Philopatry as a Tool to Define Tentative Closed Migration Cycles and Conservation Areas for Large Pelagic Fishes in the Pacific
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Large Pelagic Species Migrations
3.1.1. Skipjack (Katsuwonus pelamis)
3.1.2. Yellowfin (Thunnus albacares)
3.1.3. Bigeye Tuna (Thunnus obesus)
3.1.4. Albacore (Thunnus alalunga)
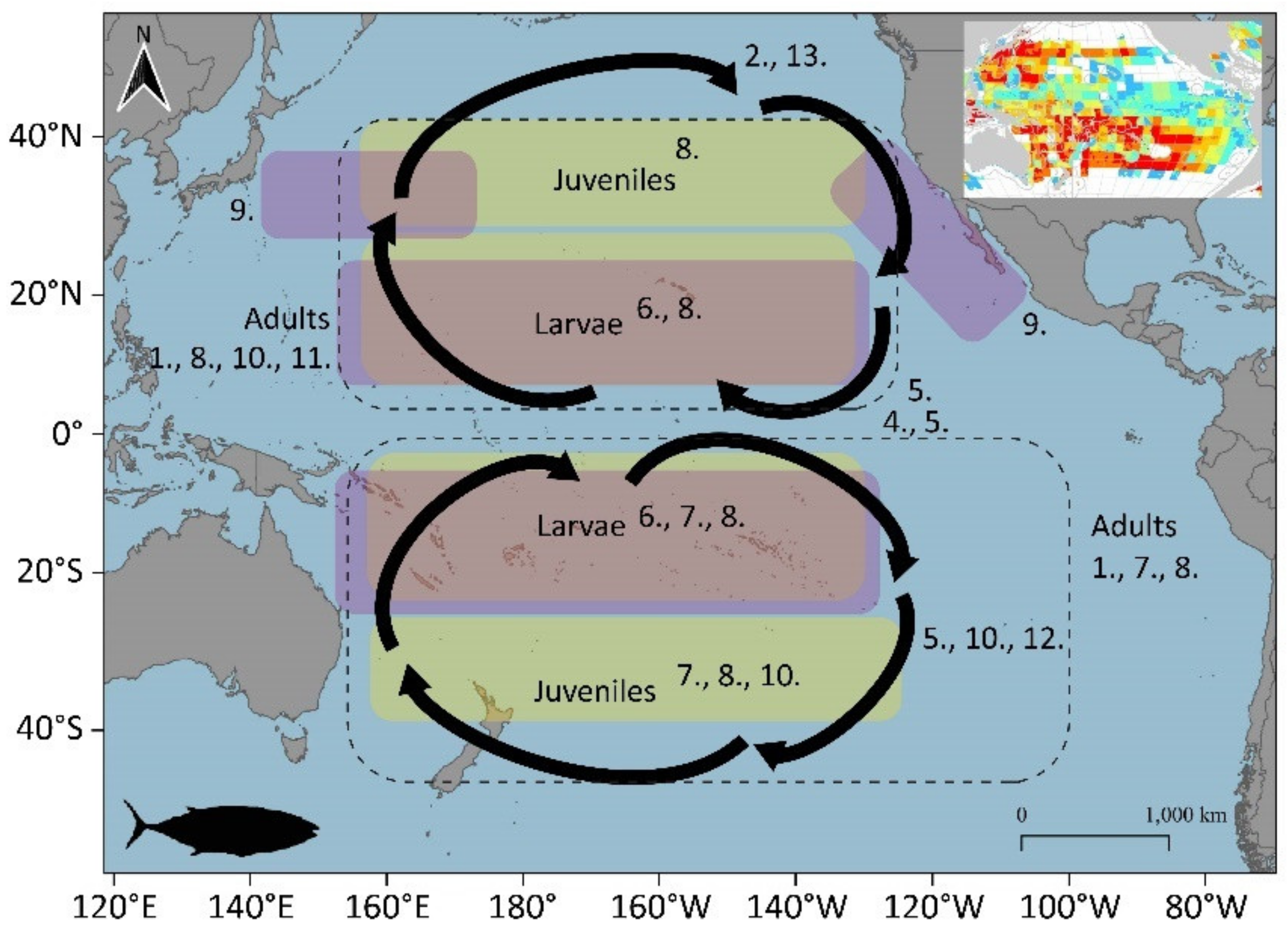
3.1.5. Pacific Bluefin Tuna (Thunnus orientalis)
3.1.6. Swordfish (Xiphias gladius)
3.1.7. Common Dolphinfish (Coryphaena hippurus)

3.1.8. Striped Marlin (Kajikia audax)
3.1.9. Black Marlin (Istiompax indica)
3.1.10. Wahoo (Acanthocybium solandri)
3.1.11. Indo-Pacific Sailfish (Istiophorus platypterus)
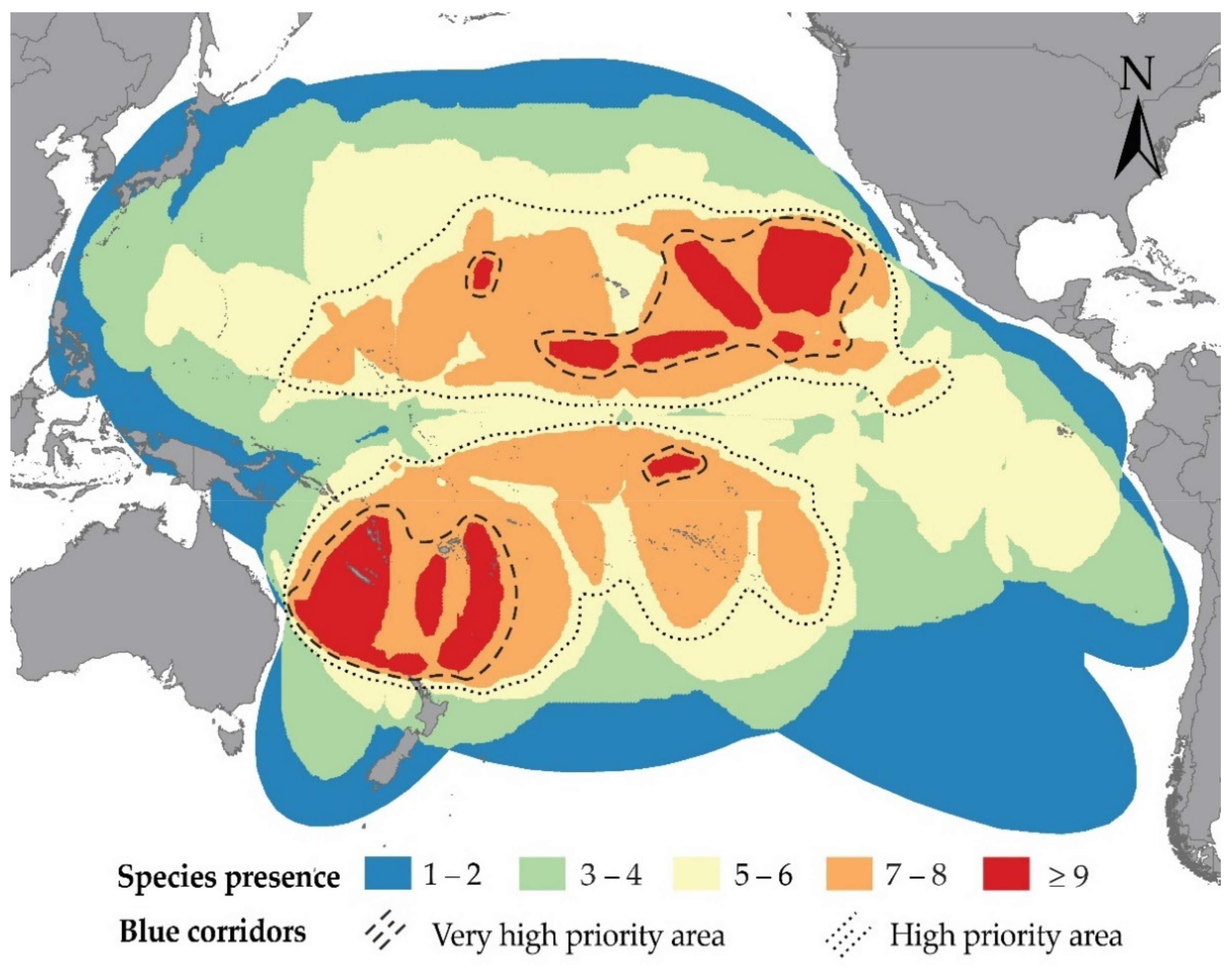
3.2. Potential Blue Corridors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allan, J.C.; Beazley, K.F.; Metaxas, A. Ecological criteria for designing effective MPA networks for large migratory pelagics: Assessing the consistency between IUCN best practices and scholarly literature. Mar. Policy 2021, 127, 104219. [Google Scholar] [CrossRef]
- Briscoe, D.; Maxwell, S.; Kudela, R.; Crowder, L.; Croll, D. Are we missing important areas in pelagic marine conservation? Redefining conservation hotspots in the ocean. Endanger. Species Res. 2016, 29, 229–237. [Google Scholar] [CrossRef]
- Hyrenbach, K.D.; Forney, K.A.; Dayton, P.K. Marine protected areas and ocean basin management. Aquat. Conserv. 2000, 10, 437–458. [Google Scholar] [CrossRef]
- Grantham, H.S.; Game, E.T.; Lombard, A.T.; Hobday, A.J.; Richardson, A.J.; Beckley, L.E.; Pressey, R.L.; Huggett, J.A.; Coetzee, J.C.; van der Lingen, C.D.; et al. Accommodating dynamic oceanographic processes and pelagic biodiversity in marine conservation planning. PLoS ONE 2011, 6, e16552. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Zeller, D.; Watson, R.; Alder, J.; Pauly, D. Potential costs and benefits of marine reserves in the high seas. Mar. Ecol. Prog. Ser. 2007, 345, 305–310. [Google Scholar] [CrossRef]
- Relano, V.; Palomares, M.L.D.; Pauly, D. Comparing the Performance of Four Very Large Marine Protected Areas with Different Levels of Protection. Sustainability 2021, 13, 9572. [Google Scholar] [CrossRef]
- Le Manach, F.; Chavance, P.; Cisneros-Montemayor, A.M.; Lindop, A.; Padilla, A.; Schiller, L.; Zeller, D.; Pauly, D. Global catches of large pelagic fishes, with emphasis on the High Seas. In Global Atlas of Marine Fisheries: A Critical Appraisal of Catches and Ecosystem Impacts; Pauly, D., Zeller, D., Eds.; Island Press: Washington, DC, USA, 2016; pp. 34–45. [Google Scholar]
- Crespo, G.O.; Dunn, D.C.; Reygondeau, G.; Boerder, K.; Worm, B.; Cheung, W.; Tittensor, D.P.; Halpin, P.N. The environmental niche of the global high seas pelagic longline fleet. Sci. Adv. 2018, 4, eaat3681. [Google Scholar] [CrossRef] [PubMed]
- Game, E.T.; Grantham, H.S.; Hobday, A.J.; Pressey, R.L.; Lombard, A.T.; Beckley, L.E.; Gjerde, K.; Bustamante, R.; Possingham, H.P.; Richardson, A.J. Pelagic protected areas: The missing dimension in ocean conservation. Trends Ecol. Evol. 2009, 24, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Boerder, K.; Schiller, L.; Worm, B. Not all who wander are lost: Improving spatial protection for large pelagic fishes. Mar. Policy 2019, 105, 80–90. [Google Scholar] [CrossRef]
- Lascelles, B.; di Sciara, G.N.; Agardy, T.; Cuttelod, A.; Eckert, S.; Glowka, L.; Hoyt, E.; Llewellyn, F.; Louzao, M.; Ridoux, V.; et al. Migratory marine species: Their status, threats and conservation management needs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 111–127. [Google Scholar] [CrossRef]
- Pauly, D.; Keskin, Ç. Temperature constraints shaped the migration routes of mackerel (Scomber scombrus) in the Black Sea. Acta Adriat. 2017, 58, 339–346. [Google Scholar] [CrossRef]
- Pauly, D. Gasping Fish and Panting Squids: Oxygen, Temperature and the Growth of Water-Breathing Animals, 2nd ed.; Excellence in Ecology (22); International Ecology Institute: Oldendorf/Luhe, Germany, 2019. [Google Scholar]
- Boyce, D.; Tittensor, D.; Worm, B. Effects of temperature on global patterns of tuna and billfish richness. Mar. Ecol. Prog. Ser. 2008, 355, 267–276. [Google Scholar] [CrossRef]
- Cury, P. Obstinate nature: An ecology of individuals. Thoughts on reproductive behavior and biodiversity. Can. J. Fish. Aquat. Sci. 1994, 51, 1664–1673. [Google Scholar] [CrossRef]
- Cury, P. Obstinate nature. ICES J. Mar. Sci. 2019, 76, 384–391. [Google Scholar] [CrossRef]
- Cury, P.; Pauly, D. Obstinate Nature; Odile Jacob: Paris, France, 2021. [Google Scholar]
- Hernández, C.M.; Witting, J.; Willis, C.; Thorrold, S.R.; Llopiz, J.K.; Rotjan, R.D. Evidence and patterns of tuna spawning inside a large no-take Marine Protected Area. Sci. Rep. 2019, 9, 10772. [Google Scholar] [CrossRef]
- Llopiz, J.K.; Hobday, A.J. A global comparative analysis of the feeding dynamics and environmental conditions of larval tunas, mackerels, and billfishes. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 113–124. [Google Scholar] [CrossRef]
- Margulies, D. Assessment of the nutritional condition of larval and early juvenile tuna and Spanish mackerel (Pisces: Scombridae) in the Panama Bight. Mar. Biol. 1993, 115, 317–330. [Google Scholar] [CrossRef]
- Sanchez-Velasco, L.; Contreras-Arredondo, I.; Esqueda-Escarcega, G. Diet composition of Euthynnus lineatus and Auxis sp. larvae (Pisces: Scombridae) in the Gulf of California. Bull. Mar. Sci. 1999, 65, 687–698. [Google Scholar]
- Young, J.; Davis, T. Feeding ecology of larvae of southern bluefin, albacore and skipjack tunas (Pisces: Scombridae) in the eastern Indian Ocean. Mar. Ecol. Prog. Ser. 1990, 61, 17–29. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Eguchi, M.; Miyashita, S. Pacific bluefin tuna, Thunnus orientalis, larvae utilize energy and nutrients of microbial loop. Aquaculture 2007, 267, 83–93. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.; Ahrens, R.; Alder, J.; Buszowski, J.; Christensen, L.; Cheung, W.L.; Dunne, J.; Froese, R.; Karpouzi, V.; et al. Database-driven models of the world’s Large Marine Ecosystems. Ecol. Model. 2009, 220, 1984–1996. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D.; Palomares, M.L.D. (Eds.) Sea around Us Concepts, Design and Data. 2020. Available online: http://www.seaaroundus.org/citation-policy/ (accessed on 30 November 2021).
- Pauly, D.; Zeller, D. (Eds.) Global Atlas of Marine Fisheries: A Critical Appraisal of Catches and Ecosystem Impacts; Island Press: Washington, DC, USA, 2016; Volume xii, 497p. [Google Scholar]
- Pauly, D.; Zeller, D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef]
- Coulter, A.; Cashion, T.; Cisneros-Montemayor, A.M.; Popov, S.; Tsui, G.; le Manach, F.; Schiller, L.; Palomares, M.L.D.D.; Zeller, D.; Pauly, D. Using harmonized historical catch data to infer the expansion of global tuna fisheries. Fish. Res. 2020, 221, 105379. [Google Scholar] [CrossRef]
- Derrick, B.; Khalfallah, M.; Relano, V.; Zeller, D.; Pauly, D. (Eds.) Updating to 2018 the 1950–2010 Marine Catch Reconstructions of the Sea Around Us: Part I—Africa, Antarctica, Europe and the North Atlantic. Fisheries Centre Research Report. 2020, Volume 28, 321p. Available online: https://rua.ua.es/dspace/bitstream/10045/114285/1/Sola_et_al_2020_Spain_Med_Cadiz_FCRR.pdf (accessed on 29 November 2021).
- Derrick, B.; Khalfallah, M.; Relano, V.; Zeller, D.; Pauly, D. (Eds.) Updating to 2018 the 1950–2010 Marine Catch Reconstructions of the Sea Around Us. Part II: The Americas and Asia-Pacific. Fisheries Centre Research Report. 2020, Volume 28, 408p. Available online: http://hdl.handle.net/2429/77673 (accessed on 29 November 2021).
- Hernandez-H, A.; Ramirez-R, N. Spawning seasonality and length at maturity of sailfish (Istiophorus platypterus) off the Pacific coast of Mexico. Bull. Mar. Sci. 1998, 63, 459–468. [Google Scholar]
- Vincent, M.; Ducharme-Barth, N.; Hampton, J. Stock Assessment of Skipjack Tuna in the Western and Central Pacific Ocean; WCPFC-SC15-2019/SA-WP-05; Western and Central Pacific Fisheries Commission: Pohnpei, Federated States of Micronesia, 2019. [Google Scholar]
- Arrizabalaga, H.; Dufour, F.; Kell, L.; Merino, G.; Ibaibarriaga, L.; Chust, G.; Irigoien, X.; Santiago, J.; Murua, H.; Fraile, I.; et al. Global habitat preferences of commercially valuable tuna. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 102–112. [Google Scholar] [CrossRef]
- Kiyofuji, H.; Aoki, Y.; Kinoshita, J.; Ohashi, S.; Fujioka, K. A Conceptual Model of Skipjack Tuna in the Western and Central Pacific Ocean (WCPO) for the Spatial Structure Configuration; WCPFC-SC15-2019/SA-WP-11; Western and Central Pacific Fisheries Commission: Pohnpei, Federated States of Micronesia, 2019; 23p. [Google Scholar]
- Itano, D.G. The Reproductive Biology of Yellowfin Tuna (Thunnus albacares) in Hawaiian Waters and the Western Tropical Pacific Ocean: Project Summary; Joint Institute for Marine and Atmospheric Research, University of Hawaii: Honolulu, HI, USA, 2000; 69p. [Google Scholar]
- Farley, J.H.; Williams, A.J.; Hoyle, S.D.; Davies, C.R.; Nicol, S.J. Reproductive dynamics and potential annual fecundity of South Pacific albacore tuna (Thunnus alalunga). PLoS ONE 2013, 8, e60577. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Honma, M.; Ueyanagi, S.; Kikawa, S. Average Distribution of Larvae of Oceanic Species of Scombrid Fishes, 1956–1981; Far Seas Fisheries Research Laboratory, S Series 12; J-GLOBAL: Chūō, Tokyo, 1985; 99p. [Google Scholar]
- Moore, B.R.; Adams, T.; Allain, V.; Bell, J.D.; Bigler, M.; Bromhead, D.; Clark, S.; Davies, C.; Evans, K.; Faasili, U., Jr.; et al. Defining the stock structures of key commercial tunas in the Pacific Ocean II: Sampling considerations and future directions. Fish. Res. 2020, 230, 105524. [Google Scholar] [CrossRef]
- Schaefer, K.M.; Fuller, D.W.; Miyabe, N. Reproductive biology of bigeye tuna (Thunnus obesus) in the eastern and central Pacific Ocean. Bull. Inter-Am. Trop. Tuna Comm. 2005, 23, 3–20. [Google Scholar]
- Schaefer, K.M.; Fuller, D.W. Spatiotemporal variability in the reproductive dynamics of skipjack tuna (Katsuwonus pelamis) in the eastern Pacific Ocean. Fish. Res. 2019, 209, 1–13. [Google Scholar] [CrossRef]
- Bell, J.D.; Ganachaud, A.; Gehrke, P.C.; Griffiths, S.P.; Hobday, A.J.; Hoegh-Guldberg, O.; Johnson, J.E.; le Borgne, R.; Lehodey, P.; Lough, J.M.; et al. Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Change 2013, 3, 591–599. [Google Scholar] [CrossRef]
- Harley, S.; Williams, P. A Compendium of Fisheries Indicators for Bigeye, Skipjack, Yellowfin, and South Pacific Albacore Tunas; WCPFC-SC9-2013/SA-WP-06; Western and Central Pacific Fisheries Commission: Pohnpei, Federated States of Micronesia, 2013; 35p. [Google Scholar]
- Fonteneau, A.; Hallier, J.P. Fifty years of dart tag recoveries for tropical tuna: A global comparison of results for the western Pacific, eastern Pacific, Atlantic, and Indian Oceans. Fish. Res. 2015, 163, 7–22. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertignac, M.; Hampton, J.; Lewis, A.; Picaut, J. El Niño Southern Oscillation and tuna in the western Pacific. Nature 1997, 389, 715–718. [Google Scholar] [CrossRef]
- Prince, E.D.; Goodyear, C.P. Hypoxia-based habitat compression of tropical pelagic fishes. Fish. Oceanogr. 2006, 15, 451–464. [Google Scholar] [CrossRef]
- Bertrand, A. Le système ton—Environnement en Polynésie française: Caractérisation de l’habitat pélagique, étude de la distribution et de la capturabilité de thons, par méthodes acoustiques et halieutiques. Ph.D. Thesis, École nationale supérieure agronomique de Rennes, Rennes, France, 1999; 315p. [Google Scholar]
- Wells, R.D.; Rooker, J.R.; Itano, D.G. Nursery origin of yellowfin tuna in the Hawaiian Islands. Mar. Ecol. Prog. Ser. 2012, 461, 187–196. [Google Scholar] [CrossRef][Green Version]
- Schaefer, K.M.; Fuller, D.W.; Block, B.A. Movements, behavior, and habitat utilization of yellowfin tuna (Thunnus albacares) in the Pacific Ocean off Baja California, Mexico, determined from archival tag data analyses, including unscented Kalman filtering. Fish. Res. 2011, 112, 22–37. [Google Scholar] [CrossRef]
- Harrison, A.L.; Costa, D.P.; Winship, A.J.; Benson, S.R.; Bograd, S.J.; Antolos, M.; Carlisle, A.B.; Dewar, H.; Dutton, P.H.; Jorgensen, S.J.; et al. The political biogeography of migratory marine predators. Nat. Ecol. Evol. 2018, 2, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Block, B.A.; Jonsen, I.D.; Jorgensen, S.J.; Winship, A.J.; Shaffer, S.A.; Bograd, S.J.; Hazen, E.L.; Foley, D.G.; Breed, G.A.; Harrison, A.L.; et al. Tracking apex marine predator movements in a dynamic ocean. Nature 2011, 475, 86–90. [Google Scholar] [CrossRef]
- FAO. World Review of Highly Migratory Species and Straddling Stocks; FAO Fisheries Technical Paper No. 337; FAO: Rome, Italy, 1994; 70p. [Google Scholar]
- Bremer, J.A.; Stéquert, B.; Robertson, N.W.; Ely, B. Genetic evidence for inter-oceanic subdivision of bigeye tuna (Thunnus obesus) populations. Mar. Biol. 1998, 132, 547–557. [Google Scholar] [CrossRef]
- Chiang, H.C.; Hsu, C.C.; Lin, H.D.; Ma, G.C.; Chiang, T.Y.; Yang, H.Y. Population structure of bigeye tuna (Thunnus obesus) in the South China Sea, Philippine Sea and western Pacific Ocean inferred from mitochondrial DNA. Fish. Res. 2006, 79, 219–225. [Google Scholar] [CrossRef]
- Hanamoto, E. Effect of oceanographic environment on bigeye tuna distribution. Bull. Jpn. Soc. Fish. Oceanogr. 1987, 51, 201–216. [Google Scholar]
- Whitelaw, A.W.; Unnithan, V.K. Synopsis of the Distribution, Biology and Fisheries of the Bigeye Tuna (Thunnus obesus, Lowe) with a Bibliography; CSIRO Marine Laboratories: Clayton, Australia, 1997; Volume 228, 62p. [Google Scholar]
- Lehodey, P.; Senina, I.; Sibert, J.; Bopp, L.; Calmettes, B.; Hampton, J.; Murtugudde, R. Preliminary forecasts of Pacific bigeye tuna population trends under the A2 IPCC scenario. Prog. Oceanogr. 2010, 86, 302–315. [Google Scholar] [CrossRef]
- Zhu, G.; Dai, X.; Xu, L.; Zhou, Y. Reproductive biology of Bigeye Tuna, Thunnus obesus, (Scombridae) in the eastern and central tropical Pacific Ocean. Environ. Biol. Fishes 2010, 88, 253–260. [Google Scholar] [CrossRef]
- Hampton, J.; Bigelow, K.; Labelle, M. A Summary of Current Information on the Biology, Fisheries and Stock Assessment of Bigeye Tuna (Thunnus obesus) in the Pacific Ocean, with Recommendations for Data Requirements and Future Research; Oceanic Fisheries Programme Technical Report No. 36; Secretariat of the Pacific Community: Noumea, New Caledonia, 1998; 46p. [Google Scholar]
- Sibert, J.R.; Musyl, M.K.; Brill, R.W. Horizontal movements of bigeye tuna (Thunnus obesus) near Hawaii determined by Kalman filter analysis of archival tagging data. Fish. Oceanogr. 2003, 12, 141–151. [Google Scholar] [CrossRef]
- Lewis, A.D. South Pacific Albacore Tuna Stock Structure: A Review of Available Information; Working Paper No. 5; South Pacific Commission: Noumea, New Caledonia, 1990; 13p, Available online: www.fao.org/3/T1817E/T1817E10.htm (accessed on 30 November 2021).
- Dhurmeea, Z.; Zudaire, I.; Chassot, E.; Cedras, M.; Nikolic, N.; Bourjea, J.; West, W.; Appadoo, C.; Bodin, N. Reproductive biology of albacore tuna (Thunnus alalunga) in the Western Indian Ocean. PLoS ONE 2016, 11, e0168605. [Google Scholar] [CrossRef]
- Arnold, G.P. Fish migration, horizontal. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Thorpe, S.A., Turekian, K.K., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 402–410. [Google Scholar] [CrossRef]
- Murray, T. A review of the biology and fisheries for albacore, Thunnus alalunga, in the south Pacific Ocean. In Interactions of Pacific Tuna Fisheries; Shomura, R.S., Majkowski, J., Langi, S., Eds.; FAO Fisheries Technical Paper. No. 336; FAO: Rome, Italy, 1993; Volume 2, pp. 188–206. [Google Scholar]
- Childers, J.; Snyder, S.; Kohin, S. Migration and behavior of juvenile North Pacific albacore (Thunnus alalunga). Fish. Oceanogr. 2011, 20, 157–173. [Google Scholar] [CrossRef]
- Uosaki, K. Preliminary results obtained from tagging of North Pacific albacore with archival tag. Collect. Vol. Sci. Pap. ICCAT 2004, 56, 1496–1503. [Google Scholar]
- Fujioka, K.; Masujima, M.; Boustany, A.M.; Kitagawa, T. Horizontal movements of Pacific bluefin tuna. In Biology and Ecology of Bluefin Tuna; Kitagawa, T., Kimura, S., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 101–122. [Google Scholar]
- Boustany, A.M.; Matteson, R.; Castleton, M.; Farwell, C.; Block, B.A. Movements of Pacific bluefin tuna (Thunnus orientalis) in the Eastern North Pacific revealed with archival tags. Prog. Oceanogr. 2010, 86, 94–104. [Google Scholar] [CrossRef]
- Madigan, D.J.; Baumann, Z.; Carlisle, A.B.; Hoen, D.K.; Popp, B.N.; Dewar, H.; Snodgrass, O.E.; Block, B.A.; Fisher, N.S. Reconstructing transoceanic migration patterns of Pacific bluefin tuna using a chemical tracer toolbox. Ecology 2014, 95, 1674–1683. [Google Scholar] [CrossRef]
- Kitagawa, T.; Boustany, A.M.; Farwell, C.J.; Williams, T.D.; Castleton, M.R.; Block, B.A. Horizontal and vertical movements of juvenile bluefin tuna (Thunnus orientalis) in relation to seasons and oceanographic conditions in the eastern Pacific Ocean. Fish. Oceanogr. 2007, 16, 409–421. [Google Scholar] [CrossRef]
- Secor, D.H. Migration Ecology of Marine Fishes; John Hopkins University Press: Baltimore, MD, USA, 2015; 304p. [Google Scholar]
- Itoh, T.; Tsuji, S.; Nitta, A. Migration patterns of young Pacific bluefin tuna (Thunnus orientalis) determined with archival tags. Fish. Bull. 2003, 101, 514–534. [Google Scholar]
- Fournier, D.; Sibert, J.; Majkowski, J.; Hampton, J. MULTIFAN a Likelihood-Based Method for Estimating Growth Parameters and Age Composition from Multiple Length Frequency Data Sets Illustrated using Data for Southern Bluefin Tuna (Thunnus maccoyii). Can. J. Fish. Aquat. Sci. 1990, 45, 301–317. [Google Scholar] [CrossRef]
- Palko, J.B.; Beardsley, G.L.; Richards, W.J. Synopsis of the Biological Data on Dolphin-Fishes, Coryphaena hippurus and Coryphaena equiselis Linnaeus; NOAA Technical Report NMFS Circular 443, FAO Fisheries Synopsis No. 130; National Marine Fisheries: Silver Spring, MD, USA, 1982; 28p. [Google Scholar]
- Braun, C.D.; Kaplan, M.B.; Horodysky, A.Z.; Llopiz, J.K. Satellite telemetry reveals physical processes driving billfish behavior. Anim. Biotelem. 2015, 3, 2. [Google Scholar] [CrossRef]
- Su, N.J.; Chang, C.H.; Hu, Y.T.; Chiang, W.C.; Tseng, C.T. Modeling the Spatial Distribution of Swordfish (Xiphias gladius) Using Fishery and Remote Sensing Data: Approach and Resolution. Remote Sens. 2020, 12, 947. [Google Scholar] [CrossRef]
- Evans, K.; Kolody, D.; Abascal, F.; Holdsworth, J.; Maru, P.; Sippel, T. Spatial Dynamics of Swordfish in the South Pacific Ocean Inferred from Tagging Data. In Proceedings of the Scientific Committee Eighth Regular Session, Busan, Korea, 7–15 August 2012; Western and Central Pacific Fisheries Commission: Busan, Korea, 2012. [Google Scholar]
- Evans, K.; Abascal, F.; Kolody, D.; Sippel, T.; Holdsworth, J.; Maru, P. The horizontal and vertical dynamics of swordfish in the South Pacific Ocean. J. Exp. Mar. Biol. Ecol. 2014, 450, 55–67. [Google Scholar] [CrossRef]
- Sakagawa, G.T.; Bell, R.R. Swordfish, Xiphias gladius. In Summary Report of the Billfish Stock Assessment Workshop: Pacific Resources; Shomura, R.S., Ed.; NOAA Technical Memorandum NMFS-SWC5; US Department of Commerce: Seattle, WA, USA, 1980; pp. 43–55. [Google Scholar]
- Davies, N.; Pilling, G.; Harley, S.; Hampton, J. Stock assessment of swordfish (Xiphias gladius) in the southwest Pacific Ocean. In Proceedings of the Scientific Committee Ninth Regular Session, Pohnpei, Federated States of Micronesia, 6–14 August 2013; Western and Central Pacific Fisheries Commission: Pohnpei, Federated States of Micronesia, 2013. [Google Scholar]
- Bremer, J.A.R.; Hinton, M.G.; Greig, T.W. Evidence of spatial genetic heterogeneity in Pacific swordfish (Xiphias gladius) revealed by the analysis of ldh-A sequences. Bull. Mar. Sci. 2006, 79, 493–503. [Google Scholar]
- Hinton, M.G. Status of swordfish stocks in the eastern Pacific Ocean estimated using data from Japanese tuna longline fisheries. Mar. Freshw. Res. 2003, 54, 393–399. [Google Scholar] [CrossRef]
- Reeb, C.A.; Arcangeli, L.; Block, B.A. Structure and migration corridors in Pacific populations of the Swordfish Xiphias gladius, as inferred through analyses of mitochondrial DNA. Mar. Biol. 2000, 136, 1123–1131. [Google Scholar] [CrossRef]
- Kasapidis, P.; Magoulas, A.; García-Cortés, B.; Mejuto-Garcia, J. Stock Structure of Swordfish (Xiphias gladius) in the Pacific Ocean Using Microsatellite DNA Markers; WCPFC-SC4-2008/BI-WP-4; Western and Central Pacific Fisheries Commission: Port Moresby, Papua New Guinea, 2008; 12p. [Google Scholar]
- ISC. Report of the eighteenth meeting of the international scientific committee for tuna and tuna-like species in the North Pacific Ocean. In Proceedings of the International Scientific Committee for Tuna and Tuna-like Species in the North Pacific Ocean, Yeosu, Korea, 11–16 July 2018. [Google Scholar]
- Lessa, R.; Santana, F.M. Growth of the dolphinfish Coryphaena hippurus from north-eastern Brazil with an appraisal of the efficacy of scales and otoliths for ageing. J. Fish Biol. 2016, 89, 977–989. [Google Scholar] [CrossRef]
- Maggio, T.; Allegra, A.; Andaloro, F.; Barreiros, J.P.; Battaglia, P.; Butler, C.M.; Cuttitta, A.; Fontes, J.M.R.; Freitas, R.; Gatt, M.; et al. Historical separation and present-day structure of common dolphinfish (Coryphaena hippurus) populations in the Atlantic Ocean and Mediterranean Sea. ICES J. Mar. Sci. 2019, 76, 1028–1038. [Google Scholar] [CrossRef]
- Torrejón-Magallanes, J.; Grados, D.; Lau-Medrano, W. Spatio-temporal distribution modeling of dolphinfish (Coryphaena hippurus) in the Pacific Ocean off Peru using artisanal longline fishery data. Deep Sea Res. Part II Top. Stud. Oceanogr. 2019, 169–170, 104665. [Google Scholar] [CrossRef]
- Díaz-Jaimes, P.; Uribe-Alcocer, M.; Rocha-Olivares, A.; García-de-León, F.J.; Nortmoon, P.; Durand, J.D. Global phylogeography of the dolphinfish (Coryphaena hippurus): The influence of large effective population size and recent dispersal on the divergence of a marine pelagic cosmopolitan species. Mol. Phylogenet. Evol. 2010, 57, 1209–1218. [Google Scholar] [CrossRef]
- Marín-Enríquez, E.; Seoane, J.; Muhlia-Melo, A. Environmental modeling of occurrence of dolphinfish (Coryphaena spp.) in the Pacific Ocean off Mexico reveals seasonality in abundance, hot spots and migration patterns. Fish. Oceanogr. 2018, 27, 28–40. [Google Scholar] [CrossRef]
- Uchiyama, J.H.; Boggs, C.H. Length-weight relationships of dolphinfish, Coryphaena hippurus, and wahoo, Acanthocybium solandri: Seasonal effects of spawning and possible migration in the central North Pacific. Mar. Fish. Rev. 2006, 68, 19–29. [Google Scholar]
- Merten, W.; Appeldoorn, R.; Hammond, D. Movement dynamics of dolphinfish (Coryphaena hippurus) in the northeastern Caribbean Sea: Evidence of seasonal re-entry into domestic and international fisheries throughout the western central Atlantic. Fish. Res. 2016, 175, 24–34. [Google Scholar] [CrossRef]
- Hopper, C.N. Patterns of Pacific blue marlin reproduction in Hawaiian waters. In Planning the Future of Billfishes; Stroud, R., Ed.; National Coalition for Marine Conservation, Inc.: Savannah, GA, USA, 1990; pp. 123–136. [Google Scholar]
- Salvadeo, C.; Auliz-Ortiz, D.M.; Petatán-Ramírez, D.; Reyes-Bonilla, H.; Ivanova-Bonchera, A.; Juárez-León, E. Potential poleward distribution shift of dolphinfish (Coryphaena hippurus) along the southern California Current System. Environ. Biol. Fishes 2020, 103, 973–984. [Google Scholar] [CrossRef]
- Flores, M.S.Z.; Ortega-García, S.; Klett-Traulsen, A. Interannual and seasonal variation of dolphinfish (Coryphaena hippurus) catch rates in the southern Gulf of California, Mexico. Fish. Res. 2008, 94, 13–17. [Google Scholar] [CrossRef]
- Lasso, J.; Zapata, L. Fisheries and biology of Coryphaena hippurus (Pisces: Coryphaenidae) in the Pacific coast of Colombia and Panama. Sci. Mar. 1999, 63, 387–399. [Google Scholar] [CrossRef]
- Perle, C.R.; Snyder, S.; Merten, W.; Simmons, M.; Dacey, J.; Rodriguez-Sanchez, R.; O’Sullivan, J.; Ortega-Garcia, S. Dolphinfish movements in the Eastern Pacific Ocean of Mexico using conventional and electronic tags. Anim. Biotelem. 2020, 8, 30. [Google Scholar] [CrossRef]
- Farrell, E.R.; Boustany, A.M.; Halpin, P.N.; Hammond, D.L. Dolphinfish (Coryphaena hippurus) distribution in relation to biophysical ocean conditions in the northwest Atlantic. Fish. Res. 2014, 151, 177–190. [Google Scholar] [CrossRef]
- Lin, S.J.; Chiang, W.C.; Musyl, M.K.; Wang, S.P.; Su, N.J.; Chang, Q.X.; Ho, Y.S.; Nakamura, I.; Tseng, C.T.; Kawabe, R. Movements and Habitat Use of Dolphinfish (Coryphaena hippurus) in the East China Sea. Sustainability 2020, 12, 5793. [Google Scholar] [CrossRef]
- Palacios-Abrantes, J.; Frölicher, T.L.; Reygondeau, G.; Sumaila, U.R.; Tagliabue, A.; Wabnitz, C.C.C.; Cheung, W.W.L. Timing and magnitude of climate-driven range shifts in transboundary fish stocks challenge their management. Glob. Change Biol. 2022, 28, 2312–2326. [Google Scholar] [CrossRef] [PubMed]
- Tripp-Valdez, M.A.; de León, F.J.G.; Ortega-García, S.; Lluch-Cota, D.; López-Martínez, J.; Cruz, P. Population genetic structure of dolphinfish (Coryphaena hippurus) in the Gulf of California, using microsatellite loci. Fish. Res. 2010, 105, 172–177. [Google Scholar] [CrossRef]
- Kraul, S. Seasonal abundance of the dolphinfish, Coryphaena hippurus, in Hawaii and the tropical Pacific Ocean. Sci. Mar. 1999, 63, 261–266. [Google Scholar] [CrossRef]
- Guzman, H.M.; Díaz-Ferguson, E.; Vega, A.J.; Robles, Y.A. Assessment of the dolphinfish Coryphaena hippurus (Perciformes: Coryphaenidae) fishery in Pacific Panama. Rev. Biol. Trop. 2015, 63, 705–716. [Google Scholar] [CrossRef]
- Chang, H.Y.; Sun, C.L.; Yeh, S.Z.; Chang, Y.J.; Su, N.J.; DiNardo, G. Reproductive biology of female striped marlin Kajikia audax in the western Pacific Ocean. J. Fish Biol. 2018, 92, 105–130. [Google Scholar] [CrossRef]
- McDowell, J.R.; Graves, J.E. Population structure of striped marlin (Kajikia audax) in the Pacific Ocean based on analysis of microsatellite and mitochondrial DNA. Can. J. Fish. Aquat. Sci. 2008, 65, 1307–1320. [Google Scholar] [CrossRef]
- Lam, C.H.; Kiefer, D.A.; Domeier, M.L. Habitat characterization for striped marlin in the Pacific Ocean. Fish. Res. 2015, 166, 80–91. [Google Scholar] [CrossRef]
- Holdsworth, J.C.; Sippel, T.J.; Block, B.A. Near real time satellite tracking of striped marlin (Kajikia audax) movements in the Pacific Ocean. Mar. Biol. 2009, 156, 505–514. [Google Scholar] [CrossRef]
- Domeier, M.L.; Ortega-Garcia, S.; Nasby-Lucas, N.; Offield, P. First marlin archival tagging study suggests new direction for research. Mar. Freshw. Res. 2019, 70, 603–608. [Google Scholar] [CrossRef]
- Sippel, T.; Holdsworth, J.; Dennis, T.; Montgomery, J. Investigating behaviour and population dynamics of striped marlin (Kajikia audax) from the southwest Pacific Ocean with satellite tags. PLoS ONE 2011, 6, e21087. [Google Scholar] [CrossRef]
- Purcell, C.M.; Edmands, S. Resolving the genetic structure of striped marlin, Kajikia audax, in the Pacific Ocean through spatial and temporal sampling of adult and immature fish. Can. J. Fish. Aquat. Sci. 2011, 68, 1861–1875. [Google Scholar] [CrossRef]
- Su, N.J.; Sun, C.L.; Punt, A.E.; Yeh, S.Z.; DiNardo, G.; Chang, Y.J. An ensemble analysis to predict future habitats of striped marlin (Kajikia audax) in the North Pacific Ocean. ICES J. Mar. Sci. 2013, 70, 1013–1022. [Google Scholar] [CrossRef]
- Su, N.J.; Sun, C.L.; Punt, A.E.; Yeh, S.Z.; DiNardo, G. Environmental influences on seasonal movement patterns and regional fidelity of striped marlin Kajikia audax in the Pacific Ocean. Fish. Res. 2015, 166, 59–66. [Google Scholar] [CrossRef]
- Kurashima, A.; Ijima, H.; Semba, Y. Review of horizontal migration of swordfish, striped marlin and blue marlin using electrical tags. In Proceedings of the Billfish Working Group Workshop, Taipei, Chinese-Taipei, 30 January–3 February 2020. ISC/20/BILLWG-01/02; 16p. [Google Scholar]
- Rohner, C.A.; Bealey, R.; Fulanda, B.M.; Pierce, S.J. Movement and habitat use of striped marlin Kajikia audax in the Western Indian Ocean. J. Fish Biol. 2020, 97, 1415–1427. [Google Scholar] [CrossRef]
- González-Armas, R.; Klett-Traulsen, A.; Hernández-Herrera, A. Evidence of billfish reproduction in the southern Gulf of California, Mexico. Bull. Mar. Sci. 2006, 79, 705–717. [Google Scholar]
- Piner, K.R.; Lee, H.H.; Kimoto, A.; Taylor, I.G.; Kanaiwa, M.; Sun, C.L. Population dynamics and status of striped marlin (Kajikia audax) in the western and central northern Pacific Ocean. Mar. Freshw. Res. 2013, 64, 108–118. [Google Scholar] [CrossRef]
- Kopf, R.K.; Davie, P.S.; Bromhead, D.B.; Young, J.W. Reproductive biology and spatiotemporal patterns of spawning in striped marlin Kajikia audax. J. Fish Biol. 2012, 81, 1834–1858. [Google Scholar] [CrossRef]
- Humphreys, R.; Brodziak, J. Reproductive maturity of striped marlin (Kajikia audax), in the central North Pacific off Hawaii. In Proceedings of the Billfish Working Group Workshop, Honolulu, HI, USA, 8–15 May 2019. [Google Scholar]
- Acosta-Pachón, T.A.; Martínez-Rincón, R.O.; Hinton, M.G. Habitat preferences of striped marlin (Kajikia audax) in the eastern Pacific Ocean. Fish. Oceanogr. 2017, 26, 615–624. [Google Scholar] [CrossRef]
- Kopf, R.K.; Davie, P.S.; Bromhead, D.; Pepperell, J.G. Age and growth of striped marlin (Kajikia audax) in the Southwest Pacific Ocean. ICES J. Mar. Sci. 2011, 68, 1884–1895. [Google Scholar] [CrossRef]
- Shimose, T.; Ashidaand, H.; Yokawa, K. Sex ratio and reproductive condition of four istiophorid billfishes in tropical regions of the eastern North Pacific Ocean: With special reference to striped marlin Kajikia audax (Philippi, 1887). J. Appl. Ichthyol. 2013, 29, 1247–1251. [Google Scholar] [CrossRef]
- Lien, Y.H.; Su, N.J.; Sun, C.L.; Punt, A.E.; Yeh, S.Z.; DiNardo, G. Spatial and environmental determinants of the distribution of Striped Marlin (Kajikia audax) in the western and central North Pacific Ocean. Environ. Biol. Fishes 2014, 97, 267–276. [Google Scholar] [CrossRef]
- Chiang, W.C.; Chang, C.T.; Madigan, D.J.; Carlisle, A.B.; Musyl, M.K.; Chang, Y.C.; Hsu, H.H.; Su, N.J.; Sun, C.L.; Ho, Y.S.; et al. Stable isotope analysis reveals feeding ecology and trophic position of black marlin off eastern Taiwan. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 175, 104821. [Google Scholar] [CrossRef]
- Collette, B.; McDowell, J.; Graves, J. Phylogeny of recent billfishes (Xiphioidei). Bull. Mar. Sci. 2006, 79, 455–468. [Google Scholar]
- Sun, C.L.; Chang, H.Y.; Liu, T.Y.; Yeh, S.Z.; Chang, Y.J. Reproductive biology of the black marlin, Istiompax indica, off southwestern and eastern Taiwan. Fish. Res. 2015, 166, 12–20. [Google Scholar] [CrossRef]
- Chiang, W.C.; Musyl, M.K.; Sun, C.L.; DiNardo, G.; Hung, H.M.; Lin, H.C.; Chen, S.C.; Yeh, S.-Z.; Chen, W.Y.; Kuo, C.L. Seasonal movements and diving behaviour of black marlin (Istiompax indica) in the northwestern Pacific Ocean. Fish. Res. 2015, 166, 92–102. [Google Scholar] [CrossRef]
- Nakamura, I. FAO Species Catalogue Vol. 5. Billfishes of the World. An Annotated and Illustrated Catalogue of Marlins, Sailfishes, Spearfishes and Swordfishes Known to Date; FAO Fisheries Synopsis; FAO: Rome, Italy, 1985; 65p. [Google Scholar]
- Domeier, M.L.; Speare, P. Dispersal of adult black marlin (Istiompax indica) from a Great Barrier Reef spawning aggregation. PLoS ONE 2012, 7, e31629. [Google Scholar] [CrossRef]
- Farchadi, N.; Hinton, M.G.; Thompson, A.R.; Yin, Z.Y. Habitat Preferences of Blue Marlin (Makaira nigricans) and Black Marlin (Istiompax indica) in the Eastern Pacific Ocean. Master’s Thesis, University of San Diego, San Diego, CA, USA, 2018; 87p. Available online: digital.sandiego.edu/theses/32 (accessed on 30 November 2021).
- Kleiber, P.; Hinton, M.G.; Uozumi, Y. Stock assessment of blue marlin (Makaira nigricans) in the Pacific using MULTIFAN-CL. Mar. Freshw. Res. 2003, 54, 349–360. [Google Scholar] [CrossRef]
- Ortiz, M.; Prince, E.D.; Serafy, J.E.; Holts, D.B.; Davy, K.B.; Pepperell, J.G.; Lowry, M.B.; Holdsworth, J.C. Global overview of the major constituent-based billfish tagging programs and their results since 1954. Mar. Freshw. Res. 2003, 54, 489–507. [Google Scholar] [CrossRef]
- Pepperell, J.C. Brief synopsis of the biology of the blue marlin (Makaira nigricans), with reference to the Indian Ocean. In IOTC Proceedings No. 3; IOTC-2000-WPB-10; Indian Ocean Tuna Commission, FAO: Victoria Mahé, Seychelles, 2000; pp. 214–227. [Google Scholar]
- Graves, J.E.; McDowell, J.R. Stock structure of the world’s istiophorid billfishes: A genetic perspective. Mar. Freshw. Res. 2003, 54, 287–298. [Google Scholar] [CrossRef]
- Hill, N.J.; Tobin, A.J.; Reside, A.E.; Pepperell, J.G.; Bridge, T.C. Dynamic habitat suitability modelling reveals rapid poleward distribution shift in a mobile apex predator. Glob. Change Biol. 2016, 22, 1086–1096. [Google Scholar] [CrossRef]
- Williams, S.M.; Bennett, M.B.; Pepperell, J.G.; Morgan, J.A.; Ovenden, J.R. Spatial genetic subdivision among populations of the highly migratory black marlin Istiompax indica within the central Indo-Pacific. Mar. Freshw. Res. 2016, 67, 1205–1214. [Google Scholar] [CrossRef]
- Williams, S.M.; Holmes, B.J.; Tracey, S.R.; Pepperell, J.R.; Domeier, M.L.; Bennett, M.B. Environmental influences and ontogenetic differences in vertical habitat use of black marlin (Istiompax indica) in the southwestern Pacific. R. Soc. Open Sci. 2017, 4, 170694. [Google Scholar] [CrossRef]
- Williams, S.M. The Global Biology, Ecology and Phylogenetic Status of Black Marlin (Istiompax indica). Ph.D. Thesis, University of Queensland, Brisbane, QLD, Australia, 2018. [Google Scholar]
- Widodo, A.A.; Satria, F.; Nugraha, B. Size and fishing ground of wahoo (Acanthocybium solandri Cuvier, 1832) from catch data of tuna longline operated in Indian ocean. Indones. Fish. Res. J. 2012, 18, 101–106. [Google Scholar] [CrossRef][Green Version]
- Sepulveda, C.A.; Aalbers, S.A.; Ortega-Garcia, S.; Wegner, N.C.; Bernal, D. Depth distribution and temperature preferences of wahoo (Acanthocybium solandri) off Baja California Sur, Mexico. Mar. Biol. 2011, 158, 917–926. [Google Scholar] [CrossRef]
- Gao, C.; Tian, S.; Kindong, R.; Dai, X. Biology and environmental preferences of wahoo, Acanthocybium solandri (Cuvier, 1832), in the Western and Central Pacific Ocean (WCPO). J. Mar. Sci. Eng. 2020, 8, 184. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. 2021. Available online: www.fishbase.org (accessed on 25 November 2021).
- Zischke, M.T. A review of the biology, stock structure, fisheries and status of wahoo (Acanthocybium solandri), with reference to the Pacific Ocean. Fish. Res. 2012, 199–120, 13–22. [Google Scholar] [CrossRef]
- Feeney, R.F.; Lea, R.N. Records of wahoo, Acanthocybium solandri (Scombridae), from California. Bull. South. Calif. Acad. Sci. 2016, 115, 198–200. [Google Scholar] [CrossRef][Green Version]
- Garber, A.F.; Tringali, M.D.; Franks, J.S. Population genetic and phylogeographic structure of wahoo, Acanthocybium solandri, from the western central Atlantic and central Pacific Oceans. Mar. Biol. 2005, 147, 205–214. [Google Scholar] [CrossRef]
- Oyafuso, Z.S.; Toonen, R.J.; Franklin, E.C. Temporal and spatial trends in prey composition of wahoo Acanthocybium solandri: A diet analysis from the central North Pacific Ocean using visual and DNA bar-coding techniques. J. Fish Biol. 2016, 88, 1501–1523. [Google Scholar] [CrossRef]
- Perelman, J.N.; Schmidt, K.N.; Haro, I.; Tibbetts, I.R.; Zischke, M.T. Feeding dynamics, consumption rates and daily ration of wahoo Acanthocybium solandri in Indo-Pacific waters. J. Fish Biol. 2017, 90, 1842–1860. [Google Scholar] [CrossRef]
- Dawson, T.P.; Irving, R.A. Developing a fisheries management plan for the Pitcairn Islands Marine Reserve. In Marine Protected Areas; Elsevier: Amsterdam, The Netherlands, 2020; pp. 271–283. [Google Scholar] [CrossRef]
- Chiang, W.C.; Musyl, M.K.; Sun, C.L.; Chen, S.-Y.; Chen, W.-Y.; Liu, D.-C.; Su, W.-C.; Yeh, S.-Z.; Fu, S.-C.; Huang, T.-L. Vertical horizontal movements of sailfish (Istiophorus platypterus) near Taiwan determined using pop-up satellite tags. J. Exp. Mar. Biol. Ecol. 2011, 397, 129–135. [Google Scholar] [CrossRef]
- IATTC. Report on the Tuna Fishery, Stocks, and Ecosystem in the Eastern Pacific Ocean in 2019. 2019. Available online: https://www.iattc.org/PDFFiles/FisheryStatusReports/_English/No-18-2020_Tunas%20billfishes%20and%20other%20pelagic%20species%20in%20the%20eastern%20Pacific%20Ocean%20in%202019.pdf (accessed on 30 November 2021).
- Lu, C.P.; Bremer, J.R.A.; McKenzie, J.L.; Chiang, W.C. Analysis of sailfish (Istiophorus platypterus) population structure in the North Pacific Ocean. Fish. Res. 2015, 166, 33–38. [Google Scholar] [CrossRef]
- Molony, B. Summary of the biology, ecology and stock status of billfishes in the WCPFC, with a review of major variables influencing longline fishery performance. In Proceedings of the 1st Meeting of the Scientific Committee of the Western and Central Pacific Fisheries Commission (WCPFC-SC1), Noumea, New Caledonia, 8–19 August 2005. [Google Scholar]
- Arizmendi-Rodríguez, D.I.; Abitia-Cárdenas, L.A.; Galván-Magaña, F.; Trejo-Escamilla, I. Food habits of sailfish Istiophorus platypterus off Mazatlan, Sinaloa, Mexico. Bull. Mar. Sci. 2006, 79, 777–791. [Google Scholar]
- Rosas-Alayola, J.; Hernández-Herrera, A.; Galvan-Magaña, F.; Abitia-Cárdenas, L.A.; Muhlia-Melo, A.F. Diet composition of sailfish (Istiophorus platypterus) from the southern Gulf of California, Mexico. Fish. Res. 2002, 57, 185–195. [Google Scholar] [CrossRef]
- Martin, G.; Makinen, A.; Andersson, Å.; Dinesen, G.E.; Kotta, J.; Hansen, J.; Herkül, K.; Ockelmann, K.W.; Nilsson, P.; Korpinen, S. Literature Review of the “Blue Corridors” Concept and Its Applicability to the Baltic Sea; BALANCE Interim Report No. 4; BALANCE: Shinjuku, Tokyo, 2006; 67p. [Google Scholar]
- Pendoley, K.L.; Schofield, G.; Whittock, P.A.; Ierodiaconou, D.; Hays, G.C. Protected species use of a coastal marine migratory corridor connecting marine protected areas. Mar. Biol. 2014, 161, 1455–1466. [Google Scholar] [CrossRef]
- Breen, P.; Posen, P.; Righton, D. Temperate Marine Protected Areas and highly mobile fish: A review. Ocean Coast. Manag. 2015, 105, 75–83. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.A.; McBride, M.F.; Bode, M.; Possingham, H.P. Prioritizing global conservation efforts. Nature 2006, 440, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Morato, T.; Hoyle, S.D.; Allain, V.; Nicol, S.J. Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc. Natl. Acad. Sci. USA 2010, 107, 9707–9711. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M.; Goodell, W.; Giddens, J.; Easton, E.E.; Wagner, D. Deep-sea biodiversity at the extremes of the Salas y Gómez and Nazca ridges with implications for conservation. PLoS ONE 2021, 16, e0253213. [Google Scholar] [CrossRef]
- Hooker, S.K.; Cañadas, A.; Hyrenbach, K.D.; Corrigan, C.; Polovina, J.J.; Reeves, R.R. Making protected area networks effective for marine top predators. Endanger. Species Res. 2011, 13, 203–218. [Google Scholar] [CrossRef]
- Carr, M.H.; Neigel, J.E.; Estes, J.A.; Andelman, S.; Warner, R.R.; Largier, J.L. Comparing marine and terrestrial ecosystems: Implications for the design of coastal marine reserves. Ecol. Appl. 2003, 13 (Suppl. 1), 90–107. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Gaymer, C.F. Progress, opportunities and challenges for marine conservation in the Pacific Islands. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 221–231. [Google Scholar] [CrossRef]
- Hanich, Q.; Campbell, B.; Bailey, M.; Molenaar, E. Research into fisheries equity and fairness—Addressing conservation burden concerns in transboundary fisheries. Mar. Policy 2015, 51, 302–304. [Google Scholar] [CrossRef]
- Collette, B.; Graves, J. Tunas and Billfishes of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Letessier, T.B.; Bouchet, P.J.; Meeuwig, J.J. Sampling mobile oceanic fishes and sharks: Implications for fisheries and conservation planning. Biol. Rev. 2017, 92, 627–646. [Google Scholar] [CrossRef]
- Rayfuse, R. Regional Fisheries Management Organizations. In The Oxford Handbook of the Law of the Sea; Rothwell, D., Elferink, A.O., Scott, K., Stephens, T., Eds.; Oxford University Press: Oxford, UK, 2015; pp. 439–462. [Google Scholar] [CrossRef]










| In Pacific EEZs (% Total Catch within EEZs) | In Pacific High Seas (% Total Catch within High Seas) |
|---|---|
| Katsuwonus pelamis (1.5) | Katsuwonus pelamis (39) |
| Thunnus albacares (0.6) | Thunnus albacares (24) |
| Thunnus obesus (0.2) | Thunnus obesus (14) |
| Thunnus alalunga (0.2) | Thunnus alalunga (6) |
| Scomberomorus commerson (0.2) | Xiphias gladius (2) |
| Scomberomorus niphonius (0.2) | Kajikia audax (0.8) |
| Coryphaena hippurus (0.08) | Coryphaena hippurus (0.7) |
| Fistularia corneta (0.07) | Thunnus orientalis (0.5) |
| Thunnus tonggol (0.06) | Thunnus maccoyii (0.1) |
| Euthynnus affinis (0.06) | Acanthocybium solandri (0.08) |
| Thunnus orientalis (0.05) | Istiompax indica (0.07) |
| Xiphias gladius (0.04) | Istiophorus platypterus (0.03) |
| Kajikia audax (0.03) | - |
| Elegatis bipinnulata (0.03) | - |
| Istiompax indica (0.01) | - |
| Scomberomorus sierra (0.007) | - |
| Acanthocybium solandri (0.005) | - |
| Istiophorus platypterus (0.005) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Relano, V.; Pauly, D. Philopatry as a Tool to Define Tentative Closed Migration Cycles and Conservation Areas for Large Pelagic Fishes in the Pacific. Sustainability 2022, 14, 5577. https://doi.org/10.3390/su14095577
Relano V, Pauly D. Philopatry as a Tool to Define Tentative Closed Migration Cycles and Conservation Areas for Large Pelagic Fishes in the Pacific. Sustainability. 2022; 14(9):5577. https://doi.org/10.3390/su14095577
Chicago/Turabian StyleRelano, Veronica, and Daniel Pauly. 2022. "Philopatry as a Tool to Define Tentative Closed Migration Cycles and Conservation Areas for Large Pelagic Fishes in the Pacific" Sustainability 14, no. 9: 5577. https://doi.org/10.3390/su14095577
APA StyleRelano, V., & Pauly, D. (2022). Philopatry as a Tool to Define Tentative Closed Migration Cycles and Conservation Areas for Large Pelagic Fishes in the Pacific. Sustainability, 14(9), 5577. https://doi.org/10.3390/su14095577







