 1868. https://doi.org/10.3390/molecules27061868
APA StyleKhan, M. W. A., Otaibi, A. A., Alsukaibi, A. K. D., Alshammari, E. M., Al-Zahrani, S. A., Sherwani, S., Khan, W. A., Saha, R., Verma, S. R., & Ahmed, N. (2022). Biophysical, Biochemical, and Molecular Docking Investigations of Anti-Glycating, Antioxidant, and Protein Structural Stability Potential of Garlic. Molecules, 27(6), 1868. https://doi.org/10.3390/molecules27061868






