Bone Marrow Mesenchymal Stromal Cells (BMMSCs) Augment Osteointegration of Dental Implants in Type 1 Diabetic Rabbits: An X-Ray Micro-Computed Tomographic Evaluation
Abstract
:1. Introduction
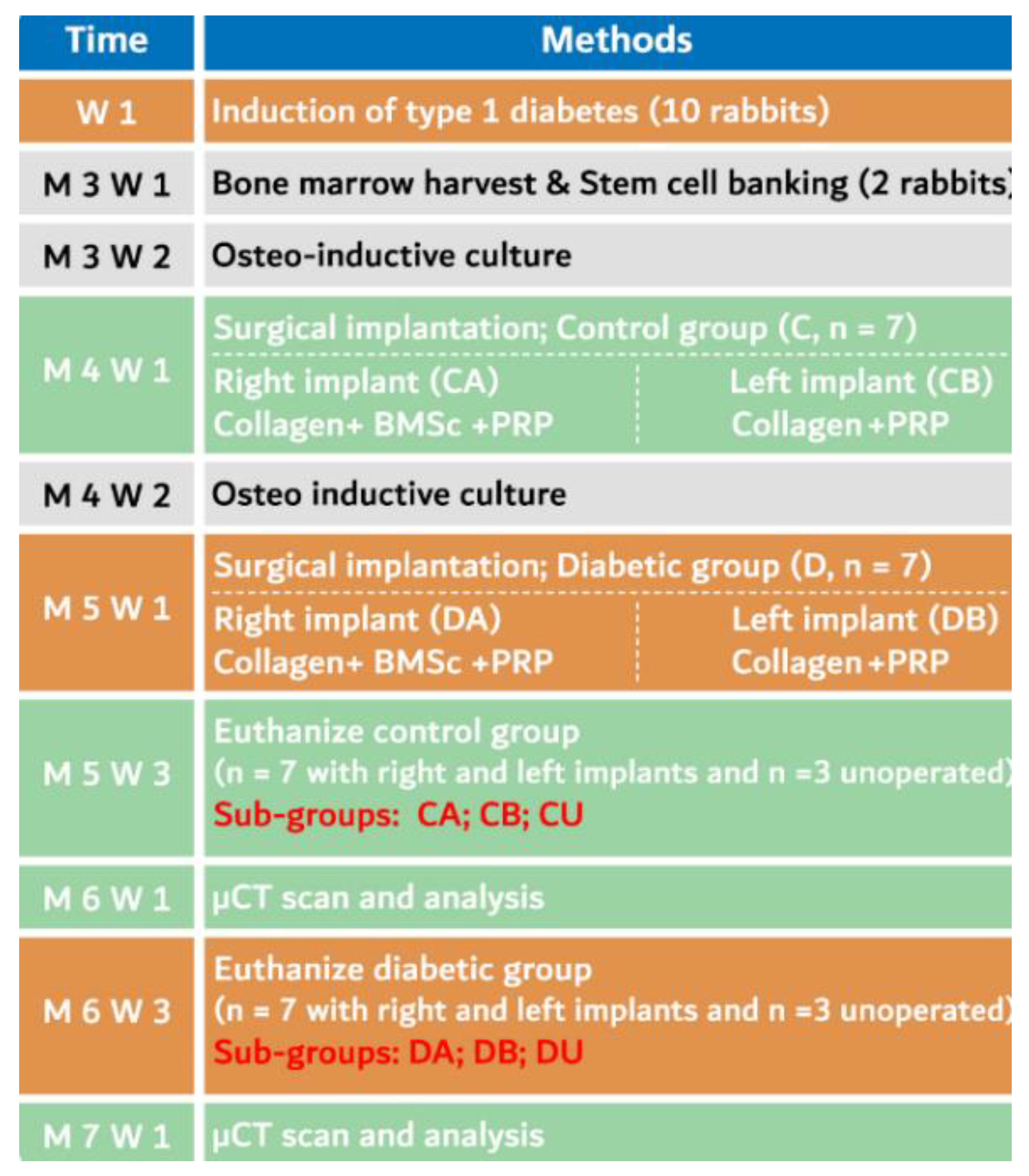
2. Materials and Methods
2.1. Animal Care
2.2. Induction of Diabetes
2.3. Autologous Platelet Rich Plasma (PRP) Preparation
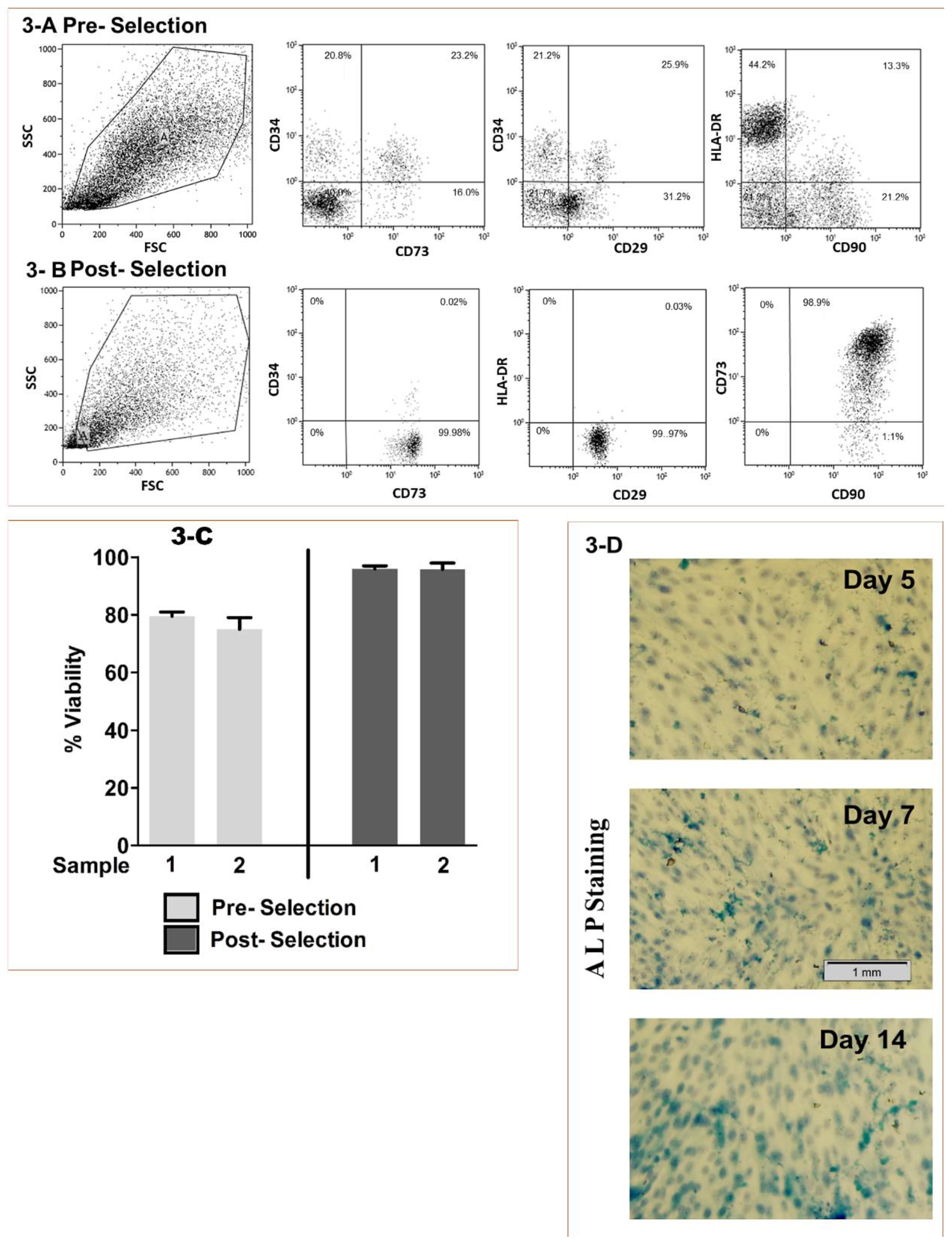
2.4. BMMSCs Sourcing, Isolation and Characterization
2.5. Viability and Osteo-induction
2.6. Osteoinduction Screening
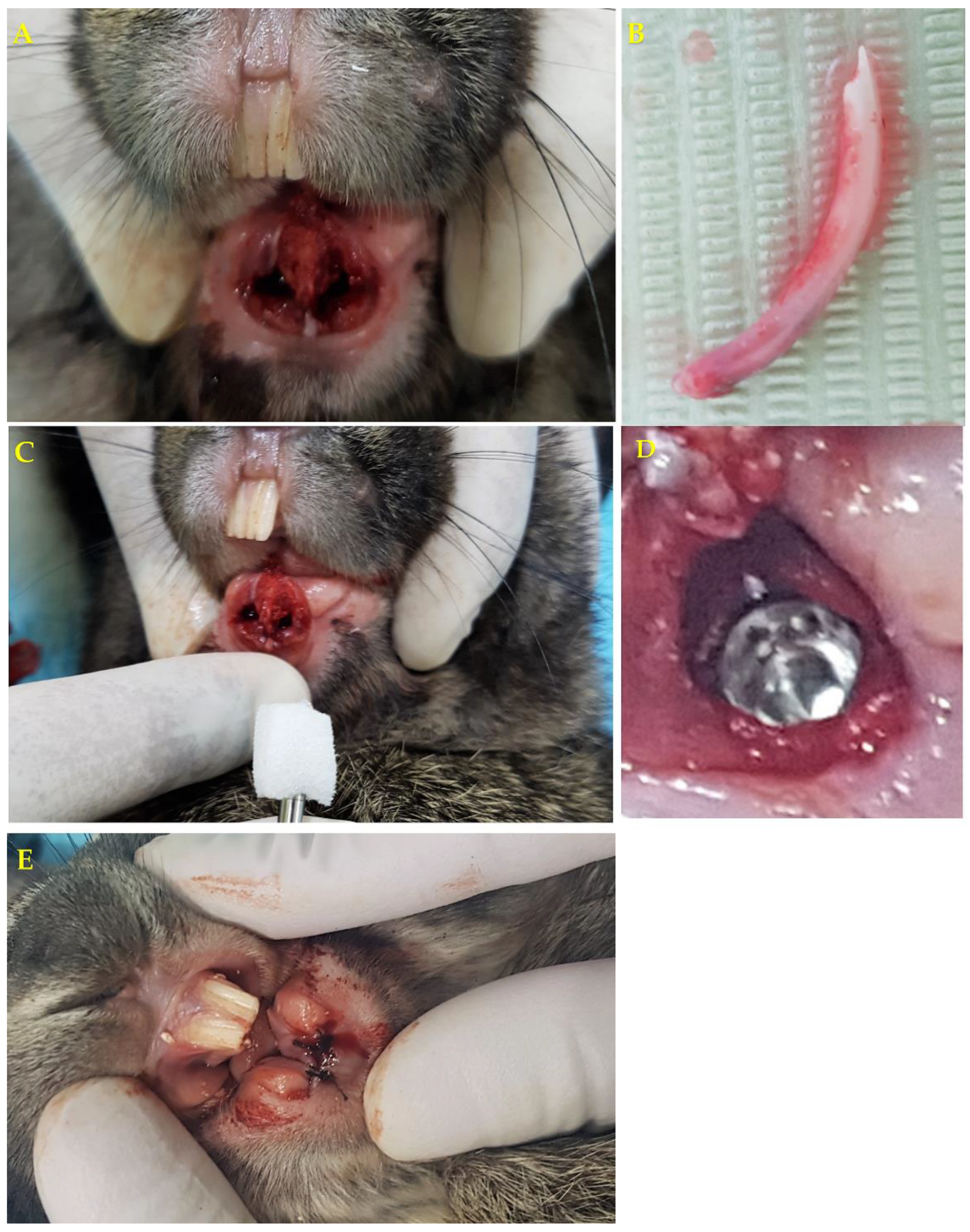
2.7. Surgical Procedure
2.8. Unoperated Animals
2.9. Animal Euthanasia and Specimen Preparation
2.10. μCT Scanning
2.11. The Micromorphometric Analysis
2.12. Statistical Analysis
3. Results
3.1. Stem Cell Processing
3.2. Unoperated Animals (Subgroup CU and DU)
3.3. Operated Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental implants and diabetes mellitus-a systematic review. Int. J. Implant. Dent. 2016, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorellini, J.P.; Chen, P.K.; Nevins, M.L. A retrospective study of dental implants in diabetic patients. Int. J. Periodontics Restor. Dent. 2000, 20, 366–373. [Google Scholar]
- Niang, P.; Ba, A.; Dia, T.S.; Tamba, B.; Gassama, B.B.C.; Diallo, B. Diabetes mellitus and early failures in oral implantology. Odontostomatol. Trop. 2011, 34, 26–32. [Google Scholar] [PubMed]
- Fahey, T.J.; Sadaty, A.; Jones, W.G.; Barber, A.; Smoller, B.; Shires, G. Diabetes impairs the late inflammatory response to wound healing. J. Surg. Res. 1991, 50, 308–313. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [Green Version]
- Nevins, M.L.; Karimbux, N.Y.; Weber, H.P.; Giannobile, W.V.; Fiorellini, J.P. Wound healing around endosseous implants in experimental diabetes. Int. J. Oral Maxillofac. Implant. 1998, 13, 620–629. [Google Scholar] [CrossRef]
- Takeshita, F.; Murai, K.; Iyama, S.; Ayukawa, Y.; Suetsugu, T. Uncontrolled Diabetes Hinders Bone Formation Around Titanium Implants in Rat Tibiae. A Light and Fluorescence Microscopy, and Image Processing Study. J. Periodontol. 1998, 69, 314–320. [Google Scholar] [CrossRef]
- Morris, H.F.; Ochi, S.; Winkler, S. Implant Survival in Patients With Type 2 Diabetes: Placement to 36 Months. Ann. Periodontol. 2000, 5, 157–165. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. Impact of Diabetes Mellitus and Glycemic Control on the Osseointegration of Dental Implants: A Systematic Literature Review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry – Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M.; Hoshina, H.; Li, M.; Arasawa, M.; Uematsu, K.; Ogawa, S.; Yamada, K.; Kawase, T.; Suzuki, K.; Ogose, A.; et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: Coordinated activation of bone formation and resorption. Bone 2012, 50, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry – Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Kemeny, D.M.; Heng, B.C.; Ouyang, H.W.; Melendez, A.J.; Cao, T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J. Immunol. 2006, 176, 2864–2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Temenoff, J.S.; Tabata, Y.; Caplan, A.; Mikos, A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials 2007, 28, 3217–3227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A. Review: Mesenchymal Stem Cells: Cell–Based Reconstructive Therapy in Orthopedics. Tissue Eng. 2005, 11, 1198–1211. [Google Scholar] [CrossRef] [Green Version]
- Reddy, S.H.R.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-cell therapy and platelet-rich plasma in regenerative medicines: A review on pros and cons of the technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar]
- Garbin, L.C.; Olver, C.S. Platelet-Rich Products and Their Application to Osteoarthritis. J. Equine Veter- Sci. 2020, 86, 102820. [Google Scholar] [CrossRef]
- Hung, B.P.; Hutton, D.L.; Kozielski, K.L.; Bishop, C.J.; Naved, B.; Green, J.J.; Caplan, A.; Gimble, J.M.; Dorafshar, A.H.; Grayson, W.L. Platelet-Derived Growth Factor BB Enhances Osteogenesis of Adipose-Derived But Not Bone Marrow-Derived Mesenchymal Stromal/Stem Cells. STEM CELLS 2015, 33, 2773–2784. [Google Scholar] [CrossRef] [Green Version]
- Jacobsson, M.; Albrektsson, T.; Chrcanovic, B.; Wennerberg, A. Osseointegration of Implants: A Biological and Clinical Overview. JSM Dent. Surg. 2017, 2, 1022. [Google Scholar]
- Stübinger, S.; Dard, M. The Rabbit as Experimental Model for Research in Implant Dentistry and Related Tissue Regeneration. J. Investig. Surg. 2013, 26, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Altug, H.A.; Tatli, U.; Coskun, A.T.; Erdogan, Ö.; Özkan, A.; Sencimen, M.; Kürkçü, M. Effects of hyperbaric oxygen treatment on implant osseointegration in experimental diabetes mellitus. J. Appl. Oral Sci. 2018, 26, e20180083. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Y.; Wen, X.-X.; Yang, X.-J.; Zhou, D.-P.; Wu, Q.; Feng, Y.; Ding, H.-J.; Lei, W.; Yu, H.; Liu, B.; et al. Ophiopogonin D improves osteointegration of titanium alloy implants under diabetic conditions by inhibition of ROS overproduction via Wnt/β-catenin signaling pathway. Biochimie 2018, 152, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Cirano, F.; Pimentel, S.; Casati, M.; Correa, M.; Pino, D.; Messora, M.; Silva, P.H.F.; Ribeiro, F.V. Effect of curcumin on bone tissue in the diabetic rat: Repair of peri-implant and critical-sized defects. Int. J. Oral Maxillofac. Surg. 2018, 47, 1495–1503. [Google Scholar] [CrossRef]
- Wang, J.; Wan, R.; Mo, Y.; Zhang, Q.; Sherwood, L.C.; Chien, S. Creating a Long-Term Diabetic Rabbit Model. Exp. Diabetes Res. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Pazzini, J.; De Nardi, A.B.; Huppes, R.R.; Gering, A.P.; Ferreira, M.G.; Silveira, C.P.; Luzzi, M.C.; Santos, R. Method to obtain platelet-rich plasma from rabbits (Oryctolagus cuniculus ). Pesqui. Veterinária Bras. 2016, 36, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Husam Almanseekanaa, L.; Mansoor Jasim, A.; Mohammed, R. Isolation and characterization of bone marrowmesenchymal stem cells from rat and rabbit, a modified method. Karbala J. Pharm. Sci. 2012, 3, 44–51. [Google Scholar]
- Tan, S.-L.; Ahmad, T.S.; Selvaratnam, L.; Kamarul, T. Isolation, characterization and the multi-lineage differentiation potential of rabbit bone marrow-derived mesenchymal stem cells. J. Anat. 2013, 222, 437–450. [Google Scholar] [CrossRef]
- Yamada, Y.; Ueda, M.; Hibi, H.; Nagasaka, T. Translational research for injectable tissue-engineered bone regeneration using mesenchymal stem cells and platelet-rich plasma: From basic research to clinical case study. Cell Transplant. 2004, 13, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Eleotério, R.B.; Sepulveda, R.; Reis, E.C.; Valente, F.L.; Borges, A.P.B. Isolation, expansion and differentiation of mesenchymal stromal cells from rabbits’ bone marrow. Pesqui. Veterinária Bras. 2016, 36, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Zhao, J.-L.; Mao, M.; Chen, J.; Dong, Z.-W.; Liu, Y.-P. Scaffold-Based Delivery of Bone Marrow Mesenchymal Stem Cell Sheet Fragments Enhances New Bone Formation In Vivo. J. Oral Maxillofac. Surg. 2017, 75, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.W.; Shernoff, A.F.; Tarlow, J.L.; A Colwell, J.; Scheetz, J.P.; Bingham, S.F. Dental endosseous implant assessments in a type 2 diabetic population: A prospective study. Int. J. Oral Maxillofac. Implant. 2000, 15, 811–818. [Google Scholar]
- Barbosa, M.A.L.; Filho, L.I.; Iwaki, L.C.V.; Natali, M.R.M.; Takeshita, W.; Sabio, S. Histologic and histomorphometric study of bone repair around short dental implants inserted in rabbit tibia, associated with tricalcium phosphate graft bone. Acta Sci. Heal. Sci. 2014, 36, 257. [Google Scholar] [CrossRef] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Kumararama, S.S.; Mishra, S.K.; Chowdhary, R. Evaluation of bone stimulation by different designs of microthreaded implants in enhancing osseointegration: An in vivo animal study supported by a numerical analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.S.; Kontogiorgos, E.; Dechow, P.C.; Kerns, D.G.; Nelson, C.J.; Opperman, L. Comparison of the effects of phosphate-coated and sandblasted acid-etched titanium implants on osseointegration: A microcomputed tomographic examination in the canine model. Int. J. Oral Maxillofac. Implant. 2012, 27, 1069–1080. [Google Scholar]
- Zheng, R.; Park, Y.; Cho, J.; Kim, S.; Heo, S.; Koak, J.; Lee, J. Bone Regeneration at Dental Implant Sites with Suspended Stem Cells. J. Dent. Res. 2014, 93, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2007, 8, 301–316. [Google Scholar] [CrossRef]
- Lazarus, H.M.; E Haynesworth, S.; Gerson, S.L.; Rosenthal, N.S.; I Caplan, A. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant. 1995, 16, 557–564. [Google Scholar]
- Richardson, S.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Dimarino, A.M.; Caplan, A.; Bonfield, T. Mesenchymal Stem Cells in Tissue Repair. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninu, A.R.; Maiti, S.K.; Kumar, S.; Sangeeta, P.; Kritaniya, D.; Gupta, S.; Saxena, A.; Kumar, N. Isolation, proliferation, characterization and in vivo osteogenic potential of bone-marrow derived mesenchymal stem cells (rBMSC) in rabbit model. Indian J. Exp. Biol. 2017, 55, 79–87. [Google Scholar] [PubMed]
- Yu, M.; Zhou, W.; Song, Y.; Yu, F.; Li, D.; Na, S.; Zou, G.; Zhai, M.; Xie, C. Development of mesenchymal stem cell-implant complexes by cultured cells sheet enhances osseointegration in type 2 diabetic rat model. Bone 2011, 49, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Y.; Feng, Y.; Ma, Z.-S.; Li, X.; Wang, J.; Wang, L.; Lei, W. The promotion of osteointegration under diabetic conditions using chitosan/hydroxyapatite composite coating on porous titanium surfaces. Biomaterials 2014, 35, 7259–7270. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Zhang, X.; Wu, Y.; Li, L.; Yin, Q.; Zheng, L.; Zhang, H.; Sun, C. Streptozotocin-Induced Diabetic Rat–Derived Bone Marrow Mesenchymal Stem Cells Have Impaired Abilities in Proliferation, Paracrine, Antiapoptosis, and Myogenic Differentiation. Transplant. Proc. 2010, 42, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Lee, M.-S.; Tsai, T.-L.; Leiferman, E.M.; Trask, D.J.; Squire, M.W.; Li, W.-J. Bone Morphogenetic Protein-6 Attenuates Type 1 Diabetes Mellitus-Associated Bone Loss. STEM CELLS Transl. Med. 2019, 8, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Tan, N.; Zhou, Y.; Wei, H.; Ren, S.; Yu, F.; Chen, H.; Jia, C.; Yang, G.; Song, Y. Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus. Int. J. Nanomed. 2017, 12, 7089–7101. [Google Scholar] [CrossRef] [Green Version]
- Irina-Maria, G.; Stoian, I.M. Implant surgery in healthy compromised patients-review of literature. J. Med. Life 2014, 7, 7–10. [Google Scholar]
- Zavan, B.; Ferroni, L.; Gardin, C.; Sivolella, S.; Piattelli, A.; Mijiritsky, E. Release of VEGF from Dental Implant Improves Osteogenetic Process: Preliminary In Vitro Tests. Materials 2017, 10, 1052. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.-C.; Seol, Y.-J.; A Cirelli, J.; Pellegrini, G.; Jin, Q.; Franco, L.M.; Goldstein, S.A.; Chandler, L.A.; Sosnowski, B.; Giannobile, W.V. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2009, 17, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Gabet, Y.; Kohavi, D.; Kohler, T.; Baras, M.; Müller, R.; Bab, I. Trabecular Bone Gradient in Rat Long Bone Metaphyses: Mathematical Modeling and Application to Morphometric Measurements and Correction of Implant Positioning. J. Bone Miner. Res. 2007, 23, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campillo, V.-E.; Langonnet, S.; Pierrefeu, A.; Chaux-Bodard, A.-G. Anatomic and histological study of the rabbit mandible as an experimental model for wound healing and surgical therapies. Lab. Anim. 2014, 48, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Pothuaud, L.; Van Rietbergen, B.; Mosekilde, L.; Beuf, O.; Levitz, P.; Benhamou, C.L.; Majumdar, S. Combination of topological parameters and bone volume fraction better predicts the mechanical properties of trabecular bone. J. Biomech. 2002, 35, 1091–1099. [Google Scholar] [CrossRef]
- Ulrich, D.; Van Rietbergen, B.; Laib, A.; Rüegsegger, P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone 1999, 25, 55–60. [Google Scholar] [CrossRef]
- Yeom, H.; Blanchard, S.; Kim, S.; Zunt, S.; Chu, T.-M.G. Correlation Between Micro-Computed Tomography and Histomorphometry for Assessment of New Bone Formation in a Calvarial Experimental Model. J. Craniofacial Surg. 2008, 19, 446–452. [Google Scholar] [CrossRef]
- Zhou, H.-Z.; Li, Y.-D.; Liu, L.; Chen, X.-D.; Wang, W.-Q.; Ma, G.-W.; Su, Y.-C.; Qi, M.; Shi, B. Early osseointegration of implants with cortex-like TiO2 coatings formed by micro-arc oxidation: A histomorphometric study in rabbits. Acta Acad. Med. Wuhan 2017, 37, 122–130. [Google Scholar] [CrossRef]
- Choi, J.-Y.C.; Choi, C.; Yeo, I.-S. Spiral scanning imaging and quantitative calculation of the 3-dimensional screw-shaped bone-implant interface on micro-computed tomography. J. Periodontal Implant. Sci. 2018, 48, 202–212. [Google Scholar] [CrossRef]


| Group/Subgroups | Parameters | Right Side | Left Side | n | 95% CI for Mean Difference | r | t | p* | Df | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | L | U | |||||||
| Unoperated (CU + DU) | BV/TV | 33.31 | 4.45 | 32.98 | 3.74 | 6 | −1.06 | 1.72 | 0.962 | 0.605 | 0.57 | 5 |
| TbTh | 419.51 | 96.82 | 402.98 | 70.49 | 6 | −38.46 | 71.52 | 0.850 | 0.773 | 0.48 | 5 | |
| Control (Rt side = CA) (Lt side = CB) | BV/TV | 35.68 | 5.81 | 32.09 | 5.37 | 7 | 2.92 | 4.26 | 0.995 | 13.17 | 0.00 | 6 |
| TbTh | 436.83 | 101.57 | 382.05 | 86.26 | 7 | 24.15 | 85.41 | 0.951 | 4.38 | 0.01 | 6 | |
| BIC | 68.12 | 6.20 | 59.80 | 6.18 | 7 | 6.34 | 10.29 | 0.941 | 10.31 | 0.00 | 6 | |
| Diabetic (Rt side = DA) (Lt side = DB) | BV/TV | 31.65 | 4.25 | 28.89 | 4.16 | 7 | 1.70 | 3.82 | 0.963 | 6.38 | 0.00 | 6 |
| TbTh | 371.88 | 71.88 | 340.44 | 63.55 | 7 | 8.79 | 54.09 | 0.942 | 3.40 | 0.02 | 6 | |
| BIC | 66.68 | 5.42 | 56.75 | 7.15 | 7 | 3.99 | 15.87 | 0.506 | 4.09 | 0.01 | 6 | |
| Parameters | Groups | 95% CI for Mean Difference | t | p* | df | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CU | DU | ||||||||||
| M | SD | n | M | SD | n | L | U | ||||
| BV/TV | 34.15 | 3.85 | 6 | 32.31 | 4.10 | 6 | −3.28 | 6.95 | 0.798 | 0.443 | 10 |
| TbTh | 430.54 | 100.94 | 6 | 391.95 | 58.50 | 6 | −67.53 | 144.71 | 0.810 | 0.437 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah Alqahtani, N.; C. Chandramoorthy, H.; Shaik, S.; Syed, J.; Chowdhary, R.; Antony, L. Bone Marrow Mesenchymal Stromal Cells (BMMSCs) Augment Osteointegration of Dental Implants in Type 1 Diabetic Rabbits: An X-Ray Micro-Computed Tomographic Evaluation. Medicina 2020, 56, 148. https://doi.org/10.3390/medicina56040148
Abdullah Alqahtani N, C. Chandramoorthy H, Shaik S, Syed J, Chowdhary R, Antony L. Bone Marrow Mesenchymal Stromal Cells (BMMSCs) Augment Osteointegration of Dental Implants in Type 1 Diabetic Rabbits: An X-Ray Micro-Computed Tomographic Evaluation. Medicina. 2020; 56(4):148. https://doi.org/10.3390/medicina56040148
Chicago/Turabian StyleAbdullah Alqahtani, Nabeeh, Harish C. Chandramoorthy, Sharaz Shaik, Jamaluddin Syed, Ramesh Chowdhary, and Leoney Antony. 2020. "Bone Marrow Mesenchymal Stromal Cells (BMMSCs) Augment Osteointegration of Dental Implants in Type 1 Diabetic Rabbits: An X-Ray Micro-Computed Tomographic Evaluation" Medicina 56, no. 4: 148. https://doi.org/10.3390/medicina56040148





