A Comparative Analysis between Subpectoral versus Prepectoral Single Stage Direct-to-Implant Breast Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
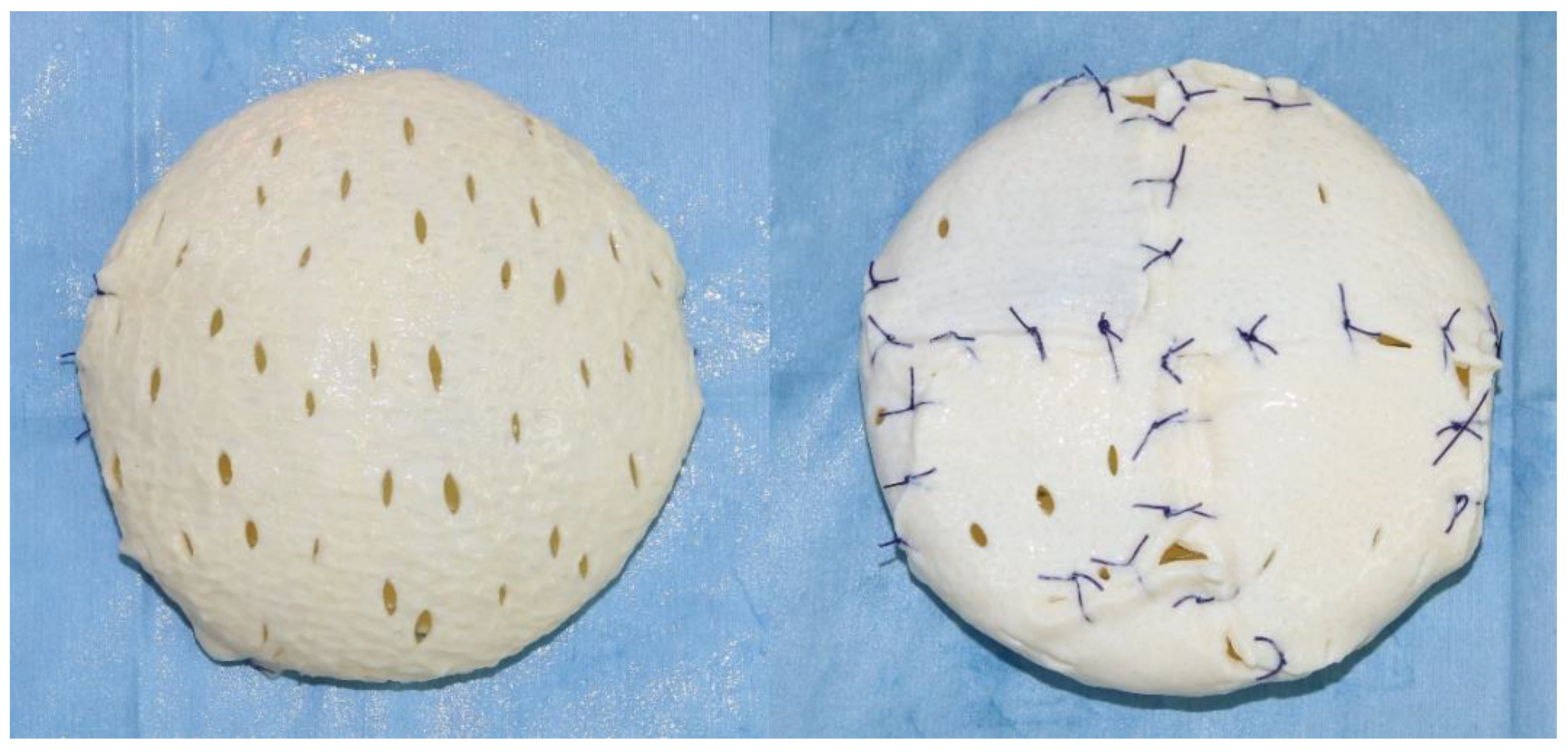
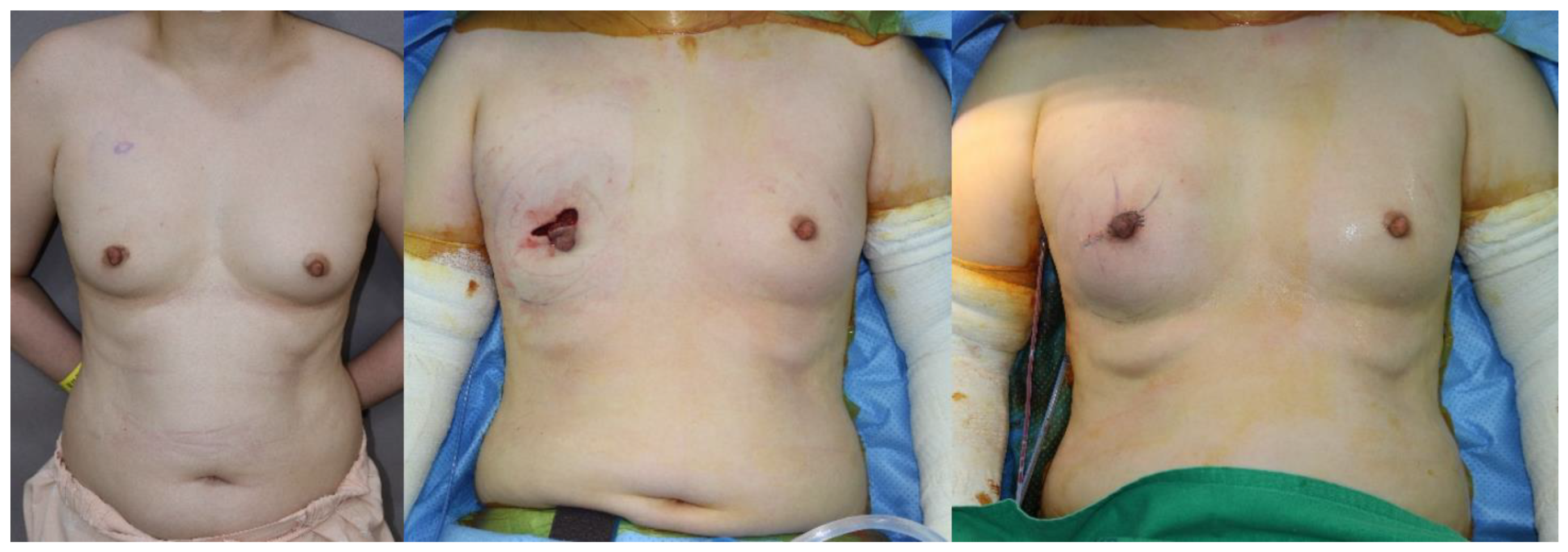
2.2. Surgical Technique
2.3. Pain Assessment
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Operative Data
3.2. Surgical Outcome and Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Society of Plastic Surgeons. 2019 Plastic Surgery Statistics Report. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2019/plastic-surgery-statistics-full-report-2019.pdf (accessed on 9 September 2020).
- Macadam, A.S.; Lennox, P.A. Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Can. J. Plast. Surg. 2012, 20, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, C.A. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann. Plast. Surg. 2006, 57, 1–5. [Google Scholar] [CrossRef]
- Salzberg, C.A.; Ashikari, A.Y.; Berry, C.; Hunsicker, L.M. Acellular dermal matrix-assisted direct-to-implant breast reconstruction and capsular contracture: A 13-year experience. Plast. Reconstr. Surg. 2016, 138, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Spear, S.L.; Parikh, P.M.; Reisin, E.; Menon, N.G. Acellular dermis-assisted breast reconstruction. Aesthetic. Plast. Surg. 2008, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Nguyen, T.J.; Shahabi, A.; Hwang, B.H.; Chan, L.S.; Wong, A.K. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann. Plast. Surg. 2012, 68, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Vardanian, A.J.; Clayton, J.L.; Roostaeian, J.; Shirvanian, V.; Da Lio, A.; Lipa, J.E.; Crisera, C.; Festekjian, J.H. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast. Reconstr. Surg. 2011, 128, 403e–410e. [Google Scholar] [CrossRef] [PubMed]
- Fracol, M.; Feld, L.N.; Chiu, W.K.; Kim, J.Y.S. An overview of animation deformity in prosthetic breast reconstruction. Gland. Surg. 2019, 8, 95–101. [Google Scholar] [CrossRef]
- Cattelani, L.; Polotto, S.; Arcuri, M.F.; Pedrazzi, G.; Linguadoca, C.; Bonati, E. One-step prepectoral breast reconstruction with dermal matrix-covered implant compared to submuscular implantation: Functional and cost evaluation. Clin. Breast. Cancer. 2018, 18, e703–e711. [Google Scholar] [CrossRef]
- Baker, B.G.; Irri, R.; MacCallum, V.; Chattopadhyay, R.; Murphy, J.; Harvey, J.R. A Prospective comparison of short-term outcomes of subpectoral and prepectoral strattice-based immediate breast reconstruction. Plast. Reconstr. Surg. 2018, 141, 1077–1084. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Choi, M.; Karp, N.S. Mastectomy flap thickness and complications in nipple-sparing mastectomy: Objective evaluation using magnetic resonance imaging. Plast. Reconstr. Surg. Glob. Open. 2017, 5, e1439. [Google Scholar] [CrossRef]
- Fredericks, S. A 10-year experience with subcutaneous mastectomy. Clin. Plast. Surg. 1975, 2, 347–357. [Google Scholar] [CrossRef]
- Radovan, C. Breast reconstruction after mastectomy using the temporary expander. Plast. Reconstr. Surg. 1982, 69, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Prpic, I. Reconstruction of the breast after mastectomy for carcinoma. Aesthetic. Plast. Surg. 1981, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Salibian, A.A.; Frey, J.D.; Karp, N.S. Strategies and considerations in selecting between subpectoral and prepectoral breast reconstruction. Gland. Surg. 2019, 8, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Margulies, I.G.; Salzberg, C.A. The use of acellular dermal matrix in breast reconstruction: Evolution of techniques over 2 decades. Gland. Surg. 2019, 8, 3–10. [Google Scholar] [CrossRef]
- Becker, H.; Fregosi, N. The impact of animation seformity on quality of life in post-mastectomy reconstruction patients. Aesthet. Surg. J. 2017, 37, 531–536. [Google Scholar] [CrossRef]
- Walia, G.S.; Aston, J.; Bello, R.; Mackert, G.A.; Pedreira, R.A.; Cho, B.H.; Carl, H.M.; Rada, E.M.; Rosson, G.D.; Sacks, J.M. Prepectoral versus subpectoral tissue expander placement: A clinical and quality of life outcomes study. Plast. Reconstr. Surg. Glob. Open. 2018, 6, e1731. [Google Scholar] [CrossRef]
- Chandarana, M.N.; Jafferbhoy, S.; Marla, S.; Soumian, S.; Narayanan, S. Acellular dermal matrix in implant-based immediate breast reconstructions: A comparison of prepectoral and subpectoral approach. Gland. Surg. 2018, 7 (Suppl. 1), S64–S69. [Google Scholar] [CrossRef]
- Potter, S.; Browning, D.; Savović, J.; Holcombe, C.; Blazeby, J.M. Systematic review and critical appraisal of the impact of acellular dermal matrix use on the outcomes of implant-based breast reconstruction. Br. J. Surg. 2015, 102, 1010–1025. [Google Scholar] [CrossRef]
- Bernini, M.; Calabrese, C.; Cecconi, L.; Santi, C.; Gjondedaj, U.; Roselli, J.; Nori, J.; Fausto, A.; Orzalesi, L.; Casella, D. Subcutaneous direct-to-implant breast reconstruction: Surgical, functional, and aesthetic results after long-term follow-up. Plast. Reconstr. Surg. Glob. Open. 2015, 3, e574. [Google Scholar] [CrossRef]
- Vu, M.M.; Oliveira, G.S.D., Jr.; Mayer, K.E.; Blough, J.T.; Kim, J.Y.S. A prospective study assessing complication rates and patient-reported outcomes in breast reconstructions using a novel, deep dermal human acellular dermal matrix. Plast. Reconstr. Surg. Glob. Open. 2015, 3, e585. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Park, S.O.; Chang, H.; Jin, U.S. Inhibition mechanism of acellular dermal matrix on capsule formation in expander-implant breast reconstruction after postmastectomy radiotherapy. Ann. Surg. Oncol. 2018, 25, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Casella, D.; Bernini, M.; Bencini, L.; Roselli, J.; Lacaria, M.T.; Martellucci, J.; Banfi, R.; Calabrese, C.; Orzalesi, L. TiLoop(R) Bra mesh used for immediate breast reconstruction: Comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur. J. Plast. Surg. 2014, 37, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.; Harless, C.; Jacobson, S.R. Revisiting an old place: Single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast. J. 2017, 23, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Sigalove, S. Options in acellular dermal matrix-device assembly. Plast. Reconstr. Surg. 2017, 140, 39S–42S. [Google Scholar] [CrossRef] [PubMed]
- Schnarrs, R.H.; Carman, C.M.; Tobin, C.; Chase, S.A.; Rossmeier, K.A. Complication rates with human acellular dermal matrices: Retrospective review of 211 consecutive breast reconstructions. Plast. Reconstr. Surg. Glob. Open. 2016, 4, e1118. [Google Scholar] [CrossRef]
- Nadeem, R. Prepectoral implant-based breast reconstruction; complete acellular dermal matrix wrap or anterior circumferential cover. Breast. J. 2018, 24, 223–224. [Google Scholar] [CrossRef]
- Downs, R.K.; Hedges, K. An alternative technique for immediate direct-to-implant breast reconstruction—A case series. Plast. Reconstr. Surg. Glob. Open. 2016, 4, e821. [Google Scholar] [CrossRef]

| Prepectoral | Subpectoral | p-Value | |
|---|---|---|---|
| No. of patients (%) | 53 (31.7) | 114 (68.3) | |
| Age, mean ± SD, yrs | 47.68 ± 7.45 | 46.56 ± 9.65 | 0.41 |
| BMI, mean ± SD, kg/m2 | 23.92 ± 3.61 | 22.65 ± 2.81 | 0.01 |
| Hypertension (%) | 3 (5.7) | 14 (12.3) | 0.19 |
| Diabetes (%) | 2 (3.8) | 3 (2.6) | 0.65 * |
| Adjuvant chemotherapy (%) | 17 (32.1) | 49 (43.0) | 0.18 |
| Neoadjuvant chemotherapy (%) | 3 (5.7) | 12 (10.5) | 0.39 * |
| Adjuvant radiation therapy (%) | 6 (11.3) | 21 (18.4) | 0.25 |
| Cancer laterality | 0.87 | ||
| No. of right (%) | 30 (56.6) | 63 (55.3) | |
| No. of left (%) | 23 (43.3) | 51 (44.7) | |
| Cancer stage | 0.40 | ||
| No. of stage I (%) | 39 (73.6) | 76 (66.7) | |
| No. of stage II (%) | 11 (20.8) | 24 (21.0) | |
| No. of stage III (%) | 3 (5.7) | 14 (10.2) | |
| No. of stage IV (%) | 0 (0) | 0 | |
| Mastectomy type | 0.99 | ||
| No. of nipple-sparing (%) | 47 (88.7) | 101 (88.6) | |
| No. of skin-sparing (%) | 6 (11.3) | 13 (11.4) | |
| Mastectomy specimen weight, mean ± SD, g | 285.9 ± 116.9 | 302.2 ± 173.0 | 0.57 |
| Inserted implant volume, mean ± SD, cc | 249.0 ± 104.8 | 268.1 ± 103.0 | 0.27 |
| Inserted ADM size, mean ± SD, cm2 | 261.7 ± 71.1 | 119.7 ± 36.6 | <0.01 † |
| Prepectoral | Subpectoral | p-Value | |
|---|---|---|---|
| Average days until drain removed, mean ± SD, days | 11.09 ± 4.82 | 14.93 ± 5.57 | <0.01 † |
| Pain scale, mean ± SD | |||
| At 12 hrs after surgery | 4.49 ± 1.93 | 4.10 ± 1.28 | 0.18 |
| At 24 hrs after surgery | 2.66 ± 1.82 | 2.36 ± 1.38 | 0.29 |
| At 7 days after surgery | 1.08 ± 1.19 | 0.80 ± 1.07 | 0.14 |
| Complication | |||
| Seroma, n (%) | 6 (11.3) | 14 (12.3) | 0.86 |
| Infection, n (%) | 5 (9.4) | 1 (9.6) | 0.97 |
| Hematoma, n (%) | 0 (0) | 1 (0.9) | >0.99 * |
| Skin necrosis, n (%) | 2 (3.8) | 4 (8.8) | 0.34 * |
| Capsular contracture, n (%) | 2 (3.8) | 4 (3.5) | >0.99 * |
| Implant loss, n (%) | 5 (9.4) | 7 (6.1) | 0.52 * |
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Hemovac Duration | β | t | p | VIF | β | t | p | VIF |
| Constant | 1.068 | 0.287 | −1.857 | 0.065 | ||||
| Age | 0.045 | 0.597 | 0.551 | 1.098 | 0.039 | 0.562 | 0.575 | 1.099 |
| BMI | 0.142 | 1.649 | 0.101 | 1.435 | 0.242 | 2.935 | 0.004 | 1.519 |
| Resection weight | 0.296 | 3.545 | 0.001 | 1.339 | 0.231 | 2.945 | 0.004 | 1.374 |
| Insertion plane | 0.356 | 5.157 | <0.001 † | 1.064 | ||||
| ΔF(p) | 9.857 (<0.001 †) | 15.202 (<0.001 †) | ||||||
| R2 (adj. R2) | 0.154 (0.138) | 0.273 (0.255) | ||||||
| Pain score at 12 h after surgery | ||||||||
| Constant | 3.729 | <0.001 | 3.899 | <0.001 | ||||
| Age | 0.023 | 0.278 | 0.781 | 1.098 | 0.025 | 0.301 | 0.764 | 1.099 |
| BMI | 0.053 | 0.565 | 0.573 | 1.435 | 0.021 | 0.221 | 0.825 | 1.519 |
| Resection weight | −0.089 | −0.980 | 0.328 | 1.339 | −0.068 | −0.748 | 0.456 | 1.374 |
| Insertion plane | −0.113 | −1.404 | 0.162 | 1.064 | ||||
| ΔF(p) | 0.398 (0.755) | 0.793 (0.531) | ||||||
| R2 (adj. R2) | 0.007 (−0.011) | 0.019 (−0.005) | ||||||
| Pain score at 24 h after surgery | ||||||||
| Constant | 2.001 | 0.047 | 2.205 | −0.029 | ||||
| Age | −0.013 | −0.157 | 0.876 | 1.098 | −0.012 | −0.141 | 0.888 | 1.099 |
| BMI | 0.076 | 0.811 | 0.418 | 1.435 | 0.054 | 0.561 | 0.576 | 1.519 |
| Resection weight | −0.109 | −1.206 | 0.229 | 1.339 | −0.095 | −1.037 | 0.301 | 1.374 |
| Insertion plane | −0.078 | −0.969 | 0.334 | 1.064 | ||||
| ΔF(p) | 0.507 (0.678) | 0.615 (0.653) | ||||||
| R2 (adj. R2) | 0.009 (−0.009) | 0.015 (−0.009) | ||||||
| Pain score at 7 days after surgery | ||||||||
| Constant | 0.999 | 0.319 | 1.681 | 0.095 | ||||
| Age | 0.022 | 0.269 | 0.788 | 1.098 | 0.024 | 0.295 | 0.769 | 1.099 |
| BMI | −0.003 | −0.028 | 0.978 | 1.435 | −0.037 | −0.389 | 0.698 | 1.519 |
| Resection weight | 0.026 | 0.292 | 0.771 | 1.339 | 0.049 | 0.534 | 0.594 | 1.374 |
| Insertion plane | −0.124 | −1.539 | 0.126 | 1.064 | ||||
| ΔF(p) | 0.059 (0.981) | 0.637 (0.637) | ||||||
| R2 (adj. R2) | 0.001 (−0.017) | 0.015 (−0.009) | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Hong, S.E. A Comparative Analysis between Subpectoral versus Prepectoral Single Stage Direct-to-Implant Breast Reconstruction. Medicina 2020, 56, 537. https://doi.org/10.3390/medicina56100537
Kim J-H, Hong SE. A Comparative Analysis between Subpectoral versus Prepectoral Single Stage Direct-to-Implant Breast Reconstruction. Medicina. 2020; 56(10):537. https://doi.org/10.3390/medicina56100537
Chicago/Turabian StyleKim, Jeong-Hoon, and Seung Eun Hong. 2020. "A Comparative Analysis between Subpectoral versus Prepectoral Single Stage Direct-to-Implant Breast Reconstruction" Medicina 56, no. 10: 537. https://doi.org/10.3390/medicina56100537
APA StyleKim, J.-H., & Hong, S. E. (2020). A Comparative Analysis between Subpectoral versus Prepectoral Single Stage Direct-to-Implant Breast Reconstruction. Medicina, 56(10), 537. https://doi.org/10.3390/medicina56100537





