Treatment Effect of CT-Guided Periradicular Injections in Context of Different Contrast Agent Distribution Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients and Procedure
2.3. Assessment of Technical Success
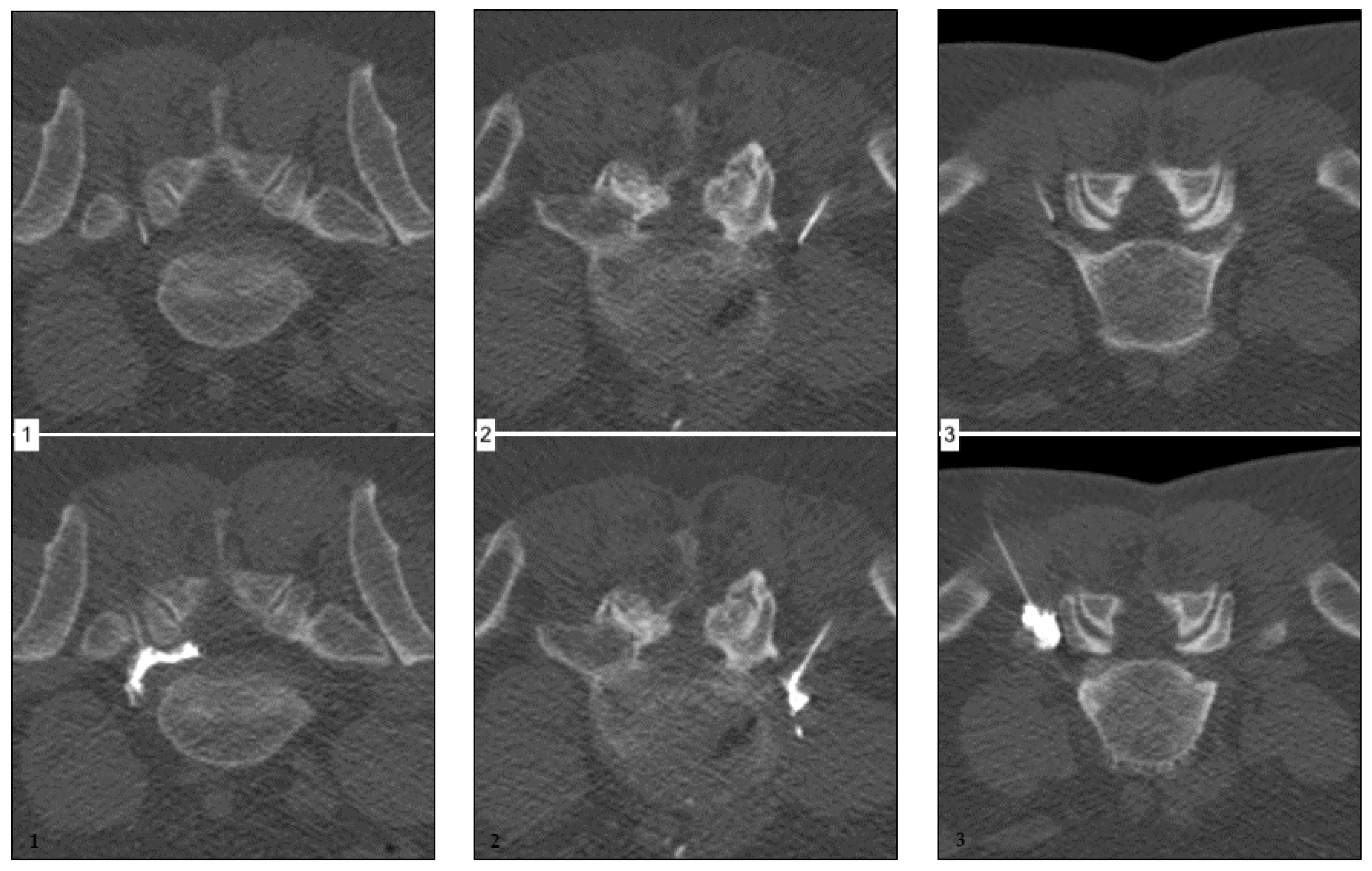
- without primarily apparent nerve root contact or;
- spatially confined, non-circumferential spread along the nerve root or;
- extensive surface contact with at least partially circumferential distribution, including the intraspinal portion of the nerve root.
2.4. Statistical Analysis
2.4.1. Whole Collective Analysis
2.4.2. Subgrouping according to First-Line Therapy
2.4.3. Subgrouping according to the Duration of Predominantly Radicular Backpain Prior to CT-Guided Percutaneous Therapy
3. Results
3.1. General Findings and Remarks
- 29.8% had LBP accompanied by a diffuse radiating component;
- 55.3% had LBP and dermatome-related radiating pain with matching dysesthesia;
- 14.3% had LBP and dermatome-related radiating pain with additional motor deficits. In greater detail, of those 23 patients:
- 8.07% (n = 13) had a L4 syndrome with accompanying knee extensor muscle weakness;
- 6.21% (n = 10) had a L5 syndrome with accompanying foot drop;
- 0.6% (one patient) suffered from dermatome-matching dysesthesia only.
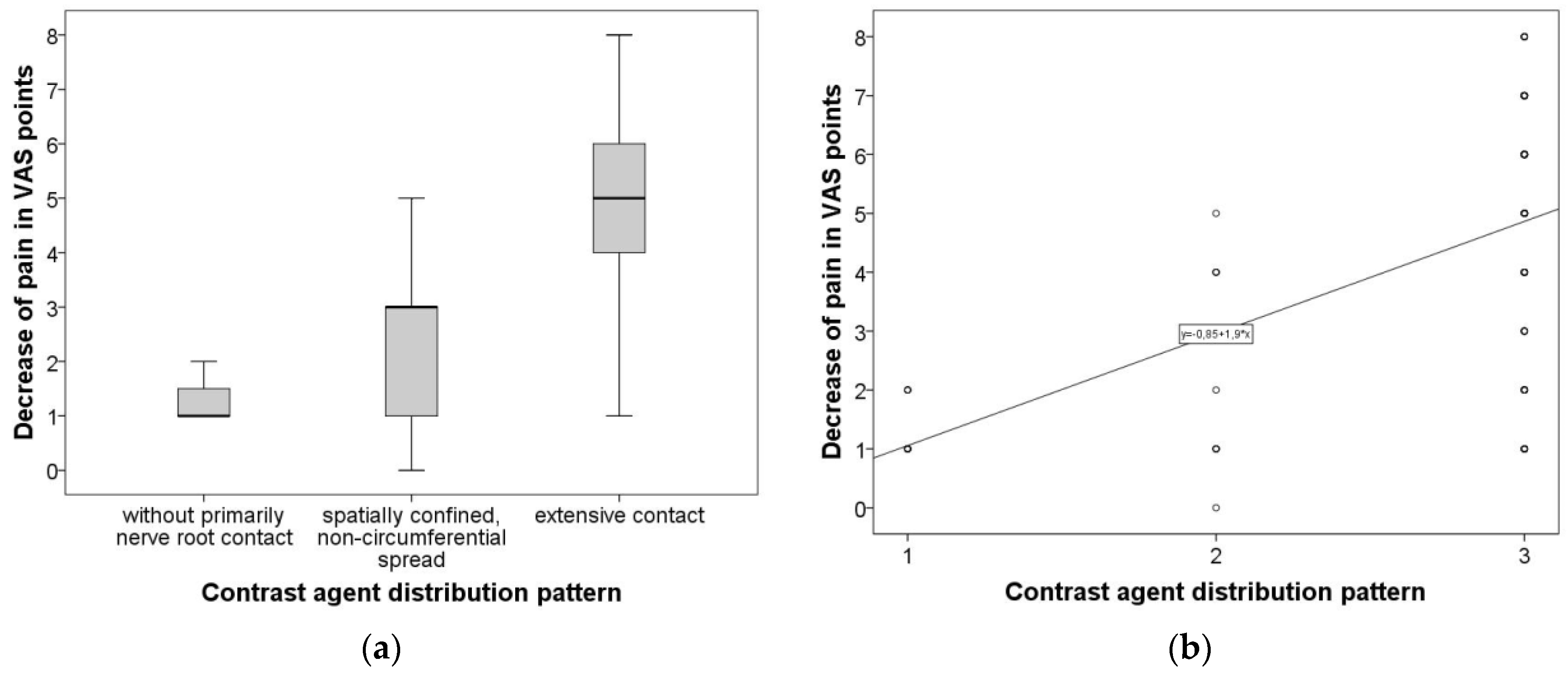
3.2. Overall Group Comparison and Correlative Analysis
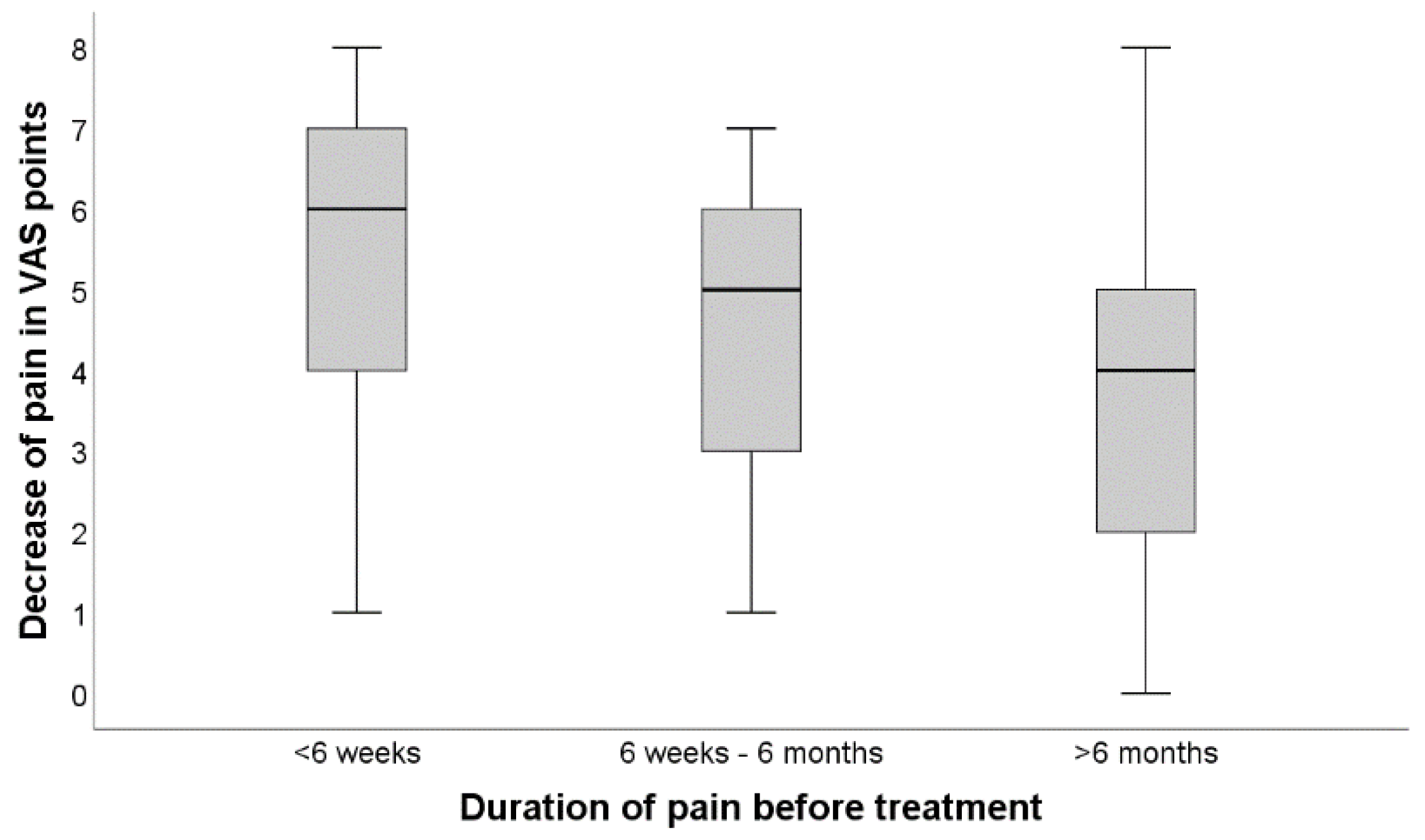
3.3. Subgrouping according to Temporal Extent of Predominantly Radicular Backpain Prior to CT-Guided Percutaneous Therapy
3.3.1. Group Comparison and Correlative Analysis in Subgroups Accounting for Different First-Line Therapies
3.3.2. Subgroup Comparison and Correlative Analysis in Subgroups accounting for Pain Duration between Onset and CT-Guided Percutaneous Treatment
4. Discussion
- application of contrast agent in periradicular infiltrations is helpful for improving the distribution of corticosteroids along the affected nerve root;
- a circumferential distribution is associated with good treatment efficacy;
- periradicular infiltrations, as a complementary treatment for both conservative therapy and surgery, help to significantly reduce pain for the majority of patients in the early phase and potentially up to six months, only a minority experiences permanent and sufficient pain relief
- in comparison to the natural course, periradicular infiltrations improve outcomes and are a safe, effective and, therefore, justifiable complementary treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maselli, F.; Palladino, M.; Barbari, V.; Storari, L.; Rossettini, G.; Testa, M. The diagnostic value of Red Flags in thoracolumbar pain: A systematic review. Disabil. Rehabil. 2020, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Finucane, L.M.; Downie, A.; Mercer, C.; Greenhalgh, S.M.; Boissonnault, W.G.; Pool-Goudzwaard, A.L.; Beneciuk, J.M.; Leech, R.L.; Selfe, J. International Framework for Red Flags for Potential Serious Spinal Pathologies. J. Orthop. Sports Phys. Ther. 2020, 50, 350–372. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.T.; Tavares, S.P.; Robertson, S.C.; Sharp, R.; Marshall, R.W. Conservatively treated massive prolapsed discs: A 7-year follow-up. Ann. R. Coll. Surg. Engl. 2010, 92, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyo, R.A.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef]
- Azharuddin, A.; Aryandono, T.; Magetsari, R.; Dwiprahasto, I. Predictors of the conservative management outcomes in patients with lumbar herniated nucleus pulposus: A prospective study in Indonesia. Asian J. Surg. 2022, 45, 277–283. [Google Scholar] [CrossRef]
- Weber, H. Lumbar disc herniation: A controlled, prospective study with ten years of observation. Spine 1983, 8, 131–140. [Google Scholar] [CrossRef]
- Kim, C.H.; Choi, Y.; Chung, C.K.; Kim, K.J.; Shin, D.A.; Park, Y.K.; Kwon, W.K.; Yang, S.H.; Lee, C.H.; Park, S.B.; et al. Nonsurgical treatment outcomes for surgical candidates with lumbar disc herniation: A comprehensive cohort study. Sci. Rep. 2021, 11, 3931. [Google Scholar] [CrossRef]
- Peul, W.C.; Van Den Hout, W.B.; Brand, R.; Thomeer, R.T.W.M.; Koes, B.W. Prolonged conservative care versus early surgery in patients with sciatica caused by lumbar disc herniation: Two year results of a randomised controlled trial. BMJ 2008, 336, 1355–1358. [Google Scholar] [CrossRef] [Green Version]
- Deyo, R.A.; Mirza, S.K. Clinical Practice. Herniated Lumbar Intervertebral Disk. N. Engl. J. Med. 2016, 374, 1763–1772. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Hao, X.; Guo, X.; Wang, L. Spontaneous regression of herniated lumbar discs: Report of one illustrative case and review of the literature. Clin. Neurol. Neurosurg. 2016, 143, 86–89. [Google Scholar] [CrossRef]
- Elkholy, A.R.; Farid, A.M.; Shamhoot, E.A. Spontaneous Resorption of Herniated Lumbar Disk: Observational Retrospective Study in 9 Patients. World Neurosurg. 2019, 124, 453–459. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, P.; Jiang, H.; Li, X.; Qian, X.; Yu, Z.; Zhu, Y.; Liu, J. Conservative Treatment for Giant Lumbar Disc Herniation: Clinical Study in 409 Cases. Pain Physician 2021, 24, E639–E648. [Google Scholar]
- Haro, H. Translational research of herniated discs: Current status of diagnosis and treatment. J. Orthop. Sci. 2014, 19, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Autio, R.A.; Karppinen, J.; Niinimaki, J.; Ojala, R.; Kurunlahti, M.; Haapea, M. Determinants of spontaneous resorption of intervertebral disc herniations. Spine 2006, 31, 1247–1252. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakamura, T.; Sei, A.; Kikuchi, T.; Takagi, K.; Matsukawa, A. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: An experimental study using a new hernia model. Spine 2005, 30, 55–61. [Google Scholar] [CrossRef]
- Kobayashi, S.; Meir, A.; Kokubo, Y.; Uchida, K.; Takeno, K.; Miyazaki, T. Ultrastructural analysis on lumbar disc herniation using surgical specimens: Role of neovascularization and macrophages in hernias. Spine 2009, 34, 655–662. [Google Scholar] [CrossRef]
- Peul, W.C.; van Houwelingen, H.C.; van den Hout, W.B.; Brand, R.; Eekhof, J.A.; Tans, J.T.; Thomeer, R.T.; Koes, B.W.; Leiden-The Hague Spine Intervention Prognostic Study Group. Surgery versus prolonged conservative treatment for sciatica. N. Engl. J. Med. 2007, 356, 2245–2256. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Hanscom, B.; Skinner, J.S.; Abdu, W.A.; Hilibrand, A.S.; Boden, S.D.; Deyo, R.A. Surgical vs nonoperative treatment for lumbar disk herniation: The Spine Patient Outcomes Research Trial (SPORT): A randomized trial. JAMA 2006, 296, 2441–2450. [Google Scholar] [CrossRef] [Green Version]
- Vroomen, P.C.; de Krom, M.C.; Knottnerus, J.A. Predicting the outcome of sciatica at short-term follow-up. Br. J. Gen. Pract. 2002, 52, 119–123. [Google Scholar]
- Gossner, J. Safety of CT-Guided Lumbar Nerve Root Infiltrations. Analysis of a Two-Year Period. Interv. Neuroradiol. 2014, 20, 533–537. [Google Scholar] [CrossRef] [Green Version]
- Cyteval, C.; Fescquet, N.; Thomas, E.; Decoux, E.; Blotman, F.; Taourel, P. Predictive Factors of Efficacy of Periradicular Corticosteroid Injections for Lumbar Radiculopathy. Am. J. Neuroradiol. 2005, 27, 978–982. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbergleit, R.; Mehta, B.A.; Sanders, W.P.; Talati, S.J. Imaging guided injection techniques with fluoroscopy and CT for spinal pain management. RadioGraphics 2001, 21, 927–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, N.; Salaria, M.; Salaria, A.Q. Comparison of fluoroscopic Guided Transforaminal Epidural Injections of Steroid and Local Anaesthetic with Conservative Management in Patients with Chronic Lumbar Radiculopathies. Anesth. Essays Res. 2017, 11, 17–22. [Google Scholar] [PubMed] [Green Version]
- Johnson, B.A.; Schellhas, K.P.; Pollei, S.R. Epidurography and therapeutic epidural injections: Technical considerations and experience with 5334 cases. Am. J. Neuroradiol. 1999, 20, 697–705. [Google Scholar]
- Krämer, J.; Blettner, M.; Hammer, G.P. Image-guided injection therapy in the lumbar spine. Dtsch. Arztebl. Int. 2008, 105, 596–598. [Google Scholar] [CrossRef]
- White, A.H.; Derby, R.; Wynne, G. Epidural injections for the diagnosis and treatment of low-back pain. Spine 1980, 5, 78–86. [Google Scholar] [CrossRef]
- Bartynski, W.S.; Grahovac, S.Z.; Rothfus, W.E. Incorrect needle position during lumbar epidural steroid administration: Inaccuracy of loss of air pressure resistance and requirement of fluoroscopy and epidurography during needle insertion. Am. J. Neuroradiol. 2005, 26, 502–505. [Google Scholar]
- Pfirrmann, C.W.; Oberholzer, P.A.; Zanetti, M.; Boos, N.; Trudell, D.J.; Resnick, D.; Hodler, J. Selective nerve root blocks for the treatment of sciatica: Evaluation of injection site and effectiveness—A study with patients and cadavers. Radiology 2001, 221, 704–711. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Tapping, C.R.; Bartlett, R.; Maliakal, P. Factors that affect the efficacy of fluoroscopically guided selective spinal nerve root block in the treatment of radicular pain: A prospective cohort study. Can. Assoc. Radiol. J. 2013, 64, 370–375. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.; Nagda, J.; Aner, M.; Simopoulos, T. Cervical Epidural Contrast Spread Patterns in Fluoroscopic Antero-Posterior, Lateral, and Contralateral Oblique View: A Three-Dimensional Analysis. Pain Med. 2017, 18, 1027–1039. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Kim, S.H.; Lee, I.S.; Choi, J.A.; Choi, J.Y.; Hong, S.H.; Kang, H.S. Therapeutic effect and outcome predictors of sciatica treated using transforaminal epidural steroid injection. AJR Am. J. Roentgenol. 2006, 187, 1427–1431. [Google Scholar] [CrossRef]
- Jeong, H.S.; Lee, J.W.; Kim, S.H.; Myung, J.S.; Kim, J.H.; Kang, H.S. Effectiveness of transforaminal epidural steroid injection by using a preganglionic approach: A prospective randomized controlled study. Radiology 2007, 245, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, T.J.; Peterson, C.K.; Zeimpekis, K.G.; Bensler, S.; Sutter, R.; Pfirrmann, C.W.A. Fluoroscopy-guided versus CT-guided Lumbar Steroid Injections: Comparison of Radiation Exposure and Outcomes. Radiology 2019, 290, 752–759. [Google Scholar] [CrossRef]
- Cunha, C.; Silva, A.J.; Pereira, P.; Vaz, R.; Gonçalves, R.M.; Barbosa, M.A. The inflammatory response in the regression of lumbar disc herniation. Arthritis Res. Ther. 2018, 20, 251. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; Liu, J.T.; Jiang, H.; Mo, W.; Yu, P.F.; Li, X.C.; Xue, R.R. Incidence of Spontaneous Resorption of Lumbar Disc Heniation: A Meta-Analysis. Pain Physician 2017, 20, E45–E52. [Google Scholar]
- Reinhold, A.K.; Rittner, H.L. Barrier function in the peripheral and central nervous system—A review. Pflug. Arch. 2017, 469, 123–134. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Shi, X.Q.; Martin, H.C.; Huang, H.; Luheshi, G.; Rivest, S.; Zhang, J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain 2014, 155, 954–967. [Google Scholar] [CrossRef]
- Isami, K.; Haraguchi, K.; So, K.; Asakura, K.; Shirakawa, H.; Mori, Y.; Nakagawa, T.; Kaneko, S. Involvement of TRPM2 in peripheral nerve injury-induced infiltration of peripheral immune cells into the spinal cord in mouse neuropathic pain model. PLoS ONE 2013, 8, e66410. [Google Scholar] [CrossRef] [Green Version]
- Moreau, N.; Mauborgne, A.; Bourgoin, S.; Couraud, P.O.; Romero, I.A.; Weksler, B.B.; Villanueva, L.; Pohl, M.; Boucher, Y. Early alterations of Hedgehog signaling pathway in vascular endothelial cells after peripheral nerve injury elicit blood-nerve barrier disruption, nerve inflammation, and neuropathic pain development. Pain 2016, 157, 827–839. [Google Scholar] [CrossRef]
- Shimizu, F.; Sano, Y.; Saito, K.; Abe, M.A.; Maeda, T.; Haruki, H.; Kanda, T. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem. Res. 2012, 37, 401–409. [Google Scholar] [CrossRef]
- Kashiwamura, Y.; Sano, Y.; Abe, M.; Shimizu, F.; Haruki, H.; Maeda, T.; Kawai, M.; Kanda, T. Hydrocortisone enhances the function of the blood-nerve barrier through the up-regulation of claudin-5. Neurochem. Res. 2011, 36, 849–855. [Google Scholar] [CrossRef]
- Huang, T.L.; Wen, Y.T.; Chang, C.H.; Chang, S.W.; Lin, K.H.; Tsai, R.K. Early Methylprednisolone Treatment Can Stabilize the Blood-Optic Nerve Barrier in a Rat Model of Anterior Ischemic Optic Neuropathy (rAION). Investig. Ophthalmol. Vis. Sci. 2017, 58, 1628–1636. [Google Scholar] [CrossRef] [Green Version]
- Moen, A.; Lind, A.L.; Thulin, M.; Kamali-Moghaddam, M.; Røe, C.; Gjerstad, J.; Gordh, T. Inflammatory Serum Protein Profiling of Patients with Lumbar Radicular Pain One Year after Disc Herniation. Int. J. Inflam. 2016, 2016, 3874964. [Google Scholar] [CrossRef]
- Botwin, K.P.; Gruber, R.D.; Bouchlas, C.G.; Torres-Ramos, F.M.; Sanelli, J.T.; Freeman, E.D.; Slaten, W.K.; Rao, S. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: An outcome study. Am. J. Phys. Med. Rehabil. 2002, 81, 898–905. [Google Scholar] [CrossRef]
- Lurie, J.; Tomkins-Lane, C. Management of lumbar spinal stenosis. BMJ 2016, 352, h6234. [Google Scholar] [CrossRef]
- Fritz, J.M.; Lurie, J.D.; Zhao, W.; Whitman, J.M.; Delitto, A.; Brennan, G.P.; Weinstein, J.N. Associations between physical therapy and long-term outcomes for individuals with lumbar spinal stenosis in the SPORT study. Spine J. 2014, 14, 1611–1621. [Google Scholar] [CrossRef] [Green Version]
- Krahulik, D.; Vaverka, M.; Hrabalek, L.; Pohlodek, D.; Jablonsky, J.; Valosek, J.; Zapletalova, J. Periradicular corticosteroid infiltration for radicular pain—Comparison of Diprophos and Depomedrone and ozone effects. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sucuoğlu, H.; Soydaş, N. Does paravertebral ozone injection have efficacy as an additional treatment for acute lumbar disc herniation? A randomized, double-blind, placebo-controlled study. J. Back Musculoskelet. Rehabil. 2021, 34, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ekşi, M.Ş.; Özcan-Ekşi, E.E.; Orhun, Ö.; Turgut, V.U.; Pamir, M.N. Proposal for a new scoring system for spinal degeneration: Mo-Fi-Disc. Clin. Neurol. Neurosurg. 2020, 198, 106120. [Google Scholar] [CrossRef]

| Age | 58.69 (15.1); Range 20–83 |
|---|---|
| Gender | 90 male; 71 female |
| BMI | 66 normal; 70 overweight; 25 obese |
| Working ability | 21.7% yes; 42.9% no; 35.4% retired |
| Treatment strategy prior to PRT | 19.3% pain medication only 49.7% meds and physio 31.1% surgery |
| Current mobility | 74.5% unaffected; 25.5% limited |
| N | % | |
|---|---|---|
| Disc herniation (bulge or protrusion) | 58 | 36 |
| Osteoligamentous degeneration (e.g., FH, LSS 1) | 41 | 25.5 |
| Degeneration and disc herniation | 51 | 31.7 |
| Spondylolisthesis | 6 | 3.7 |
| Multisegmental herniations | 1 | 0.6 |
| Degenerative aggravation in context of old fracture in osteoporosis | 2 | 1.2 |
| Post-traumatic (fracture) kyphosis | 1 | 0.6 |
| No causative MRI finding for LBP | 1 | 0.6 |
| Segment | N | % | Left | Right |
|---|---|---|---|---|
| L2 | 6 | 3.7 | 3 | 3 |
| L3 | 18 | 11.2 | 9 | 9 |
| L4 | 23 | 14.3 | 10 | 13 |
| L5 | 78 | 48.4 | 43 | 35 |
| S1 | 36 | 22.4 | 18 | 18 |
| Contrast Distribution Pattern | N | % |
|---|---|---|
| Without primarily apparent nerve root contact | 15 | 9.3 |
| Spatially confined, non-circumferential spread along the nerve root | 17 | 10.6 |
| Extensive surface contact with at least partially circumferential distribution, including the intra-spinal portion of the nerve root and epidural space | 126 | 78.3 |
| Median | Range | |
|---|---|---|
| VAS before treatment | 6.91 | 2–9 |
| VAS after treatment | 2.68 | 0–9 |
| Difference after PRT in VAS points | 4.30 | 0–8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuschel, V.; Scherlach, C.; Pfeifle, C.; Krause, M.; Struck, M.F.; Hoffmann, K.-T.; Schob, S. Treatment Effect of CT-Guided Periradicular Injections in Context of Different Contrast Agent Distribution Patterns. Diagnostics 2022, 12, 787. https://doi.org/10.3390/diagnostics12040787
Reuschel V, Scherlach C, Pfeifle C, Krause M, Struck MF, Hoffmann K-T, Schob S. Treatment Effect of CT-Guided Periradicular Injections in Context of Different Contrast Agent Distribution Patterns. Diagnostics. 2022; 12(4):787. https://doi.org/10.3390/diagnostics12040787
Chicago/Turabian StyleReuschel, Vera, Cordula Scherlach, Christian Pfeifle, Matthias Krause, Manuel Florian Struck, Karl-Titus Hoffmann, and Stefan Schob. 2022. "Treatment Effect of CT-Guided Periradicular Injections in Context of Different Contrast Agent Distribution Patterns" Diagnostics 12, no. 4: 787. https://doi.org/10.3390/diagnostics12040787
APA StyleReuschel, V., Scherlach, C., Pfeifle, C., Krause, M., Struck, M. F., Hoffmann, K.-T., & Schob, S. (2022). Treatment Effect of CT-Guided Periradicular Injections in Context of Different Contrast Agent Distribution Patterns. Diagnostics, 12(4), 787. https://doi.org/10.3390/diagnostics12040787






