Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review
Abstract
1. Introduction
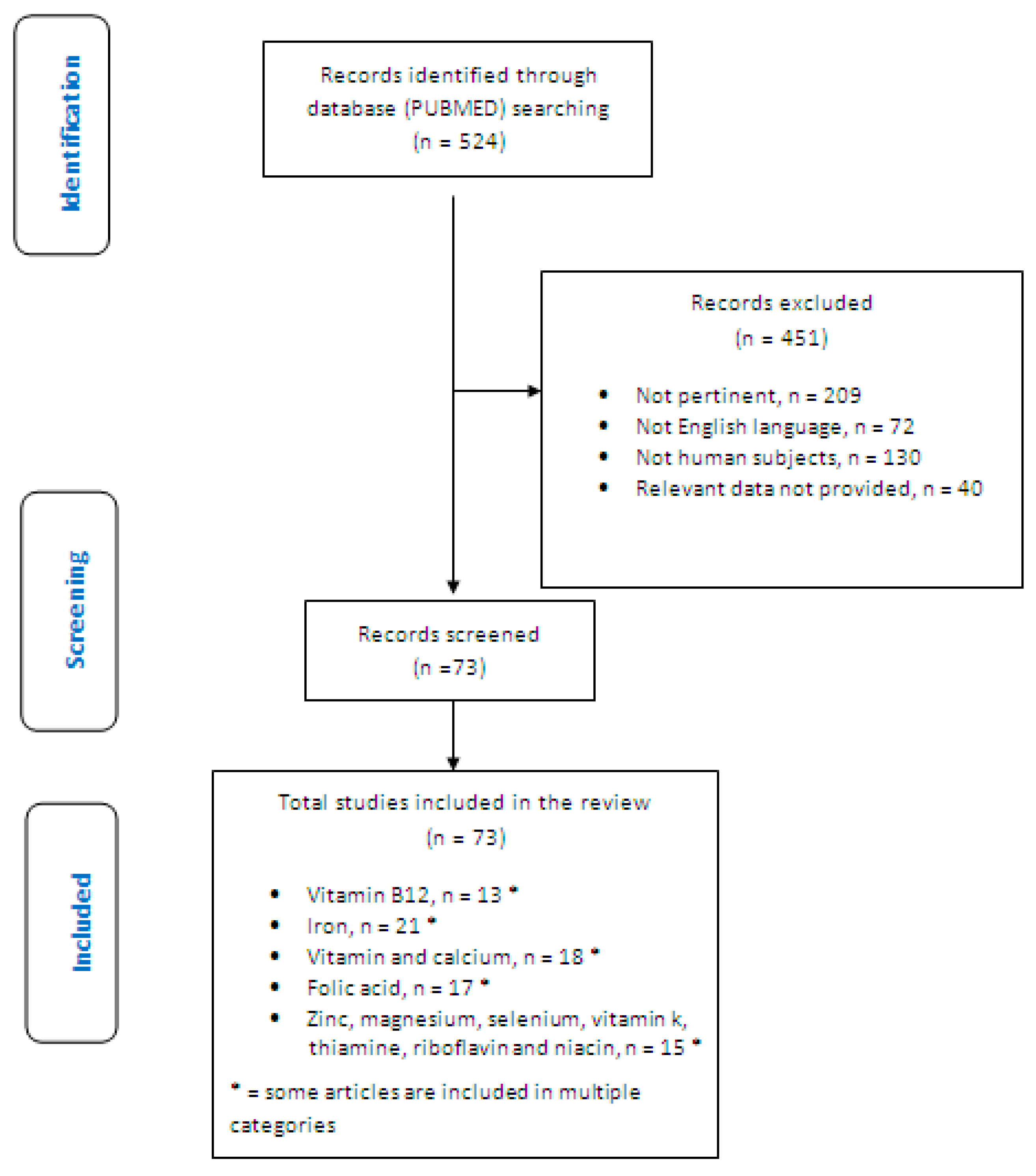
2. Materials and Methods
- Configuration of a working group: three operators skilled in clinical nutrition (one acting as a methodological operator and two participating as clinical operators).
- Formulation of the revision question on the basis of considerations made in the abstract: “the state of the art on nutritional deficiencies in celiac subjects on LTGFD therapy with good compliance; “good compliance” was defined as those patients who had been apparently carefully compliant with the GFD for a at least one year based on dietary history, and this was supported by the absence of coeliac antibodies (if present at diagnosis), or having a healed duodenal biopsy if previous coeliac serology was unavailable”.
- Identification of relevant studies: a research strategy was planned on PubMed (Public MedIine run by the National Center of Biotechnology Information (NCBI) of the National Library of Medicine of Bathesda (USA)) as follows: (a) Definition of the keywords (celiac disease; vitamin B12; iron; folic acid; vitamin D; calcium; zinc; magnesium; LTGFD therapy; LTGFDWGC), allowing the definition of the interest field of the documents to be searched, grouped in quotation marks (“…”) and used separately or in combination; (b) use of: the Boolean (a data type with only two possible values: true or false) AND operator, that allows the establishments of logical relations among concepts; (c) Research modalities: advanced search; (d) Limits: time limits: papers published in the last 20 years; humans; adults; languages: English; (e) Manual search performed by the senior researchers experienced in clinical nutrition through the revision of reviews and individual articles on management of inflammation and oxidative stress by dietary approach in celiac patients published in journals qualified in the Index Medicus.
- Analysis and presentation of the outcomes: we create paragraphs about different micronutrients, and the data extrapolated from the “revised studies” were collocated in tables; in particular, for each study we specified the author and year of publication and study characteristics.
- The analysis was carried out in the form of a narrative review of the reports. At the beginning of each section, the keywords considered and the type of studies chosen are reported. We evaluated, as is suitable for the narrative review, studies of any design which considered the nutritional deficiencies in celiac adult subjects on LTGFD therapy with good compliance.
3. Results
3.1. Vitamin B12
3.2. Iron
3.3. Folic Acid
3.4. Vitamin D and Calcium
3.4.1. Vitamin D
3.4.2. Calcium
3.5. Other Micronutrients
3.5.1. Zinc
3.5.2. Magnesium
3.5.3. Selenium
3.5.4. Vitamin K
3.5.5. Niacin, Riboflavin and Thiamin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The Gluten-Free Diet: Safety and Nutritional Quality. Nutrients 2010, 2, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac Disease. Nat. Rev. Dis. Primers 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Sanders, D.S.; Green, P.H.R. Coeliac Disease. Lancet 2018, 391, 70–81. [Google Scholar] [CrossRef]
- De Silvestri, A.; Capittini, C.; Poddighe, D.; Valsecchi, C.; Marseglia, G.; Tagliacarne, S.C.; Scotti, V.; Rebuffi, C.; Pasi, A.; Martinetti, M.; et al. HLA-DQ Genetics in Children with Celiac Disease: A Meta-Analysis Suggesting a Two-Step Genetic Screening Procedure Starting with HLA-DQ β Chains. Pediatr. Res. 2018, 83, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Capittini, C.; De Silvestri, A.; Rebuffi, C.; Tinelli, C.; Poddighe, D.; Capittini, C.; De Silvestri, A.; Rebuffi, C.; Tinelli, C.; Poddighe, D. Relevance of HLA-DQB1*02 Allele in the Genetic Predisposition of Children with Celiac Disease: Additional Cues from a Meta-Analysis. Medicina (B. Aires) 2019, 55, 190. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG Clinical Guidelines: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef]
- I.C.G.T. Coeliac Disease; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten Free Diet and Nutrient Deficiencies: A Review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Ciacci, C.; Ciclitira, P.; Hadjivassiliou, M.; Kaukinen, K.; Ludvigsson, J.F.; McGough, N.; Sanders, D.S.; Woodward, J.; Leonard, J.N.; Swift, G.L. The Gluten-Free Diet and Its Current Application in Coeliac Disease and Dermatitis Herpetiformis. United Eur. Gastroenterol. J. 2015, 3, 121–135. [Google Scholar] [CrossRef]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete Recovery of Intestinal Mucosa Occurs Very Rarely in Adult Coeliac Patients despite Adherence to Gluten-Free Diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef]
- Lebwohl, B.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.R.; Ludvigsson, J.F. Predictors of Persistent Villous Atrophy in Coeliac Disease: A Population-Based Study. Aliment. Pharmacol. Ther. 2014, 39, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.M.; Weir, D.C.; DeGroote, M.; Mitchell, P.D.; Singh, P.; Silvester, J.A.; Leichtner, A.M.; Fasano, A. Value of IgA TTG in Predicting Mucosal Recovery in Children with Celiac Disease on a Gluten-Free Diet. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Peraaho, M.; Lindfors, K.; Partanen, J.; Woolley, N.; Pikkarainen, P.; Karvonen, A.-L.; Laasanen, T.; Sievaneh, H.; Maki, M.; et al. Persistent Small Bowel Mucosal Villous Atrophy without Symptoms in Coeliac Disease. Aliment. Pharmacol. Ther. 2007, 25, 1237–1245. [Google Scholar] [CrossRef]
- Egger, M.; Dickersin, K.; Smith, G.D. Problems and Limitations in Conducting Systematic Reviews. In Systematic Reviews in Health Care; BMJ Publishing Group: London, UK, 2008; pp. 43–68. [Google Scholar]
- Kupper, C. Dietary Guidelines and Implementation for Celiac Disease. Gastroenterology 2005, 128 (Suppl. 1), S121–S127. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate Nutrient Supplementation in Celiac Disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Penagini, F.; Dilillo, D.; Meneghin, F.; Mameli, C.; Fabiano, V.; Zuccotti, G.V. Gluten-Free Diet in Children: An Approach to a Nutritionally Adequate and Balanced Diet. Nutrients 2013, 5, 4553–4565. [Google Scholar] [CrossRef]
- Harper, J.W.; Holleran, S.F.; Ramakrishnan, R.; Bhagat, G.; Green, P.H.R. Anemia in Celiac Disease Is Multifactorial in Etiology. Am. J. Hematol. 2007, 82, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Dahele, A.; Ghosh, S. Vitamin B12 Deficiency in Untreated Celiac Disease. Am. J. Gastroenterol. 2001, 96, 745–750. [Google Scholar] [CrossRef]
- Dickey, W.; Ward, M.; Whittle, C.R.; Kelly, M.T.; Pentieva, K.; Horigan, G.; Patton, S.; McNulty, H. Homocysteine and Related B-Vitamin Status in Coeliac Disease: Effects of Gluten Exclusion and Histological Recovery. Scand. J. Gastroenterol. 2008, 43, 682–688. [Google Scholar] [CrossRef]
- Halfdanarson, T.R.; Litzow, M.R.; Murray, J.A. Hematologic Manifestations of Celiac Disease. Blood 2007, 109, 412–421. [Google Scholar] [CrossRef]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of Poor Vitamin Status in Coeliac Patients on a Gluten-Free Diet for 10 Years. Aliment. Pharmacol. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Svensson, M.; Tholstrup, J.; Hultberg, B. Clinical Trial: B Vitamins Improve Health in Patients with Coeliac Disease Living on a Gluten-Free Diet. Aliment. Pharmacol. Ther. 2009, 29, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Indicators for Assessing Folate and Vitamin B-12 Status and for Monitoring the Efficacy of Intervention Strategies. Am. J. Clin. Nutr. 2011, 94, 666S–672S. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Welander, A.; Lassila, R.; Ekbom, A.; Montgomery, S.M. Risk of Thromboembolism in 14,000 Individuals with Coeliac Disease. Br. J. Haematol. 2007, 139, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S. Are B Vitamins a Risk Factor for Venous Thromboembolism? Yes. J. Thromb. Haemost. 2006, 4, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Bolaman, Z.; Kadikoylu, G.; Yukselen, V.; Yavasoglu, I.; Barutca, S.; Senturk, T. Oral versus Intramuscular Cobalamin Treatment in Megaloblastic Anemia: A Single-Center, Prospective, Randomized, Open-Label Study. Clin Ther. 2003, 25, 3124–3134. [Google Scholar] [CrossRef]
- Vidal-Alaball, J.; Butler, C.C.; Cannings-John, R.; Goringe, A.; Hood, K.; McCaddon, A.; McDowell, I.; Papaioannou, A. Oral Vitamin B12 versus Intramuscular Vitamin B12 for Vitamin B12 Deficiency. Cochrane Database Syst. Rev. 2005, 3. [Google Scholar] [CrossRef] [PubMed]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional Consequences of Celiac Disease and the Gluten-Free Diet. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef]
- Fasano, A.; Berti, I.; Gerarduzzi, T.; Not, T.; Colletti, R.B.; Drago, S.; Elitsur, Y.; Green, P.H.R.; Guandalini, S.; Hill, I.D.; et al. Prevalence of Celiac Disease in At-Risk and Not-at-Risk Groups in the United States: A Large Multicenter Study. Arch. Intern. Med. 2003, 163, 286–292. [Google Scholar] [CrossRef]
- Malterre, T. Digestive and Nutritional Considerations in Celiac Disease: Could Supplementation Help? Altern. Med. Rev. 2009, 14, 247–257. [Google Scholar]
- Haapalahti, M.; Kulmala, P.; Karttunen, T.J.; Paajanen, L.; Laurila, K.; Mäki, M.; Mykkänen, H.; Kokkonen, J. Nutritional Status in Adolescents and Young Adults with Screen-Detected Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.; Sano, K.; Lebwohl, B.; Diamond, B.; Green, P.H.R. Changing Presentation of Adult Celiac Disease. Dig. Dis. Sci. 2003, 48, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Kolho, K.L.; Färkkilä, M.A.; Savilahti, E. Undiagnosed Coeliac Disease Is Common in Finnish Adults. Scand. J. Gastroenterol. 1998, 33, 1280–1283. [Google Scholar] [PubMed]
- Kapur, G.; Patwari, A.K.; Narayan, S.; Anand, V.K. Iron Supplementation in Children with Celiac Disease. Indian J. Pediatr. 2003, 70, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Severi, C.; Chistolini, A.; Antonelli, G.; Lahner, E.; Marcheggiano, A.; Iannoni, C.; Monarca, B.; Delle Fave, G. Efficacy of Gluten-Free Diet Alone on Recovery from Iron Deficiency Anemia in Adult Celiac Patients. Am. J. Gastroenterol. 2001, 96, 132–137. [Google Scholar] [CrossRef]
- Hopper, A.D.; Leeds, J.S.; Hurlstone, D.P.; Hadjivassiliou, M.; Drew, K.; Sanders, D.S. Are Lower Gastrointestinal Investigations Necessary in Patients with Coeliac Disease? Eur. J. Gastroenterol. Hepatol. 2005, 17, 617–621. [Google Scholar] [CrossRef]
- Oxford, E.C.; Nguyen, D.D.; Sauk, J.; Korzenik, J.R.; Yajnik, V.; Friedman, S.; Ananthakrishnan, A.N. Impact of Coexistent Celiac Disease on Phenotype and Natural History of Inflammatory Bowel Diseases. Am. J. Gastroenterol. 2013, 108, 1123–1129. [Google Scholar] [CrossRef]
- Aspuru, K.; Villa, C.; Bermejo, F.; Herrero, P.; López, S.G. Optimal Management of Iron Deficiency Anemia Due to Poor Dietary Intake. Int. J. Gen. Med. 2011, 4, 741–750. [Google Scholar] [CrossRef]
- Mimura, E.C.M.; Breganó, J.W.; Dichi, J.B.; Gregório, E.P.; Dichi, I. Comparison of Ferrous Sulfate and Ferrous Glycinate Chelate for the Treatment of Iron Deficiency Anemia in Gastrectomized Patients. Nutrition 2008, 24, 663–668. [Google Scholar] [CrossRef]
- Pineda, O.; Ashmead, H.D. Effectiveness of Treatment of Iron-Deficiency Anemia in Infants and Young Children with Ferrous Bis-Glycinate Chelate. Nutrition 2001, 17, 381–384. [Google Scholar] [CrossRef]
- Fishbane, S.; Kowalski, E.A. The Comparative Safety of Intravenous Iron Dextran, Iron Saccharate, and Sodium Ferric Gluconate. Semin. Dial. 2000, 13, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg, S.; Stoinov, S.; Simanenkov, V.; Dudar, L.V.; Karnafel, W.; Garcia, L.C.; Sambuelli, A.M.; D’Haens, G.; Gasche, C. A Novel Intravenous Iron Formulation for Treatment of Anemia in Inflammatory Bowel Disease: The Ferric Carboxymaltose (FERINJECT) Randomized Controlled Trial. Am. J. Gastroenterol. 2008, 103, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Friedrisch, J.R.; Cançado, R.D. Intravenous Ferric Carboxymaltose for the Treatment of Iron Deficiency Anemia. Rev. Bras. Hematol. Hemoter. 2015, 37, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.F.; McIntyre, A.S.; Scott, B.B. Guidelines for the Management of Iron Deficiency Anaemia. British Society of Gastroenterology. Gut 2000, 46 (Suppl. 3–4), IV1–IV5. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.A.; Marrazzo, S.; Gangemi, P.; Battaglia, E.; Giancotti, L.; Miniero, R. Oral Iron Absorption Test with Ferrous Bisglycinate Chelate in Children with Celiac Disease. Minerva Pediatr. 2019, 71, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Vilppula, A.; Kaukinen, K.; Luostarinen, L.; Krekelä, I.; Patrikainen, H.; Valve, R.; Luostarinen, M.; Laurila, K.; Mäki, M.; Collin, P. Clinical Benefit of Gluten-Free Diet in Screen-Detected Older Celiac Disease Patients. BMC Gastroenterol. 2011, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T. Folate, Iron, and Dietary Fiber Contents of the Gluten-Free Diet. J. Am. Diet. Assoc. 2000, 100, 1389–1396. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Trends in Wheat-Flour Fortification with Folic Acid and Iron--Worldwide, 2004 and 2007. MMWR. Morb. Mortal. Wkly. Rep. 2008, 57, 8–10. [Google Scholar]
- Howard, M.R.; Turnbull, A.J.; Morley, P.; Hollier, P.; Webb, R.; Clarke, A. A Prospective Study of the Prevalence of Undiagnosed Coeliac Disease in Laboratory Defined Iron and Folate Deficiency. J. Clin. Pathol. 2002, 55, 754–757. [Google Scholar] [CrossRef]
- Hallert, C.; Tobiasson, P.; Walan, A. Serum Folate Determinations in Tracing Adult Coeliacs. Scand. J. Gastroenterol. 1981, 16, 263–267. [Google Scholar] [CrossRef]
- Sategna-Guidetti, C.; Grosso, S.B.; Grosso, S.; Mengozzi, G.; Aimo, G.; Zaccaria, T.; Di Stefano, M.; Isaia, G.C. The Effects of 1-Year Gluten Withdrawal on Bone Mass, Bone Metabolism and Nutritional Status in Newly-Diagnosed Adult Coeliac Disease Patients. Aliment. Pharmacol. Ther. 2000, 14, 35–43. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Cazzato, I.A.; Danese, S.; Fagiuoli, S.; Gionchetti, P.; Annicchiarico, B.E.; D’Aversa, F.; Gasbarrini, A. Folate in Gastrointestinal Health and Disease. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 376–385. [Google Scholar] [PubMed]
- Patwari, A.K.; Anand, V.K.; Kapur, G.; Narayan, S. Clinical and Nutritional Profile of Children with Celiac Disease. Indian Pediatr. 2003, 40, 337–342. [Google Scholar] [PubMed]
- Kemppainen, T.A.; Kosma, V.M.; Janatuinen, E.K.; Julkunen, R.J.; Pikkarainen, P.H.; Uusitupa, M.I. Nutritional Status of Newly Diagnosed Celiac Disease Patients before and after the Institution of a Celiac Disease Diet--Association with the Grade of Mucosal Villous Atrophy. Am. J. Clin. Nutr. 1998, 67, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Tighe, P.; Ward, M.; McNulty, H.; Finnegan, O.; Dunne, A.; Strain, J.; Molloy, A.M.; Duffy, M.; Pentieva, K.; Scott, J.M. A Dose-Finding Trial of the Effect of Long-Term Folic Acid Intervention: Implications for Food Fortification Policy. Am. J. Clin. Nutr. 2011, 93, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Saibeni, S.; Lecchi, A.; Meucci, G.; Cattaneo, M.; Tagliabue, L.; Rondonotti, E.; Formenti, S.; De Franchis, R.; Vecchi, M. Prevalence of Hyperhomocysteinemia in Adult Gluten-Sensitive Enteropathy at Diagnosis: Role of B12, Folate, and Genetics. Clin. Gastroenterol. Hepatol. 2005, 3, 574–580. [Google Scholar] [CrossRef]
- Pantaleoni, S.; Luchino, M.; Adriani, A.; Pellicano, R.; Stradella, D.; Ribaldone, D.G.; Sapone, N.; Isaia, G.C.; Di Stefano, M.; Astegiano, M. Bone Mineral Density at Diagnosis of Celiac Disease and after 1 Year of Gluten-Free Diet. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Szymczak, J.; Bohdanowicz-Pawlak, A.; Waszczuk, E.; Jakubowska, J. Low Bone Mineral Density in Adult Patients with Coeliac Disease. Endokrynol. Pol. 2012, 63, 270–276. [Google Scholar] [CrossRef]
- García-Porrúa, C.; González-Gay, M.A.; Avila-Alvarenga, S.; Rivas, M.J.; Soilan, J.; Penedo, M. Coeliac Disease and Osteomalacia: An Association Still Present in Western Countries. Rheumatology 2000, 39, 1435. [Google Scholar] [CrossRef]
- McNicholas, B.A.; Bell, M. Coeliac Disease Causing Symptomatic Hypocalcaemia, Osteomalacia and Coagulapathy. BMJ Case Rep. 2010, 2010, bcr0920092262. [Google Scholar] [CrossRef]
- Sahebari, M.; Sigari, S.Y.; Heidari, H.; Biglarian, O. Osteomalacia Can Still Be a Point of Attention to Celiac Disease. Clin. Cases Miner. Bone Metab. 2011, 8, 14–15. [Google Scholar] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Shab-Bidar, S.; Bours, S.; Geusens, P.P.M.M.; Kessels, A.G.H.; van den Bergh, J.P.W. Serum 25(OH)D Response to Vitamin D3 Supplementation: A Meta-Regression Analysis. Nutrition 2014, 30, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Duerksen, D.R.; Ali, M.; Leslie, W.D. Dramatic Effect of Vitamin D Supplementation and a Gluten-Free Diet on Bone Mineral Density in a Patient with Celiac Disease. J. Clin. Densitom. 2012, 15, 120–123. [Google Scholar] [CrossRef]
- Muzzo, S.; Burrows, R.; Burgueño, M.; Ríos, G.; Bergenfreid, C.; Chavez, E.; Leiva, L. Effect of Calcium and Vitamin D Supplementation on Bone Mineral Density of Celiac Children. Nutr. Res. 2000, 20, 1241–1247. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and Celiac Disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U. Pathologic Bone Alterations in Celiac Disease: Etiology, Epidemiology, and Treatment. Nutrition 2014, 30, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kavak, U.S.; Yüce, A.; Kocak, N.; Demir, H.; Saltik, I.N.; Gürakan, F.; Ozen, H. Bone Mineral Density in Children with Untreated and Treated Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Staun, M.; Jarnum, S. Measurement of the 10,000-Molecular Weight Calcium-Binding Protein in Small-Intestinal Biopsy Specimens from Patients with Malabsorption Syndromes. Scand. J. Gastroenterol. 1988, 23, 827–832. [Google Scholar] [CrossRef]
- Pazianas, M.; Butcher, G.P.; Subhani, J.M.; Finch, P.J.; Ang, L.; Collins, C.; Heaney, R.P.; Zaidi, M.; Maxwell, J.D. Calcium Absorption and Bone Mineral Density in Celiacs after Long Term Treatment with Gluten-Free Diet and Adequate Calcium Intake. Osteoporos. Int. 2005, 16, 56–63. [Google Scholar] [CrossRef]
- Larussa, T.; Suraci, E.; Nazionale, I.; Leone, I.; Montalcini, T.; Abenavoli, L.; Imeneo, M.; Pujia, A.; Luzza, F. No Evidence of Circulating Autoantibodies against Osteoprotegerin in Patients with Celiac Disease. World J. Gastroenterol. 2012, 18, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Larussa, T.; Suraci, E.; Imeneo, M.; Marasco, R.; Luzza, F. Normal Bone Mineral Density Associates with Duodenal Mucosa Healing in Adult Patients with Celiac Disease on a Gluten-Free Diet. Nutrients 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Rujner, J.; Socha, J.; Syczewska, M.; Wojtasik, A.; Kunachowicz, H.; Stolarczyk, A. Magnesium Status in Children and Adolescents with Coeliac Disease without Malabsorption Symptoms. Clin. Nutr. 2004, 23, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Breedon, C. Medical Center Aunt Cathy’s Guide to: Thinking About OTHER Nutrition Issues in Celiac Disease. Available online: http://www.mnsna.org/wp-content/uploads/2010/07/Aunt-C-Celiac-Disease-short-OtherNutr-Issues-Sanf-this-12-no-date.pdf (accessed on 3 July 2019).
- Stazi, A.V.; Trinti, B. Selenium Status and Over-Expression of Interleukin-15 in Celiac Disease and Autoimmune Thyroid Diseases. Ann. Ist. Super. Sanita 2010, 46, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Hinks, L.J.; Inwards, K.D.; Lloyd, B.; Clayton, B.E. Body Content of Selenium in Coeliac Disease. Br. Med. J. 1984, 288, 1862–1863. [Google Scholar] [CrossRef]
- Faerber Emily Community Rotation. Selenium Supplement Information for Your Gluten-Free Patients. 2011. Available online: http://depts.washington.edu/nutr/wordpress/wp-content/uploads/2015/03/Selenium_2012.pdf (accessed on 3 July 2019).
- Mager, D.R.; Qiao, J.; Turner, J. Vitamin D and K Status Influences Bone Mineral Density and Bone Accrual in Children and Adolescents with Celiac Disease. Eur. J. Clin. Nutr. 2012, 66, 488–495. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Gibson, P.R. Nutritional Inadequacies of the Gluten-Free Diet in Both Recently-Diagnosed and Long-Term Patients with Coeliac Disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef]
- Thompson, T. Thiamin, Riboflavin, and Niacin Contents of the Gluten-Free Diet: Is There Cause for Concern? J. Am. Diet. Assoc. 1999, 99, 858–862. [Google Scholar] [CrossRef]
| Authors | Type of Study | Country and Year | Results |
|---|---|---|---|
| [2] | Review | Italy, 2010 | Common nutrient deficiencies in celiac subjects at diagnosis are: iron, calcium, magnesium, vitamin D, zinc, folate, niacin, vitamin B12, riboflavin, calorie/protein, and fiber. Deficiencies in folate, niacin, and vitamin B12 may occur after LTGFD. |
| [9] | Review | Italy, 2016 | Low levels of fibers, folate, vitamin B12, vitamin D, calcium, iron, zinc and magnesium are common at diagnosis stage. In some subsets of treated celiac disease (CD) patients they can persist. |
| [16] | Review | USA, 2005 | Deficiencies in fiber, iron, calcium, vitamin D, magnesium, zinc, folate, niacin, vitamin B12, and riboflavin can occur at time of diagnosis. Deficiencies in fiber, iron, calcium, vitamin D, and magnesium can persist after following a GFD. Diet and gluten-free products are often low in B vitamins, calcium, vitamin D, iron, zinc, magnesium, and fiber. |
| [17] | Review | Italy, 2013 | Reduced levels of iron, folate, vitamin B12, and vitamin D are common at the time of diagnosis. After GFD low levels of folate, vitamin B12 and vitamin D can persist. |
| [18] | Review | Italy, 2013 | Common deficiencies at diagnosis include: fiber, iron, calcium, vitamin D, magnesium, zinc, folate, niacin, and vitamin B12. Deficiencies of fiber, iron, calcium, vitamin D, magnesium, zinc, folate, niacin, vitamin B12 may persist after following a GFD. Deficiencies of fiber, folate, niacin, vitamin B12, and riboflavin may persist after LTGFD. |
| Nutrient | Route of Administration | Dosage and Sources |
|---|---|---|
| Vitamin B12 | Oral preferable to intramuscular | |
| Iron | Oral preferable to intravenous |
|
| Folic acid | Oral preferable to parenteral | |
| Vitamin D—Calcium | Oral preferable to parenteral |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina 2019, 55, 337. https://doi.org/10.3390/medicina55070337
Rondanelli M, Faliva MA, Gasparri C, Peroni G, Naso M, Picciotto G, Riva A, Nichetti M, Infantino V, Alalwan TA, et al. Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina. 2019; 55(7):337. https://doi.org/10.3390/medicina55070337
Chicago/Turabian StyleRondanelli, Mariangela, Milena A. Faliva, Clara Gasparri, Gabriella Peroni, Maurizio Naso, Giulia Picciotto, Antonella Riva, Mara Nichetti, Vittoria Infantino, Tariq A. Alalwan, and et al. 2019. "Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review" Medicina 55, no. 7: 337. https://doi.org/10.3390/medicina55070337
APA StyleRondanelli, M., Faliva, M. A., Gasparri, C., Peroni, G., Naso, M., Picciotto, G., Riva, A., Nichetti, M., Infantino, V., Alalwan, T. A., & Perna, S. (2019). Micronutrients Dietary Supplementation Advices for Celiac Patients on Long-Term Gluten-Free Diet with Good Compliance: A Review. Medicina, 55(7), 337. https://doi.org/10.3390/medicina55070337





