Ethyl Rosmarinate Prevents the Impairment of Vascular Function and Morphological Changes in L-NAME-Induced Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ethyl Rosmarinate
2.2. Animals
2.3. Effect of Ethyl Rosmarinate on Blood Pressure, Heart Rate, and Body Weight
2.4. Effect of Ethyl Rosmarinate on Vascular Reactivity
2.5. Effect of Ethyl Rosmarinate on Morphological Changse of the Aorta
2.6. Effect of Ethyl Rosmarinate on the Expression of eNOS
2.7. Effect of Ethyl Rosmarinate on Vasorelaxation Associated with eNOS Pathway
2.8. Statistical Analysis
3. Results
3.1. Chronic Effects of Ethyl Rosmarinate on SBP, Heart Rate, and Body Weight
3.2. Ethyl Rosmarinate Attenuated Hypertension-Induced Endothelial Dysfunction
3.2.1. Effect of Ethyl Rosmarinate on Endothelium-Dependent Induced Vasorelaxation
3.2.2. Effect of Ethyl Rosmarinate on Endothelium-Independent Induced Vasorelaxation
3.2.3. Effect of Ethyl Rosmarinate on α1-Adrenergic Receptor-Induced Vasoconstriction
3.3. Morphometric Analysis
3.4. Effect of Ethyl Rosmarinate on the Expression of eNOS in Endothelial Cells
3.5. Effect of Ethyl Rosmarinate on Vasorelaxation Associated with the eNOS Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puddu, P.; Puddu, G.M.; Zaca, F.; Muscari, A. Endothelial dysfunction in hypertension. Acta Cardiol. 2000, 55, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Sessa, W.C. Role of endothelial-derived nitric oxide in hypertension and renal disease. Curr. Opin. Nephrol. Hypertens. 2007, 16, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Simko, F. Is NO the king? Pathophysiological benefit with uncertain clinical impact. Physiol. Res. 2007, 56, S1–S6. [Google Scholar] [PubMed]
- Sanders, D.B.; Kelley, T.; Larson, D. The role of nitric oxide synthase/nitric oxide in vascular smooth muscle control. Perfusion 2000, 15, 97–104. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Podzuweit, T.; Bocker, W.; Samoilova, V.E.; Thomas, S.; Wellner, M.; Baba, H.A.; Robenek, H.; Schnekenburger, J.; Lerch, M.M. Vascular smooth muscle and nitric oxide synthase. FASEB J. 2002, 16, 500–508. [Google Scholar] [CrossRef]
- Zicha, J.; Dobesova, Z.; Kunes, J. Antihypertensive mechanisms of chronic captopril or N-acetylcysteine treatment in L-NAME hypertensive rats. Hypertens. Res. 2006, 29, 1021. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.S.; Park, S.; Kim, W.K. Regular exercise produced cardioprotective effects on rat’s heart with hypertension induced by L-NAME administration. Clin. Exp. Hypertens. 2009, 31, 364–375. [Google Scholar] [CrossRef]
- Alwi, N.A.N.M.; Zakaria, Z.; Karim, A.A.H.; Nordin, N.A.M.M.; Ugusman, A. Antihypertensive Effect of Piper sarmentosum in L-NAME-Induced Hypertensive Rats. Sains Malays. 2018, 47, 2421–2428. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Aeschbach, R.; Prior, E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J. Agric. Food Chem. 1996, 44, 131–135. [Google Scholar] [CrossRef]
- Osakabe, N.; Takano, H.; Sanbongi, C.; Yasuda, A.; Yanagisawa, R.; Inoue, K.I.; Yoshikawa, T. Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 2004, 21, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Swarup, V.; Ghosh, J.; Ghosh, S.; Saxena, A.; Basu, A. Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob. Agents Chemother. 2007, 51, 3367–3370. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Canadanovic-Brunet, J.; Cetkovic, G.; Djilas, S.; Tumbas, V.; Bogdanovic, G.; Mandic, A.; Markov, S.; Cvetkovic, D.; Canadanovic, V. Radical scavenging, antibacterial, and antiproliferative activities of Melissa officinalis L. extracts. J. Med. Food 2008, 11, 133–143. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Chen, W.Y.; Liao, S.L.; Chou, S.T.; Yang, C.P.; Chen, C.J. Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem. Toxicol. 2017, 108, 214–223. [Google Scholar] [CrossRef]
- Ersoy, S.; Orhan, I.; Turan, N.; Sahan, G.; Ark, M.; Tosun, F. Endothelium-dependent induction of vasorelaxation by Melissa officinalis L. ssp. officinalis in rat isolated thoracic aorta. Phytomedicine 2008, 15, 1087–1092. [Google Scholar] [CrossRef]
- Wicha, P.; Tocharus, J.; Nakaew, A.; Pantan, R.; Suksamrarn, A.; Tocharus, C. Ethyl rosmarinate relaxes rat aorta by an endothelium-independent pathway. Eur. J. Pharmacol. 2015, 766, 9–15. [Google Scholar] [CrossRef]
- Thammason, H.; Khetkam, P.; Pabuprapap, W.; Suksamrarn, A.; Kunthalert, D. Ethyl rosmarinate inhibits lipopolysaccharide-induced nitric oxide and prostaglandin E2 production in alveolar macrophages. Eur. J. Pharmacol. 2018, 824, 17–23. [Google Scholar] [CrossRef]
- Ahmad, A.; Dempsey, S.; Daneva, Z.; Azam, M.; Li, N.; Li, P.L.; Ritter, J. Role of nitric oxide in the cardiovascular and renal systems. Int. J. Mol. Sci. 2018, 19, 2605. [Google Scholar] [CrossRef]
- Holecyova, A.; Torok, J.; Bernatova, I.; Pechanova, O. Restriction of nitric oxide rather than elevated blood pressure is responsible for alterations of vascular responses in nitric oxide-deficient hypertension. Physiol. Res. 1996, 45, 317–322. [Google Scholar]
- Kopincova, J.; Puzserova, A.; Bernatova, I. L-NAME in the cardiovascular system—Nitric oxide synthase activator? Pharmacol. Rep. 2012, 64, 511–520. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, B.; Xu, B.; Mi, X.; Li, G.; Ma, C.; Xie, J.; Li, J.; Wang, Z. Rosmarinic acid alleviates the endothelial dysfunction induced by hydrogen peroxide in rat aortic rings via activation of AMPK. Oxidative Med. Cell Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dey, N.; Sellak, H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: From the regulation of tone to gene expression. J. Appl. Physiol. 2001, 91, 1421–1430. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1–R12. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Carvalho, M.H.; Khan, M.T.; Matsunaga, K. Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. J. Vasc. Res. 1987, 24, 145–149. [Google Scholar] [CrossRef]
- Tominaga, M.; Fujii, K.; Abe, I.; Takata, Y.; Kobayashi, K.; Fujishima, M. Hypertension and ageing impair acetylcholine-induced vasodilation in rats. J. Hypertens. 1994, 12, 259–268. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; Zhao, J.; Coey, U.; Green, J. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J. Appl. Physiol. 2005, 98, 629–632. [Google Scholar] [CrossRef]
- Lataro, R.M.; Silva, C.A.A.; Tefe-Silva, C.; Prado, C.M.; Salgado, H.C. Acetylcholinesterase inhibition attenuates the development of hypertension and inflammation in spontaneously hypertensive rats. Am. J. Hypertens. 2015, 28, 1201–1208. [Google Scholar] [CrossRef]
- Martin, G.; Bolofo, M.; Giles, H. Inhibition of endothelium-dependent vasorelaxation by arginine analogues: A pharmacological analysis of agonist and tissue dependence. Br. J. Pharmacol. 1992, 105, 643–652. [Google Scholar] [CrossRef][Green Version]
- Su, C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur. J. Pharmacol. 1985, 114, 93–96. [Google Scholar]
- Kieler-Jensen, N.; Lundin, S.; Ricksten, S.E. Vasodilator therapy after heart transplantation: Effects of inhaled nitric oxide and intravenous prostacyclin, prostaglandin E1, and sodium nitroprusside. J. Heart Lung Transplant. 1995, 14, 436–443. [Google Scholar] [CrossRef]
- Diniz, M.C.; Olivon, V.C.; Tavares, L.D.; Simplicio, J.A.; Gonzaga, N.A.; de Souza, D.G.; Bendhack, L.M.; Tirapelli, C.R.; Bonaventura, D. Mechanisms underlying sodium nitroprusside-induced tolerance in the mouse aorta: Role of ROS and cyclooxygenase-derived prostanoids. Life Sci. 2017, 176, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.H.; Wang, L.Y.; Zhang, B.B.; Hu, Q.M.; Wang, P.; He, B.Q.; Bao, G.H.; Liang, J.Y.; Wu, F.H. Ethyl Rosmarinate Protects High Glucose-Induced Injury in Human Endothelial Cells. Molecules 2018, 23, 3372. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Yoshimi, H.; Takata, S.; Watanabe, T.; Kumagai, S.; Nakajima, K.; Sakakibara, S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1988, 154, 868–875. [Google Scholar] [CrossRef]
- Van Renterghem, C.; Vigne, P.; Barhanin, J.; Schmid Alliana, A.; Frelin, C.; Lazdunski, M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem. Biophys. Res. Commun. 1988, 157, 977–985. [Google Scholar] [CrossRef]
- Giaid, A.; Yanagisawa, M.; Langleben, D.; Michel, R.P.; Levy, R.; Shennib, H.; Kimura, S.; Masaki, T.; Duguid, W.P.; Stewart, D.J. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N. Engl. J. Med. 1993, 328, 1732–1739. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular endothelin in hypertension. Vasc. Pharmacol. 2005, 43, 19–29. [Google Scholar] [CrossRef]
- Kohan, D.E. Endothelin, hypertension, and chronic kidney disease: New insights. Curr. Opin. Nephrol. Hypertens. 2010, 19, 134. [Google Scholar] [CrossRef]
- Houde, M.; Desbiens, L.; D’Orleans-Juste, P. Endothelin-1: Biosynthesis, Signaling and Vasoreactivity. Adv. Pharmacol. 2016, 77, 143–175. [Google Scholar]

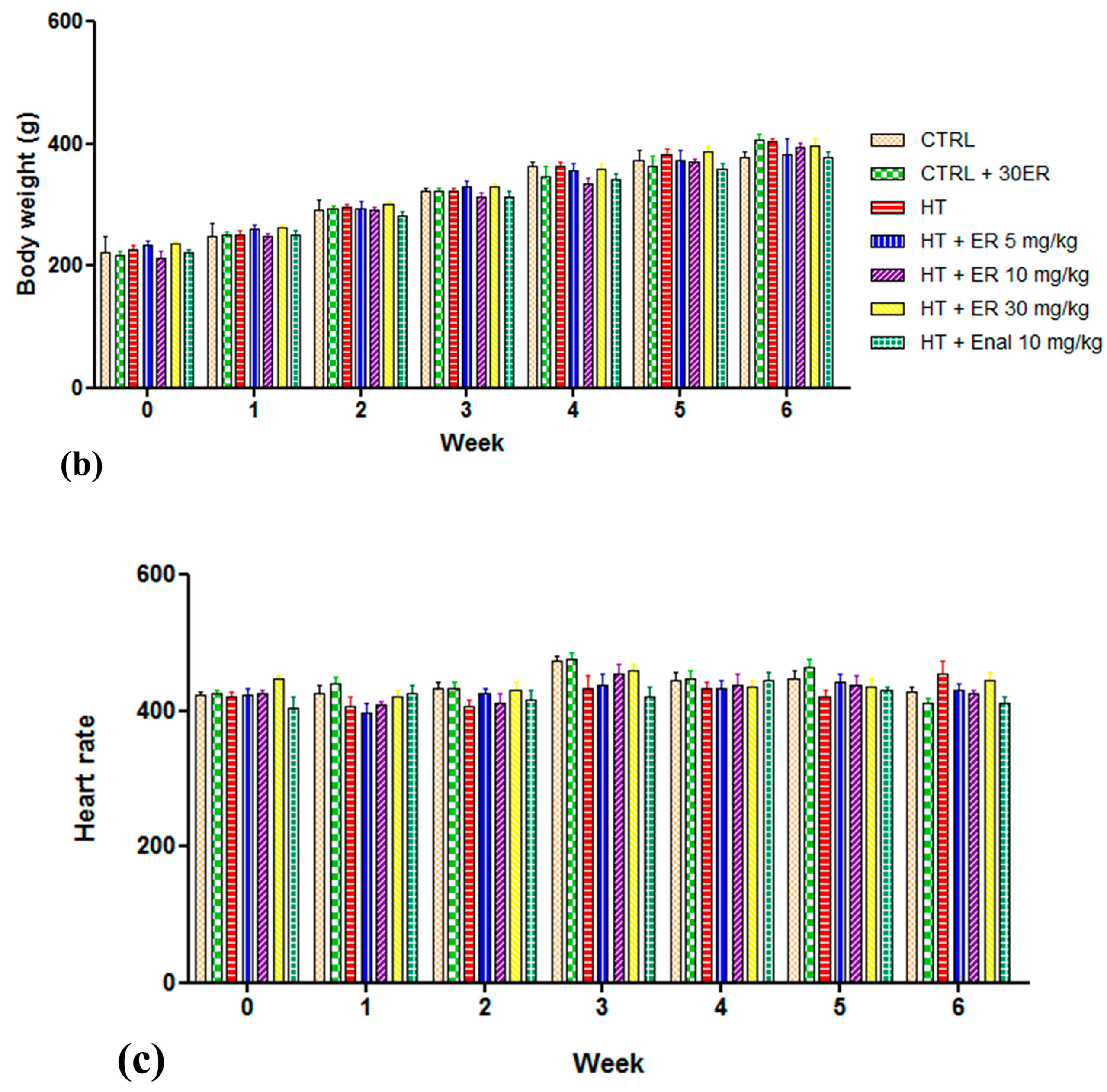



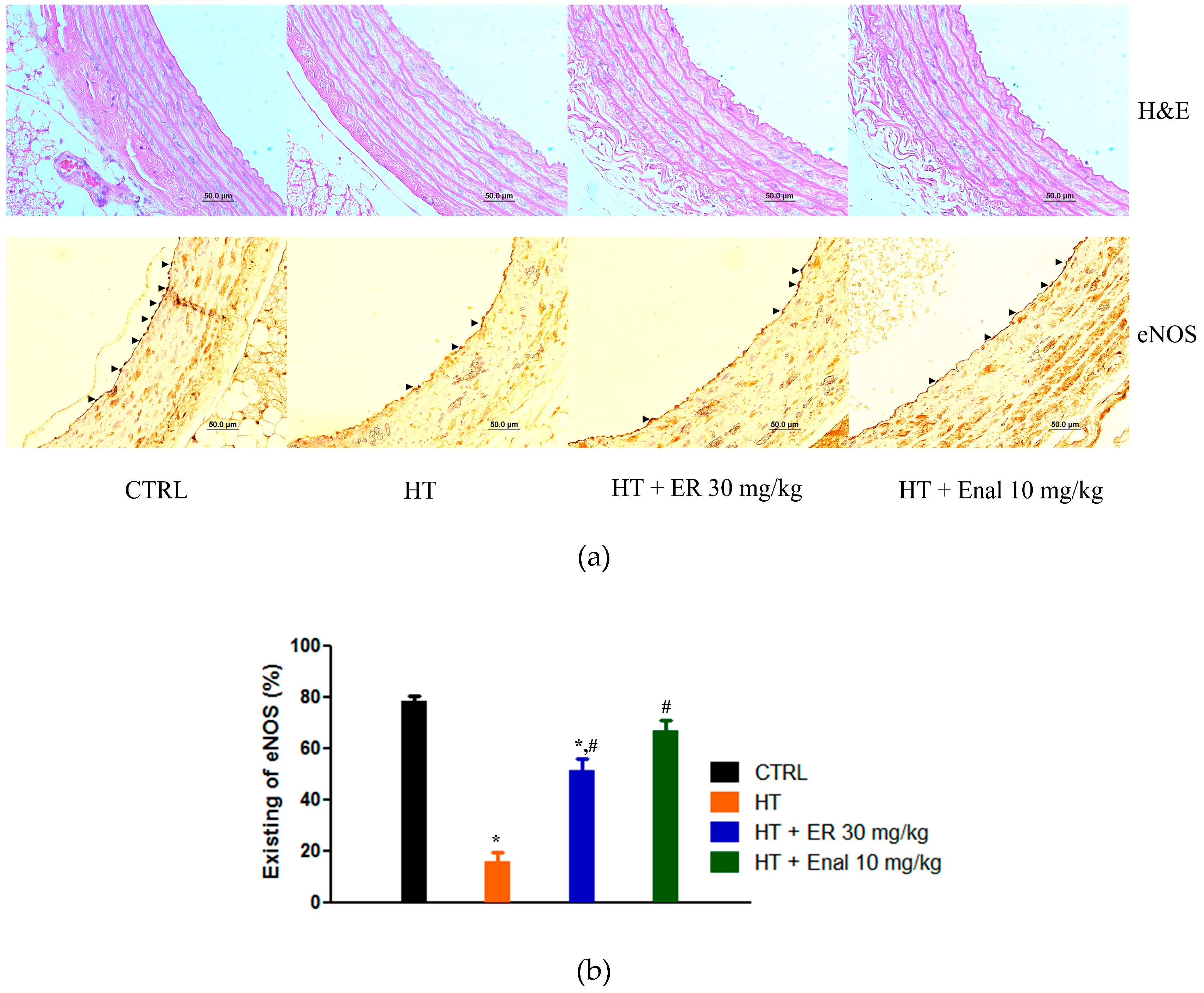

| Parameters | CTRL | ER (30 mg/kg) | HT | HT + ER (5 mg/kg) | HT + ER (15 mg/kg) | HT + ER (30 mg/kg) | HT + Enal (10 mg/kg) |
|---|---|---|---|---|---|---|---|
| SBP (mmHg) | 114.7 ± 0.5 | 114.4 ± 0.4 | 145.8 ± 0.3* | 127.9 ± 0.5# | 124.2 ± 1.0# | 116.1 ± 0.6# | 114.2 ± 0.3# |
| Heart rate (beats/min) | 429.3 ± 4.9 | 411.8 ± 7.5 | 455.3 ± 17.9 | 430.8 ± 10.0 | 426.9 ± 4.0 | 446.1 ± 11.4 | 412.4 ± 9.1 |
| Body weight (g) | 378.3 ± 8.5 | 406.3 ± 9.2 | 404.5 ± 5.3 | 382.8 ± 26.8 | 394.8 ± 7.9 | 396.0 ± 12.6 | 378.3 ± 8.5 |
| %Emax | CTRL | ER (30 mg/kg) | HT | HT + ER (5 mg/kg) | HT + ER (15 mg/kg) | HT + ER (30 mg/kg) | HT + Enal (10 mg/kg) |
|---|---|---|---|---|---|---|---|
| Ach | 100.2 ± 3.0 | 102.9 ± 1.4 | 5.2 ± 1.5* | 20.6 ± 0.4* | 51.0 ± 0.7* | 80.4 ± 0.5 | 103.1 ± 3.1 |
| SNP | 105.5 ± 1.1 | 105.7 ± 2.7 | 102.7 ± 3.3 | 102.3 ± 1.3 | 101.7 ± 0.6 | 106.9 ± 4.5 | 107.8 ± 2.8 |
| PE | 45.4 ± 2.2 | 47.4 ± 0.6 | 84.6 ± 0.8* | 78.4 ± 0.3* | 49.5 ± 1.1 | 47.7 ± 1.7 | 48.5 ± 3.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantan, R.; Tocharus, J.; Nakaew, A.; Suksamrarn, A.; Tocharus, C. Ethyl Rosmarinate Prevents the Impairment of Vascular Function and Morphological Changes in L-NAME-Induced Hypertensive Rats. Medicina 2019, 55, 777. https://doi.org/10.3390/medicina55120777
Pantan R, Tocharus J, Nakaew A, Suksamrarn A, Tocharus C. Ethyl Rosmarinate Prevents the Impairment of Vascular Function and Morphological Changes in L-NAME-Induced Hypertensive Rats. Medicina. 2019; 55(12):777. https://doi.org/10.3390/medicina55120777
Chicago/Turabian StylePantan, Rungusa, Jiraporn Tocharus, Archawin Nakaew, Apichart Suksamrarn, and Chainarong Tocharus. 2019. "Ethyl Rosmarinate Prevents the Impairment of Vascular Function and Morphological Changes in L-NAME-Induced Hypertensive Rats" Medicina 55, no. 12: 777. https://doi.org/10.3390/medicina55120777
APA StylePantan, R., Tocharus, J., Nakaew, A., Suksamrarn, A., & Tocharus, C. (2019). Ethyl Rosmarinate Prevents the Impairment of Vascular Function and Morphological Changes in L-NAME-Induced Hypertensive Rats. Medicina, 55(12), 777. https://doi.org/10.3390/medicina55120777





