Non-Celiac Gluten Sensitivity: A Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Gluten Related Disorders (GRDs)
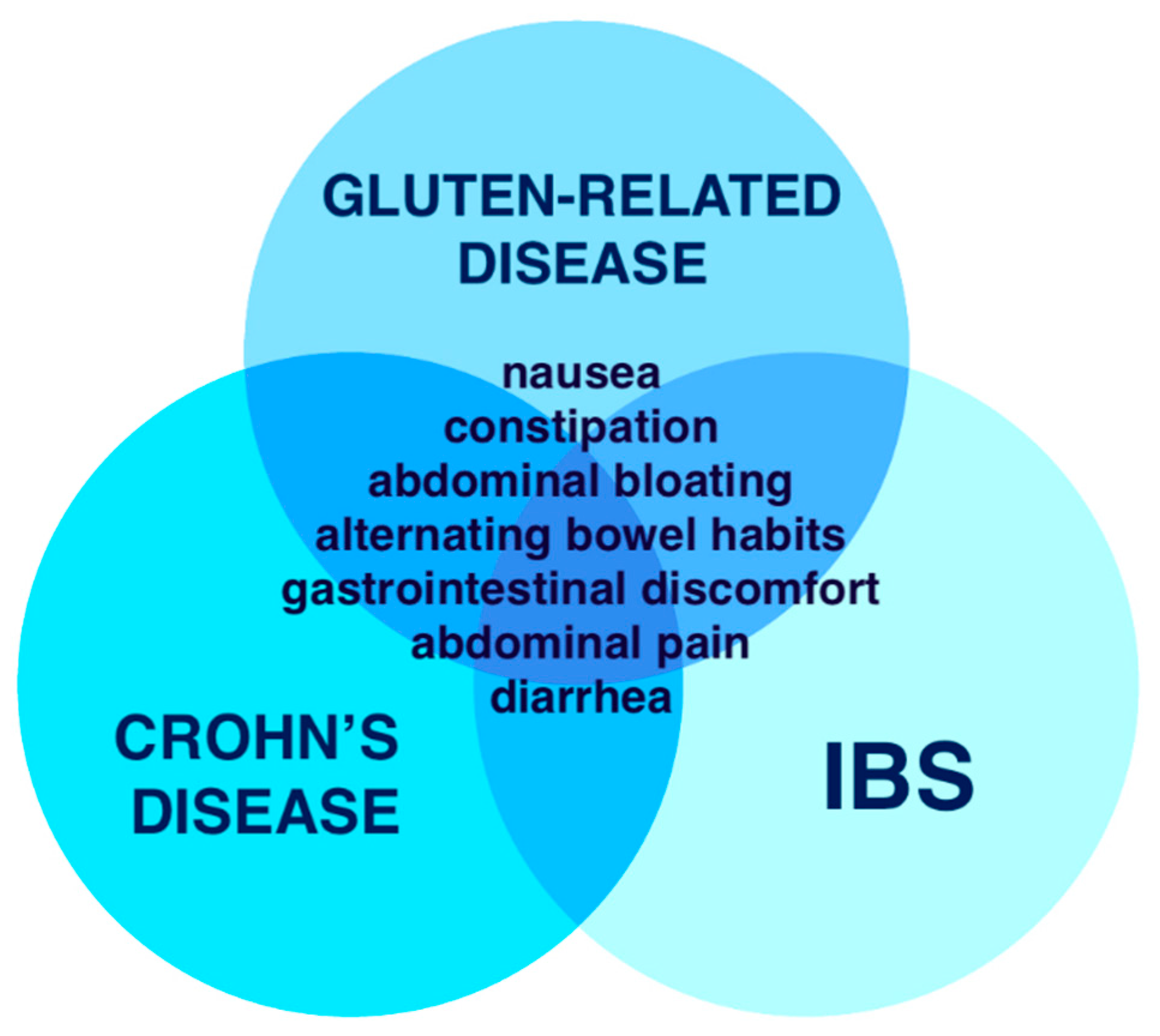
2.2. Epidemiology of Gluten Related Disorders (GRDs)
2.3. Gluten
2.4. Amylase-Tripsin Inhibitors (ATIs)
2.5. Fermentable Oligo-, Di- and Mono-Saccharides and Polyols (FODMAPs)
2.6. The Salerno Experts’ Criteria of NCGS
3. Results
4. Discussion
5. Conclusions
- Symptoms of non-celiac gluten sensitivity are similar to gluten-related disease, irritable bowel syndrome and Crohn’s disease.
- With Salerno Experts’ Criteria of non-celiac gluten sensitivity it is possible to diagnose patients properly and give them advice about nutritional treatment.
Author Contributions
Funding
Conflicts of Interest
References
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Alvey, C.; Anderson, C.M.; Freeman, M. Wheat Gluten and Coeliac Disease. Arch. Dis Child. 1957, 32, 434–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, A.L. The Gluten-Free Diet: Fad or Necessity? Diabetes Spectr. Publ. Am. Diabetes Assoc. 2017, 30, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5084031/ (accessed on 28 January 2019). [CrossRef] [PubMed]
- Ortiz, C.; Valenzuela, R.; Lucero, A.Y. Celiac disease, non celiac gluten sensitivity and wheat allergy: Comparison of 3 different diseases triggered by the same food. Rev. Chil. Pediatría 2017, 88, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.R.; Cremon, C.; Stanghellini, V.; Barbara, G. Recent advances in understanding non-celiac gluten sensitivity. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-Celiac Gluten Sensitivity: The New Frontier of Gluten Related Disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef] [PubMed]
- Cooper, B.T.; Holmes, G.K.; Ferguson, R.; Thompson, R.A.; Allan, R.N.; Cooke, W.T. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology 1980, 79, 801–806. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Piscitelli, D.; Giorgio, F.; Amoruso, A.; Iannone, A.; Principi, M.; Di Leo, A. Biological markers for non-celiac gluten sensitivity: A question awaiting for a convincing answer. Gastroenterol. Hepatol. Bed Bench. 2018, 11, 203–208. [Google Scholar]
- Leccioli, V.; Oliveri, M.; Romeo, M.; Berretta, M.; Rossi, P. A New Proposal for the Pathogenic Mechanism of Non-Coeliac/Non-Allergic Gluten/Wheat Sensitivity: Piecing Together the Puzzle of Recent Scientific Evidence. Nutrients 2019, 9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5707675/ (accessed on 28 January 2019). [CrossRef] [PubMed]
- Tanveer, M.; Ahmed, A. Non-Celiac Gluten Sensitivity: A Systematic Review. J. Coll Physicians Surg.--Pak. Jcpsp 2019, 29, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pr. J. Am. Gastroenterol. Assoc. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Giambalvo, O.; La Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 2017, 9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5537885/ (accessed on 28 January 2019). [CrossRef] [PubMed]
- Cabrera-Chávez, F.; Dezar, G.V.A.; Islas-Zamorano, A.P.; Espinoza-Alderete, J.G.; Vergara-Jiménez, M.J.; Magaña-Ordorica, D.; Ontiveros, N. Prevalence of Self-Reported Gluten Sensitivity and Adherence to a Gluten-Free Diet in Argentinian Adult Population. Nutrients 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- DiGiacomo, D.V.; Tennyson, C.A.; Green, P.H.; Demmer, R.T. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: Results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand. J. Gastroenterol. 2013, 48, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, T.; Nijeboer, P.; IJssennagger, C.E.; Sanders, D.S.; Mulder, C.J.J.; Bouma, G. Prevalence and Characterization of Self-Reported Gluten Sensitivity in The Netherlands. Nutrients 2016, 8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5133100/ (accessed on 28 January 2019). [CrossRef]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK study assessing the population prevalence of self-reported gluten sensitivity and referral characteristics to secondary care. Eur J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R.; Study Group for Non-Celiac Gluten Sensitivity. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef]
- Reese, I.; Schäfer, C.; Kleine-Tebbe, J.; Ahrens, B.; Bachmann, O.; Ballmer-Weber, B.; Beyer, K.; Bischoff, S.C.; Blümchen, K.; Dölle, S.; et al. Non-celiac gluten/wheat sensitivity (NCGS)-a currently undefined disorder without validated diagnostic criteria and of unknown prevalence: Position statement of the task force on food allergy of the German Society of Allergology and Clinical Immunology (DGAKI). Allergo J. Int. 2018, 27, 147–151. [Google Scholar] [PubMed]
- Biesiekierski, J.R. What is gluten? J. Gastroenterol. Hepatol. 2017, 32 (Suppl 1), 78–81. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.-J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S. Extending our knowledge of fermentable, short-chain carbohydrates for managing gastrointestinal symptoms. Nutr. Clin. Pr. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2013, 28, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, W.; Schuppan, D.; Schink, M.; Schwappacher, R.; Wirtz, S.; Agaimy, A.; Neurath, M.F.; Zopf, Y. Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin. Nutr. Edinb Scotl. 2019, 38, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Gayam, S.; Kupec, J.T. The Role of a Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyol Diet in Nonceliac Gluten Sensitivity. Gastroenterol. Res. Pract. 2018, 2018, 1561476. [Google Scholar] [CrossRef] [PubMed]
- Catassi, G.; Lionetti, E.; Gatti, S.; Catassi, C. The Low FODMAP Diet: Many Question Marks for a Catchy Acronym. Nutrients 2017, 9, 292. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Guandalini, S.; Polanco, I. Nonceliac gluten sensitivity or wheat intolerance syndrome? J. Pediatr. 2015, 166, 805–811. [Google Scholar] [CrossRef]
- Volta, U.; Caio, G.; De Giorgio, R.; Henriksen, C.; Skodje, G.; Lundin, K.E. Non-celiac gluten sensitivity: A work-in-progress entity in the spectrum of wheat-related disorders. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Slim, M.; Rico-Villademoros, F.; Calandre, E.P. Psychiatric Comorbidity in Children and Adults with Gluten-Related Disorders: A Narrative Review. Nutrients 2018, 10, 875. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Brusca, I.; Mansueto, P.; Pirrone, G.; Barrale, M.; Di Prima, L.; Ambrosiano, G.; Iacono, G.; Lospalluti, M.L.; La Chiusa, S.M.; et al. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. Off. Clin. Pr. J. Am. Gastroenterol. Assoc. 2010, 8, 254–260. [Google Scholar] [CrossRef]
- Vojdani, A.; Perlmutter, D. Differentiation between Celiac Disease, Nonceliac Gluten Sensitivity, and Their Overlapping with Crohn’s Disease: A Case Series. Case Rep. Immunol. 2013, 2013, 248482. [Google Scholar] [CrossRef] [PubMed]
- Makharia, A.; Catassi, C.; Makharia, G.K. The Overlap between Irritable Bowel Syndrome and Non-Celiac Gluten Sensitivity: A Clinical Dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5707740/ (accessed on 27 January 2019). [CrossRef] [PubMed]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Tovoli, F.; De Giorgio, R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014, 14, 26. [Google Scholar] [CrossRef]
- Makhlouf, S.; Messelmani, M.; Zaouali, J.; Mrissa, R. Cognitive impairment in celiac disease and non-celiac gluten sensitivity: Review of literature on the main cognitive impairments, the imaging and the effect of gluten free diet. Acta Neurol. Belg. 2018, 118, 21–27. [Google Scholar] [CrossRef]
- Busby, E.; Bold, J.; Fellows, L.; Rostami, K. Mood Disorders and Gluten: It’s Not All in Your Mind! A Systematic Review with Meta-Analysis. Nutrients 2018, 10, 1708. [Google Scholar] [CrossRef]
- Rodrigo, L.; Hernández-Lahoz, C.; Lauret, E.; Rodriguez-Peláez, M.; Soucek, M.; Ciccocioppo, R.; Kruzliak, P. Gluten ataxia is better classified as non-celiac gluten sensitivity than as celiac disease: A comparative clinical study. Immunol. Res. 2016, 64, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Rao, D.G.; Grìnewald, R.A.; Aeschlimann, D.P.; Sarrigiannis, P.G.; Hoggard, N.; Aeschlimann, P.; Mooney, P.D.; Sanders, D.S. Neurological Dysfunction in Coeliac Disease and Non-Coeliac Gluten Sensitivity. Am. J. Gastroenterol. 2016, 111, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Capannolo, A.; Viscido, A.; Barkad, M.A.; Valerii, G.; Ciccone, F.; Melideo, D.; Frieri, G.; Latella, G. Non-Celiac Gluten Sensitivity among Patients Perceiving Gluten-Related Symptoms. Digestion 2015, 92, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the Presence of Non-Celiac Gluten Sensitivity in Patients with Functional Gastrointestinal Symptoms: Results from a Multicenter Randomized Double-Blind Placebo-Controlled Gluten Challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Baschè, R.; Ferraresi, A.; Ricci, C.; Lanzarotto, F.; Marullo, M.; Villanacci, V.; Hidalgo, A.; Lanzini, A. Randomised clinical study: Gluten challenge induces symptom recurrence in only a minority of patients who meet clinical criteria for non-coeliac gluten sensitivity. Aliment. Pharm. Ther. 2015, 42, 968–976. [Google Scholar] [CrossRef]
- Hollon, J.; Puppa, E.L.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Shahbazkhani, B.; Sadeghi, A.; Malekzadeh, R.; Khatavi, F.; Etemadi, M.; Kalantri, E.; Rostami-Nejad, M.; Rostami, K. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2015, 7, 4542–4554. [Google Scholar] [CrossRef]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328.e1-3. [Google Scholar] [CrossRef]
- Skodje, G.I.; Sarna, V.K.; Minelle, I.H.; Rolfsen, K.L.; Muir, J.G.; Gibson, P.R.; Veierød, M.B.; Henriksen, C.; Lundin, K.E.A. Fructan, Rather Than Gluten, Induces Symptoms in Patients with Self-Reported Non-Celiac Gluten Sensitivity. Gastroenterology 2018, 154, 529–539.e2. [Google Scholar] [CrossRef]
- Di Sabatino, A.; Volta, U.; Salvatore, C.; Biancheri, P.; Caio, G.; De Giorgio, R.; Di Stefano, M.; Corazza, G.R. Small Amounts of Gluten in Subjects with Suspected Nonceliac Gluten Sensitivity: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Clin. Gastroenterol. Hepatol. Off. Clin. Pr. J. Am. Gastroenterol. Assoc. 2015, 13, 1604–1612.e3. [Google Scholar] [CrossRef]
- Rosinach, M.; Fernández-Bañares, F.; Carrasco, A.; Ibarra, M.; Temiño, R.; Salas, A.; Esteve, M. Double-Blind Randomized Clinical Trial: Gluten versus Placebo Rechallenge in Patients with Lymphocytic Enteritis and Suspected Celiac Disease. PLoS ONE 2016, 11, e0157879. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; D’Alcamo, A.; Iacono, G.; Soresi, M.; Iacobucci, R.; Arini, A.; Geraci, G.; Fayer, F.; Cavataio, F.; La Blasca, F.; et al. Persistence of Nonceliac Wheat Sensitivity, Based on Long-term Follow-up. Gastroenterology 2017, 153, 56–58.e3. [Google Scholar] [CrossRef] [PubMed]
- Roncoroni, L.; Bascuñán, K.A.; Vecchi, M.; Doneda, L.; Bardella, M.T.; Lombardo, V.; Scricciolo, A.; Branchi, F.; Elli, L. Exposure to Different Amounts of Dietary Gluten in Patients with Non-Celiac Gluten Sensitivity (NCGS): An Exploratory Study. Nutrients 2019, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Brusca, I.; Mansueto, P.; Soresi, M.; D’Alcamo, A.; Ambrosiano, G.; Pepe, I.; Iacono, G.; Lospalluti, M.L.; La Chiusa, S.M.; et al. Fecal assays detect hypersensitivity to cow’s milk protein and gluten in adults with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. Off. Clin. Pr. J. Am. Gastroenterol. Assoc. 2011, 9, 956–971.e3. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1907. [Google Scholar] [CrossRef]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological Tests in Gluten Sensitivity (Nonceliac Gluten Intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef]
- Carroccio, A.; D’Alcamo, A.; Cavataio, F.; Soresi, M.; Seidita, A.; Sciumè, C.; Geraci, G.; Iacono, G.; Mansueto, P. High Proportions of People with Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology 2015, 149, 596–603.e1. [Google Scholar] [CrossRef]
- Infantino, M.; Manfredi, M.; Meacci, F.; Grossi, V.; Severino, M.; Benucci, M.; Bellio, E.; Bellio, V.; Nucci, A.; Zolfanelli, F.; et al. Diagnostic accuracy of anti-gliadin antibodies in Non-Celiac Gluten Sensitivity (NCGS) patients. Clin. Chim. Acta 2015, 451, 135–141. [Google Scholar] [CrossRef]
- Norsa, L.; Shamir, R.; Zevit, N.; Verduci, E.; Hartman, C.; Ghisleni, D.; Riva, E.; Giovannini, M. Cardiovascular disease risk factor profiles in children with celiac disease on gluten-free diets. World J. Gastroenterol. 2013, 19, 5658–5664. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Vidgen, E.; Augustin, L.S.; van Erk, M.; Geelen, A.; Parker, T.; Faulkner, D.; Vuksan, V.; Josse, R.G.; et al. High-protein diets in hyperlipidemia: Effect of wheat gluten on serum lipids, uric acid, and renal function. Am. J. Clin. Nutr. 2001, 74, 57–63. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Vuksan, V.; Augustin, L.S.; Mehling, C.; Parker, T.; Vidgen, E.; Lee, B.; Faulkner, D.; Seyler, H.; et al. Effect of wheat bran on serum lipids: Influence of particle size and wheat protein. J. Am. Coll Nutr. 1999, 18, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef] [PubMed]
- Niland, B.; Cash, B.D. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non–Celiac Disease Patients. Gastroenterol Hepatol. 2018, 14, 82–91. [Google Scholar]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. Edinb. Scotl. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Grant, C.; Grehn, S.; Grännö, C.; Hultén, S.; Midhagen, G.; Ström, M.; Svensson, H.; Valdimarsson, T. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment. Pharm. Ther. 2002, 16, 1333–1339. [Google Scholar] [CrossRef]
- Tovoli, F.; Granito, A.; Negrini, G.; Guidetti, E.; Faggiano, C.; Bolondi, L. Long term effects of gluten-free diet in non-celiac wheat sensitivity. Clin. Nutr. Edinb. Scotl. 2019, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]


| Celiac Disease | NCGS | Wheat Allergy | |
|---|---|---|---|
| Prevalence | 0.5–1.7% | no population studies | 0.5–9% in children |
| Pathogenesis | autoimmune | non-specific immune response | IgE mediated response |
| DQ2-DQ8 HLA haplotypes | positive in 95% cases | positive in 50% cases | negative |
| Serological markers | IgA anti-EMA, IgA anti-tTG, IgG anti-DGP, IgA anti-gliadin | IgA/IgG anti-gliadin in 50% cases | specific IgE antibodies against wheat and gliadin |
| Duodenal biopsy * | Marsh I to IV with domination of Marsh III and IV | Marsh 0-II, but according to some experts Marsh III might also be in NCGS | Marsh 0-II |
| Duodenal villi atrophy | present | absent | might be present or absent |
| References | Study Group | Exclusion Criteria | Methods | Findings | Comments |
|---|---|---|---|---|---|
| Biesiekierski et al. 2013 | IBS patients fulfilling Rome III criteria in NCGS criteria, on GFD for 6 weeks | CD, IBD, age < 16, serious GI disease (cirrhosis), psychiatric disorders, alcohol abuse, NSAIDs and immunosuppressive treatment | GFD for 6 weeks, next 2-weeks diet low in FODMAPs, then 3 days one of the groups—high gluten 16 g, low gluten 2 g gluten or 14 g whey protein, control for 2 weeks washout period and crossover to another group for 3 days. The primary outcome: GI symptoms measured by using 100-mm VAS scoring. The secondary outcome: Fatigue measured by Daily-Fatigue Impact Scale (D-FIS), gliadin-specific T-cell response, biomarkers. | The primary outcome: Gluten-specific responses only in 8% of patients, 16% had worsening of overall GI symptoms in high gluten diet | Limitations: The nocebo effect was present independent of substances which was delivered |
| The secondary outcome: Fatigue measured by D-FIS was lower in the low FOTMAPs diet, no significant difference in biomarkers, physical activity or sleep was observed, only one patient had gliadin-specific T-cell response. | |||||
| Capannolo et al. 2014 | Individuals with gluten related symptoms | CD and WA | NCGS finding: on the basis of the disappearance of the symptoms within GFD 6 month, followed by 1month GD. | CD patients: 26 (6.63%); WA patients: 2 (0.51%); NCGS patients: 27 (6.88%). Patients with no change of symptoms after GFD 337 (85.9%) | Limitations: Lack of blindness in GFD challenge |
| Missing evaluation of possible influence by other food components | |||||
| Symptoms in 74% NCGS patients: | |||||
| Intestinal: abdominal pain, diarrhea, constipation, alternating bowel function, epigastric pain | |||||
| Extra intestinal: malaise, chronic fatigue, headache, anxiety, confused mind, depression, joint/muscle pain, resembling fibromyalgia, weight loss, anemia, dermatitis, rash | |||||
| Related disorders in NCGS patients: lactose intolerance, autoimmune thyroiditis, type 1 diabetes, psoriasis, sarcoidosis | |||||
| Zanini et al. 2015 | Individuals on gluten-free diet (GFD) on their own initiative | CD, non-strict adherence to a GFD, symptomatic on GFD | The primary outcome: the ability of the participants to correctly identify flour containing gluten. GSRS questionnaire was performed | Only 34% (12 participants) correctly identified gluten- containing flour fulfilling the clinical diagnostic criteria for NCGS. | The gluten-free flour used in this test contained FODMAP |
| Hollon et al. 2015 | Individuals with Active CD, CD in Remission, Gluten Sensitivity (GS) | Positive CD serology, abnormal duodenal histopathology, unresponsive to gluten open challenge | GS finding: on the basis of the disappearance of the symptoms within GFD; non-blinded gluten challenge (10 g) for a minimum of 2 months before endoscopy | Increase of gut permeability after PT-gliadin ex-vivo administration in all three study groups, and in control group | Limitations: Lack of blindness in GFD challenge—possible placebo-response; Lack of GFD challenge in the control group—possible individuals with undiagnosed GS/CD |
| Shahbazkhani et al. 2015 | Individuals with newly diagnosed IBS based on the Rome III criteria | Patients with CD, GFD introduced ever in medical history, self-exclusion of wheat from the diet, IBD, diabetes, concurrent drugs for depression/anxiety, NSAI drugs intake, abnormal levels of: glucose, urea, creatinine, sodium, potassium, hemoglobin, erythrocyte sedimentation rate, thyroid function tests | GS finding: IBS diagnosed patients responding to gluten challenge by means of statistically significant worsening of symptoms after gluten meal packet. Patients previously following strict GFD, continued gluten challenge for 6 weeks | Significant increase for following symptoms after gluten-containing meal challenge: bloating, abdominal pain, stool consistence, tiredness, nausea | Limitations: Packets containing gluten meal in the form of powder—not recommended according to Salerno criteria |
| Pros: double-blind randomized placebo-controlled trial | |||||
| Di Sabatino et al. 2015 | Suspected NCGS individuals | CD, WA | Individuals were randomly assigned to groups given gluten or placebo for 1 week, each via gastro-soluble capsules. After a 1 week of gluten-free diet, participants crossed over to the other group. | Gluten group: significantly increased overall symptoms (intestinal symptoms: abdominal bloating and pain, extra-intestinal symptoms: foggy mind, depression, aphthous stomatitis) vs. placebo group. | Limitation: small study group |
| Elli et al. 2016 | Individuals with functional gastroenterological symptoms with enrolled on 3-week-long GFD | CD, WA, IBS psychiatric disorders, major abdominal surgery, diabetes mellitus, systemic autoimmune diseases, previous anaphylactic episodes, any systemic disorders, pregnant, breast feeding women, GFD in previous 6 months, patients on pharmacological therapy | Phase 1. GFD response individuals: questionnaire and next 3-week GFD. Patients with significantly improvement carried on to next phase. Phase 2. 98 subjects. GFD response patients—maintain strict GFD and underwent placebo-controlled double-blind gluten challenge with crossover. Patients were randomized to take gluten in capsules or placebo (rice starch) for 7 days. Total duration: 21 days: 7 days on gluten or placebo, 7 days wash-out, 7 days on gluten or placebo. | 28 individuals from phase 2 reported a symptomatic relapse and deterioration of quality of life. 14 patients responded to the placebo ingestion. About 14 patients responding to gluten withdrawal showed a symptomatic relapse during the gluten challenge—they are suspected to have NCGS. | Strengths: The blinding of patients and doctors, and the crossover design. |
| Weaknesses: arbitrary choice of timing and gluten dosage, the protocol did not make use of a scheduled diet besides GFD, other diet variables cannot be excluded (ATIs). Symptomatic deterioration was also observed in placebo group. | |||||
| Rosinach et al. 2016 | Individuals with clinical GI symptoms and clinical and histological remission after GFD | Age < 18, CD, NSAIDs and Olmesartan immunosuppressive treatment in last month, immunosuppressive therapy, parasitic or H. pylori infection, AD, pregnant or breastfeeding women, participation in other randomized controlled trials in the last 4 weeks, serious GI diseases and GI surgery, severe comorbidities, failure to comply with the protocol requirements | Patients were randomly assigned to gluten group (20 g/day, n = 11) and placebo (n = 7). Clinical symptoms were measured by VAS, quality of life using GIQLI. Scientists examined the presence of gamma/delta+ cells and transglutaminase deposits. | 91% of patients with clinical relapse during gluten challenge compared to 28.5% after placebo. Worsening results in clinical scores and GIQLI was observed in patients on gluten diet, but not in the placebo | Limitations: a small study group |
| primary end-point: disease relapse after 6 months | |||||
| Carroccio et al. 2017 | Induviduals with NCWS | Data collecting from a previous study of NCWS. | 88% subjects improved after a diagnosis of NCWS; 145 of 148 patients on strict GFD (98%) had reduced symptoms, compared to 30 of 52 patients who was not on GFD, 20 (from 22) subjects who repeated DBPC challenge reacted to wheat. NCWS is a persistent condition. | Limitations: not thoroughly discussed exclusion criteria | |
| Skodje et al. 2018 | Individuals self-reported NCGS on gluten-free diet (GFD) on their own initiative for at least 6 months | Exclusion criteria: CD, WA, IBD, gastrointestinal comorbidity, allergy to nuts and sesame, alcohol abuse, pregnancy, breast feeding, women in fertile age without using contraception, long travel distance, considerable infection, patients on immunosuppressive agents’ therapy | GFD for 6 months, next 7 days on one of three diets challenge (gluten 5,7g, fructans 2,1g and placebo), 7 days washout, then crossover to next diet. The primary outcome: gastrointestinal symptoms measured by GSRS-IBS. The secondary outcome: daily GI symptoms measured by VAS, life quality depends on symptoms by SF-36, depression and anxiety symptoms measured by Hospital Anxiety and Depression Scale, Fatigue measured by Giessen Subjective Complaint List and VAS | The primary outcome: overall GSRS-IBS higher in the fructans group (38,6) than in the gluten group (33,1) and placebo (34,3). The secondary outcome: overall GI symptoms measured by VAS higher in FODMAPs diet, decreased vitality and greater weakness in the group of patients receiving fructans | Limitations: high nocebo response |
| Roncoroni et al. 2019 | Individuals with NCGS criteria, complaining about functional GI symptoms | CD, WA, IBD, adult age (<18 years old), positive anti-tissue transglutaminase IgA, psychiatric disorders, major abdominal surgery, diabetes, GFD for previous six months, autoimmune diseases and systemic disorders, pregnancy, breast feeding, experience of anaphylaxis and patients during pharmacotherapy | GFD for 3 weeks, then exposure to diets with gradually increasing the amount of gluten: low-gluten diet (3.5–4 g gluten/day, week 1, n = 22 + 2 dropped out patients), mid-gluten diet (6.7–8 g gluten/day, week 2, n = 14), and a high-gluten diet (10–13 g gluten/day, week 3, n = 8). Patients without GI symptoms on a previous diet were classified into more gluten- containing diet. Patients with GI symptoms were shifted back to a well-tolerated diet. Daily GI symptoms measured by VAS, life quality depends on symptoms by SF-36 | Different reactions of patients after the introduction of gluten. | Limitation: a small study group |
| References | Study Group | Exclusion Criteria | Methods | Findings | Comments |
|---|---|---|---|---|---|
| Carroccio et al. 2011 | Individuals who fulfilled Rome II criteria for IBS | Individuals with organic diseases | Symptom severity questionnaire was analyzed, fecal samples were assayed, and levels of specific immunoglobulin E were measured. Patients were observed for 4 weeks, placed on an elimination diet (without cow’s milk and derivatives, wheat, egg, tomato, and chocolate) for 4 weeks, and kept a diet diary. Those who reported improvements after the elimination diet period were then diagnosed with food hypersensitivity (FH), based on the results of a double-blind, placebo-controlled, oral food challenge (with cow’s milk proteins and then with wheat proteins). | 40 of patients with IBS (25%) were found to have FH. Levels of fecal ECP and tryptase were significantly higher among patients with IBS and FH than those without FH. The ECP assay was the most accurate assay for diagnosis of FH, showing 65% sensitivity and 91% specificity. | Limitations: recruitment of patients not in line with Salerno criteria. |
| Carroccio et al. 2012 | Individuals with non-celiac wheat sensitivity (NCWS), | IgA deficiency, self-exclusion of wheat from the diet, lack of DBPC-challenge method in the diagnosis | A review of the clinical charts of patients with IBS-like presentation, diagnosed with WS challenge in the years 2001-2011. | 1/3 IBS patients who underwent DBPC wheat challenge were really suffering from WS. WS group: higher frequency of anemia, weight loss, self-reported wheat intolerance, coexistent atopy, and food allergy in infancy than the IBS controls, higher frequency of positive serum assays for IgG/IgA anti-gliadin and cytometric basophil activation in “in vitro” assay, eosinophil infiltration of the duodenal and colon mucosa. Two groups with distinct clinical characteristics were identified: WS alone (with similar to CD clinical features) and WS with multiple food hypersensitivity (clinical features similar to those found in allergic patients) | Limitations: recruitment of patients not in line with Salerno criteria. |
| Volta et al. 2012 | Individuals with GS (NCGS) | CD, WA | Retrospective evaluation of collected samples from GS (study group) and CD (control group) individuals. Assessment of IgG/IgA AGA, IgA EmA, IgA tTGA, IgG DGP-AGA. HLA DQ2/DQ8 presence was assessed | GS is characterized by IgG AGA positivity (50%), although is less common comparing to CD. IgA AGA are rare. GS patients were lacking EmA, tTGA, and DGP-AGA. | Limitations: not thoroughly described exclusion criteria for study group |
| Caio et al. 2014 | Individuals with NCGS with simultaneous AGA IgG positivity | CD, WA | AGA of both IgG and IgA classes were assayed by ELISA in 44 NCGS and 40 CD patients after 6 months of gluten-free diet. | AGA IgG in NCGS patients disappear after introduction of GFD. | |
| Carroccio et al. 2015 | NCWS patients of the retrospective cohort study | Incomplete clinical charts were excluded from retrospective study; for both studies: EmA in the culture medium of the duodenal biopsies, self-exclusion of wheat from the diet and refusal to reintroduce it before entering the study, other organic gastrointestinal diseases. | NCWS patients—tTG IgG, EmA IgA and IgG negative, absence of intestinal villous atrophy and WA. Patient medical records were reviewed to identify those with autoimmune disease (AD). CD or IBS served as controls. Serum samples were collected from all subjects and ANA levels were measured by immunofluorescence analysis. Participants completed a questionnaire and their medical records were reviewed to identify those with ADs. Individuals were randomly assigned to groups given gluten or placebo for 1 week, each via gastro-soluble capsules. After a 1 week of gluten-free diet, participants crossed over to the other group. | Patients with NCWS were more likely to be ANA positive than both patients with CD and IBS, in both the retrospective and prospective studies. Patients with NCWS showed a frequency of AD similar to CD, but significantly higher than IBS controls, in both the retrospective and prospective studies. NCWS or CD are more likely to be ANA-positive, have DQ2/DQ8 haplotypes and AD compared with patients with IBS. | Limitations: selection bias of the tertiary centers conducting research; evaluation of the duodenal histology; not in line with Salerno criteria |
| NCWS patients of the prospective study | |||||
| Infantino et al. 2015 | Induviduals with suspected NCGS | CD, WA | Evaluation of collected samples from GS (study group), CD and healthy (control group) individuals. Assessment of IgG/IgA AGA, IgA EmA, IgA tTGA, IgG/IgA DGP-AGA. HLA DQ2/DQ8 presence was assessed | Statistically significant correlation between AGA IgG and NCGS were found. However, AGA IgG still remains to be weak NCGS marker. | Limitations: recruitment of patients not in line with Salerno criteria; small study group |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszkowska, A.; Pawlicka, M.; Mroczek, A.; Bałabuszek, K.; Nieradko-Iwanicka, B. Non-Celiac Gluten Sensitivity: A Review. Medicina 2019, 55, 222. https://doi.org/10.3390/medicina55060222
Roszkowska A, Pawlicka M, Mroczek A, Bałabuszek K, Nieradko-Iwanicka B. Non-Celiac Gluten Sensitivity: A Review. Medicina. 2019; 55(6):222. https://doi.org/10.3390/medicina55060222
Chicago/Turabian StyleRoszkowska, Anna, Marta Pawlicka, Anna Mroczek, Kamil Bałabuszek, and Barbara Nieradko-Iwanicka. 2019. "Non-Celiac Gluten Sensitivity: A Review" Medicina 55, no. 6: 222. https://doi.org/10.3390/medicina55060222
APA StyleRoszkowska, A., Pawlicka, M., Mroczek, A., Bałabuszek, K., & Nieradko-Iwanicka, B. (2019). Non-Celiac Gluten Sensitivity: A Review. Medicina, 55(6), 222. https://doi.org/10.3390/medicina55060222





