The Morphopathogenetic Aspects of Intraabdominal Adhesions in Children under One Year of Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunohistochemical Analysis
- TGFβ (code orb7087, rabbit, working dilution 1:100, Biorbyt Ltd., Cambridge, UK),
- FGF-2 (code ab16828, rabbit, working dilution 1:200, Abcam, Cambridge, UK),
- FGFR1 (code ab10646, rabbit, working dilution 1:100, Abcam, Cambridge, UK),
- PGP 9.5 (code 439273A, rabbit, working dilution 1:100, ZYMED Laboratories, Invitrogen Corporation, Carlsbad, CA, USA),
- CgA (code 910216A, rabbit, working dilution 1:100, Invitrogen Corporation, Carlsbad, CA, USA),
- IL-1α (code sc-9983, mouse, working dilution 1:50, Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
- IL-4 (code orb10908, rabbit, working dilution 1:100, Biorbyt Ltd., Cambridge, UK),
- IL-6 (code LS-B1582, mouse, working dilution 1:50, LifeSpan BioSciences, Inc., Seattle, WA, USA),
- IL-7 (code orb48420, rabbit, working dilution 1:100, Biorbyt Ltd., Cambridge, UK),
- IL-8 (code orb39299, rabbit, working dilution 1:100, Biorbyt Ltd., Cambridge, UK),
- IL-10 (code ab34843, rabbit, working dilution 1:400, Abcam, Cambridge, UK),
- TNFα (code sc-52250, mouse, working dilution 1:100, Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
- MMP-2 (code orb11061, rabbit, working dilution 1:100, Biorbyt Ltd., Cambridge, UK), and
- TIMP-2 (code sc-21735, mouse, working dilution 1:50, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies.
- 0—no positive structures in the visual field;
- 0/+—occasionally positive structures in the visual field;
- +—few positive structures in the visual field;
- +/++—few to moderate positive structures in the visual field;
- ++—moderate positive structures in the visual field;
- ++/+++—moderate to numerous positive structures in the visual field;
- +++/++++—numerous to abundant positive structures in the visual field;
- ++++—abundant positive structures in the visual field.
2.2. Data Analysis
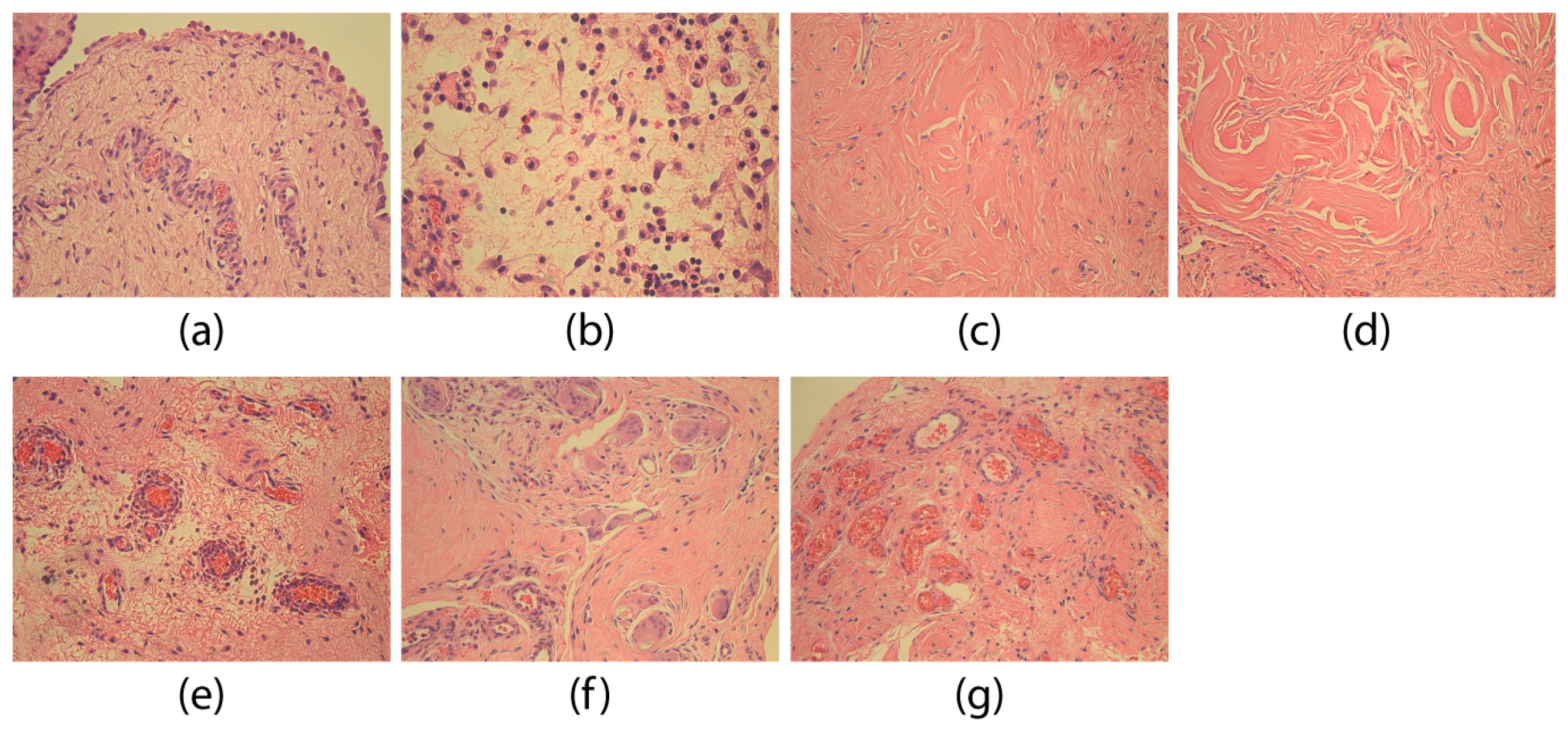
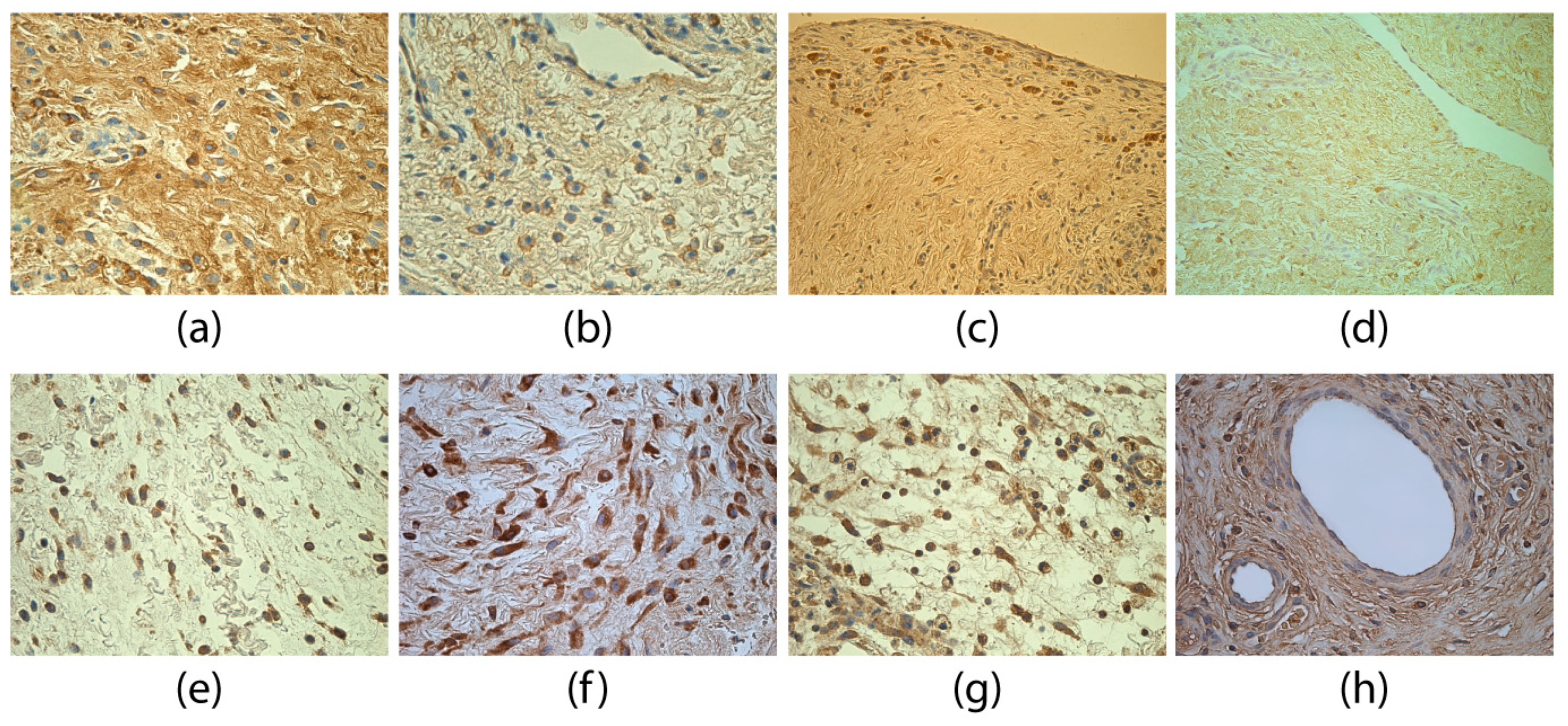
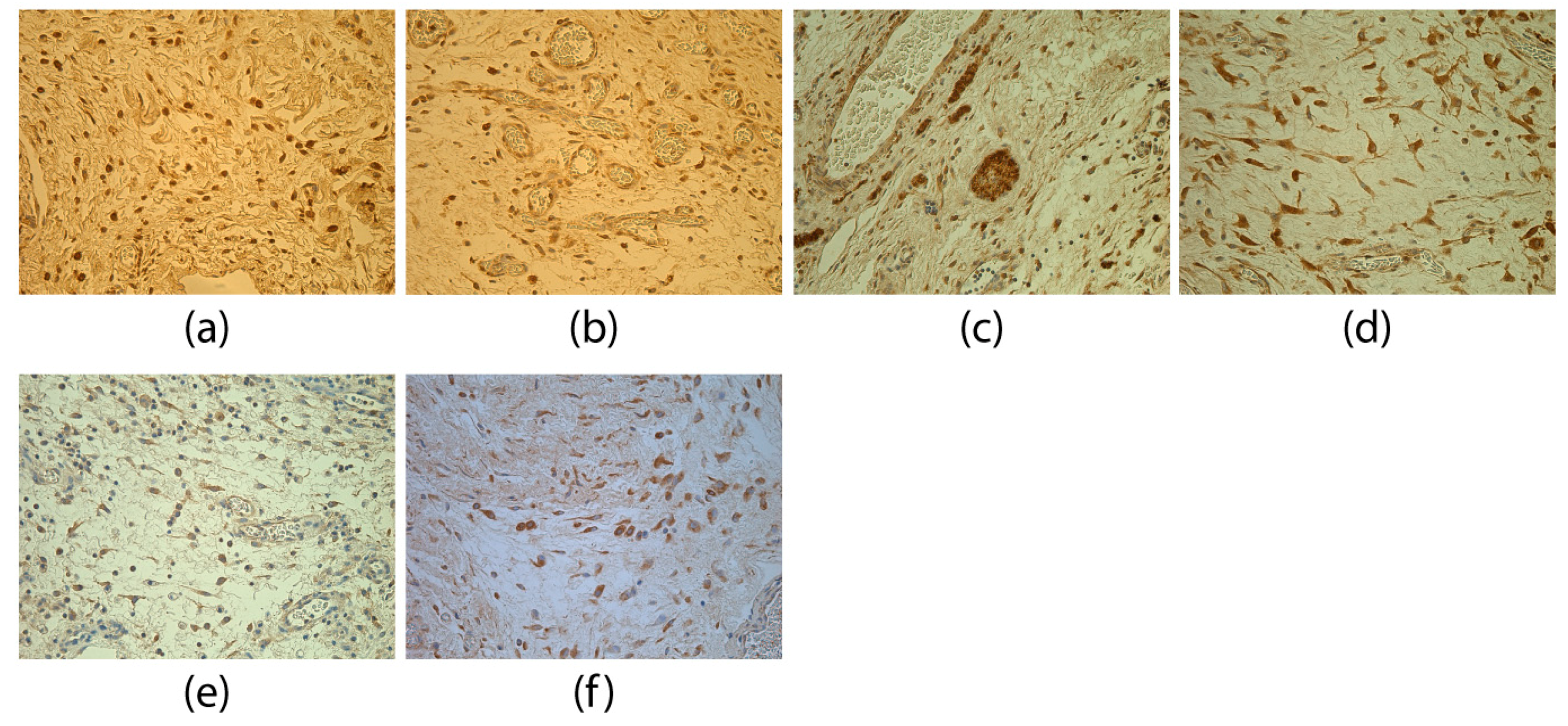
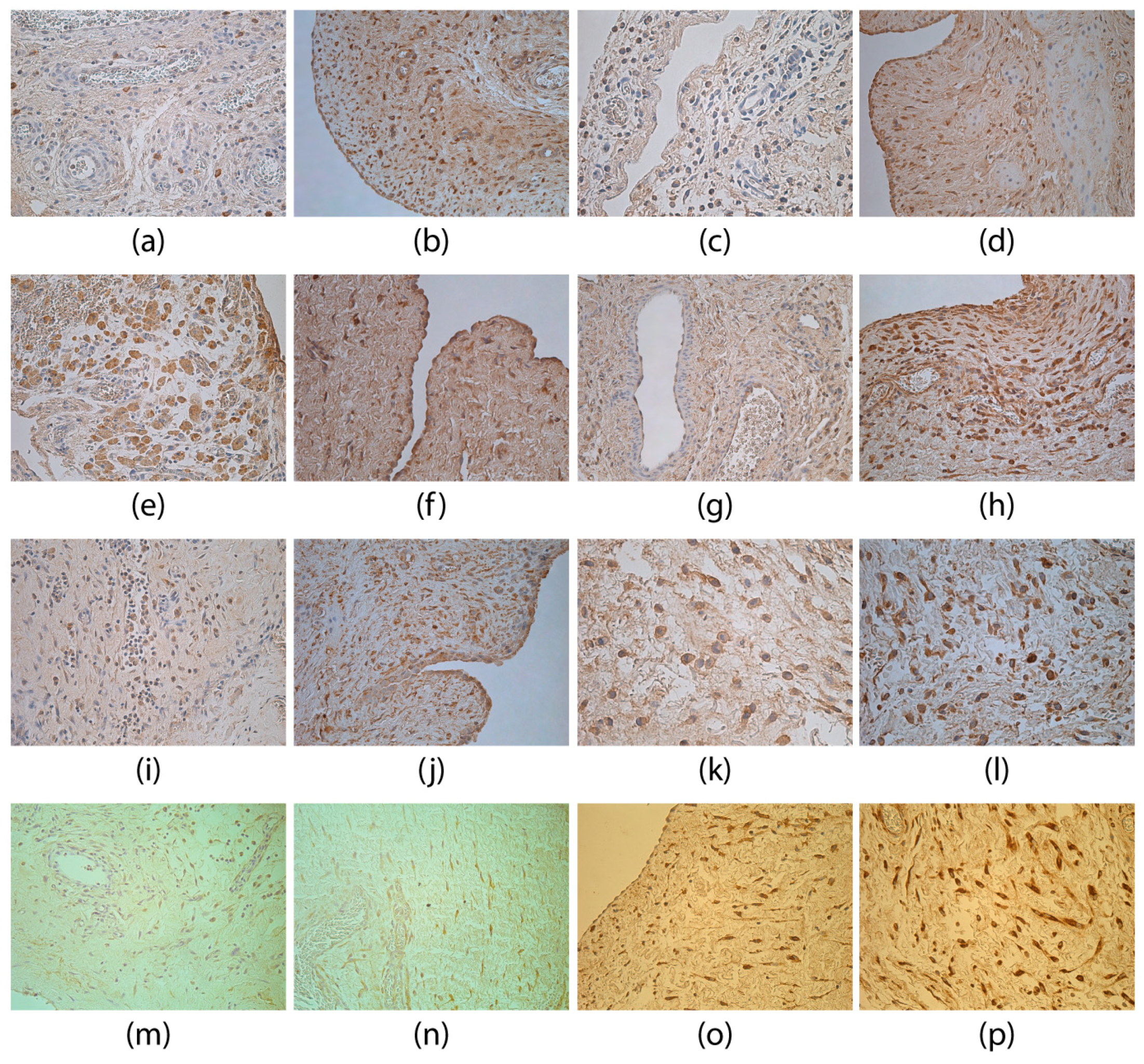
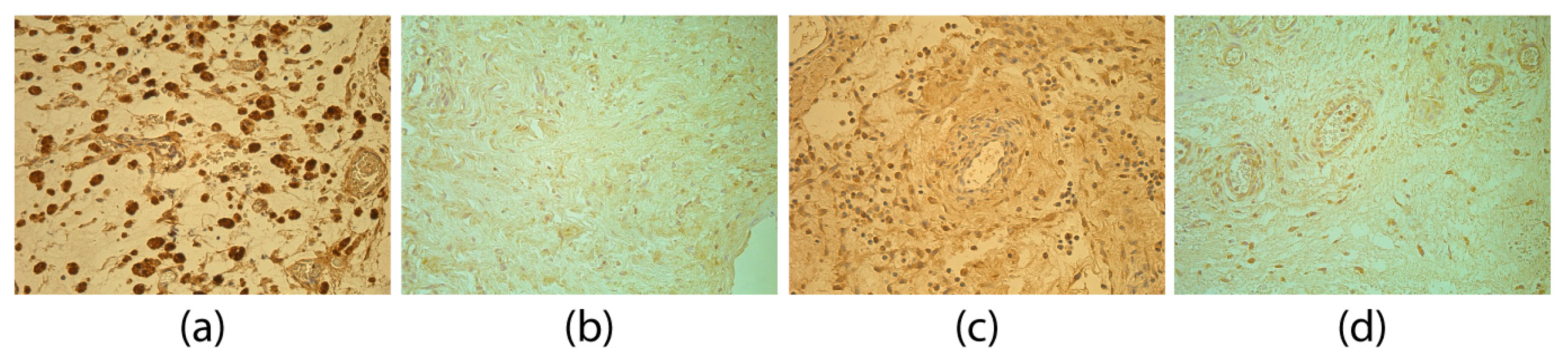
3. Results
4. Discussion
5. Conclusions
- The increase in TGFβ-containing structures, as well as disbalance between MMP-2 and TIMP-2 in adhesion tissues gives evidence of the growth/regenerative potential of loose connective tissue and proves increased fibrosis in the pathogenesis of intraabdominal adhesions.
- Less detected FGF-2 and more prominent FGR1 findings point out a compensatory receptor stimulation in response to the lacking same factor in adhesion tissues.
- The decrease in PGP 9.5-positive structures indicate hypoxic injury and tissue ischemia and proves the stimulation of neoangiogenesis.
- An unpronounced IL-1 and marked IL-10 finding indicate the dominating local tissue protection reaction, the decrease in IL-4-positive structures could be the direct cause of giant cells, but the decrease of IL-8-positive structures could confirm a delayed chemotaxis of inflammatory cells and the prolongation of the inflammatory process.
- Similar findings of CgA in both groups show the unspecific role of these factors in morphopathogenesis of adhesions.
Author Contributions
Funding
Conflicts of Interest
References
- Basbug, M.; Bulbuller, N.; Camci, C.; Ayten, R.; Aygen, E.; Ozercan, I.H.; Arikanoglu, Z.; Akbulut, S. The effect of antivascular endothelial growth factor on the development of adhesion formation in laparotomizes rats: Experimental study. Gastroenterol. Res. Pract. 2011, 2011, 578691. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.H. Prevention of peritoneal adhesions: A promising role for gene therapy. World J. Gastroenterol. 2011, 17, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Brüggmann, D.; Tchartchian, G.; Wallwiener, M.; Münstedt, K.; Tinneberg, H.; Hackethal, A. Intra-abdominal adhesions definition, origin, significance in surgical practice, and treatment options. Dtsch. Arztebl. Int. 2010, 107, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Tae-Jung, S.; Ji-Woong, C.H. Small bowel obstruction caused by an anomalous congenital band in an infant. Korean J. Pediatr. 2008, 51, 219–221. [Google Scholar] [CrossRef]
- Sozen, S.; Emir, S.; Yazar, F.M.; Altinsoy, H.B.; Topuz, O.; Vurdem, U.E.; Cetinkunar, S.; Özkan, Z.; Güzel, K. Small bowel obstruction due to anomalous congenital peritoneal bands—Case series in adults. Bratisl. Med. J. 2012, 113, 186–189. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Tsilioni, I.; Spyridakis, M.-E.; Kiropoulos, T.; Oikonomidi, S.; Koukoulis, G.; Tepetes, K. Matrix Metaloproteinase-2 and -9 Serum Levels as Potential Markers of Intraperitoneal Adhesions. J. Investig. Surg. 2013, 26, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Ansaloni, L.; Manfredi, R.; Campanati, L.; Poiasina, E.; Bertoli, P.; Capponi, M.G.; Sarelli, M.; Di Saverio, S.; Cucchi, M.; et al. Peritoneal adhesion index (PAI): Proposal of a score for the "ignored iceberg" of medicine and surgery. World J. Emerg. Surg. 2013, 8. [Google Scholar] [CrossRef]
- Chegini, N. TGF-beta system: The principal profibrotic mediator of peritoneal adhesion formation. Semin. Reprod. Med. 2008, 26, 298–312. [Google Scholar] [CrossRef]
- Sun, H.-J.; Cai, W.-W.; Gong, L.-L.; Wang, X.; Zhu, X.-X.; Wan, M.-Y.; Wang, P.-Y.; Qiu, L.-Y. FGF-2-mediated FGFR1 signaling in human microvascular endothelial cells is activated by vaccarin to promote angiogenesis. Biomed. Pharmacother. 2017, 95, 144–152. [Google Scholar] [CrossRef]
- Meyer, M.; Müller, A.-K.; Yang, J.; Moik, D.; Ponzio, G.; Ornitz, D.M.; Grose, R.; Werner, S. FGF receptors 1 and 2 are key regulators of keratinocyte migration in vitro and in wounded skin. J. Cell Sci. 2012, 125, 5690–5701. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Liakakos, T.; Thomakos, N.; Fine, P.M.; Dervenis, C.; Young, R.L. Peritoneal adhesions: Etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig. Surg. 2001, 18, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Bayhan, Z.; Zeren, S.; Kocak, F.E.; Kocak, C.; Akcılar, R.; Kargı, E.; Tiryaki, C.; Yaylak, F.; Akcılar, A. Antiadhesive and anti-inflammatory effects of pirfenidone in postoperative intra-abdominal adhesion in an experimental rat model. J. Surg. Res. 2016, 201, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Chen, X.; Wang, G.; Fan, L.; Wang, K.; Li, X. Effect of Resveratrol on the Prevention of Intra-Abdominal Adhesion Formation in a Rat Model. Cell. Physiol. Biochem. 2016, 39, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, Y.; Li, Z.; Zhang, T.; Zeng, L. Evaluation of breviscapine on prevention of experimentally induced abdominal adhesions in rats. Am. J. Surg. 2016, 211, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Keegan, A.D.; Heller, N.M.; Rook, G.A.W.; Shea-Donohue, T.; Atamas, S.P. Regulation of inflammation by interleukin-4: A review of “alternatives”. J. Leukoc. Biol. 2012, 92, 753–764. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; Van Laar, J.M. Interleukin-6 (IL-6) Trans Signaling Drives a STAT3-dependent Pathway That Leads to Hyperactive Transforming Growth Factor-β (TGF-β) Signaling Promoting SMAD3 Activation and Fibrosis via Gremlin Protein. J. Biol. Chem. 2014, 289, 9952–9960. [Google Scholar] [CrossRef]
- Vallée, A.; LeCarpentier, Y.; Guillevin, R.; Vallée, J.-N. Interactions between TGF-β1, canonical WNT/β-catenin pathway and PPAR γ in radiation-induced fibrosis. Oncotarget 2017, 8, 90579–90604. [Google Scholar] [CrossRef]
- De Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.A.; Mulero, V.; Calado, Â. Cxcl8 (Interleukin-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Seidler, S.; Gassler, N.; Nattermann, J.; Luedde, T.; Trautwein, C.; Tacke, F. Interleukin-8 Is Activated in Patients with Chronic Liver Diseases and Associated with Hepatic Macrophage Accumulation in Human Liver Fibrosis. PLoS ONE 2011, 6, e21381. [Google Scholar] [CrossRef]
- Shmarov, V.A.; Malashchenko, V.V.; Meniailo, M.E.; Gazatova, N.D.; Todosenko, N.M.; Melashchenko, O.B.; Goncharov, A.G.; Seledtsov, V.I. Direct effects of interleukin-7 on the function of human T cells in vitro. Eur. Cytokine Netw. 2016, 27, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, X.; Shen, G.; Wang, W.; Li, J.; Zhao, J.; Wei, Y.-Q.; Edwards, C.K. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci. Rep. 2016, 6, 24249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onishi, A.; Akimoto, T.; Urabe, M.; Hirahara, I.; Muto, S.; Ozawa, K.; Nagata, D.; Kusano, E. Attenuation of methylglyoxal-induced peritoneal fibrosis: Immunomodulation by interleukin-10. Lab. Investig. 2015, 95, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Michaud, M.; Shankar, R.; Canosa, S.; Schwartz, M.; Madri, J.A. MMP-2: A modulator of neuronal precursor activity and cognitive and motor behaviors. Behav. Brain Res. 2017, 333, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Miyake, K.; Aoki, M.; Ogawa, R.; Akaishi, S.; Shimada, T.; Okada, T.; Hyakusoku, H. Tissue Inhibitor of Metalloproteinase-2 Suppresses Collagen Synthesis in Cultured Keloid Fibroblasts. Plast. Reconstr. Surg. Glob. Open 2015, 3, e520. [Google Scholar] [CrossRef] [PubMed]
- Ucar, E.; Borazan, A.; Semerci, E.; Binici, D.; Yaldiz, M.; Aysal, A.; Altug, E.; Kuvandik, C.; Huzmeli, C.; Yetim, T.; et al. The Effects of Interferon α2b on Chemically-Induced Peritoneal Fibrosis and on Peritoneal Tissue MMP-2 and TIMP-2 Levels in Rats. J. Int. Med. Res. 2010, 38, 187–194. [Google Scholar] [CrossRef]
- Junga, A.; Pilmane, M.; Ābola, Z.; Volrāts, O. The Distribution of Vascular Endothelial Growth Factor (VEGF), Human Beta-Defensin-2 (HBD-2), and Hepatocyte Growth Factor (HGF) in Intra-Abdominal Adhesions in Children under One Year of Age. Sci. World J. 2018, 2018, 5953095. [Google Scholar] [CrossRef]
- Stefanini, M.; De Martino, C.; Zamboni, L. Fixation of Ejaculated Spermatozoa for Electron Microscopy. Nature 1967, 216, 173–174. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Raine, L.; Fanger, H. The Use of Antiavidin Antibody and Avidin-Biotin-Peroxidase Complex in Immunoperoxidase Technics. Am. J. Clin. Pathol. 1981, 75, 816–821. [Google Scholar] [CrossRef]
- Pilmane, M.; Luts, A.; Sundler, F. Changes in neuroendocrine elements in bronchial mucosa in chronic lung disease in adults. Thorax 1995, 50, 551–554. [Google Scholar] [CrossRef]
- Riffenburgh, R.H. Chapter 11—Tests on Ranked Data. In Statistics in Medicine, 3rd ed.; Elsevier Inc.: San Diego, CA, USA, 2012; pp. 221–248. [Google Scholar]
- Riffenburgh, R.H. Chapter 21—Regression and Correlation. In Statistics in Medicine, 3rd ed.; Elsevier Inc.: San Diego, CA, USA, 2012; pp. 443–472. [Google Scholar]
- Verrecchia, F.; Mauviel, A. Transforming growth factor-β and fibrosis. World J. Gastroenterol. 2007, 13, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- Pohlers, D.; Brenmoehl, J.; Löffler, I.; Müller, C.K.; Leipner, C.; Schultze-Mosgau, S.; Stallmach, A.; Kinne, R.W.; Wolf, G. TGF-β and fibrosis in different organs—Molecular pathway imprints. Biochim. Biophys. Acta 2009, 1792, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Ghellai, A. Role of transforming growth factor beta-1 in peritonitis-induced adhesions. J. Gastrointest. Surg. 2000, 4, 316–323. [Google Scholar] [CrossRef]
- Leask, A.; Abraham, D.J. TGF-β signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Wang, P.; Shu, B.; Xu, Y.; Zhu, J.; Liu, J.; Zhou, Z.; Chen, L.; Zhao, J.; Liu, X.; Qi, S.; et al. Basic fibroblast growth factor reduces scar by inhibiting the differentiation of epidermal stem cells to myofibroblasts via the Notch1/Jagged1 pathway. Stem Cell Res. Ther. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. Fibroblast Growth Factor 2 as an Antifibrotic: Antagonism of Myofibroblast Differentiation and Suppression of Pro-Fibrotic Gene Expression. Cytokine Growth Factor Rev. 2017, 38, 49–58. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004, 20, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Guglielmotto, M.; Monteleone, D.; Vasciaveo, V.; Repetto, I.E.; Manassero, G.; Tabaton, M.; Tamagno, E. The Decrease of Uch-L1 Activity Is a Common Mechanism Responsible for Aβ 42 Accumulation in Alzheimer’s and Vascular Disease. Front. Aging Neurosci. 2017, 9, 320. [Google Scholar] [CrossRef]
- Wilson, C.L.; Murphy, L.B.; Leslie, J.; Kendrick, S.; French, J.; Fox, C.R.; Sheerin, N.S.; Fisher, A.; Robinson, J.H.; Tiniakos, D.G.; et al. Ubiquitin C-terminal hydrolase 1: A novel functional marker for liver myofibroblasts and a therapeutic target in chronic liver disease. J. Hepatol. 2015, 63, 1421–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corti, A. Chromogranin A and the Tumor Microenvironment. Cell. Mol. Neurobiol. 2010, 30, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Maciver, A.H.; McCall, M.; Shapiro, A.J. Intra-abdominal adhesions: Cellular mechanisms and strategies for prevention. Int. J. Surg. 2011, 9, 589–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mion, F.; Tonon, S.; Toffoletto, B.; Cesselli, D.; Pucillo, C.E.; Vitale, G. IL-10 production by B cells is differentially regulated by immune-mediated and infectious stimuli and requires p38 activation. Mol. Immunol. 2014, 62, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Joyce, J.A. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle 2010, 9, 4824–4835. [Google Scholar] [CrossRef] [PubMed]
- Binder, F.; Hayakawa, M.; Choo, M.K.; Sano, Y.; Park, J.M. Interleukin-4-induced β-catenin regulates the conversion of macrophages to multinucleated giant cells. Mol. Immunol. 2013, 54, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Henkels, M.K.; Frondorf, K.; Gonzalez-Meija, E.M.; Doseff, A.L.; Gomez-Cambronero, J. IL-8-induced neutrophil chemotaxis is mediated by Janus kinase 3 (JAK3). FEBS Lett. 2011, 585, 159–166. [Google Scholar] [CrossRef]
- Jin, X.; Ren, S.; Macarak, E.; Rosenbloom, J. Pathobiological mechanisms of peritoneal adhesions: The mesenchymal transition of rat peritoneal mesothelial cells induced by TGF-β1 and IL-6 requires activation of Erk1/2 and Smad2 linker region phosphorylation. Matrix Biol. 2016, 51, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [Green Version]
- Bikker, A.; Hack, C.E.; Lafeber, F.P.; Van Roon, J.A. Interleukin-7: A key mediator in T cell-driven autoimmunity, inflammation, and tissue destruction. Curr. Pharm. Des. 2012, 18, 2347–2356. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, D.L.; Zhang, X.; Liu, X.F.; Xue, F.; Yang, R.C. Interleukin-7 is decreased and maybe plays a pro-inflammatory function in primary immune thrombocytopenia. Platelets 2015, 26, 243–249. [Google Scholar] [CrossRef]
- Ambler, D.; Fletcher, N.; Diamond, M.; Saed, G. Effects of hypoxia on the expression of inflammatory markers IL-6 and TNF-a in human normal peritoneal and adhesion fibroblasts. Syst. Biol. Reprod. Med. 2012, 58, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chegini, N.; Zhao, Y.; Kotseos, K.; Ma, C.; Bennett, B.; Diamond, M.P.; Holmdahl, L.; Skinner, K. Differential expression of matrix metalloproteinase and tissue inhibitor of MMP in serosal tissue of intraperitoneal organs and adhesions. BJOG 2002, 109, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Z.; Wang, P.; Xu, M. Erythropoietin Modulates Imbalance of Matrix Metalloproteinase-2 and Tissue Inhibitor of Metalloproteinase-2 in Doxorubicin-induced Cardiotoxicity. Heart Lung Circ. 2014, 23, 772–777. [Google Scholar] [CrossRef] [PubMed]
| Factor | Group | Median | IQR | U | p |
|---|---|---|---|---|---|
| TGFβ | Adhesions | ++ | 0.5 | 50.5 | <0.001 |
| Control | +/++ | 0 | |||
| FGF-2 | Adhesions | + | 2 | 83.0 | 0.007 |
| Control | ++/+++ | 0 | |||
| FGFR1 | Adhesions | ++ | 1.125 | 81.0 | 0.006 |
| Control | + | 0.375 | |||
| PGP 9.5 | Adhesions | +/++ | 0.5 | 58.5 | 0.001 |
| Control | ++/+++ | 0.5 | |||
| CgA | Adhesions | + | 0.5 | 170.5 | 0.491 |
| Control | + | 0 | |||
| IL-1α | Adhesions | +/++ | 1.5 | 98.5 | 0.015 |
| Control | ++ | 0 | |||
| IL-4 | Adhesions | +/++ | 1 | 60.5 | 0.002 |
| Control | ++ | 0.5 | |||
| IL-6 | Adhesions | ++ | 1 | 146.5 | 0.243 |
| Control | ++/+++ | 0.5 | |||
| IL-7 | Adhesions | ++/+++ | 1 | 144.5 | 0.190 |
| Control | ++/+++ | 0.5 | |||
| IL-8 | Adhesions | + to +/++ | 1 | 40.0 | < 0.001 |
| Control | ++/+++ | 0 | |||
| IL-10 | Adhesions | ++/+++ | 0.5 | 184.0 | 0.769 |
| Control | ++/+++ | 0.5 | |||
| TNFα | Adhesions | ++ | 0.5 | 124.0 | 0.082 |
| Control | ++ | 0 | |||
| MMP-2 | Adhesions | +/++ | 1 | 174.0 | 0.654 |
| Control | +/++ | 0.75 | |||
| TIMP-2 | Adhesions | ++ | 0.5 | 112.0 | 0.036 |
| Control | ++ | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junga, A.; Pilmane, M.; Ābola, Z.; Volrāts, O. The Morphopathogenetic Aspects of Intraabdominal Adhesions in Children under One Year of Age. Medicina 2019, 55, 556. https://doi.org/10.3390/medicina55090556
Junga A, Pilmane M, Ābola Z, Volrāts O. The Morphopathogenetic Aspects of Intraabdominal Adhesions in Children under One Year of Age. Medicina. 2019; 55(9):556. https://doi.org/10.3390/medicina55090556
Chicago/Turabian StyleJunga, Anna, Māra Pilmane, Zane Ābola, and Olafs Volrāts. 2019. "The Morphopathogenetic Aspects of Intraabdominal Adhesions in Children under One Year of Age" Medicina 55, no. 9: 556. https://doi.org/10.3390/medicina55090556





