The Extent of Extemporaneous Preparation and Regulatory Framework of Extemporaneous Compounding in Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sales Volume of Extemporaneous Preparations in Latvia
- Name of the pharmacy;
- Name of the company owning the pharmacy;
- Municipality or republican city, where the pharmacy is located;
- Amount of money (in euro, without VAT), which the pharmacy obtained from the sale of extemporaneous medicinal products to natural and legal persons;
- Share in percentage of the total amount of money (in euro, without VAT), which the pharmacy obtained from the sale of extemporaneous preparations to natural and legal persons. As the total sales volume of all Latvian community pharmacies for extemporaneous preparations (in euro, without VAT) is known, each pharmacy’s share is expressed as a percentage from total sales volume. Hospital pharmacies were not included in the study.
2.2. Compliance of Latvian Laws with the Requirements of the Resolution
- Regulations of the Cabinet of Ministers No. 377 of 31 May 2005 “Procedure of Circulation of Alcohol in Pharmaceutical Companies, Veterinarian Pharmaceutical Companies, Pharmacies, Medical Institutions and Veterinary Medicine” as amended;
- Regulations of the Cabinet of Ministers No. 57 of 17 January 2006 “Regulations Regarding Procedures for the Labelling of Medicinal Products and the Requirements to Be Set for the Package Leaflet of Medicinal Products” as amended;
- Regulations of the Cabinet of Ministers No. 304 of 18 April 2006 “Regulations Regarding the Procedures for the Manufacture and Control of Medicinal Products, the Requirements for the Qualification and Professional Experience of a Qualified Person and the Procedures for the Issuance of the Certificate of Good Manufacturing Practice to a Medicinal Products Manufacturing Undertaking” as amended;
- Regulations of the Cabinet of Ministers No. 376 of 9 May 2006 “Procedures for the Registration of Medicinal Products” as amended;
- Regulations of the Cabinet of Ministers No. 416 of 26 June 2007 “Procedures Regarding the Distribution and Quality Control of Medicinal Products” as amended;
- Regulations of the Cabinet of Ministers No. 288 of 23 March 2010 “Regulations Regarding Operating of Pharmacies” as amended;
- Regulations of the Cabinet of Ministers No. 610 of 2 August 2011 “Criteria for the Location of Pharmacies and Pharmacy Branches” as amended;
- Regulations of the Cabinet of Ministers No. 800 of 19 October 2011 “Procedures for the Licensing of Pharmaceutical Activity” as amended;
- Regulations of the Cabinet of Ministers No. 344 of 25 June 2013 “Procedures for Importing and Distributing Active Substances” as amended.
2.3. Ethical Approval
3. Results
3.1. Overview of Latvian Pharmacies, Which Had a Special Operation Condition “Preparation of Medicinal Products in the Pharmacy” in the Annex to Their Licence in 2017
3.2. Compliance of Latvian Laws with the Requirements of the Resolution
4. Discussion
5. Conclusions
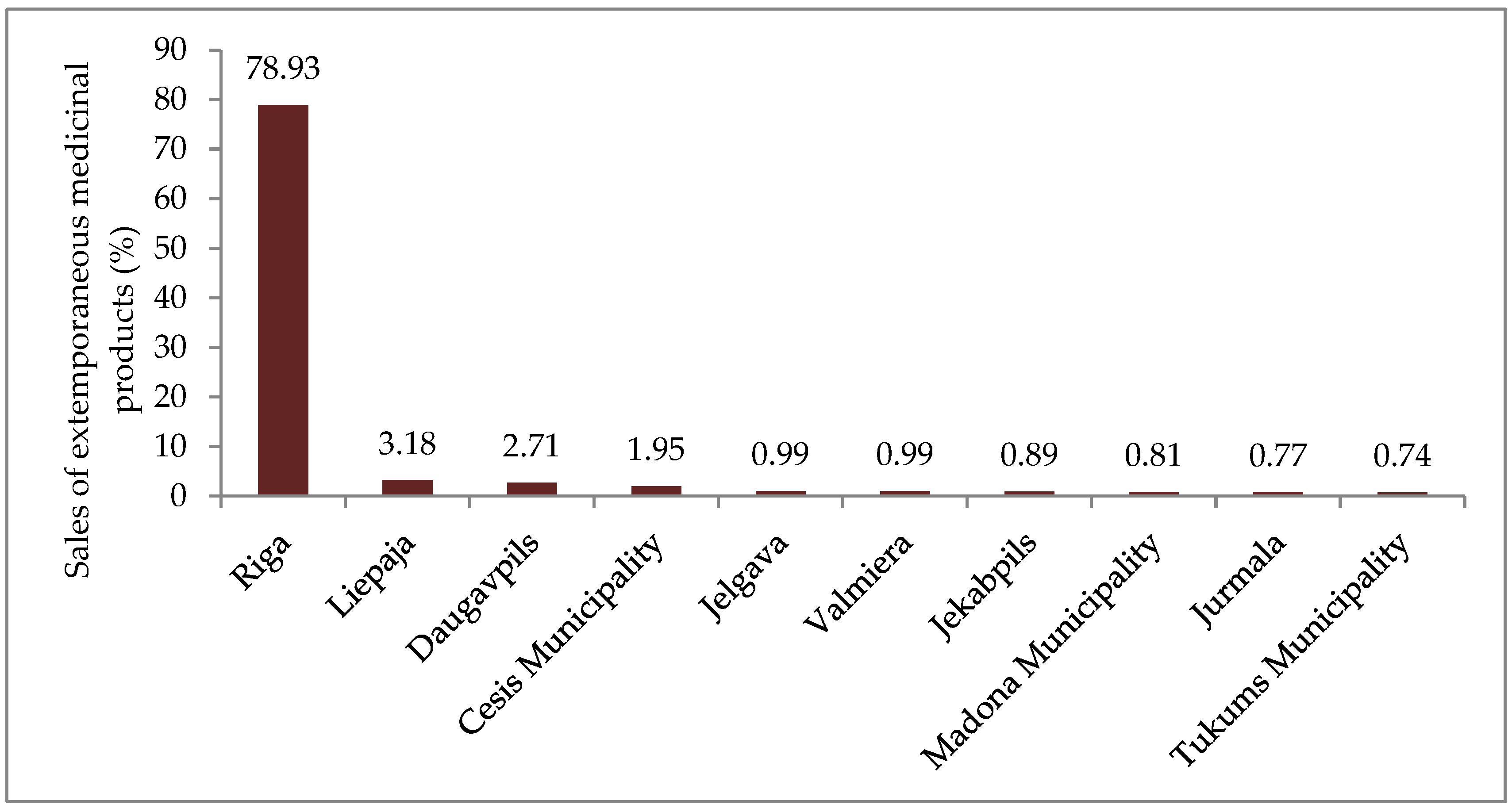
- The survey results demonstrate that the service “preparation of medicinal products in a pharmacy” is offered in all Latvian statistical regions. The total number of compounding pharmacies (280 or 36.5% of all community pharmacies) evidence that the service is needed. The income of pharmacies of different regions from sale of extemporaneous preparations compounded by them shows that most of the extemporaneous medicinal products are compounded in the Riga statistical region (78.93%) and amounts in other regions are considerably smaller.
- Latvia implemented recommendations of the Council of Europe only partially. Latvian regulatory enactments meet Paragraphs 7, 10, 12 of the resolution. Paragraphs 3, 4, 5, 8, 11, 13 of the resolution are partially described in the Latvian regulatory enactments. Paragraphs 6, 9 of the resolution are not described in the Latvian regulatory enactments. The Latvian example highlights a necessity for European Union countries to compare their national legislation with the requirements of the resolution’s last version and, if necessary, implement relevant amendments.
Author Contributions
Funding
Conflicts of Interest
References
- Pharmaceutical Preparations. In European Pharmacopoeia 9.5. Supplement; Council of Europe: Strasbourg, France, 2018; pp. 5569–5571.
- Zaid, A.N.; Al-Ramahi, R.; Shahed, Q.; Saleh, B.; Elaraj, J. Determinants and Frequency of Pharmaceutical Compounding in Pharmacy Practice in Palestine. Int. J. Pharm. Pract. 2012, 20, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Logrippo, S.; Ricci, G.; Sestili, M.; Cespi, M.; Ferrara, L.; Palmieri, G.F.; Ganzetti, R.; Bonacucina, G.; Blasi, P. Oral Drug Therapy in Elderly with Dysphagia: Between a Rock and a Hard Place! Clin. Interv. Aging 2017, 12, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Gudeman, J.; Jozwiakowski, M.; Chollet, J.; Randell, M. Potential Risks of Pharmacy Compounding. Drugs R D 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Falconer, J.R.; Steadman, K.J. Extemporaneously Compounded Medicines. Aust. Prescr. 2017, 40, 5–8. [Google Scholar] [CrossRef] [PubMed]
- The Pew Charitable Trusts. U.S. Illnesses and Deaths Associated With Compounded or Repackaged Medications, 2001–2017; The PEW Charitable Trusts: Philadelphia, PA, USA, 2017; Available online: https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2017/us-illnesses-and-deaths-associated-with-compounded-medications-or-repackaged-medications (accessed on 15 June 2019).
- Valizadeh, S.; Rasekhi, M.; Hamishehkar, H.; Asadollahi, M.; Hamishehkar, H. Medication Errors in Oral Dosage Form Preparation for Neonates: The Importance of Preparation Technique. J. Res. Pharm. Pract. 2015, 4, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chemists Fined after Baby Death. Available online: http://news.bbc.co.uk/2/hi/health/660055.stm (accessed on 15 June 2019).
- Perez, M. Intoxication mortelle avec un produit amaigrissant. Le Figaro, 15 October 2007. [Google Scholar]
- Carvalho, M.; Tuleau, C.; Taylor, K.M.G. Current Compounding Practices in Europe. Int. J. Pharm. Compd. 2008, 12, 94–99. [Google Scholar] [PubMed]
- Minghetti, P.; Pantano, D.; Gennari, C.G.M.; Casiraghi, A. Regulatory Framework of Pharmaceutical Compounding and Actual Developments of Legislation in Europe. Health Policy 2014, 117, 328–333. [Google Scholar] [CrossRef]
- Council of Europe Committee of Ministers Resolution CM/Res(2016)1 on Quality and Safety Assurance Requirements for Medicinal Products Prepared in Pharmacies for the Special Needs of Patients. Available online: https://search.coe.int/cm/Pages/result_details.aspx?ObjectId=090000168065c132 (accessed on 15 June 2019).
- Scheepers, H. Impact of the Council of Europe Resolution on Quality and Safety Assurance Requirements for Medicinal Products Prepared in Pharmacies for the Special Needs of Patients. In Pharmacy Preparations: European Quality Standards and Regulation; Datawyse/Universitaire Pers Maastricht: Maastricht, The Netherlands, 2017; pp. 43–60. [Google Scholar]
- Ministru kabineta. gada 28. aprīļa rīkojums Nr. 271 “Par Latvijas Republikas statistiskajiem reģioniem un tajos ietilpstošajām administratīvajām vienībām” ar grozījumiem. Latvijas Vēstnesis 2004, 69, 3017. [Google Scholar]
- Ministru kabineta. gada 23. marta noteikumi Nr. 288 “Aptieku darbības noteikumi” ar grozījumiem. Latvijas Vēstnesis 2010, 51–52, 4243–4244. [Google Scholar]
- Ministru kabineta. gada 18. aprīļa noteikumi Nr. 304 “Noteikumi par zāļu ražošanas un kontroles kārtību, par zāļu ražošanu atbildīgās amatpersonas kvalifikācijas prasībām un profesionālo pieredzi un kārtību, kādā zāļu ražošanas uzņēmumam izsniedz labas ražošanas prakses sertifikātu” ar grozījumiem. Latvijas Vēstnesis 2006, 70, 3438. [Google Scholar]
- Farmaceitiskās Inspekcijas Konvencija Farmaceitiskās Inspekcijas Sadarbības Shēma—Valsts Valodas Centra Tulkojums. Available online: https://vvc.gov.lv/image/catalog/dokumenti/PE-010-4-GUIDE-TO-GOOD-PRACTICES-1.doc (accessed on 15 June 2019).
- Zāļu valsts aģentūra. Darbības virzienu raksturojums. In Zāļu valsts aģentūras darbības stratēģija 2017–2019. gadam; Zāļu valsts aģentūra: Rīga, Latvija, 2017; pp. 15–19. [Google Scholar]
- gada 10. aprīļa Farmācijas likums ar grozījumiem. Latvijas Vēstnesis 1997, 103, 818.
- Ministru kabineta. gada 31. maija noteikumi Nr. 377 “Spirta aprites kārtība farmaceitiskās darbības uzņēmumos, veterinārfarmaceitiskās darbības uzņēmumos, aptiekās, ārstniecības iestādēs un veterinārmedicīnā” ar grozījumiem. Latvijas Vēstnesis 2005, 88, 3246. [Google Scholar]
- Ministru kabineta. gada 25. jūnija noteikumi Nr. 344 “Aktīvo vielu importēšanas un izplatīšanas kārtība” ar grozījumiem. Latvijas Vēstnesis 2013, 123, 4929. [Google Scholar]
- Ministru kabineta. gada 9. maija noteikumi Nr. 376 “Zāļu reģistrēšanas kārtība” ar grozījumiem. Latvijas Vēstnesis 2006, 97, 3465. [Google Scholar]
- Ministru kabineta. gada 17. janvāra noteikumi Nr. 57 “Noteikumi par zāļu marķēšanas kārtību un zāļu lietošanas instrukcijai izvirzāmajām prasībām” ar grozījumiem. Latvijas Vēstnesis 2006, 14, 3382. [Google Scholar]
- Ministru kabineta. gada 19. oktobra noteikumi Nr. 800 “Farmaceitiskās darbības licencēšanas kārtība” ar grozījumiem. Latvijas Vēstnesis 2011, 170, 4568. [Google Scholar]
- Ministru kabineta. gada 2. augusta noteikumi Nr. 610 “Aptieku un aptieku filiāļu izvietojuma kritēriji” ar grozījumiem. Latvijas Vēstnesis 2011, 125, 4523. [Google Scholar]
- Ministru kabineta. gada 5. marta noteikumi Nr. 102 “Aptieku un aptieku filiāļu izvietojuma kritēriji” (zaudējis spēku). Latvijas Vēstnesis 2002, 47, 2622. [Google Scholar]
- Ministru Kabineta Noteikumu Projekta “Aptieku Darbības Noteikumi” Sākotnējās Ietekmes Novērtējuma Ziņojums (Anotācija). Available online: http://tap.mk.gov.lv/doc/2016_09/VManot_260916_aptieku_darb.923.docx (accessed on 15 June 2019).
- State Agency of Medicines. Pharmacy Map. Available online: https://www.zva.gov.lv/en/pharmacy-map (accessed on 15 June 2019).
- Ministru kabineta. gada 26. jūnija noteikumi Nr. 416 “Zāļu izplatīšanas un kvalitātes kontroles kārtība”. Latvijas Vēstnesis 2007, 104, 3680. [Google Scholar]
- Reis, M.; Carvalho, M.; Rodrigues, A. Compounding Practices in a Portuguese Community Pharmacy. Int. J. Pharm. Compd. 2014, 18, 392–395. [Google Scholar]
- Federal Union of German Associations of Pharmacists (ABDA). Pharmacies’ Provision, Supply and Services. In German Pharmacies, Figures, Data, Facts, 2018; ABDA: Berlin, Germany, 2018; pp. 12–13. [Google Scholar]
- Herborg, H.; Sørensen, E.W.; Frøkjær, B. Pharmaceutical Care in Community Pharmacies: Practice and Research in Denmark. Ann. Pharmacother. 2007, 41, 681–689. [Google Scholar] [CrossRef]
- Bell, J.S.; Väänänen, M.; Ovaskainen, H.; Närhi, U.; Airaksinen, M.S. Providing Patient Care in Community Pharmacies: Practice and Research in Finland. Ann. Pharmacother. 2007, 41, 1039–1046. [Google Scholar] [CrossRef]
- Carvalho, M. Compounding in Spain. In Compounding Practices in Europe: Extemporaneously Compounded Oral Medicines in European Hospital Pharmacies; UCL School of Pharmacy: London, UK, 2013; p. 243. [Google Scholar]
- Database of Central Statistical Bureau of Latvia. Available online: http://data1.csb.gov.lv/pxweb/en/iedz/iedz__iedzskaits__ikgad/ISG020.px/ (accessed on 9 August 2019).
- Ministru kabineta. gada 7. augusta rīkojums Nr. 394 “Par konceptuālo ziņojumu ”Par veselības aprūpes sistēmas reformu”. Latvijas Vēstnesis 2017, 157, 5984. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiseļova, O.; Mauriņa, B.; Šidlovska, V.; Zvejnieks, J. The Extent of Extemporaneous Preparation and Regulatory Framework of Extemporaneous Compounding in Latvia. Medicina 2019, 55, 531. https://doi.org/10.3390/medicina55090531
Kiseļova O, Mauriņa B, Šidlovska V, Zvejnieks J. The Extent of Extemporaneous Preparation and Regulatory Framework of Extemporaneous Compounding in Latvia. Medicina. 2019; 55(9):531. https://doi.org/10.3390/medicina55090531
Chicago/Turabian StyleKiseļova, Olga, Baiba Mauriņa, Venta Šidlovska, and Jānis Zvejnieks. 2019. "The Extent of Extemporaneous Preparation and Regulatory Framework of Extemporaneous Compounding in Latvia" Medicina 55, no. 9: 531. https://doi.org/10.3390/medicina55090531
APA StyleKiseļova, O., Mauriņa, B., Šidlovska, V., & Zvejnieks, J. (2019). The Extent of Extemporaneous Preparation and Regulatory Framework of Extemporaneous Compounding in Latvia. Medicina, 55(9), 531. https://doi.org/10.3390/medicina55090531




