Celiac-Related Autoantibodies and IL-17A in Bulgarian Patients with Dermatitis Herpetiformis: A Cross-Sectional Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Serum Samples
2.2. Immune Serology Testing
2.2.1. Immunoenzyme Testing
- anti-tTG antibodies (Anti-Tissue Transglutaminase Screen IgA + IgG, Orgentec Diagnostika GmbH, cut-off value > 10 U/mL);
- anti-DGP antibodies (Quanta Lite Celiac DGP Screen IgA + IgG, Inova Diagnostics, Inc., San Diego, USA, cut-off > 15 U/mL);
- AAA (Quanta Lite F-Actin IgA ELISA, Inova Diagnostics, Inc., San Diego, USA, cut-off > 20 U/mL);
- IL-17A (Human IL-17A ELISA kit, Diaclone, GenProbe, France, sensitivity < 2.3 pg/mL).
2.2.2. Immunoblot Testing
2.3. Statistical Analysis
3. Results
3.1. Serum Levels of the Celiac Disease-Related Autoantibodies and the Pro-Inflammatory Cytokine IL-17A
3.2. Performance Characteristics of the Celiac-Related Antibodies Tested in DH Patients
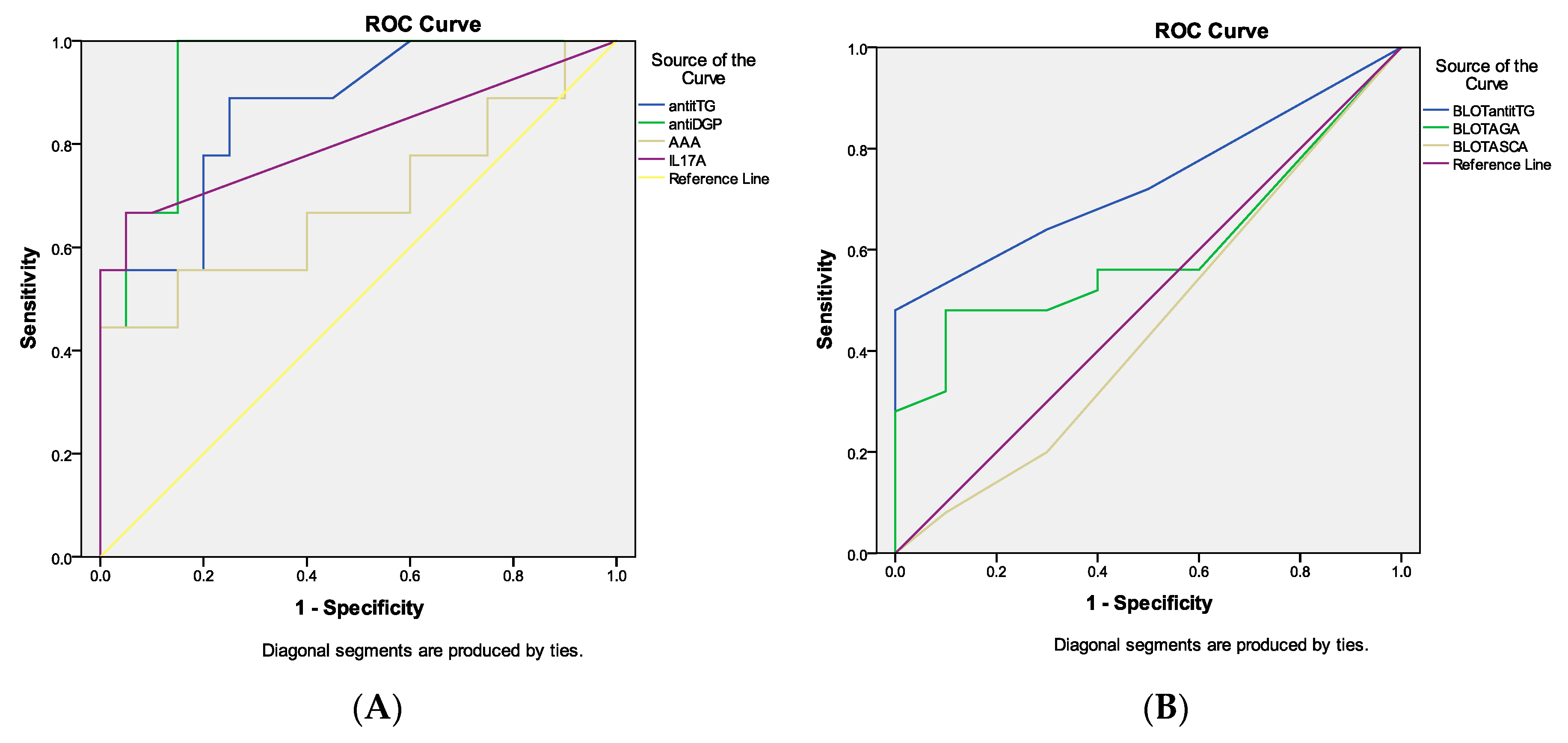
3.3. ROC Curve Analysis of the Celiac Disease-Related Antibodies and IL-17A in DH Patients
3.4. Correlation between Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Antiga, E.; Caproni, M. The diagnosis and treatment of dermatitis herpetiformis. Clin. Cosmet. Investig. Dermatol. 2015, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Antiga, E.; Melani, L.; Fabbri, P. The Italian Group for Cutaneous Immunopathology. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Reunala, T.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Collin, P. Dermatitis Herpetiformis: A Common Extraintestinal Manifestation of Coeliac Disease. Nutrients 2018, 10, 602. [Google Scholar] [CrossRef] [PubMed]
- Sárdy, M.; Kárpáti, S.; Merkl, B.; Paulsson, M.; Smyth, N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 2002, 195, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Salmi, T.T.; Hervonen, K.; Kaukinen, K.; Reunala, T. Dermatitis herpetiformis: A cutaneous manifestation of coeliac disease. Ann. Med. 2017, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bonciani, D.; Verdelli, A.; Bonciolini, V.; D’Errico, A.; Antiga, E.; Fabbri, P.; Caproni, M. Dermatitis herpetiformis: From the genetics to the development of skin lesions. Clin. Dev. Immunol. 2012, 2012, 239691. [Google Scholar] [CrossRef]
- Reunala, T. Dermatitis herpetiformis: Coeliac disease of the skin. Ann. Med. 1998, 30, 416–418. [Google Scholar] [CrossRef]
- Savilahti, E.; Reunala, T.; Mäki, M. Increase of lymphocytes bearing the gamma/delta T cell receptor in the jejunum of patients with dermatitis herpetiformis. Gut 1992, 33, 206–211. [Google Scholar] [CrossRef]
- Alonso-Llamazares, J.; Gibson, L.E.; Rogers, R.S. Clinical, pathologic, and immunopathologic features of dermatitis herpetiformis: Review of the Mayo Clinic experience. Int. J. Dermatol. 2007, 46, 910–919. [Google Scholar] [CrossRef]
- Mazzarella, G. Effector and suppressor T cells in celiac disease. World J. Gastroenterol. 2015, 21, 7349–7356. [Google Scholar] [CrossRef]
- Berger, E. Zur Allergischen Pathogenese der Cöliakie; Bibliotheca Paediatrica; S Karger Ag: Basel, Switzerland, 1958; pp. 1–55. [Google Scholar]
- Rossi, T.M.; Tjota, A. Serologic indicators of celiac disease. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Seah, P.P.; Fry, L.; Hoffbrand, A.V.; Holborow, E.J. Tissue antibodies in dermatitis herpetiformis and adult coeliac disease. Lancet 1971, 1, 834–836. [Google Scholar] [CrossRef]
- Chorzelski, T.P.; Beutner, E.H.; Sulej, J.; Tchorzewska, H.; Jablonska, S.; Kumar, V.; Kapuscinska, A. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br. J. Dermatol. 1984, 111, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Ehnis, T.; Bauer, M.; Donner, P.; Volta, U.; Riecken, E.O.; Schuppan, D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 1997, 3, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Caja, S.; Mäki, M.; Kaukinen, K.; Lindfors, K. Antibodies in celiac disease: Implications beyond diagnostics. Cell. Mol. Immunol. 2011, 8, 103–109. [Google Scholar] [CrossRef]
- Korponay-Szabó, I.R.; Laurila, K.; Szondy, Z.; Halttunen, T.; Szalai, Z.; Dahlbom, I.; Rantala, I.; Kovács, J.B.; Fésüs, L.; Mäki, M. Missing endomysial and reticulin binding of coeliac antibodies in transglutaminase 2 knockout tissues. Gut 2003, 52, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molberg, O.; Mcadam, S.N.; Körner, R.; Quarsten, H.; Kristiansen, C.; Madsen, L.; Fugger, L.; Scott, H.; Norén, O.; Roepstorff, P.; et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998, 4, 713–717. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Dähnrich, C.; Probst, C.; Komorowski, L.; Stöcker, W.; Schlumberger, W.; Zillikens, D.; Rose, C. Novel assay for detecting celiac disease-associated autoantibodies in dermatitis herpetiformis using deamidated gliadin-analogous fusion peptides. J. Am. Acad. Dermatol. 2012, 66, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Sugai, E.; Smecuol, E.; Niveloni, S.; Vázquez, H.; Label, M.; Mazure, R.; Czech, A.; Kogan, Z.; Mauriño, E.; Bai, J.C. Celiac disease serology in dermatitis herpetiformis. Which is the best option for detecting gluten sensitivity? Acta Gastroenterol. Latinoam. 2006, 36, 197–201. [Google Scholar]
- Fuertes, I.; Mascaró, J.M.; Bombí, J.A.; Iranzo, P. A Retrospective Study of Clinical, Histological, and Immunological Characteristics in Patients with Dermatitis Herpetiformis. The Experience of Hospital Clinic de Barcelona, Spain between 1995 and 2010 and a Review of the Literature. Actas Dermo-Sifiliográficas Engl. Ed. 2011, 102, 699–705. [Google Scholar] [CrossRef]
- Dahele, A.; Gosh, S. The role of serological tests in redefining coeliac disease. Proc. R. Coll. Phys. Edinb. 2000, 30, 100–113. [Google Scholar]
- Shahid, M.; Drenovska, K.; Velikova, T.; Vassileva, S. Dermatitis herpetiformis in Bulgaria: Report of 78 patients. J. Investig. Dermatol. 2017, 137, S280. [Google Scholar] [CrossRef]
- Clarindo, M.V.; Possebon, A.T.; Soligo, E.M.; Uyeda, H.; Ruaro, R.T.; Empinotti, J.C. Dermatitis herpetiformis: Pathophysiology, clinical presentation, diagnosis and treatment. An. Bras. Dermatol. 2014, 89, 865–877. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Jaskowski, T.D.; Donaldson, M.R.; Hull, C.M.; Wilson, A.R.; Hill, H.R.; Zone, J.J.; Book, L.S. Novel Screening Assay Performance in Pediatric Celiac Disease and Adult Dermatitis Herpetiformis. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 19–23. [Google Scholar] [CrossRef]
- Desai, A.M.; Krishnan, R.S.; Hsu, S. Medical pearl: Using tissue transglutaminase antibodies to diagnose dermatitis herpetiformis. J. Am. Acad. Dermatol. 2005, 53, 867–868. [Google Scholar] [CrossRef]
- Porter, W.M.; Unsworth, D.J.; Lock, R.J.; Hardman, C.M.; Baker, B.S.; Fry, L. Tissue transglutaminase antibodies in dermatitis herpetiformis. Gastroenterology 1999, 117, 749–750. [Google Scholar] [CrossRef]
- Dieterich, W.; Schuppan, D.; Laag, E.; Bruckner-Tuderman, L.; Reunala, T.; Kárpáti, S.; Zágoni, T.; Riecken, E.O. Antibodies to Tissue Transglutaminase as Serologic Markers in Patients with Dermatitis Herpetiformis. J. Investig. Dermatol. 1999, 113, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Jarzabek-Chorzelska, M.; Sulej, J.; Rajadhyaksha, M.; Jablonska, S. Tissue Transglutaminase and Endomysial Antibodies—Diagnostic Markers of Gluten-Sensitive Enteropathy in Dermatitis Herpetiformis. Clin. Immunol. 2001, 98, 378–382. [Google Scholar] [CrossRef]
- Koop, I.; Ilchmann, R.; Izzi, L.; Adragna, A.; Koop, H.; Barthelmes, H. Detection of autoantibodies against tissue transglutaminase in patients with celiac disease and dermatitis herpetiformis. Am. J. Gastroenterol. 2000, 95, 2009–2014. [Google Scholar] [CrossRef]
- Caproni, M.; Cardinali, C.; Renzi, D.; Calabrò, A.; Fabbri, P. Tissue transglutaminase antibody assessment in dermatitis herpetiformis. Br. J. Dermatol. 2001, 144, 196–197. [Google Scholar] [CrossRef]
- Sárdy, M.; Csikós, M.; Geisen, C.; Preisz, K.; Kornseé, Z.; Tomsits, E.; Töx, U.; Hunzelmann, N.; Wieslander, J.; Kárpáti, S.; et al. Tissue transglutaminase ELISA positivity in autoimmune disease independent of gluten-sensitive disease. Clin. Chim. Acta Int. J. Clin. Chem. 2007, 376, 126–135. [Google Scholar] [CrossRef]
- Sugai, E.; Hwang, H.J.; Vazquez, H.; Smecuol, E.; Niveloni, S.; Mazure, R.; Maurino, E.; Aeschlimann, P.; Binder, W.; Aeschlimann, D.; et al. New Serology Assays Can Detect Gluten Sensitivity among Enteropathy Patients Seronegative for Anti-Tissue Transglutaminase. Clin. Chem. 2010, 56, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.R.; Rostami, K.; Marsh, M.N. Diagnosis of coeliac disease. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 389–400. [Google Scholar] [CrossRef]
- Rostami, K.; Kerckhaert, J.; Tiemessen, R.; von Blomberg, B.M.; Meijer, J.W.; Mulder, C.J. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: Disappointing in clinical practice. Am. J. Gastroenterol. 1999, 94, 888–894. [Google Scholar] [CrossRef]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Samaşca, G.; Băican, A.; Pop, T.; Pîrvan, A.; Miu, N.; Andreica, M.; Cristea, V.; Dejica, D. IgG-F-actin antibodies in celiac disease and dermatitis herpetiformis. Rom. Arch. 2010, 69, 177–182. [Google Scholar]
- Granito, A.; Muratori, L.; Muratori, P.; Guidi, M.; Lenzi, M.; Bianchi, F.B.; Volta, U. Anti-saccharomyces cerevisiae antibodies (ASCA) in coeliac disease. Gut 2006, 55, 296. [Google Scholar]
- Velikova, T.; Spassova, Z.; Tumangelova-Yuzeir, K.; Krasimirova, E.; Ivanova-Todorova, E.; Kyurkchiev, D.; Altankova, I. Serological Update on Celiac Disease Diagnostics in Adults. Int. J. Celiac Dis. 2018, 6, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Zebrowska, A.; Wagrowska-Danilewicz, M.; Danilewicz, M.; Stasikowska-Kanicka, O.; Cynkier, A.; Sysa-Jedrzejowska, A.; Waszczykowska, E. IL-17 Expression in Dermatitis Herpetiformis and Bullous Pemphigoid. Mediat. Inflamm. 2013, 2013, 967987. [Google Scholar] [CrossRef]
- Juczynska, K.; Wozniacka, A.; Waszczykowska, E.; Danilewicz, M.; Wagrowska-Danilewicz, M.; Wieczfinska, J.; Pawliczak, R.; Zebrowska, A. Expression of the JAK/STAT Signaling Pathway in Bullous Pemphigoid and Dermatitis Herpetiformis. Mediat. Inflamm. 2017, 2017, 6716419. [Google Scholar] [CrossRef] [PubMed]
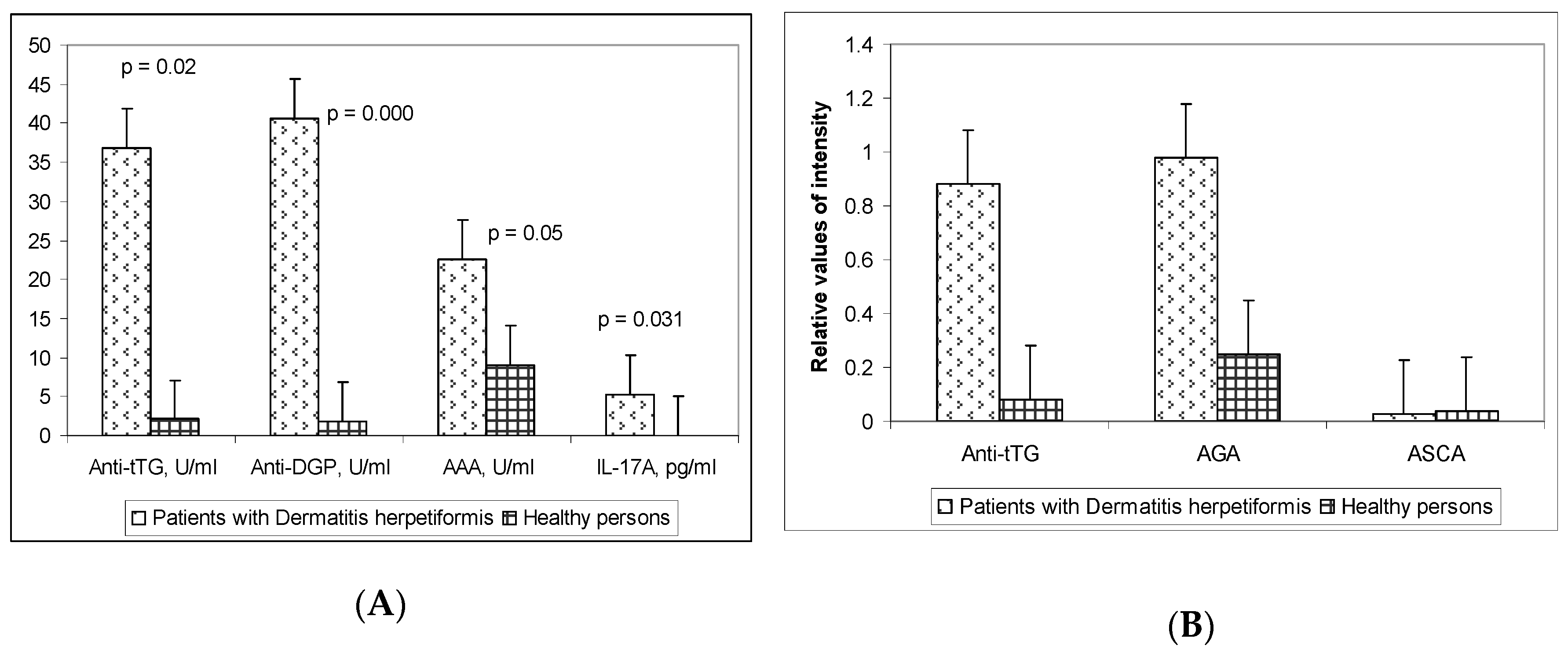
| Anti-tTG IgA + IgG (ELISA) | Anti-DGP IgA + IgG (ELISA) | AAA IgG (ELISA) | Anti-tTG IgG (Line Blot) | AGA IgG (Line Blot) | |
|---|---|---|---|---|---|
| Sensitivity | 42.3% | 46.4% | 34.7% | 46% | 50% |
| Specificity | 100% | 95% | 100% | 100% | 100% |
| PPV * | 100% | 90.9% | 100% | 100% | 100% |
| NPV ** | 57% | 57.1% | 54.1% | 59% | 60% |
| Anti-tTG (ELISA) | Anti-DGP (ELISA) | AAA (ELISA) | IL-17A (ELISA) | Anti-tTG (Line Blot) | AGA (Line Blot) | |
|---|---|---|---|---|---|---|
| Anti-tTG (ELISA) | r = 0.894 p < 0.001 | r = 0.863 p = 0.001 | r = 0.938 p < 0.001 | r = 0.520 p = 0.003 | r = 0.507 p = 0.076 | |
| anti-DGP (ELISA) | r = 0.502 p = 0.009 | r = 0.452 p = 0.031 | r = 0.532 p = 0.001 | r = 0.346 p = 0.038 | ||
| AAA (ELISA) | r = 0.692 p < 0.001 | r = 0.112 p = 0.500 | r = 0.221 p = 0.186 | |||
| IL-17A(ELISA) | r = 0.079 p = 0.676 | r = −0.222 p = 0.238 | ||||
| Anti-tTG (Line blot) | r = 0.678 p < 0.001 | |||||
| AGA (Line blot) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velikova, T.; Shahid, M.; Ivanova-Todorova, E.; Drenovska, K.; Tumangelova-Yuzeir, K.; Altankova, I.; Vassileva, S. Celiac-Related Autoantibodies and IL-17A in Bulgarian Patients with Dermatitis Herpetiformis: A Cross-Sectional Study. Medicina 2019, 55, 136. https://doi.org/10.3390/medicina55050136
Velikova T, Shahid M, Ivanova-Todorova E, Drenovska K, Tumangelova-Yuzeir K, Altankova I, Vassileva S. Celiac-Related Autoantibodies and IL-17A in Bulgarian Patients with Dermatitis Herpetiformis: A Cross-Sectional Study. Medicina. 2019; 55(5):136. https://doi.org/10.3390/medicina55050136
Chicago/Turabian StyleVelikova, Tsvetelina, Martin Shahid, Ekaterina Ivanova-Todorova, Kossara Drenovska, Kalina Tumangelova-Yuzeir, Iskra Altankova, and Snejina Vassileva. 2019. "Celiac-Related Autoantibodies and IL-17A in Bulgarian Patients with Dermatitis Herpetiformis: A Cross-Sectional Study" Medicina 55, no. 5: 136. https://doi.org/10.3390/medicina55050136
APA StyleVelikova, T., Shahid, M., Ivanova-Todorova, E., Drenovska, K., Tumangelova-Yuzeir, K., Altankova, I., & Vassileva, S. (2019). Celiac-Related Autoantibodies and IL-17A in Bulgarian Patients with Dermatitis Herpetiformis: A Cross-Sectional Study. Medicina, 55(5), 136. https://doi.org/10.3390/medicina55050136






